Abstract
Renal artery stenosis (RAS) elicits development of hypertension and post-stenotic kidney damage, which may become irresponsive to restoration of arterial patency. Rather than mere losses of blood flow or oxygen supply, irreversible intra-renal microvascular rarefaction, tubular injury, and interstitial fibrosis are now attributed to intrinsic pathways activated within the kidney, focusing attention on the kidney parenchyma as a therapeutic target. Several regenerative approaches involving delivery of reparative cells or products achieved kidney repair in experimental models of RAS, and delivery of mesenchymal stem/stromal cells has already been translated to human subjects with RAS with promising results. Ongoing development of innovative approaches in kidney disease await application, validation, and acceptance as routine clinical treatment to avert kidney damage in RAS.
Keywords: renovascular disease, hypertension, mesenchymal stem/stromal cells, regeneration
Rationale for renal protection in renovascular disease
Development of obstructions in the renal artery or arteries may evoke development of hypertension (see Glossary) and progressive loss of kidney function, or chronic kidney disease (CKD). In turn, hypertension and CKD are major independent risk factors for cardiovascular morbidity and mortality. Therefore, restoration of blood pressure (BP) control and maintenance of kidney function are critical healthcare priorities. However, clinical trials over the past two decades have shown that these goals are not often achieved upon removal of the renal artery obstruction. These findings have led to a precipitous fall in the number of revascularization procedures performed in patients with this disease, known as renal artery stenosis (RAS) or renovascular disease (RVD). Consequently, an increasing number of patients manifest deterioration of post-stenotic kidney function [1-3]. However, the mechanisms underlying kidney damage distal to RAS or its irreversibility remain obscure.
This review first describes current paradigms linked to kidney injury distal to large vessel disease (Figure 1), and recent clinical trials attempting to restore kidney function by reversing vascular obstruction. Second, it discusses the role of the renal microvessels in the pathogenesis of RVD. Third, mechanisms responsible for perturbing the renal microcirculation in RVD are examined. Finally, proposed regenerative approaches to repair the renal microvessels are discussed, as well as their potential drawbacks.
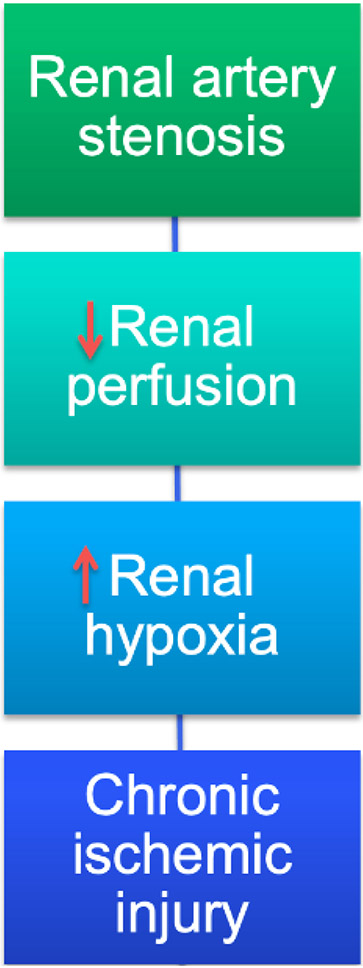
Figure 1. Traditional paradigms underpinning kidney injury distal to large vessel disease.
Renal artery stenosis is purported to injure the post-stenotic kidney by inducing hypoperfusion that leads to hypoxia, and thereby chronic ischemic injury, which have traditionally been ascribed to the upstream occlusive lesion. Recent studies have implicated intrinsic renal mechanisms as perpetuating microvascular loss and interstitial fibrosis.
RVD as a Clinical Entity
Clinical features
Hypertension is a major risk factor for cardiovascular disease and chronic kidney disease (CKD) (https://www.cdc.gov/bloodpressure/about.htm). Adjusted for age, in 2017–2018 the prevalence of hypertension among US adults aged ≥18 years was 45.4% [4]. Its prevalence increases by age and is slightly higher among men than women.
For most of the cases of hypertension in adults, the underlying etiology remains obscure, and probably includes an amalgamate of genetic, environmental, and lifestyle factors [5]. This form has been traditionally referred to as ‘essential hypertension’. A minority of cases of hypertension, 5-10%, are caused by partial obstruction in the renal artery that leads blood to the kidney. A hemodynamically significant RAS, or that expected to bear functional implications for the kidney and BP, is usually considered to involve at least 50-70% obstruction of the arterial lumen [6]. Almost 7% of adults over 65 years of age are estimated to have an obstruction of ≥60% in the renal artery [7]. Most of the obstructive lesions in the renal artery responsible for this clinical entity are caused by atherosclerosis and can evolve either in one (unilateral) or both (bilateral) kidneys. Indeed, this condition is common in individuals with atherosclerotic lesions in other vascular beds, like the coronary or peripheral vasculature. When an obstructive lesion develops in the renal artery, the kidney distal to RAS misinterprets the decrease in renal blood flow (RBF) as a fall in arterial BP, and promptly enlists to correct this emergency by elevating BP. However, because RAS is unlikely to resolve spontaneously, over time the kidney needs to adapt functionally and structurally to chronic loss of RBF and oxygen supply [8]. Therefore, the consequences of RAS include development of chronic secondary (renovascular) hypertension and kidney damage, which might become irreversible (Clinician’s Corner).
Clinician’s Corner.
Atherosclerotic renal artery stenosis (RAS) may lead to development of hypertension and chronic kidney disease, but randomized, controlled clinical trials have shown that these are not often reversed by revascularization. These findings have led to a decrease in the number of revascularization procedures performed, and in turn more patients with RAS now presenting with deterioration of post-stenotic kidney function.
Damage to the post-stenotic kidney was traditionally attributed to kidney ischemia and hypoxia secondary to an occlusive lesion in the renal artery. However, recent research findings depicted kidney injury in RAS as the culmination of complex interactions among a number of intrinsic pathological processes, including maladaptive, prolonged activation of the renin-angiotensin-aldosterone system, circulating nephrotoxic factors (oxidized lipids, cytokines, adipokines), and tissue hypoxia, which synergize to orchestrate kidney remodeling and intra-renal microvascular loss.
These findings provided the impetus to focus on the kidney parenchyma, rather that the stenosis per se, as a therapeutic target. Regenerative approaches harness endogenous repair mechanisms, involving different types of reparative cells or cellular products, to regenerate injured tissues and organ systems. Cell-based therapy has been successful in mitigating kidney injury in animal models of RAS.
This approach has been recently translated to the clinical platform. In a Phase-1a dose-escalating clinical trial, autologous adipose-tissue-derived mesenchymal stem/stromal cells (MSCs) were infused through the renal artery in 21 patients with RAS. Three months later, stenotic-kidney function improved, whereas hypoxia, renal-vein inflammatory cytokines, and systolic blood pressure fell compared to patients with RAS treated with medical therapy alone, although the renal artery was not revascularized. These findings established an exciting potential for autologous MSCs to directly attenuate injury in post-stenotic human kidneys.
