Abstract
Prenatal alcohol exposure (PAE) may result in Fetal Alcohol Spectrum Disorders (FASD). The hippocampus has been recognized as a vulnerable target to alcohol-induced developmental damage. However, the effect of prenatal exposure to alcohol on dendritic morphological adaptations throughout the hippocampal fields in the developing brain still remains largely unknown in the context of FASD. We hypothesized that chronic binge alcohol exposure during pregnancy alters dendrite arborization throughout the developing rat hippocampus. Pregnant Sprague-Dawley rats were assigned to either a pair-fed control (PF-Cont) or a binge alcohol (Alcohol) treatment group. Alcohol dams were acclimatized via a once-daily orogastric gavage of 4.5 g/kg alcohol from gestational day (GD) 5–10 and progressed to 6 g/kg alcohol from GD 11–21. Pair-fed dams similarly received isocaloric maltose dextrin. After parturition, all dams received an ad libitum diet and nursed their offspring until postnatal day (PND) 10 when the pup brains were collected for morphological analysis. PAE increased dendritic arborization and complexities of CA1, CA2/3, and DG neurons in the PND 10 rat hippocampus. The number of primary dendrites, total dendritic length, and number of dendritic branches were significantly increased following PAE, and Sholl analysis revealed significantly more intersections of the dendritic processes in PND 10 offspring following PAE compared with those in the PF-Cont group. We conclude that chronic binge PAE significantly alters hippocampal dendritic morphology in the developing hippocampus. We conjecture that this morphological alteration in postnatal rat hippocampal dendrites following chronic binge prenatal alcohol exposure may play a critical role in FASD neurobiological phenotypes.
Keywords: FASD, alcohol, fetal, hippocampus, dendrites, arborization
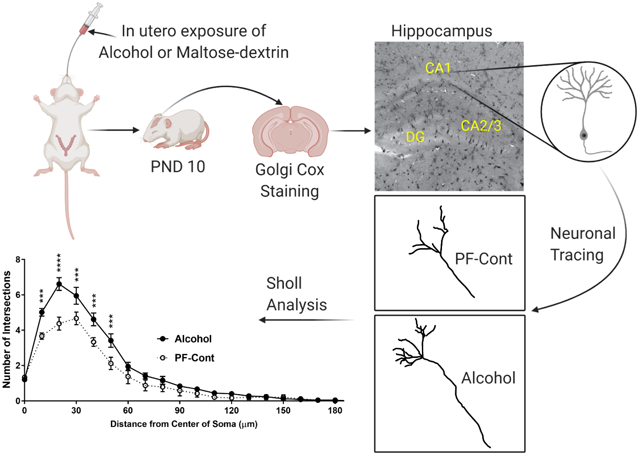
Graphical Abstract
1. Introduction
Prenatal alcohol exposure (PAE) is a leading cause of Fetal Alcohol Spectrum Disorders (FASD), which is described as an array of cognitive, neurobehavioral and physical developmental disabilities (Riley and McGee, 2005, Sokol et al., 2003, Riley et al., 2011). A recent study reported that 1 in 10 pregnant women consumed alcohol in the past month and that 1 in 33 women binge drank during pregnancy (Tan et al., 2015), with 8.4% of newborns exhibiting biochemical evidence of PAE (Bakhireva et al., 2017). The prevalence of FASD is estimated at 2–5% of school-age children in the United States (May et al., 2018, Roozen et al., 2016). PAE-induced developmental deficits can be severe and may persist for a lifetime.
The hippocampus is intricately associated with cognition, behavior, learning and memory. The developing hippocampus is profoundly vulnerable to the teratogenic effects of alcohol (Lebel et al., 2011, Archibald et al., 2001, Gautam et al., 2015). The susceptibility of the hippocampus may vary depending on the dose, duration, timing, and pattern of alcohol exposure. (Ho et al., 1972, Gil-Mohapel et al., 2010, Dudek et al., 2014, Kodituwakku, 2007, Savage et al., 2002). Alcohol exposure during hippocampal development has been shown to alter hippocampal synaptic plasticity (Bhattacharya et al., 2015, Sutherland et al., 1997, Medina, 2011, Fontaine et al., 2016), synaptic activity (Kajimoto et al., 2016, Krawczyk et al., 2016), cellular morphology (Berman and Hannigan, 2000, Ramos et al., 2002), and gene expression (Chen et al., 2013, Chater-Diehl et al., 2016).
Although the hippocampus has been recognized as a vulnerable target of alcohol-induced developmental damage, there is little knowledge related to the morphological alteration of the hippocampal neurons caused by alcohol exposure in vivo, mainly in adults and in neonates (Barnes and Walker, 1981, McMullen et al., 1984, Goeke et al., 2018). Furthermore, the effect of prenatal exposure to alcohol on morphological alteration in the hippocampal fields and the dentate gyrus remains largely unknown in the context of FASD. We recently identified using proteomics and RNA-SEQ that the developing hippocampus is a major target of alcohol exposure in utero (Davis-Anderson et al., 2018, Lunde-Young et al., 2019). Following these high throughput studies, we also recently reported major deficits in hippocampal mTOR signaling, a pathway closely associated with hippocampal neuronal development (Lee et al., 2020). As a logical next step, we herein hypothesized that chronic binge alcohol exposure during pregnancy alters developmental dendritic morphological adaptations. In the present study, utilizing Golgi-Cox staining-based morphological analyses, we evaluated dendrite arborization in the postnatal day 10 (PND) rat hippocampal formation CA1 field, CA2/3 field, and the dentate gyrus (DG) neurons following alcohol exposure in utero.
