Abstract
Objective:
To determine whether infants at higher risk of bronchopulmonary dysplasia (BPD) or death benefit more from vitamin A therapy than those at lower risk.
Study design:
Post-hoc reanalysis of a landmark phase-3 randomized controlled trial conducted from January 1996-July 1997 at 14 university-affiliated neonatal intensive care units in the United States. Data analysis performed October 2019-October 2020. Infants born weighing 401–1000 g and receiving respiratory support at 24 hours of age were assigned to intramuscular vitamin A 5000 IU or sham procedure 3 times weekly for 4 weeks. The primary outcome was BPD, defined as use of supplemental oxygen, or death at 36 weeks’ postmenstrual age. An externally validated model for predicting BPD or death was used to estimate the risk of these outcomes for each infant.
Results:
As previously reported, 222/405 (54.8%) infants assigned vitamin A therapy and 248/402 (61.7%) in the control group developed BPD or died (RR=0.89 [95% CI: 0.80–0.99]; RD=−6.9% [−13.0 - −0.7%]). Predicted individual risks of BPD or death ranged from 7.1 to 98.6% (median 61.5%; mean 60.9%). The effect of vitamin A therapy on BPD or death depended on infants’ risk of the primary outcome (p=0.03 for interaction): for example, RR=0.73 (RD=−14.5%) for infants with 25% predicted risk and RR=0.96 (RD=−1.0%) for infants with 75% risk. There was no difference in reduction of vitamin A deficiency across risk groups.
Conclusion:
Contrary to expectation, the effect of vitamin A therapy on bronchopulmonary dysplasia or death was greater for lower-risk than higher-risk infants.
Trial Registration ClinicalTrials.gov NCT01203488
Bronchopulmonary dysplasia (BPD) affects more than one-third of very-low-birth-weight infants1,2 and causes mortality, increased medical resource utilization, and impaired growth and neurodevelopment, with effects extending into adulthood.3–5 Of dozens of therapies developed to prevent BPD during the past 25 years,6 vitamin A therapy is among few to demonstrate both safety and effectiveness in several randomized controlled trials.7,8 However, vitamin A therapy has had variable uptake in clinical practice9 due to cost, potential pain from intramuscular injections, and a benefit perceived as modest (number needed to treat to benefit one patient [NNT] of 14–15) when applied to all trial-eligible infants.10–12
The NICHD Neonatal Research Network (NRN) trial was the largest and most prominent trial of vitamin A therapy. With 807 participants, it included more than twice as many participants as all other studies combined (n=358) and >5 times as many as the second-largest trial (n=154) in the most recent Cochrane Review.8 Although the trial was performed in 1996–1997, it provides the primary basis for the use of vitamin A therapy today.
Some researchers and clinicians have suggested that vitamin A therapy should be provided to only infants at particularly high risk of BPD or death.8,13 However, this approach has never been evaluated. Therefore, we undertook a reanalysis of the NICHD NRN Vitamin A Trial to test whether infants at higher risk of BPD or death benefit more than those at lower risk of these outcomes.
METHODS
We used data from the NICHD NRN Vitamin A Trial, conducted January 1996-July 1997 at 14 university-affiliated U.S. neonatal intensive care units and reported in June 1999.14 The study included 807 infants with birth weights of 401–1000 g receiving mechanical ventilation or supplemental oxygen 24 hours after birth. Infants with major congenital anomalies, congenital nonbacterial infections, terminal illness (pH<6.80 or bradycardia and hypoxia for >2 hours) or receiving vitamin A in parenteral nutrition greater than that contained in standard multivitamin formulations (>1500 IU/d) were excluded. Infants were randomized at 24–96 hours after birth to intramuscular vitamin A 5000 IU three times weekly for 4 weeks or sham procedure.
Randomization was stratified by center and birth weight group (401–750 g and 751–1000 g) and performed using randomization lists and sealed envelopes unblinded to a research pharmacist. The intervention and sham control were administered by a research nurse to maintain blinding of the care team. The study was approved by each participating center’s institutional review board and required parental consent.
To estimate risks of BPD or death for infants in the trial, we used the NICHD NRN risk prediction model with information available 24 hours after birth, when vitamin A therapy might be initiated.15 The polytomous logistic regression model estimates risks of death and of survival with no BPD, mild BPD, moderate BPD, and severe BPD by the 2001 NIH Consensus Definition.16 Risks of these outcomes sum to 100% for each infant. We estimated the risk of BPD or death as the sum of the predicted probabilities of death, moderate BPD, and severe BPD. Inclusion of death in risk prediction was necessary because death before 36 weeks’ postmenstrual age (PMA) precluded a diagnosis of BPD.17 The model was externally validated using data from the Prevention of Ventilator Induced Lung Injury Group (PreVILIG) study and the NICHD Surfactant Positive Airway Pressure and Pulse Oximetry Trial (SUPPORT).15,18 It is publicly available through an online calculator.19
Variables in the NICHD NRN risk prediction model include gestational age at birth, birth weight, infant sex and race/ethnicity, and ventilator mode and fraction of inspired oxygen (FiO2) 24 hours after birth. Gestational age at birth was determined using the best obstetric estimate, as used in deriving the prediction model.15 (This differs from Table I of the primary trial report, which reported gestational age estimated using the original Ballard examination.20,21) Race and ethnicity were obtained from medical records and defined as White, Black, or Hispanic (24 infants classified as “other” were included in the reference group, White, for risk prediction).15 Ventilator mode was defined as high-frequency ventilation, conventional mechanical ventilation, continuous positive airway pressure, and nasal cannula or oxygen hood. In the trial, no infants received high-flow nasal cannula or intermittent positive-pressure mechanical ventilation through a non-invasive interface.
Table I.
