Abstract
Objective:
The prebiotic fiber inulin has been studied in individuals undergoing hemodialysis (HD) due to its ability to reduce gut microbiota-derived uremic toxins. However, studies examining the effects of inulin on the gut microbiota and derived metabolites are limited in these patients. We aimed to assess the impact of a 4-week supplementation of inulin on the gut microbiota composition and microbial metabolites of patients on HD.
Design and Methods:
In a randomized, double-blind, placebo-controlled, crossover study, twelve HD patients (55 ± 10 y, 50% male, 58% Black American, BMI 31.6 ± 8.9 kg/m2, 33% diabetes mellitus) were randomized to consume inulin [10 g/d for females; 15 g/d for males] or maltodextrin [6 g/d for females; 9 g/d for males] for 4 weeks, with a 4-week washout period. We assessed the fecal microbiota composition, fecal metabolites (short-chain fatty acids (SCFA), phenols, and indoles), and plasma indoxyl sulfate and p-cresyl sulfate.
Results:
At baseline, factors that explained the gut microbiota variability included BMI category and type of phosphate binder prescribed. Inulin increased the relative abundance of the phylum Verrucomicrobia and its genus Akkermansia (P interaction = 0.045). Inulin and maltodextrin resulted in an increased relative abundance of the phylum Bacteroidetes and its genus Bacteroides (P time = 0.04 and 0.03, respectively). Both treatments increased the fecal acetate and propionate (P time = 0.032 and 0.027, respectively), and there was a trend toward increased fecal butyrate (P time = 0.06). Inulin did not reduce fecal p-cresol or indoles, or plasma concentrations of p-cresyl sulfate or indoxyl sulfate.
Conclusions:
A 4-week supplementation of inulin did not lead to major shifts in the fecal microbiota and gut microbiota-derived metabolites. This may be due to high variability among participants and an unexpected increase in fecal excretion of SCFA with maltodextrin. Larger studies are needed to determine the effects of prebiotic fibers on the gut microbiota and clinical outcomes to justify their use in patients on HD.
Introduction
THE GUT MICROBIOTA is the diverse community of microorganisms that resides within the gastrointestinal tract.1–3 The gut microbiota is of interest in chronic kidney disease (CKD) as it has been associated with the pathogenesis and progression of kidney dysfunction.4,5 The gut microbiota of individuals with CKD and kidney failure has been described to have a lower abundance of some commensal bacteria, such as bifidobacteria, while having a greater abundance of pathobionts such as Enterobacteriaceae and Clostridium perfringens compared to healthy controls.6,7
The unique gut microbial composition in CKD also may be accompanied by changes in bioactive microbial-derived metabolites. Individuals with kidney failure have an expansion in bacterial families possessing indole- and phenolforming enzymes,8 along with an increase in protein fermentation products derived exclusively from microbial metabolism, such as indoles (from tryptophan) and phenols (from tyrosine).9 These indoles and phenols are absorbed and transformed in the liver, including sulfation, producing indoxyl sulfate and p-cresyl sulfate, respectively, and released into the systemic circulation.9 Importantly, serum p-cresyl sulfate and indoxyl sulfate have been associated with increases in cardiovascular mortality, endothelial dysfunction, and mineral and bone disorders in individuals with CKD.10–13 Since a big proportion of these are protein-bound, the dialysis process itself is not effective for their elimination.14 Therefore, therapies that reduce the production of these uremic toxins often are sought.
In parallel to an increased capacity for the production of uremic toxins, the gut microbiota of individuals with kidney failure may exhibit decreased saccharolytic fermentation and short-chain fatty acid (SCFA)-producing capabilities, which may be a result of a low dietary fiber intake.8,15 SCFAs, particularly butyrate, have been associated with positive outcomes in healthy adults and other clinical populations.16,17 SCFAs have effects associated with kidney function, such as regulation of blood pressure and reduction of ischemia-reperfusion injury.18–20 However, research on SCFAs production and its effects on kidney failure is limited.
Diet is a main determinant of the gut microbial composition and it has been demonstrated that the gut microbiota and derived metabolites are significantly different when animal versus plant-based diets are consumed.21 In individuals undergoing hemodialysis (HD), the dietary recommendations may be considered restrictive.22 In particular, increased intake of protein of high biological value, while limiting dietary potassium and phosphorus, may result in a diet high in animal-based foods and low in plant-based foods.23 Additionally, there is a positive association between the ratio of dietary protein-to-fiber and indoxyl sulfate and p-cresyl sulfate in individuals with CKD.11 By increasing dietary fiber and polysaccharide fermentation, protein fermentation may be spared and thus reducing the protein fermentation products, including phenols and indoles.9 Thus, reducing the protein-to-fiber ratio by supplementing dietary fiber may be a novel clinical strategy to decrease the uremic toxins of microbial origin.11 Among dietary fiber, inulin-type fructans have been shown to reduce p-cresyl sulfate in individuals undergoing HD.10 Inulin-type fructans can be found in foods and are considered prebiotics as they are selectively utilized by host microorganisms and have been shown to confer health benefits, such as increased mineral absorption, increased production of endocrine peptides, and changes in the gut microbiota in healthy and other clinical populations.24,25 Inulin-type fructans are fermented by some bacteria, particularly bifidobacteria, producing acetate and lactate through the bifidogenic shunt, which then are used by other bacteria to produce other SCFAs, predominantly butyrate.26–29 However, the effects of inulin on the gut microbial composition and other microbial metabolites have not been explored in patients on HD. Therefore, our objective was to assess the effects of a 4-week supplementation of inulin on the gut microbiota composition, fecal gut-derived metabolites (SCFAs, indoles, and phenols), and plasma concentrations of microbial metabolites in individuals undergoing HD.
Methods
We recruited individuals undergoing HD from two local dialysis clinics. Inclusion criteria included thrice-weekly HD therapy for at least 3 months and being able to provide a total of four fecal samples. Exclusion criteria included previous major gastrointestinal disease diagnosis (e.g., inflammatory bowel disease and celiac disease) or intestinal resections; antibiotic treatment 1 month prior to the start of the study; sustained hypercalcemia; and current intake of probiotics or prebiotics. Consent was obtained from each participant and all protocols were approved by our University’s Institutional Review Board in accordance with the Declaration of Helsinki. This study was registered in Clinicaltrials.gov (NCT02718885). Part of this work was published as a doctoral dissertation.30
Intervention Protocol
In a randomized, double-blind, placebo-controlled, crossover study, 13 participants were randomized using a simple randomization technique (coin toss) to the intervention (inulin) or placebo (maltodextrin). Randomization was performed by a research coordinator so that both researchers and participants were blinded to the treatment allocation. Seven participants were randomly assigned to consume the inulin supplement first, while six were assigned to consume the maltodextrin first. Participants consumed inulin (females: 10 g/day; males: 15 g/day; Orafti Synergy, Beneo, Belgium [91% inulin with a degree of polymerization of 2–60; 9% short-chain fructooligosaccharides with a degree of polymerization of 2–8]) or maltodextrin (females: 6 g/day; males: 9 g/day; Now Foods Carbogain Maltodextrin, Bloomingdale, IL) for 4 weeks, with a 4-week washout period between supplementation periods (Figure S1). The differential dose for males and females was based on the dietary reference intake for dietary fiber for males and females of 38 g/d and 25 g/d, respectively, and the intervention doses represented ~40% of the adequate intake of fiber.31 Participants received the supplements in sachets at the beginning of each week at the dialysis center (Monday or Tuesday) and were instructed to consume the supplements mixed with a fluid of their choice. The first week of the supplementation periods was considered an adaptation week, in which participants consumed half of the dose. After this first week, participants were instructed to double the dose and were suggested to split the dose in half and consume it twice a day if they had gastrointestinal symptoms, such as flatulence. Participants were asked at every HD treatment about supplement compliance, as well as the fluid used to mix the supplement, gastrointestinal symptoms, and stool consistency based on the Bristol Stool Scale.