Additional approaches being tested in experimental models include delivery of primary renal parenchymal cells, injectable hydrogel, and kidney organoids. Exciting developments in this field may introduce innovative advances and allow realizing this promising therapeutic strategy for management of patients with RAS.
In some cases, kidney injury in RVD evokes development of CKD. CKD impacts up to 17% of the European adult population [9] and 15% of US adults [https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html], driven by high prevalence of diabetes, hypertension, and obesity. Adults with CKD are at elevated risk of early death, heart disease, and stroke. In the US, Medicare spending for patients with CKD aged 65 and older represented 20% of all Medicare spending in this age group [10]. There is no cure for CKD, which might progress to require renal replacement therapy (RRT) or cause death [6]. Treatment options to reverse CKD beyond management of lifestyle are limited.
Interventions
The potential grave sequalae of RVD and the ostensible simplicity of a definitive treatment have motivated development of interventional techniques to resolve RAS. Given that the cause of kidney injury and development of secondary hypertension in patients with RVD appears self-evident (occlusive vascular disease), considerable effort has been directed towards relief of the obstruction in order to restore kidney function and BP control. The initial bypass graft surgical approach was popular in the 1970s [2] and created a new pathway for RBF to bypass the occlusion. However, in the 1990s surgery was mostly replaced in favor of a less-invasive endovascular procedure, percutaneous transluminal renal angioplasty (PTRA) and stenting (Figure 2).
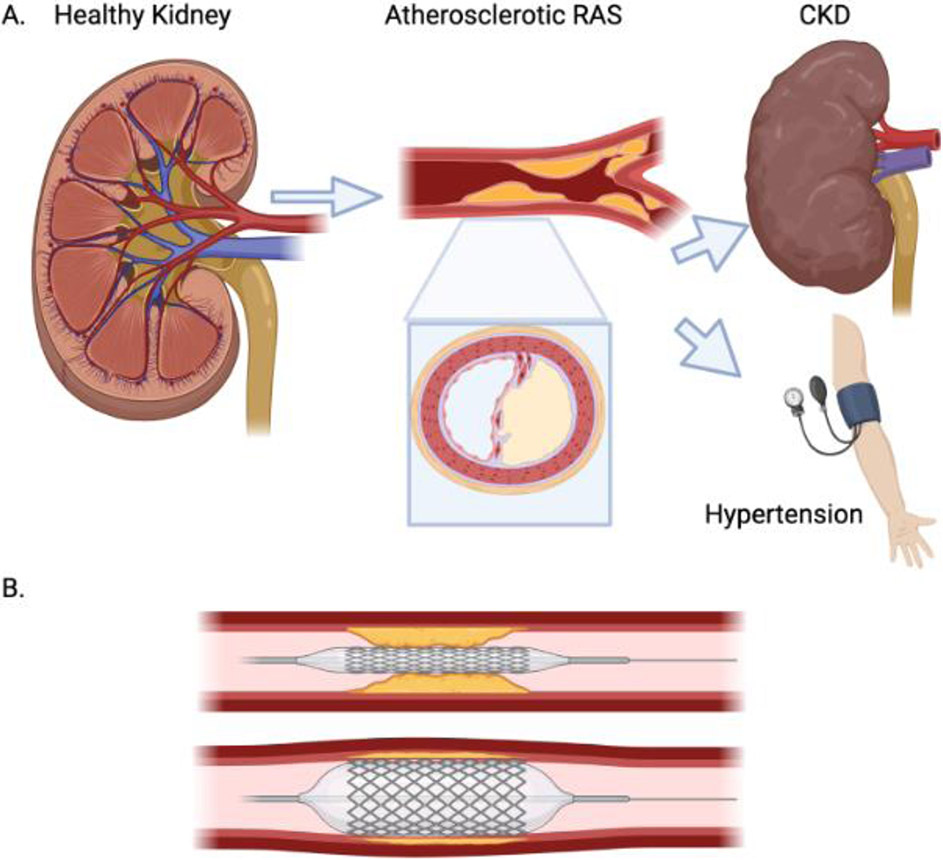
Figure 2. Development and revascularization of renal artery stenosis (RAS).
A. Development of atherosclerotic (or other) lesions in the renal artery (seen in longitudinal and cross-sections) may lead to development of hypertension and to chronic kidney disease (CKD). B. During percutaneous transluminal renal angioplasty (PTRA), a balloon (often with a stent mounted on it) (top) is inflated in the stenotic renal artery (bottom) to dilate the obstruction. The balloon is subsequently deflated and disengaged, leaving the stent engrafted in the renal artery to keep it open. Created with BioRender.com.
However, the subsequent two decades harbored clinical trials that cast doubt on the utility of either surgical or endovascular approaches. Some nonrandomized, comparative studies, single-group studies, selected case reports, and sub-group analyses [11] showed heterogeneous effects of PTRA and stenting on kidney function and BP in selected groups of patients. However, no randomized, controlled trials (RCT) detected consistently meaningful benefits for PTRA in terms of kidney function, the need for RRT, cardiovascular events, or mortality compared to medical therapy alone [2, 12] (Clinician’s Corner). The largest, most recent RCT were the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial (ISRCTN59586944)I published in 2009 [13] and the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial published in 2014 [14]. ASTRAL was a randomized, unblinded trial, in which 806 patients with atherosclerotic RVD were assigned to undergo either PTRA in addition to medical therapy or to medical therapy alone, with a median follow-up of 34 months. The primary outcome was renal function, while secondary outcomes included BP, the time to renal and major cardiovascular events, and mortality. This trial identified substantial risks (serious complications occurred in 23 revascularized patients) but no clinical benefit from PTRA [13]. In the CORAL phase-3 trial (NCT00081731)II, 947 patients with atherosclerotic RVD and either systolic hypertension or CKD were randomly assigned to similar groups (medical therapy with or without PTRA/stenting) followed for 43 months. The composite endpoint was death from cardiovascular or renal causes, myocardial infarction, stroke, hospitalization for congestive heart failure, progressive renal insufficiency, or need for RRT. The trial investigators concluded that PTRA/stenting did not confer benefit beyond medical therapy in terms of prevention of clinical events in these patients [14].
The results of these RCTs called into question the simplistic view ascribing a pivotal role to mechanical RAS alone driving progressive kidney dysfunction or hypertension. Hence, development of effective treatment strategies for RVD warrants reexamination of existing paradigms (Figure 1) and unraveling novel mechanisms underlying kidney injury.