2. Result
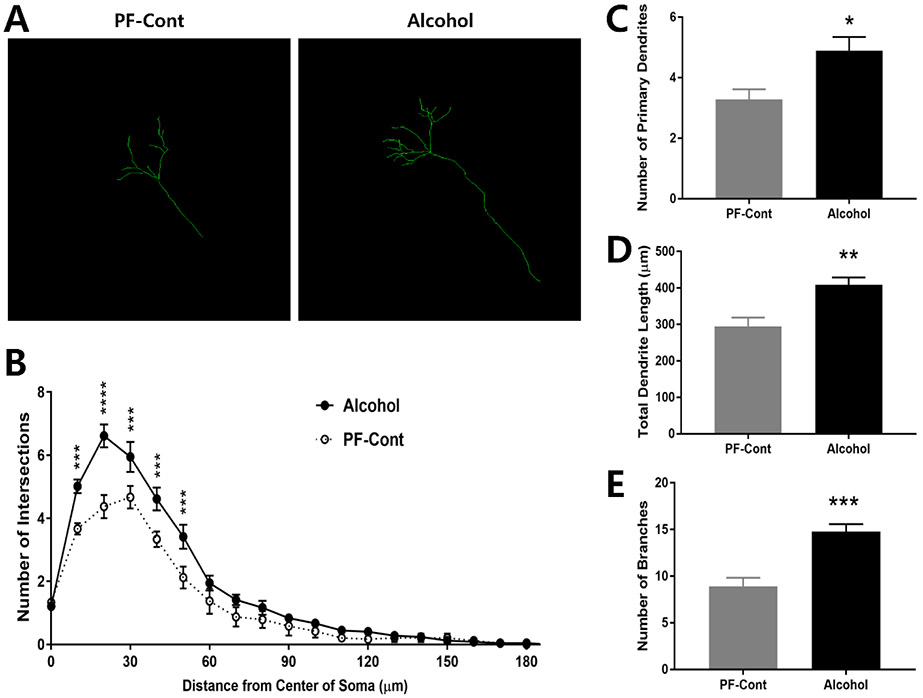
2.1. Prenatal Exposure to Alcohol Increases Dendritic Complexity of CA1 Neurons
Neuronal tracer was applied to visualize the overall dendritic branches of the CA1 neurons (Fig. 1A), and Sholl analysis was utilized to localize the effect of PAE. As shown in Fig. 1B, a significant change in the number of intersections of CA1 neurons were localized at 10-50 μm of the dendrite process from the center of soma (Fig. 1B; F(1, 5) = 19.57, P = 0.0069) in the Alcohol group compared to those in the PF-Cont group. The Alcohol group showed significantly higher number of primary dendrites (Fig. 1C; ↑ 49.15 %; PF-Cont, 3.278 ± 0.3379 vs. Alcohol, 4.889 ± 0.4527; t(10) = 2.852, P = 0.0172), total dendritic length (Fig. 1D; ↑ 38.84 %; PF-Cont, 294.3 ± 24.63 vs. Alcohol, 408.6 ± 20.51; t(10) = 3.563, P = 0.0052), and number of dendritic branches (Fig. 1E; ↑ 66.27 %; PF-Cont, 8.889 ± 0.9336 vs. Alcohol, 14.78 ± 0.7919; t(10) = 4.81, P = 0.0007) in the CA1 field neurons compared to those in the PF-Cont group.
Figure 1. Prenatal exposure to alcohol results in increased dendritic length and complexity in hippocampal CA1 neurons of postnatal day 10 (PND) offspring.
(A) Representative Z-stack confocal images illustrate dendritic branches of CA1 pyramidal neurons from PND 10 offspring following prenatal alcohol exposure (Alcohol) and their pair-fed controls (PF-Cont). (B) A 3-dimentional Sholl analysis revealed significantly more intersections of the dendritic processes at 10-50 μm from the center of soma of CA1 neurons in Alcohol rat, compared to their PF-Cont. (C) CA1 neurons from Alcohol rat exhibited increased number of the primary dendrites compared to PF-Cont. (D) The total dendrite length, and (E) the number of branches were significantly increased in the CA1 field of Alcohol compared to those in PF-Cont. Significance was established a priori at P<0.05; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. All data are presented as mean ± SEM. n=6 animals derived from 6 litters; 4 neurons/animal.