Fecal Sample Collection and Gastrointestinal Symptoms
Participants were asked to collect a complete fecal sample (Commode Specimen Collection System Sage Products, Crystal Lake, IL) at the beginning and end of both supplementation periods. For the end of the period, participants were instructed to collect and provide the fecal samples (day 21–28) to the research team. Samples were weighed, homogenized, and three-2 mL aliquots were stored at −80° C within 60 min of collection. Participants also were asked to rate consistency and ease of passage for the bowel movement. Additionally, stool consistency was scored by one member of the research team according to the Bristol Stool Scale.32 Ease of stool passage was ranked on a 5-point scale (1 = very easy, 2 = easy, 3 = neither easy nor difficult, 4 = difficult, 5 = very difficult).
DNA Extraction and Fecal Microbiota Analyses
DNA was extracted using the Powerlyzer PowerSoil DNA Isolation Kit (MO BIO, Carlsbald, CA) and quantified using a Qubit Fluorometer 3.0 using the dsDNA BR Assay Kit (ThermoFisher Scientific, Waltham, MA), while quality was assessed by electrophoresis with 2% Agarose EX-gels using the E-Gel iBase (Invitrogen, Grand Island, NY). Fluidigm Access Array was used to generate16S rRNA gene amplicons, in combination with Roche High Fidelity Fast Start Kit. Primers 515 F (5′-GTG YCAGCMGCCGCGGTAA-3′) and 806R (5′-GGA CTACNVGGGTWTCTAAT-3′) targeting a 252bp-fragment of the V4 region of the bacterial 16S rRNA were amplified.33 CS1 forward tag and CS2 reverse tag were added according to the Fluidigm protocol. Sequencing was performed through Illumina Mi-seq using V3 reagents. Relative changes in bacterial diversity (α-diversity and β-diversity) and taxonomical changes were analyzed through the open software QIIME (version 1.9.1). In short, high-quality (quality value $ 20) sequencing reads were demultiplexed. Sequences then were clustered into features, in this case, operational taxonomic units (OTUs) using UCLUST34 through a closed-reference OTU picking strategy against the Greengenes 13_8 reference OTU database with a 97% similarity threshold.35 Singletons (OTUs that were observed fewer than two times) and OTUs that had less than 0.01% of the total observation were discarded. Taxonomic identity to each OTU then was assigned using UCLUST. OTUs that had a relative abundance at any timepoint of $1% were considered for analysis. For α- and β-diversities, samples were rarified to an even sampling depth of 67,614 sequences/ sample. β-diversity was calculated using weighted and unweighted UniFrac distance measures.36
Fecal dry Matter, SCFAs, Phenols, and Indoles
SCFAs were quantified by gas chromatography according to Erwin et al.37 The concentrations of phenols and indoles were quantified by gas chromatography according to Flickinger et al.38 Fecal dry matter was measured according to the methods of the Association of Official Analytical Chemists.39
Blood Sample Collection and Plasma Metabolites
A plasma sample was obtained at the beginning of the dialysis session immediately after the fecal sample collection (BD Vacutainer Lithium plasma tube, Oakville, ON). Total plasma p-cresyl sulfate and indoxyl sulfate were measured by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) as described by de Loor et al.16
Dietary Intake
Participants were asked to maintain a constant dietary intake throughout the duration of the study. Dietary recalls covering the 48 h prior to the fecal sample collection were obtained by a trained dietitian using the modified version of the USDA 5-pass method.40 The records were analyzed for macronutrient and micronutrient composition using Nutrition Data System for Research (NDSR 2014 version, University of Minnesota, Minneapolis, MN).
Statistical Analysis
Means and standard deviations are reported unless otherwise noted. All outcomes were assessed for normality and variance using Brown-Forsythe’s test and log-transformed before analyses as appropriate. Repeated measures ANOVA was performed in a within-subjects analysis with two groups (inulin, maltodextrin) and two timepoints (pre, post), against variables of interest with significance at P < .05. As we only had two treatments, the Wilcoxon’s paired test was performed to assess any potential carryover effects (inulin-pre vs. maltodextrin-pre) in the main variables of interest (e.g., Bifidobacterium, Faecalibacterium, fecal SCFAs, and plasma indoxyl sulfate and p-cresyl sulfate).41 Statistical analysis was performed using SPSS version 25. Further statistical analysis of the microbial composition was performed through Statistical Analysis of Metagenomic Profiles (STAMP) using categorical variables (e.g., sex, body mass index [BMI] category, type of phosphate binders) using Welch’s t-test at baseline, which was corrected for multiple comparisons using Benjamini- Hochberg False Discovery Rate (FDR, q).42
Results
From the 30 individuals undergoing HD that were approached, fifteen participants were recruited from two outpatient dialysis clinics. Two participants dropped from the study before starting the intervention (one because of personal reasons and the other was transferred to another clinic). One patient was deceased before completing the study and 12 participants completed both supplementation periods (Table 1, Figure S2).
Table 1.
Participants Baseline Characteristics
| Variable | Mean ± SD |
|---|---|
|
| |
| Age (y) | 55 ± 10 |
| Gender (M/F) | 6/6 |
| African American (%) | 58.3% |
| BMI (kg/m2) | 31.62 ± 8.95 |
| Diabetes (%) | 46% |
| Serum Albumin (g/dL)* | 3.27 ± 0.25 |
| Energy (kcal/kg/d) | 22.43 ± 10.87 |
| Protein (g/kg/d) | 0.97 ± 0.50 |
| Carbohydrates (% total kcal) | 44.28 ± 6.97 |
| Fat (% total kcal) | 37.79 ± 6.14 |
| Total Dietary Fiber (g/1,000 kcal) | 6.79 ± 2.95 |
| Fecal Acetate (umol/g DM) | 193.36 ± 125.88 |
| Fecal Propionate (umol/g DM) | 57.92 ± 35.01 |
| Fecal Butyrate (umol/g DM) | 35.63 ± 27.94 |
| Fecal Indoles (ug/g DM) | 129.47 ± 90.29 |
| Fecal P-Cresol (ug/g DM) | 195.95 ± 137.09 |
| Plasma Indoxyl Sulfate (uM) | 110.65 ± 48.29 |
| Plasma P-Cresyl Sulfate (uM) | 180.81 ± 108.84 |
M, male; F, female; SD, standard deviation; BMI, body mass index.
Serum albumin was measured with a Point of Care analyzer that utilizes the bromcresol purple method with reference values of 3.3–5.5 g/dL.