Conventional paradigms underpinning kidney injury distal to RAS
The degree of RAS as the main determinant of post-stenotic kidney injury
Epidemiologic and imaging studies frequently consider lumen occlusion >50-60% as hemodynamically significant [6], but have used divergent tools and inconsistent indices to evaluate the stenosis. For example, angiographic techniques like x-rays, computed tomography, or magnetic resonance imaging (MRI) angiography might be 2D or 3D, and refer to a change in renal arterial lumen or diameter. Doppler ultrasound assesses the degree of RAS based on peak systolic velocity, which is inversely proportional to the degree of stenosis. Reductions in trans-lesion pressure or flow usually develop only at >70-80% occlusion [15], and release of renal-vein renin (an index of a noteworthy obstruction) occurs when trans-lesion pressure gradients in the renal artery fall by at least 10-20% [16]. Furthermore, an obstructive lesion (Figure 2) can generate variable gradients and post-stenotic waveforms, depending on flow characteristics. Last but not least, it became evident that the impact of RVD on renal hemodynamics and outcomes largely depends on the underlying etiology and intra-renal damage, which do not necessarily correlate with the degree of proximal stenosis [17-20]. For example, an atherosclerotic environment exerts direct effects on the tissue distal to RAS, although it likely synergizes with tissue ischemia to accelerate injury and fibrosis. Overall, the degree of RAS, which is difficult to evaluate clinically, is not necessarily a primary determinant of post-stenotic kidney injury.
A decrease in renal arterial flow as a determinant of post-stenotic kidney perfusion
Pre-clinical studies have consistently demonstrated that besides a main vessel obstruction, RVD induces intra-renal modifications that directly decrease tissue perfusion. In a swine model RAS decreased the spatial density of cortical microvessels and increased arteriolar tortuosity (an index of vascular immaturity), which were preventable by antioxidant intervention [21]. Given that the renal artery was not revascularized, increased intra-renal oxidative stress likely contributed to microvascular loss. Furthermore, superimposed atherosclerosis aggravates the fall in swine renal perfusion [22], underscoring the impact of the microenvironment on loss of RBF. Indeed, for the same degree of RAS, cortical perfusion is lower in patients with atherosclerotic RVD compared to patients with fibromuscular dysplasia secondary to a localized renal arterial wall lesion [17]. Therefore, RBF may fall in RVD regardless of the degree of RAS.
Tissue hypoxia as a driver of post-stenotic kidney injury
Although renal oxygen supply exceeds its metabolic requirements, it stands to reason that reduced blood supply would eventuate in kidney tissue hypoxia. Particularly susceptible is the outer medullary inter-bundle region, in which in extreme cases partial oxygen pressure may drop <5mmHg [23]. Remarkably, however, sampling the vein draining the post-stenotic kidney revealed preserved partial oxygen pressure and intra-renal tissue oxygenation despite a fall in RBF compared to patients with essential hypertension, possibly due to a parallel reduction in energy requirements for active solute transport [24]. Nonetheless, subsequent studies established the limits of kidney adaptation to hypoperfusion. Using blood oxygen level-dependent-MRI to assess intra-renal tissue deoxyhemoglobin, this group showed that severe vascular occlusion ultimately overwhelms renal capacity to adapt to reduced RBF, which developed overt cortical hypoxia [25]. In fact, intra-renal deoxyhemoglobin has been proposed to identify viable kidney parenchyma amenable for revascularization [26]. Nonetheless, superimposed atherosclerosis in swine RAS aggravates medullary hypoxia [22], consistent with a direct impact of the milieu on relentless post-stenotic kidney damage [27]. Additionally, reversal of hypoxia by PTRA does not necessarily blunt kidney inflammation or dysfunction [28]. Clearly, therefore, the relationship of renal hypoxia and perfusion is complex, and hypoxia might dissociate from irreversible post-stenotic kidney damage.
Kidney damage in RVD is secondary to chronic ischemia
Development and progression of RAS is a gradual and prolonged process. In 170 patients monitored with serial renal artery duplex scans for 33 months, the cumulative incidence of RAS progression increased from 35% at 3 years to 51% at 5 years [29]. Nevertheless, in human RVD levels of the “acute injury” biomarkers neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 are elevated [30]. Possibly, besides a chronic ischemia, RVD may encompass repeated acute ischemic injury episodes secondary to fluctuating physiological variables like sympathetic tone, BP, or RBF. The contribution of repeated insults to kidney deterioration is supported by the observation that repeated episodes of experimental acute kidney injury with apparent tubular recovery can eventuate in interstitial fibrosis and inflammation [31]. Thus, RVD might produce an initial hemodynamic reduction in RBF that transitions into repeated cycles of self-perpetuating tissue damage.
Mechanisms of intra-renal injury
Glomerulosclerosis is often linked to severe stenosis or comorbidities [32, 33], whereas the predominant pathologic feature in ischemic kidney damage is tubulo-interstitial changes [34, 35]. Identification of processes implicated in development of tubular epithelial cell (TEC) atrophy and interstitial fibrosis requires reconsideration of mechanisms underpinning tissue damage in RVD. Pre-existing and co-existing risk factors likely magnify, but are not solely accountable for, stenotic kidney damage in subjects with RVD [36]. Research over the past 2-3 decades depicted kidney injury in RVD as the culmination of complex interactions among a number of intrinsic pathological processes [37], including maladaptive, prolonged activation of the renin-angiotensin-aldosterone system, circulating nephrotoxic factors (oxidized lipids, cytokines, adipokines), and tissue hypoxia, which synergize to orchestrate kidney remodeling [37, 38].
This interaction is partly mediated by increased generation of reactive oxygen species (ROS), elevating oxidative-stress and cytokine release by infiltrating inflammatory cells. ROS and cytokines injure renal endothelial cells and TEC, which may enter a program of mesenchymal transition towards myofibroblasts [39-41], develop inflammation, or experience premature pre-programed death (apoptosis), necrosis [42], or cellular senescence [43]. Some of these alterations might be partly reversible until fibrosis eventuates in permanent parenchymal damage. Growth factors and cytokines (like transforming growth factor-β or plasminogen activator inhibitor-1) released from inflamed renal tubular cells, infiltrating leukocytes, and macrophages stimulate collagen deposition, regulate extracellular matrix turnover, and foster fibrosis. Alleviating activation of processes directly responsible for parenchymal damage is therefore capable of improving renal outcomes, regardless of restoration of renal arterial patency [44].
Similar processes elicit microvascular dysfunction and loss that characterize the post- stenotic kidney and ultimately produce fibrosis. Renal microvessels are vulnerable to pernicious insults that jeopardize their integrity and engender endothelial cell swelling and dysfunction. Reduced bioavailability of vasodilators and increased vasoconstrictor activity are early events that enhance renal vascular tone and imperil the protective barrier functions of the endothelium. In turn, prolonged vasoconstriction evokes lasting remodeling (e.g., thickening) and rarefaction (loss) of the microcirculation. While acute hypoxia typically stimulates new vessel formation (angiogenesis), this compensatory function is markedly encumbered during chronic ischemia, decreasing the efficiency of restoring stenotic kidney perfusion.