Infant characteristics by treatment group and predicted risk of bronchopulmonary dysplasia or death
| Risk of BPD or Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | All | ||||||
| Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | |
| Infant Characteristic | n=105 | n=96 | n=97 | n=105 | n=104 | n=98 | n=99 | n=103 | n=405 | n=402 |
| Birth weight (grams)—mean (SD) | 911 (66) | 911 (70) | 809 (84) | 810 (104) | 728 (102) | 723 (115) | 627 (86) | 640 (87) | 770 (135) | 769 (138) |
| Gestational age at birth (weeks)— mean (SD) | 27.6 (1.7) | 27.5 (1.6) | 26.1 (1.0) | 26.3 (1.2) | 25.5 (1.3) | 25.4 (1.2) | 24.1 (1.2) | 24 (1.2) | 25.8 (1.9) | 25.8 (1.8) |
| Female sex—n (%) | 65 (61.9) | 63 (65.6) | 51 (52.6) | 58 (55.2) | 46 (44.2) | 49 (50) | 41 (41.4) | 43 (41.7) | 203 (50.1) | 213 (53.0) |
| Race/ethnicity—n (%) | ||||||||||
| Black | 58 (55.2) | 55 (57.3) | 45 (46.4) | 41 (39.0) | 44 (42.3) | 41 (41.8) | 51 (51.5) | 52 (50.5) | 198 (48.9) | 189 (47.0) |
| White | 20 (19.0) | 21 (21.9) | 39 (40.2) | 39 (37.1) | 40 (38.5) | 45 (45.9) | 34 (34.3) | 32 (31.1) | 133 (32.8) | 137 (34.1) |
| Hispanic | 25 (23.8) | 18 (18.8) | 11 (11.3) | 18 (17.1) | 16 (15.4) | 10 (10.2) | 12 (12.1) | 16 (15.5) | 64 (15.8) | 62 (15.4) |
| Other | 2 (1.9) | 2 (2.1) | 2 (2.1) | 7 (6.7) | 4 (3.8) | 2 (2.0) | 2 (2.0) | 3 (2.9) | 10 (2.5) | 14 (3.5) |
| Small for gestational agea—n (%) | 16(15.2) | 16(16.7) | 9(9.3) | 13(12.4) | 14(13.5) | 18(18.4) | 11(11.1) | 7(6.8) | 50 (12.3) | 54 (13.4) |
| Antenatal corticosteroids—n (%) | 76/104 (73.1) | 69 (71.9) | 75 (77.3) | 83 (79.0) | 88 (84.6) | 75 (76.5) | 68 (68.7) | 71 (68.9) | 307/404 (76.0) | 298 (74.1) |
| Surfactant—n (%) | 73 (69.5) | 71 (74.0) | 84 (86.6) | 84 (80.0) | 90 (86.5) | 84 (85.7) | 90 (90.9) | 90 (87.4) | 337 (83.2) | 329 (81.8) |
| Apgar score ≤3—n (%) | ||||||||||
| At 1 min† | 34/104 (32.7) | 19 (19.8) | 37/96 (38.5) | 36/103 (35.0) | 52/102 (51.0) | 40/97 (41.2) | 53/98 (54.1) | 53/102 (52.0) | 176/400 (44.0) | 148/398 (37.2) |
| At 5 min | 3/104 (2.9) | 2 (2.1) | 7/96 (7.3) | 6/104 (5.8) | 15/102 (14.7) | 12/97 (12.4) | 16/98 (16.3) | 10/102 (9.8) | 41/400 (10.3) | 30/399 (7.5) |
| Intubation—n (%) | 82 (78.1) | 77 (80.2) | 91 (93.8) | 94 (89.5) | 97 (93.3) | 91 (92.9) | 94 (94.9) | 101 (98.1) | 364 (89.9) | 363 (90.3) |
| Chest compressions—n (%) | 8 (7.6) | 4 (4.2) | 15 (15.5) | 17 (16.2) | 18 (17.3) | 14 (14.3) | 21 (21.2) | 9 (8.7) | 62 (15.3) | 44 (10.9) |
| Resuscitation medicationsb—n (%) | 7 (6.7) | 8 (8.3) | 9 (9.3) | 12 (11.4) | 14 (13.5) | 8 (8.2) | 12 (12.1) | 12 (11.7) | 42 (10.4) | 40 (10.0) |
| FiO2 at 24 h of age—mean (SD) | 0.33 (0.10) | 0.34 (0.12) | 0.34 (0.11) | 0.36 (0.11) | 0.41 (0.16) | 0.40 (0.18) | 0.54 (0.25) | 0.52 (0.25) | 0.41(0.19) | 0.41 (0.19) |
| Ventilation at 24 h of age—n (%) | ||||||||||
| HFV | 0 (0) | 0 (0) | 1 (1.0) | 4 (3.8) | 16 (15.4) | 12 (12.2) | 20 (20.2) | 22 (21.4) | 37 (9.1) | 38 (9.5) |
| CMV | 78 (74.3) | 74 (77.1) | 89 (91.8) | 95 (90.5) | 88 (84.6) | 83 (84.7) | 77 (77.8) | 77 (74.8) | 332 (82.0) | 329 (81.8) |
| CPAPc | 14 (13.3) | 12 (12.5) | 4 (4.1) | 2 (1.9) | 0 (0) | 2 (2.0) | 0 (0) | 3 (2.9) | 18 (4.4) | 19 (4.7) |
| NC/Hood | 13 (12.4) | 10 (10.4) | 3 (3.1) | 4 (3.8) | 0 (0) | 1 (1.0) | 2 (2.0) | 1 (1.0) | 18 (4.4) | 16 (4.0) |
| Pre-treatment serum retinol (μg/dL)— mean (SD) | 15.1 (6.1) | 16.4 (6.4) | 16.2 (5.4) | 16.6 (6.1) | 16.7 (6.8) | 15.6 (6.3) | 17.6 (6.7) | 16.1 (5.8) | 16.2 (6.2) | 16.2 (6.2) |
| Time from birth to first treatment (h)— mean (SD) | 61 (20) | 60 (19) | 65 (21) | 63 (20) | 65 (21) | 65 (20) | 67 (19) | 67 (19) | 64 (20) | 64 (20) |
Defined as birth weight <10th percentile by gestational age (by best obstetrical estimate) and sex on the Alexander growth charts,36 consistent with a prior analysis of this dataset.31
Includes inotropes, sodium bicarbonate, calcium gluconate, calcium chloride, atropine, naloxone, and volume expanders.
Includes 7 infants who received continuous positive airway pressure (CPAP) through endotracheal tube; all others received non-invasive CPAP.
Significantly different (p<0.05) between vitamin A therapy and control groups.
All characteristics were significantly different by risk group (p<0.05) except resuscitation medications and pre-treatment serum retinol.
Quartile 1: ≤44.6%; Quartile 2: 44.7–61.5%; Quartile 3: 61.6–79.1%; Quartile 4: >79.1%. BPD = bronchopulmonary dysplasia; SD = standard deviation; FiO2 = fraction of inspired oxygen; HFV=high frequency ventilation; CMV = conventional mechanical ventilation; NC = nasal cannula
The primary outcome was the composite of BPD or death before 36 weeks’ PMA. BPD (“chronic lung disease” in the trial report) was defined as requiring oxygen at 36 weeks’ PMA. We evaluated the effect of vitamin A therapy on secondary outcomes including ventilatory support and respiratory medications at 36 weeks’ PMA and timing and cause of death. Information was available on duration of systemic corticosteroid exposure before 36 weeks’ PMA but not corticosteroid type or dosage.
Serum retinol concentrations were available for the first 300 infants to reach study day 28. Measurements were made before the first treatment and on study day 28 (2–3 days after last treatment). Because medications (e.g., glucocorticoids) influence retinol distribution between tissue and plasma, serum retinol concentration may not represent actual body stores.22 Therefore, relative dose-response to vitamin A injection was calculated on study day 28 by measuring change in serum retinol 3 hours after administering vitamin A 2000 IU per kg of body weight. It was calculated as (change in serum retinol pre- to post-injection) / (pre-injection serum retinol). Relative dose-response >10% indicated vitamin A deficiency.14
Statistical analyses
We estimated the risk of BPD or death for each infant at 24 postnatal hours using the NICHD NRN model. To describe the distribution of risk in the trial population, we calculated the extreme quartile risk ratio (calculated as [mean risk in the highest quartile of risk] / [mean risk in the lowest quartile of risk]) and median:mean risk ratio (calculated as [median predicted risk] / [mean predicted risk]).23,24 We evaluated model performance in our trial cohort by estimating the c-statistic (a measure of model discrimination) and comparing observed versus model-predicted outcomes in aggregate and across risk groups (to measure model calibration). To determine the relevance of this approach to contemporary clinical practice, we also tested the model in a cohort of infants born 2016–2018 in centers participating in the NICHD NRN (detailed in the Appendix; available at www.jpeds.com).