Phosphate Binders and BMI Category Are Key Determinants of Gut Microbiota Composition
Principal component analysis (PCA) of baseline data revealed a distinct fecal microbiota between participants prescribed calcium and non-calciumbased phosphate binders (i.e., sevelamer hydrochloride/carbonate). In participants who were prescribed sevelamer, there was a higher relative abundance of fecal unclassified Ruminococcaceae (q=0.028) and a lower relative abundance of fecal Bacteroides (q<0.022) (Figure 1). Similarly, the fecal microbiota was different depending on BMI category, with participants with a BMI ≥30 kg/m2 having a higher relative abundance of fecal Ruminococcus (q = 0.047) and unclassified Enterobacteriaceae (q = 0.006), while individuals with BMI <25 kg/m2 had a higher relative abundance of Coprococcus (q = 0.014) (Figure S4). Additionally, the fecal microbiota tended to be different between female and male participants (Figure S3). Female participants tended to have a lower relative abundance of fecal Faecalibacterium (q=0.054) (Figure S3).
Figure 1.
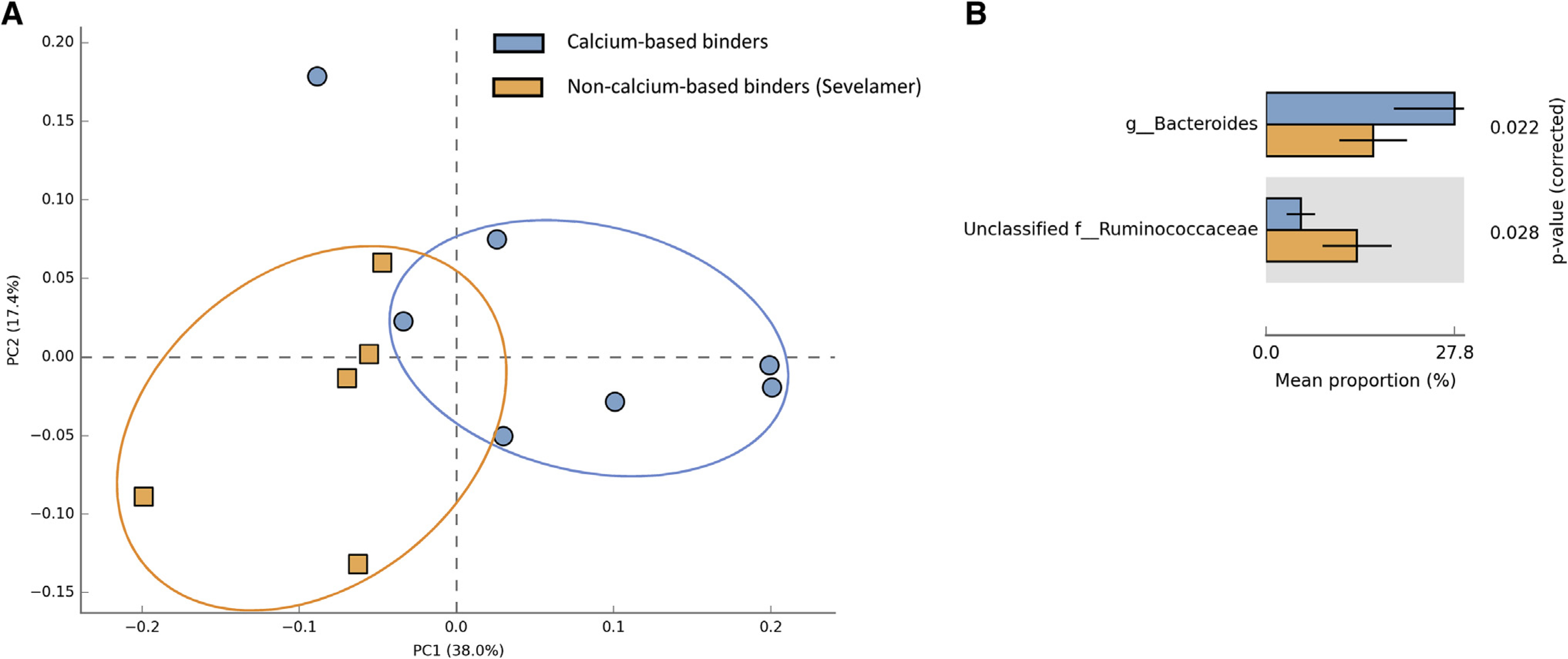
Principal component analysis (PCA) performed on the 97% OTU abundance matrix. A) There was a unique microbiota in HD patients that were prescribed sevelamer hydrochloride/carbonate (orange squares) compared to participants prescribed calcium-based binders (blue circles). B) There was a lower relative abundance of Bacteroides in those prescribed sevelamer (q = 0.022) and a higher relative abundance of unclassified Ruminococcaceae (q = 0.028).
Inulin Did Not Alter Fecal Microbial Diversity
Inulin or maltodextrin supplementation did not affect a-diversity, a metric of microbial richness within a sample. For b-diversity, or microbial diversity between samples, principal coordinate analyses of unweighted (presence vs. absence) and weighted (account for OTUs abundance) UniFrac performed on the 97% OTU abundance distance matrix did not show effects of inulin or maltodextrin (PERMANOVA P = .99 and .875, respectively; Figure S5). However, there was a high interpersonal variability, where samples from the same participant clustered together (PERMANOVA P = .001 for weighted and unweighted UniFrac; Figure S6).
Inulin Supplementation Induced Minor and Similar Modifications to the Gut Microbiota Composition to Maltodextrin
After the supplementation of inulin or maltodextrin, the phylum Bacteroidetes and its genus Bacteroides increased after both treatments (P time = 0.041 and 0.028, respectively) (Table 2). Furthermore, there was a group-by-time interaction on the Verrucomicrobia phylum and its only genus Akkermansia where it increased after inulin treatment and decreased after maltodextrin (P interaction = 0.045). There was a group-by-time trend toward significance in Ruminococcus, where it tended to decrease after inulin, while it was maintained after maltodextrin (P interaction = 0.051). Finally, we did not observe any differences in the relative abundance of genera of interest (e.g., Bifidobacterium or Faecalibacterium). However, there was a trend toward a time effect on Faecalibacterium, which increased after inulin and maltodextrin (P time = 0.079) (Table 2).
Table 2.