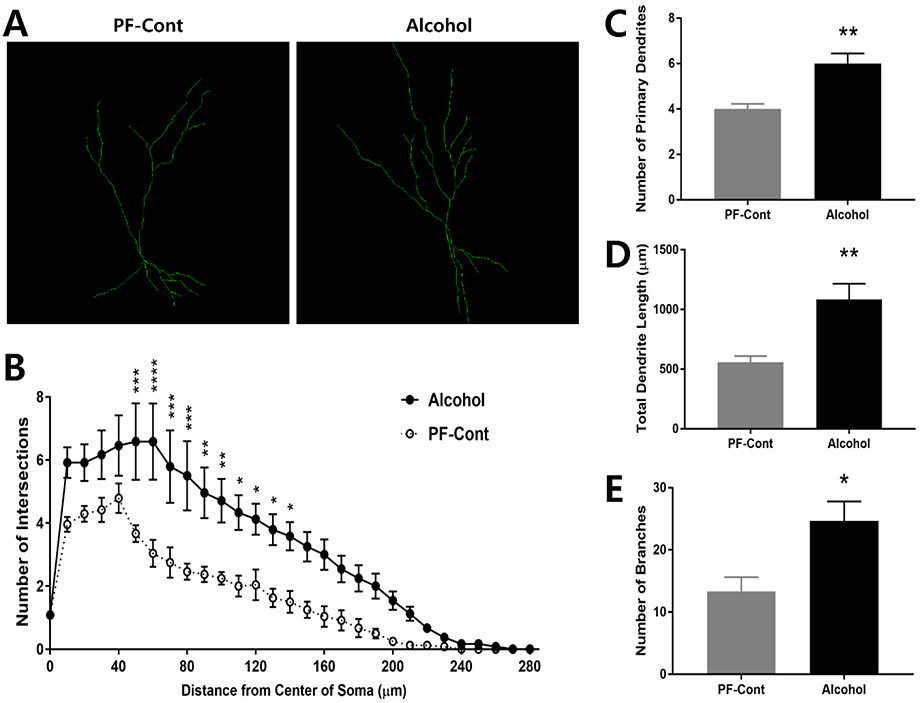
2.2. Prenatal Exposure to Alcohol Increases Dendritic Complexity of CA2/3 Neurons
Dendrite branches of CA2/3 field neurons were traced and depicted in Fig. 2A, and Sholl analysis revealed significantly more intersections of the dendritic process at 50-140 μm from the center of soma (Fig. 2B; F(1, 5) = 18.4, P = 0.0078) in CA2/3 neurons from the Alcohol group than those in the PF-Control. The Alcohol group showed a significantly increased number of primary dendrites (Fig. 2C; ↑ 50.0 %; PF-Cont, 4.0 ± 0.2236 vs. Alcohol, 6.0 ± 0.4425; t(10) = 4.034, P = 0.0024), total dendritic length (Fig. 2D; ↑ 94.26 %; PF-Cont, 557.5 ± 51.98 vs. Alcohol, 1083 ± 132.8; t(10) = 3.685, P = 0.0042), and number of dendritic branches (Fig. 2E; ↑ 84.77 %; PF-Cont, 13.33 ± 2.286 vs. Alcohol, 2463 ± 3.172; t(10) = 2.888, P = 0.0162) in CA2/3 neurons compared to those in the PF-Control group.
Figure 2. Prenatal exposure to alcohol results in increased dendritic length and complexity in hippocampal CA2/3 neurons of postnatal day 10 (PND) offspring.
(A) Representative Z-stack confocal images illustrate dendritic branches of CA2/3 pyramidal neurons from PND 10 offspring following prenatal alcohol exposure (Alcohol) and their pair-fed controls (PF-Cont). (B) A 3-dimentional Sholl analysis revealed significantly more intersections of the dendritic processes at 50-140 μm from the center of soma of CA2/3 field neurons in the Alcohol rat, compared to the PF-Cont group. (C) CA2/3 neurons from Alcohol rats exhibited increased number of the primary dendrites compared to PF-Cont. (D) The total dendrite length, and (E) the number of branches were significantly increased in the CA2/3 field of Alcohol compared to PF-Cont. Significance was established a priori at P<0.05; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. All data are presented as mean ± SEM. n=6 animals derived from 6 litters; 4 neurons/animal.
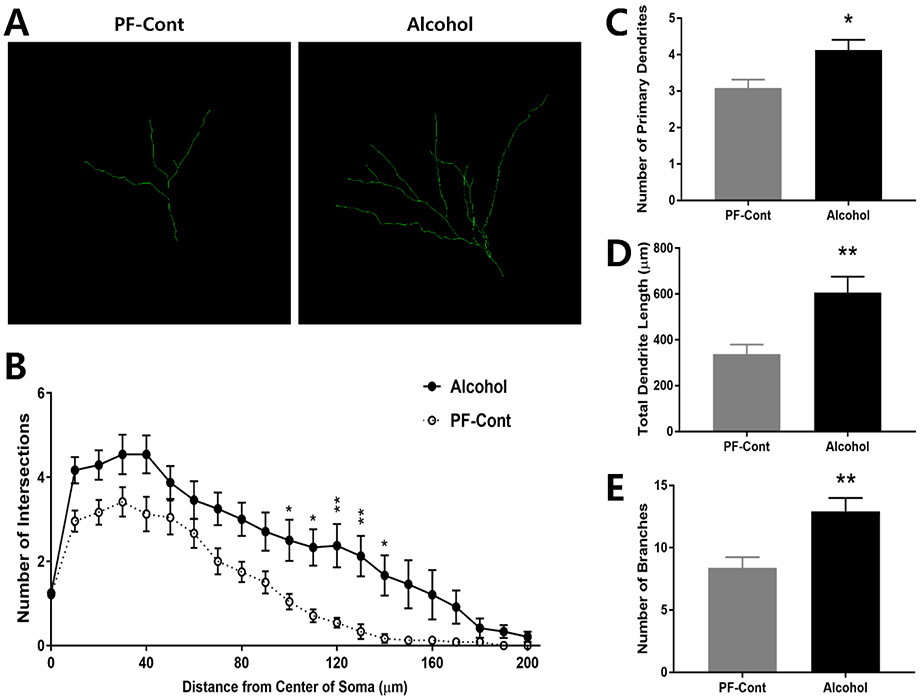
2.3. Prenatal Exposure to Alcohol Increases Dendritic Complexity of DG Neurons
Dendritic branches of the DG neurons were traced (Fig. 3A) and the number of dendritic process at 100-140 μm from the center of soma (Fig. 3B; F(1, 5) = 30.45, P = 0.0027) demonstrated more intersections in the Alcohol group compared with those in the PF-Control. The DG neurons from the Alcohol group had significant increases in the number of primary dendrites (Fig. 3C; ↑ 33.79 %; PF-Cont, 3.083 ± 02297 vs. Alcohol, 4.125 ± 0.2795; t(10) = 2.879, P = 0.0164), total dendritic length (Fig. 3D; ↑ 79.86 %; PF-Cont, 336.7 ± 42.9 vs. Alcohol, 605.6 ± 70.01; t(10) = 3.275, P = 0.0084), and number of dendritic branches (Fig. 3E; ↑ 54.27 %; PF-Cont, 8.375 ± 0.8678 vs. Alcohol, 12.92 ± 1.076; t(10) = 3.286, P = 0.0082) compared to those in the PF-Control group. Taken together, our findings reveal that prenatal alcohol exposure increased the dendritic arborization and complexity in the hippocampal CA1, CA2/3, and DG region neurons in the offspring.