For the primary analysis, to determine whether the relative effect (relative risk) of vitamin A therapy on the primary outcome of BPD or death varied by predicted risk of BPD or death, we used a log-binomial model with an interaction term for risk (continuous variable) and treatment assignment. To estimate how the absolute effect (risk difference) varied for infants with different risks of the primary outcome, we used the equivalent linear-binomial model.
For analyses of secondary outcomes, the effect of vitamin A therapy on secondary outcomes and interaction with the predicted risk of BPD or death was assessed on a relative scale. No corrections were made for multiple comparisons. Additionally, we described the sensitivity of the original trial findings to the inclusion of infants at high risk of BPD or death.
Our results were reported consistent with the Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement.25 Data were analyzed using SAS Enterprise Guide version 7.15. Statistical tests were two-sided. P-values <0.05 were considered statistically significant. Data analysis was performed October 2019-October 2020.
RESULTS
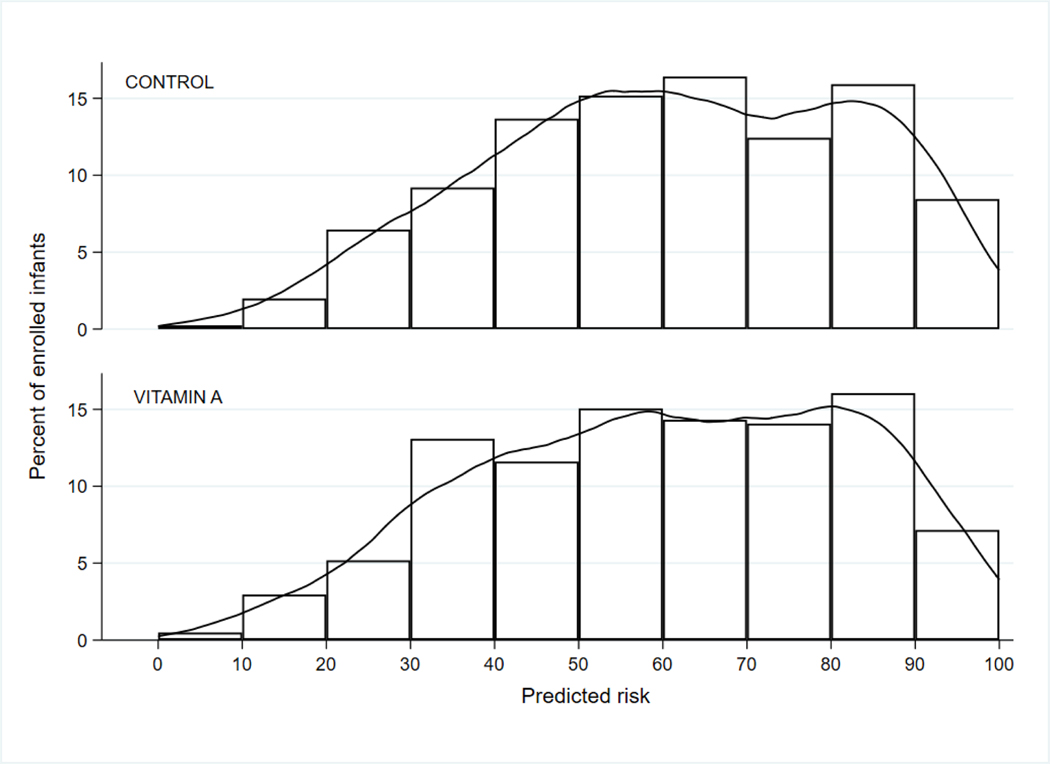
Of 1250 eligible infants, 807 were randomized (405 to vitamin A therapy, 402 to sham control) and all of these were included in the analysis (Figure 1; available at www.jpeds.com). Infant risks of BPD or death ranged from 7.1% to 98.6% with a median risk of 61.5% (interquartile range: 44.6–79.1%). Infants in the highest risk quartile had a nearly threefold greater risk of the primary outcome than infants in the lowest quartile (extreme quartile risk ratio=2.75). Mean risk was 60.9% (median:mean risk ratio=1.01), indicating that predicted risks of BPD or death were symmetrically distributed among trial participants (Figure 2; available at www.jpeds.com).
Figure 1.
CONSORT flow diagram
Figure 2.
Predicted risks of bronchopulmonary dysplasia or death by treatment arm
Predicted risks of bronchopulmonary dysplasia (BPD) or death for randomized infants ranged from 7.1% to 98.6% with a median of 61.5% (interquartile range: 44.6–79.1%) and mean of 60.9%.
Infant characteristics by model-predicted risk of BPD or death and treatment assignment are shown in Table I. Compared with infants at lower risk of BPD or death, infants at higher risk had smaller birth weights, were born earlier in gestation, and received more invasive respiratory support and FiO2, as expected from the risk-prediction model. Infants at higher risk also had lower Apgar scores and were more likely to have received surfactant, intubation, and chest compressions. There were no differences in baseline serum retinol by risk category.
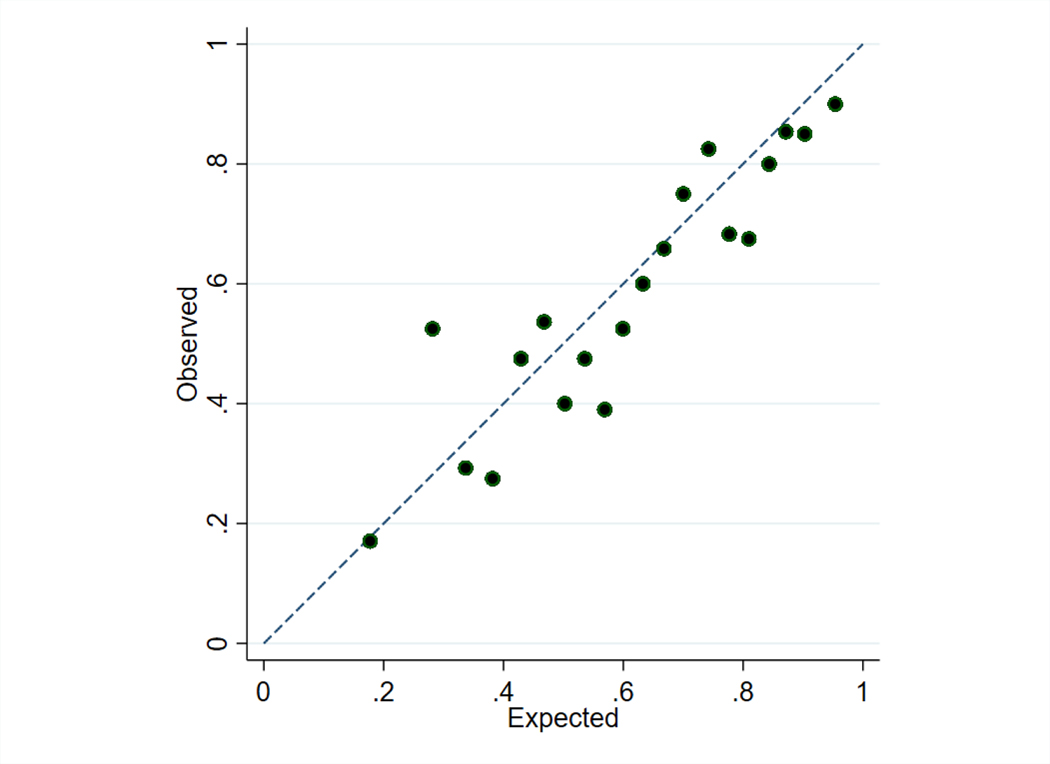
The externally developed model of BPD or death had a c-statistic of 0.72 (95% confidence interval [CI]: 0.69–0.76) in the trial cohort. This compares with c-statistics of 0.79 in the model derivation cohort (for prediction at 24 hours after birth), 0.72 in the SUPPORT validation cohort (at 24 hours after birth), and 0.74 in the PreVILIG validation cohort (at 72 hours after birth).15,18 The ratio of expected (i.e., predicted) to observed outcomes was 1.05 (Figure 3; available at www.jpeds.com). In a more recent (2016–2018) cohort of trial-eligible infants in which 31.2% of eligible infants received vitamin A therapy, the model had similar performance with a c-statistic of 0.80 (95% CI: 0.78–0.82) and ratio of expected to observed outcomes of 0.97 (online supplement).