Bacterial Taxa with ≥1% Abundance after Four Weeks of Inulin or Maltodextrin Supplementation
| Phylum (%) |
Inulin (mean ± SD) |
Maltodextrin (mean ± SD) |
Group P |
Time P |
G × T P |
||
|---|---|---|---|---|---|---|---|
| Genus (%) | Pre (n = 12) | Post (n = 12) | Pre (n = 12) | Post (n = 12) | |||
|
| |||||||
| Actinobacteria† | 3.44 ± 3.01 | 3.02 ± 2.39 | 5.69 ± 6.65 | 4.28 ± 5.12 | .213 | .167 | 1.000 |
| Bifidobacterium† | 1.43 ± 2.22 | 1.89 ± 2.23 | 3.30 ± 6.26 | 2.72 ± 5.08 | .169 | .789 | .235 |
| Collinisella | 0.88 ± 1.78 | 0.59 ± 0.96 | 1.27 ± 2.19 | 0.88 ± 1.48 | .387 | .831 | .655 |
| Bacteroidetes† | 32.02 ± 12.27 | 38.05 ± 13.60 | 27.71 ± 10.64 | 34.82 ± 15.51 | .063 | .041 | .884 |
| Bacteroides | 26.79 ± 12.63 | 32.08 ± 13.58 | 23.52 ± 11.18 | 29.86 ± 16.17 | .122 | .028 | .864 |
| Parabacteroides | 2.71 ± 1.58 | 3.00 ± 2.56 | 2.33 ± 1.90 | 3.04 ± 2.29 | .692 | .300 | .703 |
| Rikenellaceae*† | 1.66 ± 1.88 | 1.60 ± 1.77 | 1.43 ± 2.09 | 1.13 ± 1.73 | .068 | .545 | .956 |
| Firmicutes | 59.75 ± 10.98 | 54.03 ± 14.53 | 61.94 ± 12.69 | 56.47 ± 14.85 | .294 | .084 | .969 |
| Streptococcus† | 3.74 ± 7.38 | 1.55 ± 3.02 | 3.45 ± 6.51 | 2.34 ± 3.48 | .819 | .398 | .715 |
| Clostridiales | 7.33 ± 3.35 | 6.39 ± 2.82 | 9.16 ± 4.48 | 7.54 ± 4.42 | .106 | .096 | .632 |
| Lachnospiraceae* | 7.50 ± 3.48 | 5.39 ± 3.60 | 7.23 ± 3.72 | 7.21 ± 3.68 | .340 | .280 | .166 |
| Blautia† | 4.16 ± 3.91 | 4.71 ± 3.29 | 7.34 ± 5.98 | 4.49 ± 3.39 | .387 | .714 | .054 |
| Coprococcus† | 2.10 ± 1.89 | 1.55 ± 1.06 | 2.36 ± 1.92 | 2.04 ± 2.17 | .504 | .144 | .713 |
| Dorea† | 1.16 ± 1.96 | 1.05 ± 2.14 | 0.94 ± 1.27 | 2.13 ± 3.82 | .313 | .474 | .476 |
| Ruminococcus† | 3.55 ± 4.52 | 2.12 ± 2.59 | 2.45 ± 1.70 | 2.38 ± 1.93 | .560 | .207 | .051 |
| Ruminococcaceae* | 8.41 ± 5.93 | 7.02 ± 5.58 | 7.20 ± 4.87 | 6.77 ± 4.82 | .390 | .065 | .528 |
| Faecalibacterium | 5.77 ± 5.03 | 9.24 ± 7.10 | 6.96 ± 5.92 | 8.42 ± 7.90 | .848 | .079 | .429 |
| Oscillospira† | 1.75 ± 1.64 | 1.01 ± 0.68 | 1.21 ± 0.81 | 1.33 ± 1.28 | .684 | .044 | .238 |
| Ruminococcus (2)† | 4.77 ± 3.33 | 3.75 ± 4.53 | 3.76 ± 2.16 | 3.30 ± 2.52 | .482 | .158 | .487 |
| Phascolarctobacterium† | 1.02 ± 0.74 | 1.14 ± 0.95 | 0.62 ± 0.66 | 1.02 ± 1.29 | .115 | .387 | .639 |
| Erysipelotrichaceae*† | 1.61 ± 1.48 | 1.53 ± 1.91 | 1.17 ± 0.96 | 1.58 ± 2.53 | .354 | .637 | .373 |
| Eubacterium† | 2.15 ± 3.83 | 2.19 ± 2.57 | 2.60 ± 2.39 | 1.29 ± 1.21 | .399 | .132 | .098 |
| Proteobacteria† | 1.20 ± 1.18 | 2.15 ± 2.68 | 1.26 ± 1.25 | 2.04 ± 1.44 | .717 | .065 | .960 |
| Sutterella† | 0.77 ± 0.94 | 0.77 ± 0.89 | 0.87 ± 1.14 | 1.03 ± 1.42 | .442 | .840 | .586 |
| Enterobacteriaceae*† | 0.12 ± 0.17 | 1.07 ± 2.88 | 0.09 ± 0.12 | 0.49 ± 0.94 | .717 | .210 | .275 |
| Synergistetes | 1.39 ± 3.18 | 0.21 ± 0.37 | 0.87 ± 2.14 | 0.46 ± 1.07 | .769 | .110 | .415 |
| Pyramidobacter | 1.06 ± 3.16 | 0.15 ± 0.34 | 0.83 ± 2.15 | 0.46 ± 1.07 | .928 | .192 | .555 |
| Verrucomicrobia | 0.84 ± 1.68 | 1.95 ± 3.68 | 1.94 ± 2.56 | 0.99 ± 1.82 | .891 | .864 | .045 |
| Akkermansia | 0.84 ± 1.68 | 1.95 ± 3.68 | 1.94 ± 2.56 | 0.99 ± 1.82 | .891 | .864 | .045 |
G × T, group-by-time
Bold depicts statistical significance (p < .05).
Unclassified genera.
Values were transformed before analyses.
Inulin and Maltodextrin Increased Fecal SCFAs but Did Not Decrease Indole and Phenol Metabolites in Feces or Serum
Fecal acetate and propionate significantly increased after inulin and maltodextrin (P time = 0.032 and 0.027, respectively), and a numerical increase in fecal butyrate after both supplementation treatments that did not reach statistical significance (P time = 0.128) (Table 3, Figure 2).
Table 3.
Fecal Short-Chain Fatty Acids, p-Cresol, and Indoles and Plasma Uremic Toxins after Four Weeks of Inulin or Maltodextrin Supplementation
| Inulin (mean ± SD) |
Maltodextrin (mean ± SD) |
Group P |
Time P |
G × T P |
|||
|---|---|---|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post | |||
|
| |||||||
| Fecal Acetate (umol/g DM, n=9) | 209.26 ± 112.34 | 263.51 ± 116.68 | 229.71 ± 131.37 | 333.42 ± 206.19 | .227 | .032 | .401 |
| Fecal Propionate (umol/g DM, n=9) | 75.24 ± 47.74 | 80.07 ± 44.04 | 65.35 ± 34.61 | 99.42 ± 65.21 | .700 | .027 | .198 |
| Fecal Butyrate (umol/g DM, n=9) | 36.59 ± 24.68 | 49.06 ± 33.91 | 45.18 ± 27.16 | 60.10 ± 37.31 | .056 | .128 | .811 |
| Fecal Total SCFA (umol/g DM, n=9) | 323.84 ± 179.49 | 390.44 ± 179.55 | 337.49 ± 182.71 | 495.15 ± 280.92 | .215 | .030 | .282 |
| Fecal Indoles (ug/g DM, n=9) | 155.64 ± 66.61 | 132.22 ± 104.99 | 138.43 ± 69.36 | 129.51 ± 73.44 | .609 | .560 | .798 |
| Fecal P-Cresol (ug/g DM, n=9) | 250.77 ± 138.37 | 207.38 ± 148.11 | 174.73 ± 82.17 | 178.54 ± 184.61 | .111 | .677 | .483 |
| Plasma Indoxyl Sulfate (uM, n=12) | 125.86 ± 42.58 | 116.60 ± 53.79 | 115.78 ± 62.26 | 121.90 ± 62.01 | .772 | .822 | .210 |
| Plasma P-Cresyl Sulfate (uM, n=12) | 176.57 ± 103.75 | 176.00 ± 132.93 | 185.18 ± 115.17 | 162.63 ± 108.37 | .765 | .427 | .395 |
SD, standard deviation; G × T, group-by-time interaction; SCFA, short-chain fatty acids.