Figure 3. Prenatal exposure to alcohol results in increased dendritic length and complexity in hippocampal formation dentate gyrus (DG) neurons of postnatal day 10 (PND) offspring.
(A) Representative Z-stack confocal images illustrate dendritic branches of DG neurons from PND 10 offspring following prenatal alcohol exposure (Alcohol) and their pair-fed controls (PF-Cont). (B) A 3-dimentional Sholl analysis revealed significantly more intersections of the dendritic process at 10-50 μm from the center of soma of DG neurons in the Alcohol rat, compared to those in PF-Cont. (C) DG neurons from Alcohol rat exhibited increased number of the primary dendrites compared to PF-Cont. (D) The total dendrite length, and (E) the number of branches were significantly increased in DG of Alcohol rats compared with those in the PF-Cont group. Significance was established a priori at P<0.05; * P < 0.05, ** P < 0.01. All data are presented as mean ± SEM. n=6 animals derived from 6 litters; 4 neurons/animal.
3. Discussion
To our knowledge, this is the first study investigating morphological alterations related to dendritic arborization throughout the postnatal rat hippocampal formation, utilizing Golgi-cox staining and neuronal tracing following chronic binge prenatal alcohol exposure. We herein demonstrate that chronic binge prenatal alcohol exposure during pregnancy significantly increased the complexity of dendritic arborization in the hippocampal formation.
3.1. A note on the timing of alcohol exposure
Alcohol can impact the developing brain differentially depending on the ontogenetic stage. During the first trimester, alcohol can interfere with neuronal tube development and result in facial dysmorphology (Ernhart et al., 1987). During the second trimester, alcohol can alter neuronal cell proliferation and migration in the cortex and hippocampus (Gressens et al., 1992, Miller, 1995). Alcohol exposure during the third trimester can induce neuronal cell loss in the hippocampus and cerebellum (Ikonomidou et al., 2000). Our rat FASD study model utilized an exposure paradigm in which offspring were exposed to alcohol in utero during pregnancy, corresponding to the first two trimester-equivalents of human brain development. Alcohol treatments to dams were withdrawn from GD21 and rat pups were not exposed to alcohol from GD21 to PND10, corresponding to the third trimester-equivalent in humans. Measurements in the current study were conducted at the peak velocity of hippocampal growth at PND 10, corresponding to the time of parturition in women. Therefore, we were able to investigate the effect of the first two trimesters of alcohol exposure on the hippocampal neuronal morphology of neonates.
3.2. Alcohol exposure effects on hippocampus during first two trimester-equivalents
Numerous studies have reported the effect of alcohol on the number of neurons in the hippocampal formation following exposure during the first two trimester-equivalents in the rat model. PAE with a free access liquid diet (8.1 % ethanol v/v) decreased the number of CA1 pyramidal neurons, but did not alter the cell numbers in other CA fields or the dentate gyrus in adult rats (Barnes and Walker, 1981, González-Burgos et al., 2006). Maier and West demonstrated that binge-like alcohol exposure during pregnancy in rats did not affect the number of cells in the hippocampal CA1, CA3, and dentate gyrus in PND 10 pups (Maier and West, 2001, Livy et al., 2003). Although many studies have reported behavioral and cognitive deficits associated with hippocampal damage following PAE in rat models (Riley, 1990, Lobaugh et al., 1991, Berman and Hannigan, 2000, Thomas et al., 2010), stereological cell counting techniques did not demonstrate neuronal loss per se in the developing rat hippocampus. Hence, we utilized Golgi-cox staining followed by dendrite tracing to evaluate the effect of PAE on morphological alterations of dendrites in the PND 10 rat hippocampus.
3.3. Morphological alterations in the hippocampus following alcohol exposure
While many studies have analyzed morphological changes in the cerebral cortex and striatum (Whitcher and Klintsova, 2008, Rice et al., 2012, Wang et al., 2015, Hamilton et al., 2015, Cheng et al., 2018), there is little knowledge related to the morphological alterations of hippocampal neurons following alcohol exposure in vivo. Furthermore, the effect of alcohol can manifest differently depending on the time of observation as well as timing of alcohol exposure. Following chronic alcohol exposure in adult rats, the number of dendritic branches decreased but the density of CA1 and CA3 field neurons did not differ (McMullen et al., 1984). However, Goeke et al. reported that neonatal exposure to alcohol increased dendritic length and arborization of hippocampal CA1 neurons in rat pups (Goeke et al., 2018) and our study is in agreement with this finding. The current study reveals the effect of chronic binge prenatal exposure to alcohol on dendrite morphological changes in the PND 10 rat hippocampus and demonstrates increased dendritic length and arborization of hippocampal CA1, CA2/3, and DG neurons.