Peritubular capillary rarefaction, a hallmark of CKD, precedes development of fibrosis [45] and associates with microvascular dysfunction [46]. In addition to endothelial cells and capillaries, fibrotic kidneys show loss of broad size-range blood vessels [47]. Microvascular dysfunction and rarefaction that characterize stenotic kidneys in both animal models [42] and human subjects [48] correlate with loss of kidney function and vitality [49-51], and fuel a feedforward mechanism of irreversible kidney damage. Therefore, restoration of the microcirculation is at the core of attractive therapeutic approaches [52].
Regenerative therapy for RVD
Elucidation of primary deleterious drivers in RVD can advance our understanding of the pathogenesis of renal injury and support development of effective therapies. Many proposed mechanisms (endothelial dysfunction, oxidative-stress, fibrosis, inflammation, apoptosis, cellular senescence, etc.) have been targeted individually to variable degrees of success. However, the past two decades have realized a new treatment platform that exerts simultaneous multi-pronged manipulation of multiple culprit pathways. Innovative approaches mimic or harness endogenous repair mechanisms, involving different types of reparative cells or cellular products, to regenerate injured tissues and organ systems. Because the intrinsic reparative system might be dysfunctional, overwhelmed, or otherwise insufficient during disease, regenerative medicine strategies aim to replenish this system by exogenous delivery of cells harboring varying levels of pro-angiogenic, anti-inflammatory, immunomodulatory, antioxidant, and anti-fibrotic properties. Their source has been either autologous (harvested from the same subject), allogeneic (from another subject of the same species), or even xenotransplant (donor of another species), depending on the cell type and its proclivity to evoke an immune response in the host (Box 1).
Box 1: Characterization of exogenous reparative cellular products.
Endothelial progenitor cells (EPCs) are blood borne, bone-marrow-derived cells of hematopoietic origin that are mobilized endogenously in response to homing signals [87]. They express the surface markers CD34 and Flk-1, with less mature forms expressing CD133. EPCs are committed towards an endothelial lineage and are thus well-appointed to generate new microvessels [57].
Mesenchymal stem/stromal cells (MSCs) reside in the vascular fraction (stroma) of different adult tissues, including the bone-marrow, fat, and skin and can be easily isolated [88]. They self-renew and differentiate into mesenchymal cell types, possess immunomodulatory properties (and partly evade rejection by host), and are capable of tissue repair [89]. MSCs are identified by tri-lineage differentiation (e.g., towards adipocytes, chondrocytes, and osteocytes), plastic adherence in culture, and CD73+/D90+/CD105+ surface marker expression, but no CD14/CD34/CD45. MSCs show variable and modest expression of major histocompatibility complex (MHC) class-I and II molecules, and no co-stimulatory molecules CD40, CD40 ligand, CD80 and CD86. Therefore, MSCs induce only minor activation of T-cells, are considered relatively “immunoprivileged”, and their use might be feasible in allogeneic experimental settings with less concern for immune rejection [69]. This has enabled development of allogeneic ‘off-the-shelf’ commercial products (Box 2).
- Extracellular vesicles (EVs) are produced by most cell types in multicellular organisms, derive from the plasma membrane or endosomes, and range in size from 50-1,000 nm in diameter. Micro-vesicles (~50 nm to 1μm) are released through plasma membrane budding; exosomes (~40-160 nm) originate from the endosomal pathway [90]. In 2018 the International Society for Extracellular Vesicles released Minimum Information for the Study of Extracellular Vesicles (MISEV) guidelines [91], including:
- At least three positive protein markers of EVs, including at least one-transmembrane/lipid-bound protein-cytosolic protein, such as Tetraspanins (CD63, CD81, CD82); other multi-pass membrane proteins (CD47; heterotrimeric G proteins GNA); MHC class I (HLA-A/B/C, H2-K/D/Q), Integrins (ITGA/ITGB), transferrin receptor (TFR2); LAMP1/2; heparan sulfate proteoglycans including syndecans (SDC); EMMPRIN (BSG); ADAM10; GPI-anchored 5′nucleotidase CD73 (NT5E), complement-binding proteins CD55 and CD59; sonic hedgehog (SHH)
- At least one negative protein marker
- Characterization of single vesicles using two different complementary techniques, like electron or atomic force microscopy and single particle analyzers
Endothelial progenitor cells (EPCs)
Given the complexity of kidney disease mechanisms, cell-based approaches are particularly appealing in RVD. The first type of cells applied in RVD were EPCs (Box 1). The post-stenotic kidney releases ample injury signals to attract circulating EPCs [53], which evidently do not suffice to restore its microcirculation. In RAS pigs, four weeks after exogenous intrarenal infusion, autologous EPCs preserved renal microvascular function and architecture, attenuated kidney fibrosis [54, 55], and adjunctive delivery also improved PTRA outcomes [56]. These observations support this promising therapeutic intervention for preserving the kidney in RVD. Intriguingly, application of low-energy shockwave therapy over the post-stenotic kidneys enhances recruitment of circulating EPCs [57], introducing a potential tool to harness endogenous reparative systems.
Mesenchymal stem/stromal cells (MSCs)
EPC collection from peripheral blood is somewhat cumbersome, and EPCs are recognizable by the host immune system, precluding allogeneic delivery. Thus, practical considerations motivated pursuit of alternative cell types. MSCs [58] are less potent than EPCs in direct induction of angiogenesis (Box 1). However, they express angiogenic factors, robustly decrease inflammation, apoptosis, and immune activation, prominent components of renal ischemic injury, and thereby increase neovascularization both directly and indirectly [59]. Thus, MSCs have been hailed as a promising therapeutic strategy for diabetic kidney disease [60], kidney transplantation [61], and other forms of kidney injury [62]. Indeed, adipose-tissue-derived MSCs injected into the stenotic renal artery of RVD pigs decreased kidney hypoxia, fibrosis, and apoptosis, preserved the microvasculature, and improved function [42]. Delivered in conjunction with PTRA, MSCs restored renal function and improved interventional success [63]. Furthermore, repeated weekly injections of MSCs in RAS rats blunted BP, proteinuria, and sympathetic hyperactivity [64, 65], and improved contralateral kidney sodium excretion [66, 67].
Importantly, a cell-based approach has been recently translated into human subjects with RVD. In a Phase-1a dose-escalating clinical trial (NCT02266394)III, autologous adipose-tissue-derived MSCs were infused through the renal artery in 21 patients (1, 2.5 or 5.0x10^5 cells/kg, n=7 each). Three months later, stenotic-kidney RBF and GFR increased dose-dependently, whereas hypoxia, renal-vein inflammatory cytokines, and systolic BP fell compared to 18 RVD patients (matched for age, kidney function, and BP) treated with medical therapy alone [68, 69]. These findings established an exciting potential for autologous MSCs to attenuate injury in post-stenotic human kidneys [70].