Fecal excretion of SCFAs was quantified in 9 out of the 12 subjects that completed the study, as the rest of the samples were not obtained within 1 hour of the fecal sample collection.
Bold depicts statistical significance.
Figure 2.
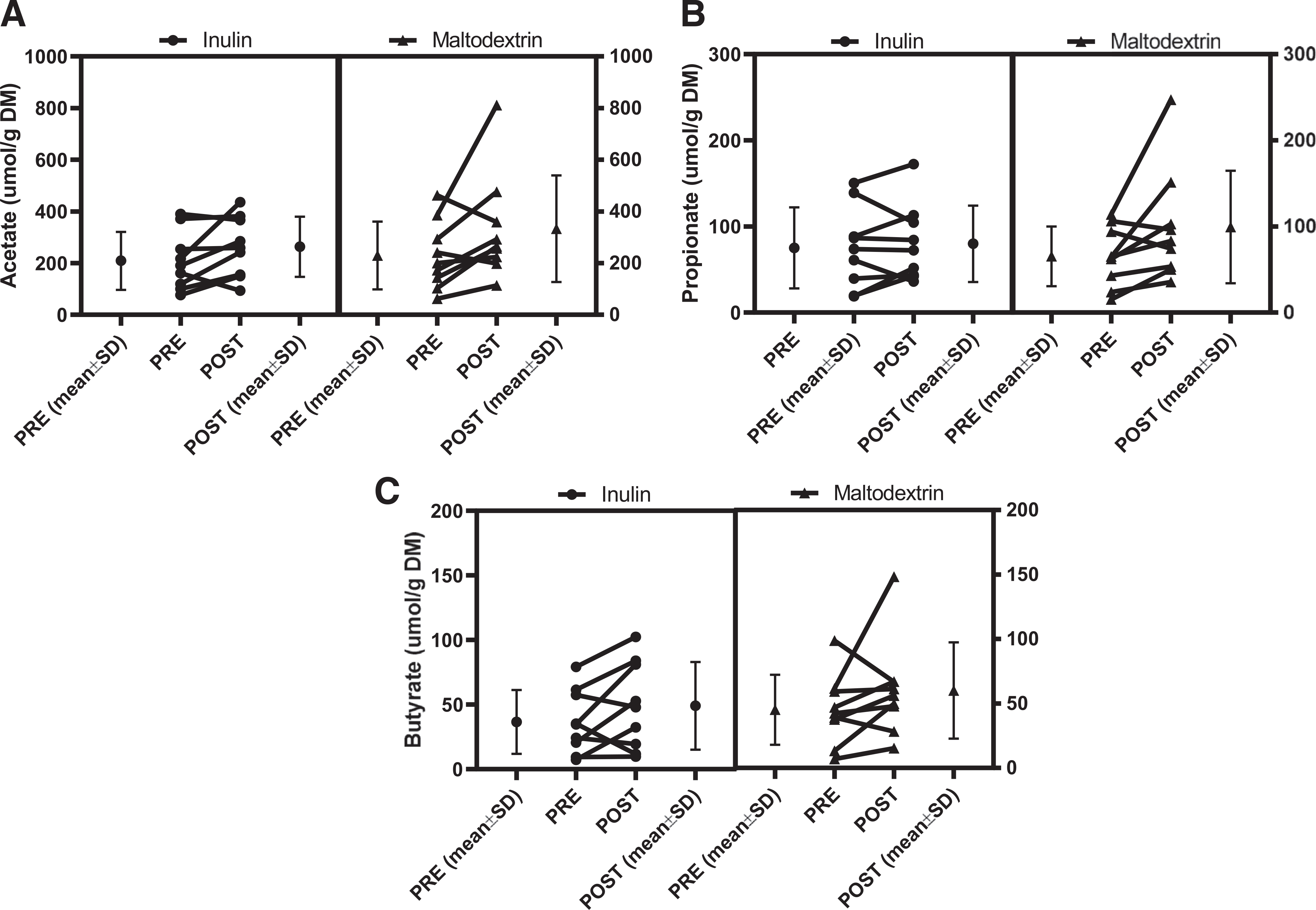
Fecal short-chain fatty acids increased after inulin and maltodextrin supplementation. The mean concentrations before and after the supplementation periods and the individual pre- and post-effects are shown. A) There was a time effect on the fecal acetate, where it increased after inulin and maltodextrin. B. There was a time effect on the fecal propionate, where it increased after inulin and maltodextrin. C) There was a similar numerical increase in fecal butyrate after inulin and maltodextrin but did not reach statistical significance.
Consumption of inulin did not alter microbiota-derived fecal p-cresol and indoles or plasma metabolites indoxyl sulfate and p-cresyl sulfate (Table 3, Figure 3). We assessed the potential carryover effect of both supplements and found no effect on the fecal SCFAs and plasma indoxyl sulfate and p-cresyl sulfate (p > .1 for all).
Figure 3.
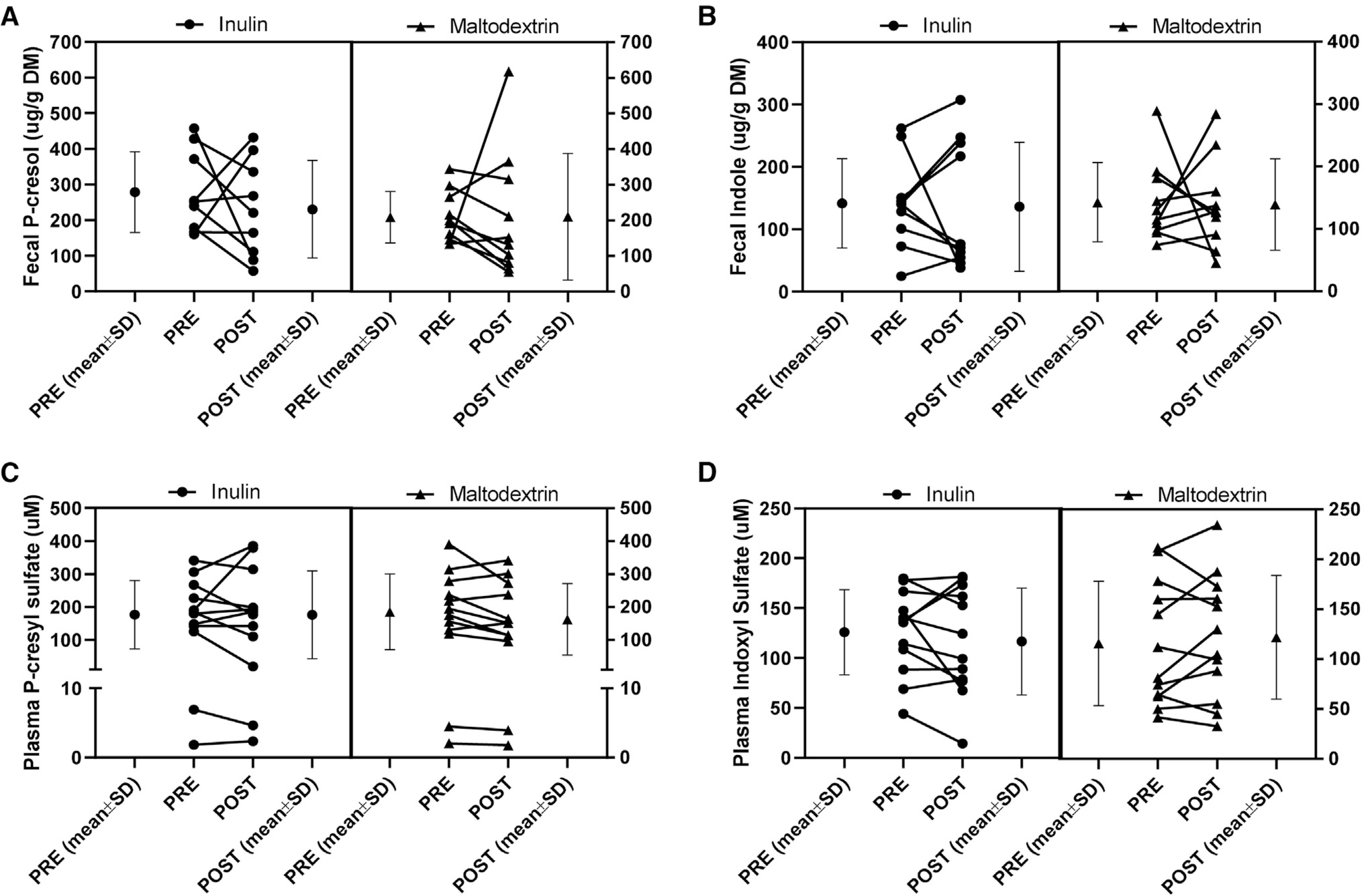
Fecal p-cresol and indole and plasma concentrations of indoxyl sulfate and p-cresyl sulfate did not change after inulin and maltodextrin supplementation. The mean concentrations before and after the supplementation periods and the individual pre- and post-effects are shown. A) Fecal p-cresol was not altered after inulin or maltodextrin. B) Fecal indole was not altered after inulin or maltodextrin supplementation. C) Plasma p-cresyl sulfate was not modified after the supplementation of inulin or maltodextrin. D) Plasma indoxyl sulfate did not change after the supplementation of inulin or maltodextrin.