Figure 3.
Risk model calibration plot
Calibration shown for infants by demi-decile of predicted risk of bronchopulmonary dysplasia or death. The dashed line provides a reference for perfect correspondence between expected (predicted risk) and observed outcomes. The ratio of expected to observed outcomes was 1.05 (slope: 0.81, intercept: −0.14).
As previously reported, of 807 infants enrolled in the NICHD NRN Vitamin A Trial, 222/405 (54.8%) infants assigned vitamin A therapy and 248/402 (61.7%) infants in the control group developed BPD or died (RR=0.89 [95% CI: 0.80–0.99] and RD=−6.9% [95% CI: −13.0 - −0.7%]).14
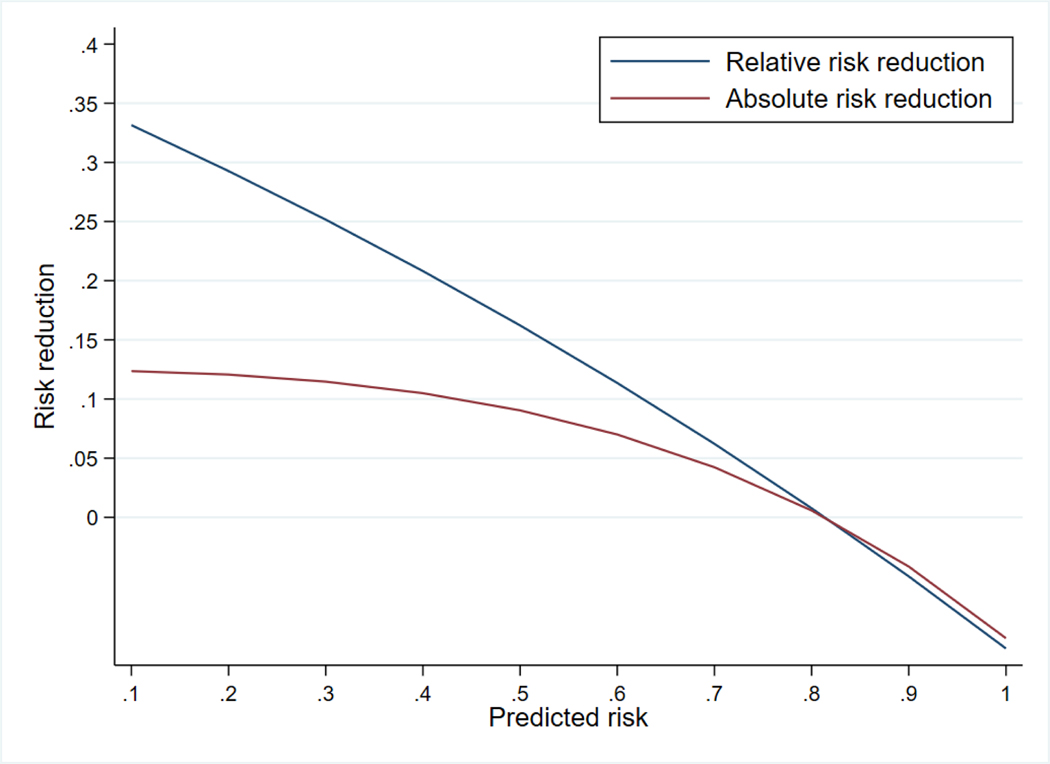
The effect of vitamin A therapy on BPD or death depended on infants’ risk of the primary outcome (p=0.03 for interaction). The effect of vitamin A was inversely related to baseline risk of BPD or death: e.g., RR=0.73 (RD=−14.5%) for infants with 25% predicted risk and RR=0.96 (RD=−1.0%) for infants with 75% risk (Table II and Figures 4 and 5; available at www.jpeds.com).
Table II.
Rates of bronchopulmonary dysplasia or death by quartile of risk
| BPD or death | ||||
|---|---|---|---|---|
|
| ||||
| Predicted probability of BPD or death | Vitamin A n (%) | Control n (%) | Relative Risk (95% CI) | Risk Difference % (95% CI) |
| Quartile 1 | 30/105 (28.5) | 39/96 (40.6) | 0.69 (0.47 – 1.00) | −12.8 (−25.7 – 0.0) |
| Quartile 2 | 39/97 (40.2) | 56/105 (53.3) | 0.84 (0.64 – 1.10) | −8.2 (−20.9 – 4.6) |
| Quartile 3 | 71/104 (68.3) | 70/98 (71.4) | 0.96 (0.81 – 1.15) | −2.7 (−14.9 – 9.4) |
| Quartile 4 | 82/99 (82.8) | 83/103 (80.6) | 1.04 (0.91 – 1.18) | 3.2 (−7.5 – 13.9) |
| All | 222/405 (54.8) | 248/402 (61.7) | 0.89 (0.80 – 0.99) | −6.9 (−13.0 - −0.7) |
|
| ||||
| Death | ||||
|
| ||||
| Predicted probability of BPD or death | Vitamin A n (%) | Control n (%) | Relative Risk (95% CI) | Risk Difference % (95% CI) |
|
| ||||
| Quartile 1 | 3/105 (2.9) | 5/96 (5.2) | 0.56 (0.13 – 2.33) | −2.3 (−7.8 – 3.3) |
| Quartile 2 | 5/97 (5.2) | 6/105 (5.7) | 0.90 (0.26 – 3.20) | −0.5 (−6.9 – 5.9) |
| Quartile 3 | 16/104 (15.4) | 16/98 (16.3) | 0.82 (0.45 – 1.52) | −3.3 (−14.1 – 7.5) |
| Quartile 4 | 35/99 (35.4) | 28/103 (27.2) | 1.26 (0.83 – 1.91) | 7.6 (−5.9 – 21.0) |
| All | 59/405 (14.6) | 55/402 (13.7) | 1.07 (0.77 – 1.48) | 0.9 (−3.8 – 5.6) |
|
| ||||
| BPD among survivors at 36 weeks’ PMA | ||||
|
| ||||
| Predicted probability of BPD or death | Vitamin A n (%) | Control n (%) | Relative Risk (95% CI) | Risk Difference % (95% CI) |
|
| ||||
| Quartile 1 | 27/102 (26.5) | 34/91 (37.4) | 0.70 (0.46 – 1.05) | −11.4 (−24.4 – 1.6) |
| Quartile 2 | 34/92 (37.0) | 50/99 (50.5) | 0.85 (0.64 – 1.14) | −6.9 (−19.7 – 5.8) |
| Quartile 3 | 55/88 (62.5) | 54/82 (65.8) | 0.94 (0.74 – 1.19) | −3.8 (−18.3 – 10.8) |
| Quartile 4 | 47/64 (73.4) | 55/75 (73.3) | 1.04 (0.84 – 1.29) | 3.2 (−12.5 – 18.8) |
| All | 163/346 (47.1) | 193/347 (55.6) | 0.86 (0.75 – 0.98) | −7.9 (−14.7 - −1.1) |
The relative risk and risk difference calculations accounted for the variables used for stratified randomization (center and birth weight strata [401–750 vs 751–1000 g]) with the Mantel-Haenszel procedure consistent with the original trial manuscript.14 Analyses of the components of the primary outcome accounting for stratified randomization were not shown in the original trial manuscript but are shown here.