MSC-derived extracellular-vesicles (EVs)
Despite their excellent safety profile, concerns about potential tumor-formation by exogenous self-replicating MSCs might limit their use (Box 2). MSCs act partly by paracrine release of growth factors, cytokines, as well as daughter EVs (Box 1) carrying a cargo of genes, proteins, lipids, and micro-RNAs resembling their parent cells, thereby modulating favorably signaling pathways in recipient cells. Being acellular, EVs do not replicate, and their preparation and preservation display some practical advantages over MSCs, such as greater stability, standardization, storage, and up-scalability. MSC-derived EVs (a mixture of various sizes) delivered into the stenotic renal artery of RVD pigs attenuate renal inflammation and fibrosis and restore function [42, 71]. However, in RAS rats EVs produced beneficial results but with lower efficacy than MSCs, with larger EVs more effective than smaller exosomes [72]. Possibly, their inability to replicate in-situ might shorten engraftment and retention of EVs within the kidney, rendering their effects more transient. Notably, given their minimal immune-reactivity and intrinsic ability to cross physical barriers, EVs are gaining traction as potential natural carrier systems for delivery of therapeutics [73]. The first pilot clinical trial applying umbilical-cord MSC-EV in patients with CKD has already shown that they were safe and improved kidney function [74].
Box 2: Potential limitations of cell-based therapy in RVDa.
- Cell preparation and conditioning
- Need to select source: fat, bone marrow, umbilical cord, embryonic, etc.
- Caveats for autologous cells:
- Inter-individual variability with varied genetic/epigenetic features
- Age/risk factor effects
- Optimized expansion, media, testing and verification
- Time consuming preparation, validation, and delivery
- Mandate pre-planning, but may need to abort if cells are not up to par
- No option for off-the-shelf product
- Caveats for allogeneic cells
- Inter-individual variability of donor cells
- Risk of host allosensitization
- Potential cell destruction due to host immune response
- Prolonged cryopreservation may yield cells with lower quality and viability
- Preconditioning may be needed to optimize cells: genetic manipulation (boost response to homing signals or adhesion molecules); hypoxia pre-conditioning; pretreatment with drugs (e.g., senolytic); selection of cell subpopulations
- Cell administration
- Localized: invasive, transient residence, but achieves higher local dose
- Systemic: low retention rates (higher dose required); transient; off-target sequestration
- Need to develop effective, sensitive, non-invasive, and robust
- Tracking over time and location
- Biomarkers to determine recipient eligibility and monitor response
- Dose response:
- Persistence of effect duration unclear
- Use of high doses may be required to achieve effects
- May necessitate repeated dosing
- Opportunities for delivery as adjunctive maneuvers
- Revascularization of the renal artery
- Deliver a cocktail of different cell types
- Combine with other therapies (pro-angiogenic, mitochondrial protection)
- Include scaffold (e.g., hydrogel, extracellular matrix)
- Use cells as therapeutic vehicle (loaded with drug, genetic material, etc.)
aModified from [3].
Kidney parenchymal cells
Considering the need to replace injured cells, delivery of primary renal parenchymal cells has been attempted in models of CKD. Mature TEC isolated by differential buoyant density show immunomodulatory and trophic properties in rat models of CKD [75], and TEC-derived EVs limit acute renal ischemic injury [76, 77]. Pericyte-like cells derived from human immature dental-pulp stem cells also restore TEC in glycerol-induced acute renal failure in rats [78]. These approaches are yet to be described in chronic RAS or RVD. Yet, in murine RAS dedifferentiated CD24+/CD133+ TEC-progenitors improve renal function and downregulate profibrotic and inflammatory gene expression [79], and their EVs confer protective effects as well [80]. Injection of human renal artery progenitor cells also improved renal function in animal models after ischemia/reperfusion injury [81]. Limitations of adult renal cell delivery, however, include their immunogenicity and limited availability, requiring invasive harvesting, complex expansion, and delivery of autologous cells with modest proliferation capacity. Thus far, these issues constrain this approach to the research arena.
Other products
Cell-based therapy is associated with several caveats (Box 2), and alternative options are being actively sought, such as introduction of biomaterials into the kidney to facilitate regeneration of glomerular and tubular structures [82]. Kidney extracellular matrix hydrogel forms an injectable scaffold for delivering adipose-derived MSCs into ischemic kidneys [83], and can serve as a robust platform for drug delivery in acute kidney injury [84]. Concurrent delivery of human MSCs and EPCs into the subcutaneous and sub-renal capsular space in mice also permits generation of joint human/murine renovascular units [85]. Both MSCs and their EVs have also been developed and employed as bio-compatible nano-carriers of drugs into target tissues (Figure 3). Exciting advances over the past few years include the use of kidney organoids, produced by directed differentiation of pluripotent stem cells, which might enable drug screening, disease modelling, and generation of tissue for renal replacement [86]. Studies are urgently needed to determine the feasibility of novel approaches to alleviate kidney ischemic injury associated with RAS and RVD.
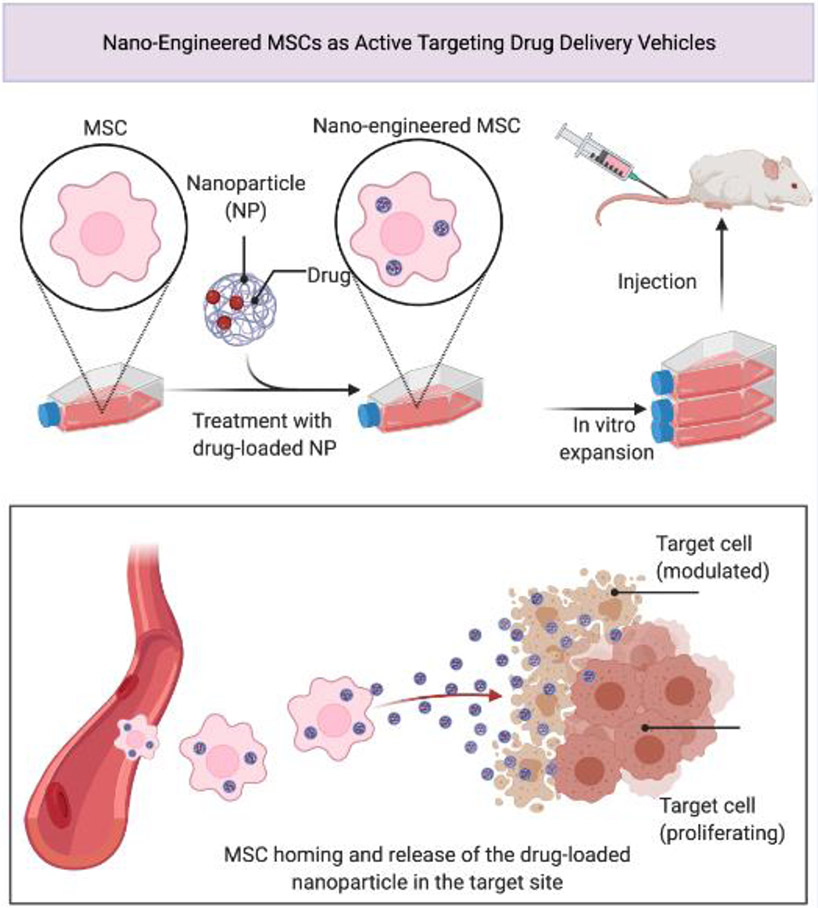
Figure 3. Nano-Engineered MSCs as Active Targeting Drug Delivery Vehicles.