Supplement Adherence, Dietary Intake, and Gastrointestinal Symptoms
Overall, there were no major shifts in dietary intake across the length of the study. However, there was an increase in dietary fiber intake after inulin supplementation, as the supplement was considered in the total dietary fiber intake (P interaction = 0.006) (Table S1). As a result, the dietary protein-to-fiber ratio was reduced after the inulin supplementation (P interaction = 0.041). This increase in dietary fiber and reduction in the protein-to-fiber ratio was not associated with a decrease in uremic toxins in plasma (data not shown). Finally, there was a time effect on total carbohydrate intake, where it was decreased after inulin and maltodextrin (P time = 0.04).
After the supplementation of inulin, there was an increase in flatulence score (P interaction = 0.026). This increase, however, was not associated with a change in compliance, where self-reported compliance after inulin was $80% (Table S2). Additionally, there was a time effect on the reflux score, where it increased after both treatments (P time = 0.027) and a group effect on the rumbling score, where the inulin group was higher overall (P group = .021) (Table S2). Finally, there were no changes in the self-reported number of bowel movements or stool consistency scored by a member of the research team (Table S2).
Discussion
In this randomized, double-blind, placebo-controlled, crossover study, a 4-week supplementation of inulin did not result in major changes in the diversity or composition of the gut microbiota, or the fecal and plasma microbial metabolites. We, however, observed that anthropometric and pharmacological variables, such as BMI category and the type of phosphate binder partially explained fecal microbiota variability.
The supplementation of inulin-type fructans has resulted in changes in the gut microbiota composition in healthy adults,41,43 individuals with obesity,44,45 diabetes,46,47 and recently in individuals with kidney failure undergoing peritoneal dialysis.48 Most studies have reported an increase in the relative abundance of Bifidobacterium, Faecalibacterium, Anaerostipes, and Roseburia.41,43,45,49 We did not observe an increase in Bifidobacterium, but we observed a trend toward an increase in the relative abundance of Faecalibacterium after inulin and maltodextrin. A failure to observe an effect on Bifidobacterium has been reported as a methodological flaw in 16S rRNA gene sequencing due to the overall low relative abundance of this genus,28 which in our participants was 2.36% (range 0–21.8%) throughout the study timepoints. Besides these genera, reductions in other genera have been reported, such as Bacteroides and Bilophila.41,45 In our study, however, we observed that the relative abundance of Bacteroides increased after inulin and maltodextrin, and that the phylum Verrucomicrobia and its genus Akkermansia increased after inulin. Finally, we observed a trend toward significance on Ruminococcus, where the relative abundance numerically decreased after inulin, while it was maintained after maltodextrin. Ruminococcus gnavus has been shown to discriminate between healthy controls and individuals with CKD.50 Interestingly, a polysaccharide produced by R. gnavus has been shown to induce the production of TNF-a in inflammatory bowel disease.51 A potential reduction of the genus Ruminococcus and its impact on inflammation in individuals with CKD and kidney failure remains to be explored.
We hypothesized that supplementation with inulin would increase the fecal excretion of SCFA, especially butyrate, compared to maltodextrin. Bifidobacteria ferment inulin-type fructans producing acetate and lactate through the bifidogenic shunt, which can be cross-fed to other bacteria to produce other SCFA, predominantly butyrate.26–29 Unfortunately, we did not measure fecal lactate levels, as it is possible that there was not enough microbial capacity to produce butyrate from lactate, as butyrate-forming enzymes are reduced in individuals with kidney failure.8 We did, however, observe a similar numerical increase in fecal butyrate after inulin and maltodextrin, which was increased by ~60% (~15uM) after both treatments.
We observed a time effect in the fecal acetate and propionate after both supplementation periods. In fact, maltodextrin supplementation led to more robust increases in fecal acetate and propionate concentrations compared to inulin supplementation, suggesting that individuals undergoing HD may have impaired digestion and absorption of maltodextrin. In vitro fermentation studies have shown that maltodextrin can be degraded by bacteria, producing lactate, acetate, and propionate.52 We decided to use maltodextrin as our control because, in theory, it is a completely digestible carbohydrate used extensively as a control for fiber supplementation studies,53 including those focused on kidney disease.54,55 Species within the Lachnospiraceae family (Eubacterium rectale) and Faecalibacterium prausnitzii can utilize maltodextrins.56 In our study, we did not observe changes in the relative abundance of the family Lachnospiraceae, but Faecalibacterium tended to increase after inulin and maltodextrin. If indeed maltodextrin is fermented by the gut microbiota in kidney failure, maltodextrin should be avoided as a placebo and the use of negative control supplements, such as cellulose, should be preferred.