BPD=bronchopulmonary dysplasia; PMA=postmenstrual age; CI=confidence interval
Figure 4.
Estimated effect of vitamin A therapy by continuous risk of bronchopulmonary dysplasia or death
The effect of vitamin A therapy varied by infants’ predicted risk of bronchopulmonary dysplasia or death. Relative risk reduction was calculated as 1-relative risk. Relative risks were estimated using a log-binomial model that included an interaction term for predicted risk (continuous) and treatment assignment (binary). Absolute risks were estimated using a linear-binomial model in the same form.
Figure 5.
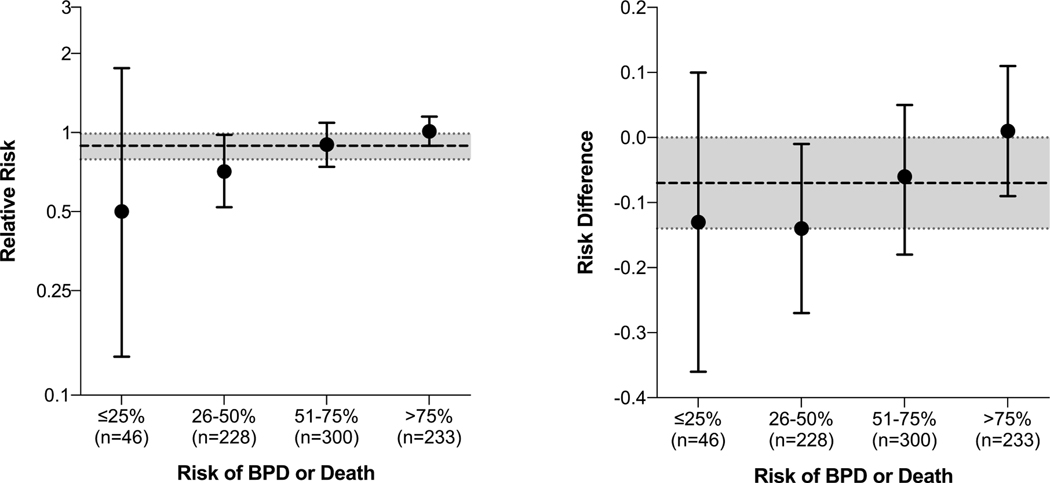
Observed effect of vitamin A therapy by risk of bronchopulmonary dysplasia or death subgroup
Circles and bars represent point estimates and 95% confidence intervals, respectively. Categories shown are defined by 25% increments of risk (rather than quartiles, as shown elsewhere in the manuscript, including Table 2). The dashed and dotted lines represent the overall estimates of effect across all risk groups, as reported in the primary report of the trial. A formal test of interaction, using risk of bronchopulmonary dysplasia (BPD) or death as a continuous variable (not categorized, as shown here), showed that the effect of vitamin A therapy depended upon infants’ risk of BPD or death (p=0.03 for interaction on the relative risk scale; p=0.04 for interaction on the risk difference scale).
When the components of the primary outcome were considered separately, there was no effect of vitamin A therapy on the rate of death regardless of predicted risk (RR=1.07 [95% CI: 0.77–1.48]). Vitamin A therapy reduced BPD among survivors (RR=0.86 [95% CI: 0.75–0.98]) with a greater effect among infants at low risk than high risk of BPD or death (p=0.01 for interaction).
For secondary outcomes, there were no differences in post-randomization risk factors for BPD, including late-onset sepsis, surgical necrotizing enterocolitis, surgical closure of the ductus arteriosus, and evidence lung injury (e.g., air leak, pulmonary hemorrhage), between infants randomized to vitamin A versus control. Vitamin A deficiency at 28 days post-randomization was less frequent among infants randomized to vitamin A regardless of risk group (Table III). Risk of BPD or death did not modify the effect of therapy on vitamin A deficiency.
Table III.