3.4. Alcohol effects on the hippocampus in children and translational perspectives
Children with FASD often show attention deficit/hyperactivity disorder (ADHD) (Lange et al., 2018) and animal models of FASD also demonstrate increased hyperactivity (Hausknecht et al., 2005, Idrus et al., 2014). Some studies have suggested that PAE induced hyperactivity is associated with altered synaptic plasticity within the cerebral cortex and striatum (Emond et al., 2009, Sonuga-Barke et al., 2016, Cheng et al., 2018) as well as alcohol-altered hippocampus-dependent behaviors in rodent FASD models (Berman and Hannigan, 2000, Thomas et al., 2008, Patten et al., 2014). Cheng et al reported that PAE increased the length and number of neuronal branches in striatum and locomotor activity in juvenile offspring (Cheng et al., 2018). Krawczyk et al. reported that PAE increased hippocampal hyperexcitability, including robust firing, accompanied by an imbalance of synaptic plasticity (Krawczyk et al., 2016). Our data in the current study are in alignment with the literature and show that morphological alterations by PAE could be linked to an increased hyperactivity observed in patients with FASD and animal models of FASD. Our results support the plausible conclusion that PAE induces an abnormal development of apical dendrites in hippocampal pyramidal neurons during particular periods of gestation, resulting in premature maturation. These particular neural changes can result in disrupted neuroplasticity and a lack of the ability of neurons, throughout developmental stages, to respond to external and internal stimuli, as well as alter synaptic and neural architecture, affecting later stages of development (Goeke et al., 2018). Similar to this current study, previous literature showed that adult rats exposed to drugs experience a change in neurons, stemming from rising dendritic arborization (Kolb and Gibb, 2015), and this increase in dendritic arborization has been proposed to disrupt neuronal plasticity (Kolb et al., 2003).
4. Mechanistic Perspectives and Conclusions
We recently reported that gestational chronic binge alcohol exposure altered hippocampal transcriptome (Lunde-Young et al., 2019), proteome (Davis-Anderson et al., 2018), and signaling pathways, including the mammalian target of rapamycin (mTOR) pathway following first two trimester-equivalent alcohol exposure (Lee et al., 2020). Since mTOR plays a crucial role in regulating protein synthesis in response to synaptic formation and plasticity, abnormalities in mTOR activity may be associated with the observed morphological alterations in hippocampal neurons in the present study. Future studies are warranted to determine the mechanism(s) underlying alcohol-induced dysregulation of hippocampal molecular signaling and the mechanistic relationship with hippocampal neurodevelopment in the context of FASD. Based on the present study, we conclude that gestational chronic binge alcohol exposure significantly alters hippocampal dendritic morphology in the developing hippocampus in rats. Thus, we conjecture that these morphological alterations in postnatal rat hippocampal dendrites following chronic binge prenatal alcohol exposure, may have a significant impact on FASD neurobiological pathology.
5. Materials & Methods
5.1. Animals
All experimental procedures were in accordance with National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee at Texas A&M University. Experimental procedures and the prenatal alcohol exposure paradigm were conducted as described previously (Naik et al., 2016, Davis-Anderson et al., 2018, Lunde-Young et al., 2019, Lee et al., 2020, Naik et al., 2020). Briefly, timed-pregnant Sprague Dawley rats purchased from Charles River (Wilmington, MA) were housed in a temperature-controlled room (23°C) with a 12-hour light/dark cycle. Animals were assigned to either a pair-fed control (PF-Cont) group (n=6 dams) or a binge alcohol treatment (Alcohol) group (n=6 dams) on gestational day (GD) 4. Alcohol group animals were acclimatized via a once-daily orogastric gavage of 4.5 g/kg (22.5% wt/v) ethanol (peak blood alcohol concentration (BAC), 216 mg/dl) from GD 5–10, and progressed to 6.0 g/kg (28.5% wt/v) ethanol (peak BAC, 289 mg/dl) from GD 11–20 (Davis-Anderson et al., 2018, Subramanian et al., 2014). The exposure paradigm utilized in this study is modeled after alcohol consumption patterns in pregnant women and is commonly utilized to study FASD phenotypes in animal models (Caetano et al., 2006, Church and Gerkin, 1988, Cudd et al., 2002, Thomas et al., 2008, Thomas et al., 2010). All animals were weighed prior to the start of the study, and each Alcohol group animal was yoked with a PF-Cont group animal of similar weight for the duration of the study. The PF-Cont group animals received isocalorically matched (50% wt/v) maltose dextrin once-daily on each exposure day to account for calories that the Alcohol group received from ethanol. The Alcohol group animal received an ad libitum diet and daily feed intake was measured. The PF-Cont group animal was fed the same amount of diet consumed by the Alcohol group animal until pups were delivered. Water was available at all times throughout the experiment for both the treatment groups. From the day following parturition, both PF-Cont and Alcohol group animals were provided ad libitum diet and were nursing their offspring until postnatal day (PND) 10. No animal was treated with alcohol during this period of time.
5.2. Brain tissue preparation and sectioning
On PND 10, each pup was weighed and one male pup was selected at the median body weight within a litter (n=6 pup brains). The selected pups were deeply anesthetized with isoflurane in the anesthesia chamber and sacrificed. Whole brains were extracted under a dissection microscope via craniotomy and washed in double distilled water. The brain tissues were immediately immersed in Golgi-Cox impregnation solution (FD Rapid GolgiStain Kit, FD NeuroTechnologies Inc., Columbia, MD) and processed according to the manufacturer’s instructions. Briefly, after impregnation for 10 days followed by cryoprotection for 4 days at room temperature in the dark, the brain tissues were frozen in chilled isopentane and sectioned at 100 μm in a coronal plane using a Leica CM1860 cryostat (Leica Biosystems, Buffalo Grove, IL). Brain sections were mounted on gelatin coated glass slides and dried overnight at room temperature. The sections were stained for 10 min in developing solution, then dehydrated in serial concentrations of ethanol. Following cleaning in xylene, the sections were coverslipped with Permount (Fisher Scientific, Ottawa, ON) and stored at room temperature in the dark.