The reduction of uremic toxins through the modulation of the gut microbiota is a topic of interest to the nephrology community. In a non-randomized study by Meijers et al.,10 a 4-week supplementation of 20 g/d of oligofructose-enriched inulin (same type of supplement as in the current study) resulted in a 20% reduction in circulating p-cresyl sulfate, without a change in indoxyl sulfate. In our study, we did not observe an effect of inulin on plasma p-cresyl sulfate or indoxyl sulfate. However, the dose utilized in the current study was 25–50% lower than reported by Meijers et al.10 Furthermore, it has been suggested that by lowering the ratio of dietary protein-to-dietary fiber, the production of these bacteria-derived uremic toxins may decrease.9 Despite having a significant reduction in the protein-to-fiber ratio after inulin supplementation (Table S1), we did not observe a decrease in these uremic toxins. Additionally, we did not observe a decrease in the fecal excretion of p-cresol and indoles, the precursors of indoxyl sulfate and p-cresyl sulfate. Specific bacterial species within the Bacteroides genus, such as Bacteroides thetaiotaomicron and Bacteroides ovatus, have been shown to express tryptophanase, the enzyme needed for the breakdown of tryptophan to indole.57 In our study, we did not find an association between the genus Bacteroides and the plasma concentration of indoxyl sulfate (data not shown). However, with our microbial analysis, we were not able to assess the relative abundance of specific bacterial species or functional capacity. Future studies should assess whether the supplementation of prebiotic fibers, including inulin, modifies bacterial species with the capacity of producing p-cresyl sulfate and indoxyl sulfate.
We observed that the fecal microbiota was different depending on the BMI category. Individuals with a BMI $30 kg/m2 had a higher relative of Ruminococcus and unclassified Enterobacteriaceae. As mentioned above, some species within the Ruminococcus genus can lead to increases in proinflammatory cytokines.51 The family Enterobacteriaceae has been shown to be increased in individuals with obesity in a large cohort of U.S. adults58 and has been shown to increase endotoxemia, leading to an increase in systemic inflammation.59
CKD-mineral and bone disorder is a highly prevalent problem in HD patients.60 Phosphate binders represent the first line of treatment for controlling hyperphosphatemia, in addition to dietary phosphate restriction and dialysis treatment.60 In our study, we observed a unique gut microbiota in those participants that were prescribed sevelamer. Sevelamer is a polymer that has a non-selective ability to bind molecules, so in addition to binding phosphate, it binds other molecules such as indoles, indoxyl sulfate, and p-cresol.9 Interestingly, we observed that participants taking sevelamer had a lower relative abundance of Bacteroides compared with participants prescribed calcium- based binders. As mentioned above, tryptophanase is expressed in some of the species within the genus Bacteroides, such as B. thetaiotaomiron and B. ovatus. As phosphate binders are ubiquitously prescribed in this clinical population, future studies should assess the effect of phosphate binders on the gut microbiota composition and metabolites produced.61
There are limitations to our study. The results of our investigation may be limited due to the small sample size and a high BMI. Additionally, our fecal analysis of the metabolites derived from the gut microbiota was performed only in 75% of our sample; however, this was to ensure confidence in our results. Furthermore, an important problem in this clinical population is adherence with treatment.62 Even though verbal compliance was >80% in all our participants, we did not measure adherence with our treatments, such as performance of a breath hydrogen test.63 Interestingly, maltodextrin exhibited traits of a dietary fiber, evidenced by an increase in fecal SCFAs after participants received the placebo treatment. This unexpected finding may have limited our ability to detect an effect of inulin on similar outcomes. Finally, we collected only one fecal sample at each timepoint, which may have limited our ability to detect a more meaningful effect of our intervention. However, we believe our results are valuable as this is the first study, to our knowledge, using inulin as a prebiotic fiber to assess its overall effects on both the gut microbiota and derived metabolites in HD patients.
Practical Application
Prebiotic fibers have been proposed to be used in kidney failure because they may reduce protein fermentation byproducts by shifting the fermentation profile toward SCFA production. In our study, a 4-week supplementation of inulin did not produce major changes in the gut microbiota composition or derived uremic toxins indoxyl sulfate or p-cresyl sulfate. While there was an increase in the fecal excretion of SCFA after inulin supplementation, these also increased after our placebo, maltodextrin, which suggests that maltodextrin should not be used as a placebo in this clinical population. Importantly, factors such as the type of phosphate binder and BMI category may have a greater impact on the composition of the gut microbiota. As phosphate binders are one of the most commonly used drugs in individuals with kidney failure, their effects on the gut microbiome should be further explored.61 Finally, due to the high variability in the composition of the gut microbiota, future studies with a larger sample size should explore the effects of prebiotic fibers on the gut microbiota and outcomes to justify their use in individuals with kidney failure undergoing HD.
Supplementary Material
Acknowledgments
We would like to thank Sonja Bjelland and Anita Matthews for helping with the coordination of the study and the staff at the CU Dialysis Clinic and Illini DaVita Dialysis Clinic, especially to Deborah Fairow, Christina Quednau, and Joetta Little.
Support and Financial Disclosures: This work was supported by a research grant from the Renal Research Institute (C2930) and the Division of Nutritional Sciences at the University of Illinois (USDA Vision 20/20 research program award). Beneo GmbH donated Orafti Synergy, but had no input in the study design, data analyses, or manuscript preparation. AB was supported by a Predoctoral Fellowship from CONACyT (Mexico’s Council of Science and Technology), a postdoctoral fellowship NIH-T32 DK120524, has received honoraria from AMGEN, research grants from Keryx Pharmaceuticals for work unrelated to the present manuscript, and is part of the AUGmeNt workgroup from the Academy of Nutrition and Dietetics. BMK has received consulting fees, research grants, and is a member of the AUGmeNt workgroup from the Academy of Nutrition and Dietetics. KRW has received funding from the National Institutes of Health and the Renal Research Institute. Part of this work was presented at the 54th European Renal Association and European Dialysis and Transplantation Association Congress64 and at the XIX International Congress on Nutrition and Metabolism in Renal Disease.65
Footnotes
Supplementary Data
Supplementary data to this article can be found online at https://doi.org/10.1053/j.jrn.2020.10.003.