The lack of specificity of therapeutic agents toward cells, and production of a plethora of undesirable side effects, led to development of novel strategies in drug delivery. Combining nanotechnology and cell-based therapy enables generation of “nano-engineered” mesenchymal stem cells (MSCs), or alternatively extracellular vesicles derived from them, which would be able to both actively target the disease site and protect the drug-loaded nanoparticle (NP) from vascular filtration and macrophage clearance. Adapted from “Nano-Engineered MSCs as Active Targeting Drug Delivery Vehicles”, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates
Concluding Remarks
Evolving paradigms suggest that irreversible tissue remodeling distal to an occlusive lesion in the renal artery results from progressive microvascular loss, TEC damage, and interstitial fibrosis, which become irresponsive to conventional therapy. Regeneration of the post-stenotic kidney by replacement of irrevocably impaired parenchymal cells and microvessels has become an attractive therapeutic option to boost or substitute for revascularization techniques. Tissue regeneration is achievable by direct implantation of reparative cells, but more commonly attainable via paracrine function of transiently engrafted cells, their functional vectors, or other biomaterials. Alas, despite compelling promise, cell-based therapy is yet to gain regulatory approval for routine treatment of kidney disease, given a number of unresolved issues (see Outstanding Questions). Prominent among them are concerns about and difficulty to ascertain the fate of injected products, uncertainties about their persistence in the tissue, or the duration of their benefits (Box 2). Nevertheless, this field evolves rapidly, as innovative regenerative solutions are identified continuously. Exciting developments in this field, both within and outside nephrology, may introduce innovative advances that would allow realizing this promising therapeutic strategy for management of patients with RVD.
Outstanding Questions.
Can injected cells be tracked? Cell fate, distribution, and migration kinetics are difficult to define in-vivo. Labeling with nanoparticles affords tracking cells using imaging, but low resolution prohibits detecting single cells or verifying viability. Bioluminescence imaging monitors longitudinally cell clusters in rodents, but not in larger animals or human subjects. Ex-vivo, prelabeled cells are counted in harvested kidneys, but low frequency and sampling errors prohibit reliable detection in biopsies. Novel noninvasive cell tracking methods are urgently needed.
How long do injected cells persist in the kidney? Reported retention times vary from days to months, and EVs even shorter, depending on the type, dose, and detection method. The duration of their beneficial effects remains to be elucidated, but in humans with RVD lasted at least 3 months.
What are the delivery routes of cell-based therapy? Cells injected systemically tend to lodge in the lung and liver, with a minority engrafting in the kidney. Intra-arterial delivery, while efficient, is invasive. However, MSCs engraft in larger numbers in ischemic kidneys expressing homing/adhesion molecules. Cells engineered to respond briskly to local cues show robust engraftment, but remain to be developed for clinical applications.
Do regenerative cells prevent or regress tissue damage? Studies employing recurring biopsies to show regression of kidney damage are lacking, but studies suggest the ability to reverse existing damage. Noninvasive biomarkers of renal fibrosis and microvascular density are needed for noninvasive monitoring, preferably of each kidney individually, such as imaging.
Can growth factors substitute for cells? Delivery of vascular endothelial or hepatocyte growth factors, or MSC paracrine factors, can achieve kidney repair, but might have off-target effects and a limited repertoire. Ongoing studies developing kidney-targeted growth factor delivery show promise for future applications.
Highlights.
Atherosclerotic renal artery stenosis (RAS) may induce renovascular hypertension and elicit development of irreversible post-stenotic kidney damage
Parenchymal injury in RAS is characterized by microvascular rarefaction, tubular injury, and interstitial fibrosis secondary to intrinsic pathways maladaptively activated within the kidney
Direct repair of the post-stenotic kidney is becoming a mainstay of restoration of kidney function in RAS, with or without revascularization of the renal artery
Regenerative approaches involving delivery of reparative cells or products can achieve kidney repair in experimental models and human subjects with RAS
Acknowledgements
Partly supported by NIH grant numbers DK120292, DK122734, and AG062104.
Glossary
- Angiogenesis
Development of new blood vessels.
- Apoptosis
Pre-programed cell death that aims to eliminate damaged cells.
- Blood pressure (BP)
The force that blood exerts against blood vessel walls, which may ultimately cause health problems. Systolic blood pressure (SBP) is the maximum arterial pressure, whereas diastolic blood pressure (DBP) is the minimum pressure. Mean arterial pressure (MAP) is the average arterial pressure throughout one cardiac cycle, systole, and diastole, and can be calculated as MAP=DBP+1/3(SBP–DBP).
- Cellular senescence
The process of deterioration with age, which also describes loss of cellular capability for division and growth.
- Chronic kidney disease (CKD)
A situation in which kidneys experience gradual loss of function and cannot filter blood adequately.
- Endothelial progenitor cells (EPCs)
See Box 2.
- Extracellular vesicles (EVs)
See Box 2.
- Fibrosis
Aberrant healing in which excessive connective tissue replaces normal parenchyma, which can become damaged and scarred.
- Hypertension
Blood pressure consistently higher than normal that can damage the heart and other organs. A normal BP level is less than 120/80mmHg (SBP/DBP). Health care professionals may consider BP consistently ≥130/80mmHg as hypertension (https://www.cdc.gov/bloodpressure/about.htm).
- Inflammation
A biological response to insults that trigger an immune reaction.
- Magnetic resonance imaging (MRI)
A medical imaging tool in which a subject placed in a strong magnetic field is exposed to high-frequency radio waves, and the response of the tissue atomic nuclei measured to generate images.
- Mesenchymal stem/stromal cells (MSCs)
See Box 2.
- Microvessels
Small arterioles, capillaries, and venules within organs, which deliver oxygen and nutrients, remove carbon-dioxide, and regulate tissue perfusion.
- Oxidative stress
An imbalance between tissue production or accumulation of free radicals and antioxidants.
- Percutaneous transluminal renal angioplasty (PTRA)
A procedure aimed at relieving renal artery obstructions, often followed by implantation of a stent to hold the renal artery open.
- Randomized, controlled trials (RCT)
A scientific study designed to reduce bias by randomly allocating subjects to groups treated differently and comparing their response.
- Reactive oxygen species (ROS)
Unstable molecules containing oxygen that easily react with other molecules and when accumulate may cause cell damage.
- Renal artery stenosis (RAS)
a narrowing of the renal artery/arteries that carry blood to one or both of the kidneys.
- Renal blood flow (RBF)
The volume of blood delivered to the kidneys per unit time.
- Renal replacement therapy (RRT)
Substitution of the blood-filtering function of the kidney in patients with renal failure.