5.3. Image Acquisition and Morphological Analysis
Digital images were captured using an Olympus BX63 stereomicroscope, ORCA-Flash 4.0 LT digital camera (HAMAMATSU, Japan), and Olympus cellSens Dimension software (Olympus, Japan) with Extended Depth of Field. Neurons were selected from the CA1 field, CA2/3 field, and the dentate gyrus (DG) of the hippocampal formation in each brain, only if they were relatively isolated from other neurons and clearly located in each field without discernable breaks. Dendrite branches were traced by ImageJ Fiji software (National Institutes of Health, Bethesda, MD) with the Simple Neurite Tracer plugin (Longair et al., 2011) on z-stacked images to visualize the structure of dendritic branches, then dendrite length and number of branches were determined for each neuron. The Sholl analysis plugin (Ferreira et al., 2014) was utilized for dendrite arborization by counting the number of crossing dendrites with concentric circles at 10 μm intervals from the center of the soma.
5.4. Statistical Analysis
For all measurements, means for each animal were analyzed by averaging across the four selected neurons per hippocampal field (n=6, 4 neurons/animal). Dendrite arborization data utilizing Sholl analysis were analyzed by mixed ANOVA with treatment group as between factor and distance from soma as within factor, followed by Sidak’s multiple comparisons test. The effects of prenatal alcohol exposure on the number of primary dendrites, total dendrite length, and the number of branches were analyzed using an unpaired two-tailed Student’s t-test. All data are presented as mean ± SEM. Significance was established a priori at P < 0.05.
Highlights.
Prenatal alcohol exposure alters dendritic morphology in PND 10 rat hippocampus
Alcohol produces maladaptation in dendritic length of developmental hippocampal CA1, CA2, and DG neurons
Alcohol increased complexity of developmental dendritic arborization in FASD model
Acknowledgements:
This study was supported by National Institutes of Health [AA19446, AA23520, AA23035, HL151497]; Texas A&M University [Tier One Program]; and Texas A&M Presidential Transformational teaching Grant (JR).
Footnotes
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ARCHIBALD SL, FENNEMA-NOTESTINE C, GAMST A, RILEY EP, MATTSON SN & JERNIGAN TL 2001. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol, 43, 148–54. [PubMed] [Google Scholar]
- BAKHIREVA LN, SHARKIS J, SHRESTHA S, MIRANDA-SOHRABJI TJ, WILLIAMS S & MIRANDA RC 2017. Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol Clin Exp Res, 41, 1004–1011. [DOI] [PubMed] [Google Scholar]
- BARNES DE & WALKER DW 1981. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Brain Res, 227, 333–40. [DOI] [PubMed] [Google Scholar]
- BERMAN RF & HANNIGAN JH 2000. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus, 10, 94–110. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYA D, DUNAWAY EP, BHATTACHARYA S, BLOEMER J, BUABEID M, ESCOBAR M, SUPPIRAMANIAM V & DHANASEKARAN M 2015. Impaired ILK Function Is Associated with Deficits in Hippocampal Based Memory and Synaptic Plasticity in a FASD Rat Model. PLOS ONE, 10, e0135700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAETANO R, RAMISETTY-MIKLER S, FLOYD LR & MCGRATH C 2006. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res, 30, 1023–30. [DOI] [PubMed] [Google Scholar]
- CHATER-DIEHL EJ, LAUFER BI, CASTELLANI CA, ALBERRY BL & SINGH SM 2016. Alteration of Gene Expression, DNA Methylation, and Histone Methylation in Free Radical Scavenging Networks in Adult Mouse Hippocampus following Fetal Alcohol Exposure. Plos One, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y, OZTURK NC & ZHOU FC 2013. DNA methylation program in developing hippocampus and its alteration by alcohol. PLoS One, 8, e60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG Y, WANG X, WEI X, XIE X, MELO S, MIRANDA RC & WANG J 2018. Prenatal Exposure to Alcohol Induces Functional and Structural Plasticity in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH MW & GERKIN KP 1988. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics, 82, 147–54. [PubMed] [Google Scholar]
- CUDD TA, CHEN WJ & WEST JR 2002. Fetal and maternal thyroid hormone responses to ethanol exposure during the third trimester equivalent of gestation in sheep. Alcohol Clin Exp Res, 26, 53–8. [PubMed] [Google Scholar]
- DAVIS-ANDERSON KL, WESSELING H, SIEBERT LM, LUNDE-YOUNG ER, NAIK VD, STEEN H & RAMADOSS J 2018. Fetal regional brain protein signature in FASD rat model. Reprod Toxicol, 76, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEK J, SKOCIC J, SHEARD E & ROVET J 2014. Hippocampal abnormalities in youth with alcohol-related neurodevelopmental disorder. J Int Neuropsychol Soc, 20, 181–91. [DOI] [PubMed] [Google Scholar]
- EMOND V, JOYAL C & POISSANT H 2009. [Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)]. Encephale, 35, 107–14. [DOI] [PubMed] [Google Scholar]
- ERNHART CB, SOKOL RJ, MARTIER S, MORON P, NADLER D, AGER JW & WOLF A 1987. Alcohol teratogenicity in the human: a detailed assessment of specificity, critical period, and threshold. Am J Obstet Gynecol, 156, 33–9. [DOI] [PubMed] [Google Scholar]