References
- 1.Fraher MH, O’Toole PW Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol. 2012;9:312–322. [DOI] [PubMed] [Google Scholar]
- 2.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stearns JC, Lynch MD, Senadheera DB, et al. Bacterial biogeography of the human digestive tract. Sci Rep. 2011;1:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent tri-methylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijers B, Evenepoel P, Anders HJ. Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol. 2019;15:531–545. [DOI] [PubMed] [Google Scholar]
- 6.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential Therapeutic Target. Am J Kidney Dis. 2016;67:483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. [DOI] [PubMed] [Google Scholar]
- 8.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;(114):S12–S19. [DOI] [PubMed] [Google Scholar]
- 10.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transpl. 2010;25:219–224. [DOI] [PubMed] [Google Scholar]
- 11.Rossi M, Johnson DW, Xu H, et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis. 2015;25:860–865. [DOI] [PubMed] [Google Scholar]
- 12.Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular Events and all Cause mortality in patients with chronic renal failure. PLoS One. 2015;10:e0132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barreto FC, Barreto DV, Canziani ME, et al. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J Bras Nefrol. 2014;36:289–296. [DOI] [PubMed] [Google Scholar]
- 14.Poesen R, Evenepoel P, de Loor H, Kuypers D, Augustijns P, Meijers B. Metabolism, protein binding, and renal Clearance of microbiota-derived p-cresol in patients with CKD. Clin J Am Soc Nephrol. 2016;11:1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luis D, Zlatkis K, Comenge B, et al. Dietary quality and adherence to dietary recommendations in patients undergoing hemodialysis. J Ren Nutr. 2016;26:190–195. [DOI] [PubMed] [Google Scholar]
- 16.de Loor H, Poesen R, De Leger W, et al. A liquid chromatography - tandem mass spectrometry method to measure a selected panel of uremic retention solutes derived from endogenous and colonic microbial metabolism. Anal Chim Acta. 2016;936:149–156. [DOI] [PubMed] [Google Scholar]
- 17.Alexander C, Swanson KS, Fahey GC Jr, Garleb KA. Perspective: physiologic importance of short-chain fatty acids from Nondigestible carbohydrate fermentation. Adv Nutr. 2019;10:576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluznick JL. Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int. 2016;90:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jama HA, Beale A, Shihata WA, Marques FZ. The effect of diet on hypertensive pathology: is there a link via gut microbiota-driven immunometabolism? Cardiovasc Res. 2019;115:1435–1447. [DOI] [PubMed] [Google Scholar]
- 20.Kaye DM, Shihata W, Jama HA, et al. Deficiency of prebiotic Fibre and Insufficient Signalling through gut metabolite Sensing receptors leads to cardiovascular disease. Circulation. 2020;141:1393–1403. [DOI] [PubMed] [Google Scholar]
- 21.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biruete A, Jeong JH, Barnes JL, Wilund KR. Modified Nutritional recommendations to Improve dietary patterns and outcomes in hemodialysis patients. J Ren Nutr. 2017;27:62–70. [DOI] [PubMed] [Google Scholar]
- 23.Kelly JT, Palmer SC, Wai SN, et al. Healthy dietary patterns and risk of mortality and ESRD in CKD: a Meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2017;12:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137(11 Suppl):2493S. [DOI] [PubMed] [Google Scholar]
- 25.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. [DOI] [PubMed] [Google Scholar]
- 26.Pokusaeva K, Fitzgerald GF, van Sinderen D. Carbohydrate metabolism in bifidobacteria. Genes Nutr. 2011;6:285–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selak M, Riviere A, Moens F, et al. Inulin-type fructan fermentation by bifidobacteria depends on the strain rather than the species and region in the human intestine. Appl Microbiol Biotechnol. 2016;100:4097–4107. [DOI] [PubMed] [Google Scholar]
- 28.Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. [DOI] [PubMed] [Google Scholar]
- 29.Chung WS, Walker AW, Louis P, et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biruete A Effects of inulin supplementation on markers of mineral and bone metabolism and the gut microbiota in hemodialysis patients. Urbana, IL, USA: University of Illinois at Urbana-Champaign; 2017. https://www.ideals.illinois.edu/bitstream/handle/2142/97577/BIRUETE-DISSERTATION-2017.pdf?isAllowed=y&sequence=1. [Google Scholar]
- 31.Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrition Requirements. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 32.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. [DOI] [PubMed] [Google Scholar]
- 33.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. [DOI] [PubMed] [Google Scholar]
- 35.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erwin ESM GJ, Emery EM. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J Dairy Sci. 1961;44:1768–1771. [Google Scholar]
- 38.Flickinger EA, Schreijen EM, Patil AR, et al. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J Anim Sci. 2003;81:2008–2018. [DOI] [PubMed] [Google Scholar]
- 39.(AOAC) AoOAC. Official methods of analysis. 17th Edition Gaithersburg, MD: Assoc. Off. Anal. Chem.; 2006. [Google Scholar]
- 40.Murphy SP. Collection and analysis of intake data from the integrated survey. J Nutr. 2003;133:585S–589S. [DOI] [PubMed] [Google Scholar]
- 41.Vandeputte D, Falony G, Vieira-Silva S, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66:1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holscher HD, Bauer LL, Gourineni V, Pelkman CL, Fahey GC Jr, Swanson KS. Agave inulin supplementation affects the fecal microbiota of healthy adults Participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:2025–2032. [DOI] [PubMed] [Google Scholar]
- 44.Salazar N, Dewulf EM, Neyrinck AM, et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr. 2015;34:501–507. [DOI] [PubMed] [Google Scholar]
- 45.Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guess ND, Dornhorst A, Oliver N, Frost GS. A Randomised crossover trial: the effect of inulin on Glucose Homeostasis in Subtypes of Prediabetes. Ann Nutr Metab. 2016;68:26–34. [DOI] [PubMed] [Google Scholar]
- 47.Dehghan P, Pourghassem Gargari B, Asghari Jafar-abadi M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition. 2014;30:418–423. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Xiong Q, Zhao J, et al. Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: a randomized crossover study. Am J Clin Nutr. 2020;111:1087–1099. [DOI] [PubMed] [Google Scholar]
- 49.Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45(Suppl):S120–S127. [DOI] [PubMed] [Google Scholar]
- 50.Lun H, Yang W, Zhao S, et al. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen. 2019;8:e00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019;116:12672–12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olano-Martin E, Mountzouris KC, Gibson GR, Rastall RA. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br J Nutr. 2000;83:247–255. [DOI] [PubMed] [Google Scholar]
- 53.Abrams SA, Griffin IJ, Hawthorne KM, et al. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82:471–476. [DOI] [PubMed] [Google Scholar]
- 54.Poesen R, Evenepoel P, de Loor H, et al. The Influence of prebiotic Arabinoxylan Oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: a randomized controlled trial. PLoS One. 2016;11:e0153893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos CI, Armani RG, Canziani MEF, et al.Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrol Dial Transpl. 2018. [DOI] [PubMed] [Google Scholar]
- 56.Warren FJ, Fukuma NM, Mikkelsen D, et al. Food Starch Structure impacts gut microbiome composition. mSphere. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devlin AS, Marcobal A, Dodd D, et al. Modulation of a circulating uremic Solute via Rational Genetic Manipulation of the gut microbiota. Cell Host Microbe. 2016;20:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters BA, Shapiro JA, Church TR, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8:9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao L The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639–647. [DOI] [PubMed] [Google Scholar]
- 60.Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, Evaluation, Prevention, and treatment of chronic kidney disease-mineral and bone disorder: Synopsis of the kidney disease: Improving Global outcomes 2017 clinical Practice Guideline Update. Ann Intern Med. 2018;168:422–430. [DOI] [PubMed] [Google Scholar]
- 61.Biruete A, Hill Gallant KM, Lindemann SR, Wiese GN, Chen NX, Moe SM. Phosphate binders and Nonphosphate effects in the gastrointestinal tract. J Ren Nutr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid H, Hartmann B, Schiffl H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res. 2009;14:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holscher HD, Doligale JL, Bauer LL, et al. Gastrointestinal tolerance and utilization of agave inulin by healthy adults. Food Funct. 2014;5:1142–1149. [DOI] [PubMed] [Google Scholar]
- 64.Biruete A, Kistler BM, Allen J, Bauer LL, Fahey GC, Swanson KS, et al. Effects of inulin supplementation on mineral metabolism and fecal short-chain fatty acid excretion in hemodialysis patients. Nephrol Dial Transpl. 2017;32(suppl_3). [Google Scholar]
- 65.Biruete A, Kistler BM, Cross TL, Allen J, de Loor H, Evenepoel P, et al. Effect of Dietary Inulin Supplementation on the Gut Microbiota and Derived Metabolites in Hemodialysis Patients. XIX International Congress on Nutrition and Metabolism in Renal Disease; Genoa, IT2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