Secondary outcomes
| Risk of BPD or Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | All | ||||||
| Outcome | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control |
| Risk factors for BPD | n=105 | n=96 | n=97 | n=105 | n=104 | n=98 | n=99 | n=103 | n=405 | n=402 |
| Late-onset sepsisa | 23 (21.9) | 34 (35.4) | 29 (29.9) | 39 (37.1) | 53 (51.0) | 46 (46.9) | 47 (48.0) | 50 (48.5) | 152 (37.6) | 169 (42.0) |
| Surgical NEC | 2 (1.9) | 4 (4.2) | 5 (5.2) | 5 (4.8) | 13 (12.6) | 9 (9.2) | 7 (7.1) | 11 (10.7) | 27 (6.7) | 29 (7.2) |
| Surgical PDA closure | 3 (2.9) | 6 (6.3) | 9 (9.3) | 8 (7.6) | 16 (15.4) | 17 (17.3) | 6 (6.1) | 20 (19.4) | 34 (8.4) | 51 (12.7) |
| Signs of lung injury | 17 (16.2) | 10 (10.4) | 26 (26.8) | 24 (22.9) | 27 (26.0) | 32 (32.7) | 51 (51.5) | 40 (38.8) | 121 (29.9) | 106 (26.4) |
| Pulmonary air leakb | 4 (3.8) | 5 (5.2) | 6 (6.2) | 9 (8.6) | 7 (6.7) | 15 (15.3) | 16 (16.2) | 11 (10.7) | 33 (8.1) | 40 (10.0) |
| Pulmonary hemorrhage | 8 (7.6) | 4 (4.2) | 6 (6.2) | 7 (6.7) | 10 (9.6) | 5 (5.1) | 16 (16.2) | 15 (14.6) | 40 (9.9) | 31 (7.7) |
| PIE | 6 (5.7) | 6 (6.3) | 22 (22.7) | 14 (13.3) | 17 (16.3) | 25 (25.5) | 42 (42.4) | 26 (25.2) | 87 (21.5) | 71 (17.7) |
| Days on mechanical ventilation (d)—mean (SD)c | 13 (13), n=88 | 15 (13), n=76 | 26 (14), n=82 | 23 (15), n=88 | 36 (18), n=81 | 32 (15), n=71 | 42 (20), n=57 | 44 (18), n=66 | 28 (20), n=308 | 28 (18), n=301 |
| Days of postnatal systemic corticosteroids (d)—mean (SD)d | 14 (13), n=26 | 21 (17), n=23 | 23 (17), n=60 | 22 (21), n=53 | 27 (20), n=63 | 25 (12), n=63 | 26 (15), n=58 | 30 (19), n=67 | 24 (18), n=207 | 26 (18), n=206 |
| Vitamin A Status e | ||||||||||
| Serum retinol (μg/dl)— median, 5th-90th %ile† | 22.2 (11.6, 65.5), n=48 | 15.9 (7.7, 35.9), n=49 | 35.3 (15.5, 73.4), n=35 | 17.4 (7.1, 73.6), n=36 | 35.6 (11.8, 81.6), n=38 | 19.5 (7.7, 74.6), n=34 | 43.7 (16.9, 101.9), n=34 | 22.7 (5.6, 58.8), n=26 | 29.8 (12.9, 83.7), n=155 | 17.8 (7.4, 63.1), n=145 |
| Relative dose–response >10% —n (%)† | 11 (23.4), n=47 | 26 (54.2), n=48 | 9 (25.7), n=35 | 16 (45.7), n=35 | 8 (21.6), n=37 | 13 (38.2), n=34 | 6 (18.2), n=33 | 10 (38.5), n=26 | 34 (22.4), n=152 | 65 (45.5), n=143 |
| Respiratory Status at 36 wk d | n=102 | n=91 | n=92 | n=99 | n=88 | n=82 | n=64 | n=75 | n=346 | n=347 |
| Respiratory support—n (%) | ||||||||||
| HFV | 0(0) | 0 (0) | 0 (0) | 1 (1.0) | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 1 (0.3) | 1 (0.3) |
| CMV | 2 (2.0) | 1 (1.1) | 6 (6.5) | 6 (6.1) | 6 (6.8) | 5 (6.1) | 9 (14.1) | 9 (12.0) | 23 (6.6) | 21 (6.1) |
| CPAPf | 3 (2.9) | 2 (2.2) | 7 (7.6) | 2 (2.0) | 7 (8.0) | 7 (8.5) | 5 (7.8) | 6 (8.0) | 22 (6.4) | 17 (4.9) |
| NC/Hood†‡ | 24 (23.5) | 35 (38.5) | 30 (32.6) | 43 (43.4) | 50 (56.8) | 46 (56.1) | 36 (56.3) | 41 (54.7) | 140 (40.5) | 165 (47.6) |
| None | 73 (71.6) | 53 (58.2) | 49 (53.3) | 47 (47.5) | 25 (28.4) | 24 (29.3) | 13 (20.3) | 19 (25.3) | 160 (46.2) | 143 (41.2) |
| FiO2—mean (SD)g | 0.51 (0.32) | 0.63 (0.35) | 0.56 (0.34) | 0.54 (0.33) | 0.56 (0.34) | 0.55 (0.33) | 0.59 (0.32) | 0.53 (0.32) | 0.56 (0.33) | 0.56 (0.33) |
| Nasal cannula flow (ml/min)—mean (SD) h,† | 350 (386) | 337 (394) | 195 (262) | 277 (296) | 300 (364) | 447 (471) | 268 (322) | 426 (408) | 278 (336) | 375 (400) |
| Mean airway pressure (cmH20)—mean (SD)i | 7.0 (0) | 8.0 (N/A) | 7.5 (1.8) | 7.9 (1.5) | 7.2 (1.5) | 9.0 (2.0) | 8.0 (2.4) | 6.2 (1.8) | 7.6 (1.9) | 7.5 (2.0) |
| Tracheostomy—n (%) | 0 (0) | 1 (1.1) | 2 (2.2) | 1 (1.0) | 2 (2.3) | 1 (1.2) | 1 (1.6) | 0 (0) | 5 (1.4) | 3 (0.9) |
| Corticosteroids—n (%) | 3 (2.9) | 7 (7.7) | 10 (10.9) | 9 (9.1) | 16 (18.2) | 5 (6.1) | 13 (20.3) | 17 (22.7) | 42 (12.1) | 38 (11.0) |
| Bronchodilators—n (%) | 10 (9.8) | 7 (7.7) | 30 (32.6) | 25 (25.3) | 24 (27.6) | 20 (24.4) | 19 (29.7) | 27 (36.0) | 83 (24.1) | 79 (22.8) |
| Diuretics—n (%) | 18 (17.6) | 17 (18.7) | 25 (27.2) | 30 (30.3) | 28 (32.2) | 23 (28.0) | 31 (48.4) | 40 (53.3) | 102 (29.6) | 110 (31.7) |
Defined as positive blood culture >72 hours after birth
Included pneumothorax, pneumopericardium, and pneumomediastinum
Mechanical ventilation includes conventional mechanical ventilation (CMV) and high-frequency ventilation (HFV) and was measured among survivors to 36 weeks’ postmenstrual age
Measured among survivors to 36 weeks’ postmenstrual age
Serum retinol level was measured for the first 300 infants to reach study day 28. Of these, 295 had complete information available to calculate a relative dose-response.
Included 2 infants who received continuous positive airway pressure (CPAP) through endotracheal tube; all others received non-invasive CPAP.
Among infants receiving any respiratory support at 36 weeks’ PMA.
Among infants on nasal cannula (NC). Reported flow rates did not exceed 2 liters per minute (2000 ml per minute). Data were not available for 15 infants.
Among infants on mechanical ventilation. Data were not available for infants receiving CPAP.
Significantly different (p<0.05) between vitamin A therapy and control groups.
Significant (p<0.05) interaction of predicted risk of bronchopulmonary dysplasia (BPD) or death on association with vitamin A therapy.
BPD=bronchopulmonary dysplasia; PDA=patent ductus arteriosus; NEC=necrotizing enterocolitis; PIE=pulmonary interstitial emphysema; FiO2 = fraction of inspired oxygen; SD = standard deviation
Among infants surviving to 36 weeks’ PMA, vitamin A therapy was associated with decreased use of nasal cannula or oxygen hood but not other respiratory modalities; the difference was greater among infants at lower risk of BPD or death (Table III). Vitamin A therapy was associated with decreased flow rates among infants using nasal cannula. There were no differences in FiO2 among infants on respiratory support or in respiratory medications use at 36 weeks’ PMA among infants who survived. There were no differences between the vitamin A and control group in the timing or causes of death among infants who died (Table IV; available at www.jpeds.com).
Table IV.