- FERREIRA TA, BLACKMAN AV, OYRER J, JAYABAL S, CHUNG AJ, WATT AJ, SJOSTROM PJ & VAN MEYEL DJ 2014. Neuronal morphometry directly from bitmap images. Nat Methods, 11, 982–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONTAINE CJ, PATTEN AR, SICKMANN HM, HELFER JL & CHRISTIE BR 2016. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: Sex, age and methodological considerations. Neurosci Biobehav Rev, 64, 12–34. [DOI] [PubMed] [Google Scholar]
- GAUTAM P, LEBEL C, NARR KL, MATTSON SN, MAY PA, ADNAMS CM, RILEY EP, JONES KL, KAN EC & SOWELL ER 2015. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum Brain Mapp, 36, 2318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIL-MOHAPEL J, BOEHME F, KAINER L & CHRISTIE BR 2010. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev, 64, 283–303. [DOI] [PubMed] [Google Scholar]
- GOEKE CM, ROBERTS ML, HASHIMOTO JG, FINN DA & GUIZZETTI M 2018. Neonatal Ethanol and Choline Treatments Alter the Morphology of Developing Rat Hippocampal Pyramidal Neurons in Opposite Directions. Neuroscience, 374, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZÁLEZ-BURGOS I, ALEJANDRE-GÓMEZ M, OLVERA-CORTÉS ME, PÉREZ-VEGA MI, EVANS S & FERIA-VELASCO A 2006. Prenatal-through-postnatal exposure to moderate levels of ethanol leads to damage on the hippocampal CA1 field of juvenile rats: a stereology and Golgi study. Neurosci Res, 56, 400–8. [DOI] [PubMed] [Google Scholar]
- GRESSENS P, LAMMENS M, PICARD JJ & EVRARD P 1992. Ethanol-induced disturbances of gliogenesis and neuronogenesis in the developing murine brain: an in vitro and in vivo immunohistochemical and ultrastructural study. Alcohol Alcohol, 27, 219–26. [PubMed] [Google Scholar]
- HAMILTON GF, CRISS KJ & KLINTSOVA AY 2015. Voluntary Exercise Partially Reverses Neonatal Alcohol-Induced Deficits in mPFC Layer II/III Dendritic Morphology of Male Adolescent Rats. Synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSKNECHT KA, ACHESON A, FARRAR AM, KIERES AK, SHEN RY, RICHARDS JB & SABOL KE 2005. Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci, 119, 302–10. [DOI] [PubMed] [Google Scholar]
- HO BT, FRITCHIE GE, IDANPAAN-HEIKKILA JE & MCISAAC WM 1972. Placental transfer and tissue distribution of ethanol-1-14 C. A radioautographic study in monkeys and hamsters. Q J Stud Alcohol, 33, 485–93. [PubMed] [Google Scholar]
- IDRUS NM, MCGOUGH NN, RILEY EP & THOMAS JD 2014. Administration of memantine during withdrawal mitigates overactivity and spatial learning impairments associated with neonatal alcohol exposure in rats. Alcohol Clin Exp Res, 38, 529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKONOMIDOU C, BITTIGAU P, ISHIMARU MJ, WOZNIAK DF, KOCH C, GENZ K, PRICE MT, STEFOVSKA V, HORSTER F, TENKOVA T, DIKRANIAN K & OLNEY JW 2000. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science, 287, 1056–60. [DOI] [PubMed] [Google Scholar]
- KAJIMOTO K, VALENZUELA CF, ALLAN AM, GE SY, GU Y & CUNNINGHAM LA 2016. Prenatal Alcohol Exposure Alters Synaptic Activity of Adult Hippocampal Dentate Granule Cells Under Conditions of Enriched Environment. Hippocampus, 26, 1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KODITUWAKKU PW 2007. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev, 31, 192–201. [DOI] [PubMed] [Google Scholar]
- KOLB B & GIBB R 2015. Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci, 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLB B, GORNY G, LI Y, SAMAHA AN & ROBINSON TE 2003. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A, 100, 10523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAWCZYK M, RAMANI M, DIAN J, FLOREZ CM, MYLVAGANAM S, BRIEN J, REYNOLDS J, KAPUR B, ZOIDL G, POULTER MO & CARLEN PL 2016. Hippocampal hyperexcitability in fetal alcohol spectrum disorder: Pathological sharp waves and excitatory/inhibitory synaptic imbalance. Experimental Neurology, 280, 70–79. [DOI] [PubMed] [Google Scholar]
- LANGE S, REHM J, ANAGNOSTOU E & POPOVA S 2018. Prevalence of externalizing disorders and Autism Spectrum Disorders among children with Fetal Alcohol Spectrum Disorder: systematic review and meta-analysis. Biochem Cell Biol, 96, 241–251. [DOI] [PubMed] [Google Scholar]
- LEBEL C, ROUSSOTTE F & SOWELL ER 2011. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev, 21, 102–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J, LUNDE-YOUNG R, NAIK V, RAMIREZ J, ORZABAL M & RAMADOSS J 2020. Chronic Binge Alcohol Exposure During Pregnancy Alters mTOR System in Rat Fetal Hippocampus. Alcohol Clin Exp Res, 44, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVY DJ, MILLER EK, MAIER SE & WEST JR 2003. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol, 25, 447–58. [DOI] [PubMed] [Google Scholar]
- LOBAUGH NJ, WIGAL T, GREENE PL, DIAZ-GRANADOS JL & AMSEL A 1991. Effects of prenatal ethanol exposure on learned persistence and hippocampal neuroanatomy in infant, weanling and adult rats. Behav Brain Res, 44, 81–6. [DOI] [PubMed] [Google Scholar]