- Renovascular disease (RVD)
A disease affecting the kidney arterial supply, consequently impairing renal perfusion. Often interchangeable with ‘RAS’, some consider RVD as RAS that is complicated by co-morbidities, or as microvascular kidney diseases alone (secondary to hypertension, diabetes, etc.).
- Stenting
Inserting a small tube, sometimes coil-shaped, into a blocked passageway to keep it open.
- Tubular epithelial cells (TEC)
A layer of cells in the renal tubule, which participate in renal function and transport of substances.
Footnotes
Resources
I. Registered in Current Controlled Trials https://www.isrctn.com/search?q=59586944
II. Registered with ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00081731
III. Registered with ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02266394
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saad A et al. (2015) Chronic renal ischemia in humans: can cell therapy repair the kidney in occlusive renovascular disease? Physiology (Bethesda) 30 (3), 175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Textor SC and Herrmann SM (2017) Evidence and Renovascular Disease: Trials and Mistrials? Am J Kidney Dis 70 (2), 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Textor SC et al. (2021) Stem Cell Therapy for Microvascular Injury Associated with Ischemic Nephropathy. Cells 10 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostchega Y et al. , Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018, NCHS Data Brief. National Center for Health Statistics, Hyattsville, MD, 2020. [PubMed] [Google Scholar]
- 5.Guarner-Lans V et al. (2020) Early Programming of Adult Systemic Essential Hypertension. Int J Mol Sci 21 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchie J and Kalra PA (2014) Atherosclerotic Renovascular Disease: Epidemiology and Clinical Manifestations. In Renal Vascular Disease (Lerman LO and Textor SC eds), pp. 3–38, Springer-Verlag. [Google Scholar]
- 7.Hansen KJ et al. (2002) Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 36 (3), 443–51. [DOI] [PubMed] [Google Scholar]
- 8.Eirin A et al. (2020) Pathophysiology of renal artery disease. In Vascular medicine: A companion to Braunwald’s Heart Disease (Third edn) (Creager MA et al. eds), pp. 300–311, Elsevier. [Google Scholar]
- 9.Bruck K et al. (2016) CKD Prevalence Varies across the European General Population. J Am Soc Nephrol 27 (7), 2135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saran R et al. (2020) US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 75 (1 Suppl 1), A6–A7. [DOI] [PubMed] [Google Scholar]
- 11.Murphy TP et al. (2016) Relationship of Albuminuria and Renal Artery Stent Outcomes: Results From the CORAL Randomized Clinical Trial (Cardiovascular Outcomes With Renal Artery Lesions). Hypertension 68 (5), 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raman G et al. (2016) Comparative Effectiveness of Management Strategies for Renal Artery Stenosis: An Updated Systematic Review. Ann Intern Med 165 (9), 635–649. [DOI] [PubMed] [Google Scholar]
- 13.Wheatley K et al. (2009) Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361 (20), 1953–62. [DOI] [PubMed] [Google Scholar]
- 14.Cooper CJ et al. (2014) Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370 (1), 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rognant N et al. (2010) Hemodynamic responses to acute and gradual renal artery stenosis in pigs. Am J Hypertens 23 (11), 1216–9. [DOI] [PubMed] [Google Scholar]
- 16.De Bruyne B et al. (2006) Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol 48 (9), 1851–5. [DOI] [PubMed] [Google Scholar]
- 17.Lerman LO et al. (1996) Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int 49 (3), 846–54. [DOI] [PubMed] [Google Scholar]
- 18.Kotliar C et al. (2012) Local and systemic cellular immunity in early renal artery atherosclerosis. Clin J Am Soc Nephrol 7 (2), 224–30. [DOI] [PubMed] [Google Scholar]
- 19.Wright JR et al. (2001) Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant 16 (4), 765–70. [DOI] [PubMed] [Google Scholar]
- 20.Khangura KK et al. (2014) Extrarenal atherosclerotic disease blunts renal recovery in patients with renovascular hypertension. J Hypertens 32 (6), 1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu XY et al. (2004) Cortical Microvascular Remodeling in the Stenotic Kidney. Role of Increased Oxidative Stress. Arterioscler Thromb Vasc Biol 24 (10), 1854–1859. [DOI] [PubMed] [Google Scholar]
- 22.Sun D et al. (2016) Early atherosclerosis aggravates renal microvascular loss and fibrosis in swine renal artery stenosis. J Am Soc Hypertens 10 (4), 325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CJ et al. (2019) Analysis of the critical determinants of renal medullary oxygenation. Am J Physiol Renal Physiol 317 (6), F1483–F1502. [DOI] [PubMed] [Google Scholar]
- 24.Gloviczki ML et al. (2010) Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 55 (4), 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloviczki ML et al. (2011) Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 58 (6), 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrysochou C et al. (2012) BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant 27 (3), 1013–9. [DOI] [PubMed] [Google Scholar]
- 27.Gloviczki ML et al. (2013) TGF Expression and Macrophage Accumulation in Atherosclerotic Renal Artery Stenosis. Clin J Am Soc Nephrol 8 (4), 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saad A et al. (2013) Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 6 (4), 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caps MT et al. (1998) Prospective study of atherosclerotic disease progression in the renal artery. Circulation 98 (25), 2866–72. [DOI] [PubMed] [Google Scholar]
- 30.Eirin A et al. (2012) Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 27 (11), 4153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatachalam MA et al. (2015) Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol 26 (8), 1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran K et al. (1992) Morphological changes and alterations in regional intrarenal blood flow induced by graded renal ischemia. J Urol 148 (2 Pt 1), 463–6. [DOI] [PubMed] [Google Scholar]
- 33.Lerman LO et al. (1999) Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10, 1455–1465. [DOI] [PubMed] [Google Scholar]
- 34.Shanley PF (1996) The pathology of chronic renal ischemia. Semin Nephrol 16 (1), 21–32. [PubMed] [Google Scholar]
- 35.Greco BA and Breyer JA (1997) Atherosclerotic ischemic renal disease. Am J Kidney Dis 29 (2), 167–87. [DOI] [PubMed] [Google Scholar]
- 36.Lerman LO et al. (1996) CT-derived intra-renal blood flow in renovascular and essential hypertension. Kidney Int. 49, 846–854. [DOI] [PubMed] [Google Scholar]
- 37.Dobrek L (2021) An Outline of Renal Artery Stenosis Pathophysiology-A Narrative Review. Life (Basel) 11 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abumoawad A et al. (2019) Tissue hypoxia, inflammation, and loss of glomerular filtration rate in human atherosclerotic renovascular disease. Kidney Int 95 (4), 948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen XJ et al. (2019) Improved renal outcomes after revascularization of the stenotic renal artery in pigs by prior treatment with low-energy extracorporeal shockwave therapy. J Hypertens 37 (10), 2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraz LR et al. (2019) Tissue-specific transcriptional regulation of epithelial/endothelial and mesenchymal markers during renovascular hypertension. Mol Med Rep 20 (5), 4467–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovisa S et al. (2020) Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Science Signaling 13 (635). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y et al. (2020) Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms. Stem Cells Dev 29 (18), 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SR et al. (2021) Increased cellular senescence in the murine and human stenotic kidney: Effect of mesenchymal stem cells. J Cell Physiol 236 (2), 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saleem M et al. (2021) The transcription factor Sox6 controls renin expression during renal artery stenosis. Kidney 360, 10.34067/KID.0002792020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehling J et al. (2016) Quantitative Micro-Computed Tomography Imaging of Vascular Dysfunction in Progressive Kidney Diseases. J Am Soc Nephrol 27 (2), 520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babickova J et al. (2017) Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91 (1), 70–85. [DOI] [PubMed] [Google Scholar]