Causes and timing of deaths
| Risk of BPD or Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | All | ||||||
| Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | Vitamin A | Control | |
| n=3 | n=5 | n=5 | n=6 | n=16 | n=16 | n=35 | n=28 | n=59 | n=55 | |
| Cause of death | ||||||||||
| Respiratory insufficiency | 0 (0) | 0 (0) | 1 (20.0) | 1 (16.7) | 3 (18.8) | 6 (37.5) | 17 (48.6) | 16 (57.1) | 21 (35.6) | 23 (41.8) |
| Before 28 postnatal days | 0 (0) | 0 (0) | 1 (20.0) | 1 (16.7) | 2 (12.5) | 6 (37.5) | 14 (40.0) | 16 (57.1) | 17 (28.8) | 23 (41.8) |
| After 28 postnatal days | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) | 0 (0) | 3 (8.6) | 0 (0) | 4 (6.8) | 0 (0) |
| Infection | 1 (33.3) | 0 (0) | 1 (20.0) | 1 (16.7) | 3 (18.8) | 4 (25.0) | 7 (20.0) | 4 (14.3) | 12 (20.3) | 9 (16.4) |
| Necrotizing enterocolitis | 0 (0) | 5 (100) | 2 (40.0) | 4 (66.7) | 4 (25.0) | 5 (31.3) | 5 (14.3) | 5 (17.9) | 11 (18.6) | 19 (34.5) |
| Intracranial bleeding | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 3 (18.8) | 0 (0) | 3 (8.6) | 2 (7.1) | 7 (11.9) | 2 (3.6) |
| Othera | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 3 (18.8) | 1 (6.3) | 3 (8.6) | 1 (3.6) | 8 (13.6) | 2 (3.6) |
| Timing of death (d)—mean (SD)a | 30 (19) | 28 (11) | 21 (17) | 31 (23) | 28 (17) | 18 (7) | 17 (13) | 17 (10) | 21 (15) | 20 (12) |
Includes infants who died of cardiac tamponade (n=2), pulmonary hemorrhage (n=1), pleural effusion (n=1), coagulopathy (n=1), and with unknown cause of death (n=5).
There were no significant differences (p<0.05) between vitamin A therapy and control group in cause of death or timing of death. Mean (SD) time of death from intracranial bleeding in the Vitamin A group was 16 (SD 8) days. Deaths from intracranial bleeding in the control group took place at 8 and 12 days.
Sensitivity of trial results to inclusion of high-risk infants
Table V shows the effect of vitamin A therapy observed if the trial had enrolled only infants with predicted risk of BPD or death less than specific thresholds. The observed effect of vitamin A therapy increased with the exclusion of infants at highest risk of BPD or death.
Table V.
Sensitivity of trial findings to exclusion of high-risk infants
| Observed Rate of BPD or Death | ||||||
|---|---|---|---|---|---|---|
| Risk of BPD or Death (%) | Proportion of Cohort Treated n (%) | Vitamin A n/N (%) | Control n/N (%) | Relative Risk (95% CI) | Risk Difference % (95% CI) | NNT |
| ≤100 | 807 (100) | 222/405 (54.8) | 248/402 (61.7) | 0.89 (0.80 – 0.99) | −6.9 (−13.0 - −0.72) | 14–15 |
| ≤90 | 744 (92.2) | 194/376 (51.6) | 220/368 (59.8) | 0.87 (0.78 – 0.98) | −7.6 (−14.0 - −1.1) | 13–14 |
| ≤80 | 615 (76.2) | 142/311 (45.7) | 168/304 (55.3) | 0.85 (0.74 – 0.98) | −8.1 (−15.4 - −0.8) | 12–13 |
| ≤70 | 508 (62.9) | 99/254 (39.0) | 132/254 (52.0) | 0.78 (0.65 – 0.93) | −11.4 (−19.5 - −3.4) | 8–9 |
| ≤60 | 384 (47.6) | 65/196 (33.2) | 87/188 (46.3) | 0.76 (0.60 – 0.96) | −10.8 (−20.1 - −1.6) | 9–10 |
| ≤50 | 262 (32.5) | 42/135 (31.1) | 57/127 (44.9) | 0.67 (0.49 – 0.92) | −14.6 (−25.9 - −3.2) | 6–7 |
Rates of the primary outcome are shown for infants with model-predicted risks of bronchopulmonary dysplasia (BPD) or death less than or equal to those specified in the first column. Comparisons including ≥75 infants per treatment arm are shown. The analysis accounted for variables used for stratified randomization (center and birth weight strata [401–750 vs 751–1000 g]) with the Mantel-Haenszel procedure consistent with the original trial manuscript.14
CI=confidence interval; NNT=Number needed to treat
DISCUSSION
In the NICHD NRN Vitamin A Trial, we found that the effect of vitamin A therapy on BPD or death was greater in infants at lower risk of BPD or death than in those at higher risk, in both relative (relative risk) and absolute (risk difference) terms. Our findings do not support targeting vitamin A therapy to only particularly high-risk infants.
As previously reported, vitamin A therapy affected the rate of BPD, defined as using supplemental oxygen at 36 weeks’ PMA, but not death.14 Notably, vitamin A therapy was associated with decreased use of oxygen by nasal cannula or hood at 36 weeks’ PMA but not other respiratory support or medications.
Our findings have important implications for both the clinical use of vitamin A therapy and the design of studies related to BPD. BPD and death have multiple etiologies.26 A single intervention may not affect all factors causing either of these outcomes. Because vitamin A therapy resulted in similar decreases in vitamin A deficiency for all infants regardless of their risk of BPD or death, we speculate that the proportion of BPD related to vitamin A deficiency may be greatest in lower-risk infants. That is, higher-risk infants developed BPD or died due to a preponderance of other risk factors for these outcomes (e.g., late-onset sepsis, surgical necrotizing enterocolitis, surgical ligation of the ductus arteriosus).27 Similar observations have been made in other areas of medicine. For example, the contribution of patent foramen ovale to cryptogenic stroke is greatest among individuals at lowest risk for stroke (e.g., young people, non-smokers).28 We observed a substantially greater effect of vitamin A therapy when the highest-risk infants, who developed BPD or died regardless of therapy, were excluded from the analysis.
Strengths of our study include the use of data from the largest clinical trial of vitamin A therapy in very-low-birth-weight infants. As a randomized trial, its results are less subject to confounding than those of observational studies on vitamin A.9 Moreover, we used an externally developed and widely available risk-prediction model validated in multiple datasets and that continues to perform well in a recent cohort. Additionally, our approach based on the PATH Statement provides important information about heterogeneity of the effect of vitamin A therapy while overcoming several limitations of conventional “one-variable-at-a-time” subgroup analyses.25 Limitations of conventional subgroup analyses include that patients have multiple attributes and belong to several subgroups or “reference classes,” a multiplicity of potential statistical comparisons by individual risk factors, and reduced statistical power to detect meaningful treatment effect heterogeneity where it exists.29,30 Previous “one-variable-at-a-time” subgroup analyses of vitamin A therapy by small-for-gestational-age status and infant birth weight group (401–750 g versus 751–1000 g) had lower power to detect differences in effect if they existed.14,31
Our study has important limitations. First, the NICHD NRN Vitamin A Trial was conducted in 1996–1997. Although this period followed introduction of routine antenatal corticosteroid and surfactant therapies, other aspects of clinical practice have since changed. Although rates of BPD did not decrease during the 2000s or 2010s,1,2 causes of BPD and death may differ in more recent populations of preterm infants. Second, we used the primary outcome of the trial, which included supplemental oxygen use at 36 weeks’ PMA, as our primary outcome, although alternative definitions of lung disease may better correlate with later respiratory sequelae of premature birth.32 Third, our study focused on respiratory outcomes and death and did not assess other potential effects of vitamin A therapy. Vitamin A may affect other organs such as the eyes and cardiovascular system.22,33 It is unclear whether the effects observed from vitamin A supplementation are secondary to correction or prevention of vitamin A deficiency versus a separate pharmacologic effect of vitamin A on developing organs. Fourth, this trial was limited to a specific course of intramuscular injections of vitamin A. Alternative doses or methods of administering vitamin A (e.g., enteral supplementation), if proven effective, may modify the balance of burden and benefit for this therapy.34,35
In conclusion, we found that the effect of vitamin A therapy to prevent BPD or death for extremely-low-birth-weight infants was less in infants with higher risk of these outcomes than in infants at lower risk of these outcomes. Our results do not support targeted administration of vitamin A therapy to higher-risk infants. Our findings have important implications for the study of BPD, for which there are few preventive therapies of proven efficacy.