- LONGAIR MH, BAKER DA & ARMSTRONG JD 2011. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics, 27, 2453–4. [DOI] [PubMed] [Google Scholar]
- LUNDE-YOUNG R, RAMIREZ J, NAIK V, ORZABAL M, LEE J, KONGANTI K, HILLHOUSE A, THREADGILL D & RAMADOSS J 2019. Hippocampal transcriptome reveals novel targets of FASD pathogenesis. Brain Behav, 9, e01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIER SE & WEST JR 2001. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol, 23, 49–57. [DOI] [PubMed] [Google Scholar]
- MAY PA, CHAMBERS CD, KALBERG WO, ZELLNER J, FELDMAN H, BUCKLEY D, KOPALD D, HASKEN JM, XU R & HONERKAMP-SMITH G 2018. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA, 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCMULLEN PA, SAINT-CYR JA & CARLEN PL 1984. Morphological alterations in rat CA1 hippocampal pyramidal cell dendrites resulting from chronic ethanol consumption and withdrawal. J Comp Neurol, 225, 111–8. [DOI] [PubMed] [Google Scholar]
- MEDINA AE 2011. Fetal alcohol spectrum disorders and abnormal neuronal plasticity. Neuroscientist, 17, 274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER MW 1995. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res, 19, 1500–9. [DOI] [PubMed] [Google Scholar]
- NAIK V, LUNDE-YOUNG R, RAMIREZ J, LEE J & RAMADOSS J 2020. Distribution of Phosphatidylethanol in Maternal and Fetal Compartments After Chronic Gestational Binge Alcohol Exposure. Alcohol Clin Exp Res, 44, 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAIK VD, LUNDE-YOUNG ER, DAVIS-ANDERSON KL, ORZABAL M, IVANOV I & RAMADOSS J 2016. Chronic binge alcohol consumption during pregnancy alters rat maternal uterine artery pressure response. Alcohol, 56, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTEN AR, FONTAINE CJ & CHRISTIE BR 2014. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr, 2, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMOS AJ, EVRARD SG, TAGLIAFERRO P, TRICARICO MV & BRUSCO A 2002. Effects of chronic maternal ethanol exposure on hippocampal and striatal morphology in offspring. Cellular and Molecular Mechanisms of Drugs of Abuse Ii: Cocaine, Substituted Amphetamines, Ghb, and Opiates, 965, 343–353. [DOI] [PubMed] [Google Scholar]
- RICE JP, SUGGS LE, LUSK AV, PARKER MO, CANDELARIA-COOK FT, AKERS KG, SAVAGE DD & HAMILTON DA 2012. Effects of exposure to moderate levels of ethanol during prenatal brain development on dendritic length, branching, and spine density in the nucleus accumbens and dorsal striatum of adult rats. Alcohol, 46, 577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILEY EP 1990. The long-term behavioral effects of prenatal alcohol exposure in rats. Alcohol Clin Exp Res, 14, 670–3. [DOI] [PubMed] [Google Scholar]
- RILEY EP, INFANTE MA & WARREN KR 2011. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev, 21, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILEY EP & MCGEE CL 2005. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood), 230, 357–65. [DOI] [PubMed] [Google Scholar]
- ROOZEN S, PETERS G-JY, KOK G, TOWNEND D, NIJHUIS J & CURFS L 2016. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcoholism: Clinical and Experimental Research, 40, 18–32. [DOI] [PubMed] [Google Scholar]
- SAVAGE DD, BECHER M, TORRE AJ & SUTHERLAND RJ 2002. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcoholism: Clinical and Experimental Research, 26, 1752–1758. [DOI] [PubMed] [Google Scholar]
- SOKOL RJ, DELANEY-BLACK V & NORDSTROM B 2003. Fetal alcohol spectrum disorder. JAMA, 290, 2996–9. [DOI] [PubMed] [Google Scholar]
- SONUGA-BARKE EJ, CORTESE S, FAIRCHILD G & STRINGARIS A 2016. Annual Research Review: Transdiagnostic neuroscience of child and adolescent mental disorders--differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J Child Psychol Psychiatry, 57, 321–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBRAMANIAN K, NAIK VD, SATHISHKUMAR K, YALLAMPALLI C, SAADE GR, HANKINS GD & RAMADOSS J 2014. Chronic binge alcohol exposure during pregnancy impairs rat maternal uterine vascular function. Alcohol Clin Exp Res, 38, 1832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND RJ, MCDONALD RJ & SAVAGE DD 1997. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus, 7, 232–8. [DOI] [PubMed] [Google Scholar]
- TAN CH, DENNY CH, CHEAL NE, SNIEZEK JE & KANNY D 2015. Alcohol use and binge drinking among women of childbearing age - United States, 2011-2013. MMWR Morb Mortal Wkly Rep, 64, 1042–6. [DOI] [PubMed] [Google Scholar]
- THOMAS JD, IDRUS NM, MONK BR & DOMINGUEZ HD 2010. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol, 88, 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS JD, SATHER TM & WHINERY LA 2008. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci, 122, 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J, CHENG Y, WANG X, ROLTSCH HELLARD E, MA T, GIL H, BEN HAMIDA S & RON D 2015. Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. J Neurosci, 35, 11634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITCHER LT & KLINTSOVA AY 2008. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse, 62, 566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]