- 47.Engel JE et al. (2019) Targeted VEGF (Vascular Endothelial Growth Factor) Therapy Induces Long-Term Renal Recovery in Chronic Kidney Disease via Macrophage Polarization. Hypertension 74 (5), 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun IO et al. (2018) Loss of Renal Peritubular Capillaries in Hypertensive Patients Is Detectable by Urinary Endothelial Microparticle Levels. Hypertension 72 (5), 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eirin A et al. (2012) Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in swine renal artery stenosis. Circ Cardiovasc Interv 5 (5), 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eirin A et al. (2011) Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol 300 (6), F1394–F1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams E and Chade AR (2020) A Boolean Model of Microvascular Rarefaction to Predict Treatment Outcomes in Renal Disease. Sci Rep 10 (1), 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chade AR et al. (2018) Systemic biopolymer-delivered vascular endothelial growth factor promotes therapeutic angiogenesis in experimental renovascular disease. Kidney Int 93 (4), 842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eirin A et al. (2013) Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 34 (7), 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chade AR et al. (2009) Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119 (4), 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chade AR et al. (2010) Endothelial Progenitor Cells Homing and Renal Repair in Experimental Renovascular Disease. Stem Cells 28 (6), 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eirin A et al. (2013) Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol 33 (5), 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y et al. (2020) Low-Energy Shockwave Treatment Promotes Endothelial Progenitor Cell Homing to the Stenotic Pig Kidney. Cell Transplant 29, 963689720917342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dominici M et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 (4), 315–7. [DOI] [PubMed] [Google Scholar]
- 59.Zhu XY et al. (2013) Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 31 (1), 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin TP et al. (2016) The Promise of Mesenchymal Stem Cell Therapy for Diabetic Kidney Disease. Curr Diab Rep 16 (5), 42. [DOI] [PubMed] [Google Scholar]
- 61.Casiraghi F and Remuzzi G (2019) Mesenchymal stromal cells in kidney transplantation. Curr Opin Nephrol Hypertens 28 (1), 40–46. [DOI] [PubMed] [Google Scholar]
- 62.Zhao L et al. (2019) Current understanding of the administration of mesenchymal stem cells in acute kidney injury to chronic kidney disease transition: a review with a focus on preclinical models. Stem Cell Res Ther 10 (1), 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eirin A et al. (2012) Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30 (5), 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliveira-Sales EB et al. (2013) Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One 8 (11), e78464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sales EB et al. (2015) Effects of mesenchymal stem cells in the renovascular hypertension. Exp Physiol, n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 66.Oliveira-Sales EB et al. (2016) Renovascular hypertension: Effects of mesenchymal stem cells in the contralateral hypertensive kidney in rats. Clin Exp Hypertens 38 (7), 586–593. [DOI] [PubMed] [Google Scholar]
- 67.Varela VA et al. (2019) Treatment with Mesenchymal Stem Cells Improves Renovascular Hypertension and Preserves the Ability of the Contralateral Kidney to Excrete Sodium. Kidney Blood Press Res 44 (6), 1404–1415. [DOI] [PubMed] [Google Scholar]
- 68.Saad A et al. (2017) Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J Am Soc Nephrol 28 (9), 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abumoawad A et al. (2020) In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int 97 (4), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sivanathan KN and Coates PT (2020) Improving human kidney function in renovascular disease with mesenchymal stem cell therapy. Kidney International 97 (4), 655–656. [DOI] [PubMed] [Google Scholar]
- 71.Eirin A et al. (2017) Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 92 (1), 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishiy C et al. (2020) Comparison of the Effects of Mesenchymal Stem Cells with Their Extracellular Vesicles on the Treatment of Kidney Damage Induced by Chronic Renal Artery Stenosis. Stem Cells Int 2020, 8814574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elsharkasy OM et al. (2020) Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev 159, 332–343. [DOI] [PubMed] [Google Scholar]
- 74.Nassar W et al. (2016) Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 20, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruce AT et al. (2015) Selected renal cells modulate disease progression in rodent models of chronic kidney disease via NF-kappaB and TGF-beta1 pathways. Regen Med 10 (7), 815–39. [DOI] [PubMed] [Google Scholar]
- 76.Dominguez JH et al. (2017) Renal Tubular Cell-Derived Extracellular Vesicles Accelerate the Recovery of Established Renal Ischemia Reperfusion Injury. J Am Soc Nephrol 28 (12), 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dominguez JM 2nd, et al. (2018) Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS One 13 (8), e0202550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barros MA et al. (2015) Immature Dental Pulp Stem Cells Showed Renotropic and Pericyte-Like Properties in Acute Renal Failure in Rats. Cell Med 7 (3), 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen XJ et al. (2021) Renovascular Disease Induces Senescence in Renal Scattered Tubular-Like Cells and Impairs Their Reparative Potency. Hypertension 77 (2), 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zou X et al. (2018) Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep 8 (1), 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pang P et al. (2017) Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney Int 91 (1), 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madl CM et al. (2018) Bioengineering strategies to accelerate stem cell therapeutics. Nature 557 (7705), 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou C et al. (2020) Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomater 115, 250–263. [DOI] [PubMed] [Google Scholar]
- 84.Zhao M et al. (2018) Control release of mitochondria-targeted antioxidant by injectable self-assembling peptide hydrogel ameliorated persistent mitochondrial dysfunction and inflammation after acute kidney injury. Drug Deliv 25 (1), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pleniceanu O et al. (2020) Successful Introduction of Human Renovascular Units into the Mammalian Kidney. J Am Soc Nephrol 31 (12), 2757–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lawlor KT et al. (2021) Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater 20 (2), 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yue Y et al. (2020) Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ Res 126 (3), 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monsanto MM et al. (2021) Hiding in plain sight: an encapsulated approach to cardiac cell therapy. Cardiovasc Res 117 (3), 648–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levy O et al. (2020) Shattering barriers toward clinically meaningful MSC therapies. Sci Adv 6 (30), eaba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conley SM et al. (2019) Metabolic Syndrome Induces Release of Smaller Extracellular Vesicles from Porcine Mesenchymal Stem Cells. Cell Transplant 28 (9-10), 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thery C et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7 (1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]