Supplementary Material
Acknowledgements
While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. RTI International, the data coordinating center for the NRN, analyzed the data for this study. Lei Li, PhD and Abhik Das, PhD had full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in the original trial. We recognize the work of the original Vitamin A protocol subcommittee and other investigators and NRN staff who made this trial possible. We also wish to thank David M. Kent, MD, MS for his helpful comments on a previous draft of this manuscript. Dr Kent declares grant funding from Gore Medical unrelated to this research.
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Research Resources (NCRR) provided grant support for the Neonatal Research Network (NRN) Vitamin A Trial and its reanalysis. The reanalysis was supported by National Institutes of Health grants F32HD098782, UG1HD087229, UG1HD068244, U24HD095254, UG1HD034216, K24HL143283, UG1HD040492, UG1HD027851, UG1HD053109. The authors declare no conflicts of interest.
Footnotes
Data Sharing:
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171(3):e164396. [DOI] [PubMed] [Google Scholar]
- 3.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11 Suppl 3:S146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaisdell CJ, Weinmann GG. NHLBI viewpoint: lung health and disease prevention research starting in childhood. Pediatr Pulmonol. 2015;50(6):604–6. [DOI] [PubMed] [Google Scholar]
- 5.Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L. Lung consequences in adults born prematurely. Thorax. 2015;70(6):574–80. [DOI] [PubMed] [Google Scholar]
- 6.Beam KS, Aliaga S, Ahlfeld SK, Cohen-Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol. 2014;34(9):705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen EA, Foglia EE, Schmidt B. Evidence-based pharmacologic therapies for prevention of bronchopulmonary dysplasia: application of the Grading of Recommendations Assessment, Development, and Evaluation methodology. Clin Perinatol. 2015;42(4):755–79. [DOI] [PubMed] [Google Scholar]
- 8.Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2016;8:CD000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolia VN, Murthy K, McKinley PS, Bennett MM, Clark RH. The effect of the national shortage of vitamin A on death or chronic lung disease in extremely low-birth weight infants. JAMA Pediatr. 2014;168(11):1039–44. [DOI] [PubMed] [Google Scholar]
- 10.Ambalavanan N, Kennedy K, Tyson J, Carlo WA. Survey of vitamin A supplementation for extremely-low-birth-weight infants: is clinical practice consistent with the evidence? J Pediatr. 2004;145(3):30–4–7. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan HC, Tabangin ME, McClendon D, Meinzen-Derr J, Margolis PA, Donovan EF. Understanding variation in vitamin A supplementation among NICUs. Pediatrics. 2010;126(2);e367–73. [DOI] [PubMed] [Google Scholar]
- 12.Couroucli XI, Placencia JL, Cates LA, Suresh GK. Should we still use vitamin A to prevent bronchopulmonary dysplasia? J Perinatol. 2016;36(8):581–5. [DOI] [PubMed] [Google Scholar]
- 13.Jensen EA, Roberts RS, Schmidt B. Drugs to prevent bronchopulmonary dysplasia: effect of baseline risk on the number needed to treat. J Pediatr. 2020;222:244–7. [DOI] [PubMed] [Google Scholar]
- 14.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med. 1999;340(25):1962–8. [DOI] [PubMed] [Google Scholar]
- 15.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41(3):861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onland W, Debray TP, Laughon MM, Miedema M, Cools F, Askie LM, et al. Clinical predictions models for bronchopulmonary dysplasia: a systematic review and external validation study. BMC Pediatr. 2013;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NICHD Neonatal Research Network. Neonatal BPD Outcome Estimator. Located at: https://neonatal.rti.org/index.cfm?fuseaction=BPDCalculator.startAccessed January 1, 2021.
- 20.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95(5 Pt 1):769–74. [DOI] [PubMed] [Google Scholar]
- 21.Donovan EF, Tyson JE, Ehrenkranz RA, Verter J, Wright LL, Korones SB, et al. Inaccuracy of Ballard scores before 28 weeks’ gestation. J Pediatr 1999;135(2 Pt 1):147–52. [DOI] [PubMed] [Google Scholar]
- 22.Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed 2005;90(2):F103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent DM, Nelson J, Dahabreh IJ, Rothwell PM, Altman DG, Hayward RA. Risk and treatment effect heterogeneity: re-analysis of individual participant data from 32 large clinical trials. Int J Epidemiol. 2016;45(6):2075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent DM, Paulus JK, van Klaveren D, D’Agostino R, Goodman S, Hayward R, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement. Ann Intern Med. 2020;172(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100(3):145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinclair JC, Haynes RB. Selecting participants that raise a clinical trial’s population attributable fraction can increase the treatment effect within the trial and reduce the required sample size. J Clin Epidemiol. 2011;64(8):893–902. [DOI] [PubMed] [Google Scholar]
- 28.Kent DM, Ruthazer R, Weimar C, Mas J-L, Serena J, Homma S, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013; 81(7):619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent DM, Rothwell PM, Ioannidis JPA, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ. 2018;364:k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Londhe VA, Nolen TL, Das A, Higgins RD, Tyson JE, Oh W, et al. Vitamin A supplementation in extremely low-birth-weight infants: subgroup analysis in small-for-gestational-age infants. Am J Perinatol. 2013;30(9):771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants: an evidence-based approach. Am J Respir Crit Care Med. 2019;200(6):751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mactier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. J Pediatr. 2012;160(6):954–9. [DOI] [PubMed] [Google Scholar]
- 34.Basu S, Khanna P, Srivastava R, Kumar A. Oral vitamin A supplementation in very low birth weight neonates: a randomized controlled trial. Eur J Pediatr. 2019;178(8):1255–65. [DOI] [PubMed] [Google Scholar]
- 35.Rakshasbhuvankar A, Simmer K, Patole SK, Stoecklin B, Nathan EA, Clarke MW, et al. Enteral vitamin A for reducing severity of bronchopulmonary dysplasia in extremely preterm infants: a randomized controlled trial. Pediatrics. 2021;147(1): e2020009985. [DOI] [PubMed] [Google Scholar]
- 36.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.