Abstract
The pre-melanosomal protein (Pmel17) is a human functional amyloid that supports melanin biosynthesis within melanocytes. This occurs in the melanosome, a membrane-bound organelle with an acidic intraluminal pH. The repeat region of Pmel17 (RPT, residues 315–444) has been previously shown to form amyloid aggregates under acidic melanosomal conditions, but not under neutral cytosolic conditions, when expressed and purified using a C-terminal hexa-histidine tag (RPT-His). Given the importance of protonation states in RPT-His aggregation, we questioned whether the histidine tag influenced the pH-dependent behavior. In this report, we generated a tagless RPT by inserting a tobacco etch virus (TEV) protease recognition sequence (ENLYGQ(G/S)) immediately upstream of a native glycine residue at position 312 in Pmel17. After purification of the fusion construct using a histidine tag, cleavage with TEV protease generated a fully native RPT (nRPT) spanning resides 312–444. We characterized the aggregation of nRPT, which formed amyloid fibrils under acidic conditions (pH ≤ 6) but not at neutral pH. Characterizing the morphologies of nRPT aggregates using transmission electron microscopy revealed a pH-dependent maturation from short, curved structures at pH 4 to paired, rod-like fibrils at pH 6. This was accompanied by a secondary structural transition from mixed random coil/β-sheet at pH 4 to canonical β-sheet at pH 6. We also show that pre-formed nRPT fibrils undergo disaggregation upon dilution into pH 7 buffer. More broadly, this strategy can be utilized to generate native amyloidogenic domains from larger proteins by utilizing intrinsic N-terminal glycine or serine residues.
Keywords: Amyloid, aggregation, disaggregation, TEM, tryptophan fluorescence
INTRODUCTION
Amyloids are a class of proteins which self-associate into micron-length filaments with a specific cross-β structure, where protein monomers form β-strands that stack laterally and form hydrogen bonds which extend parallel to the fibril growth axis [1]. The aggregation of various proteins into amyloid structures is well-recognized in numerous diseases. Of the many examples include amyloid-β and tau in Alzheimer’s disease as well as α-synuclein in Parkinson’s disease [2]. However, not all amyloid-forming proteins are associated with disease. Some proteins form amyloids that serve a distinct biological function in the fibrillar-state, making them “functional” rather than pathological [3].
Several functional amyloids have been described in humans [4, 5], one example being the pre-melanosomal protein (Pmel17), which forms an amyloid fibrillar matrix that supports melanin biosynthesis (i.e., pigmentation) within melanocytes [6]. This occurs within the melanosome, a membrane-bound organelle with an acidic intraluminal pH that goes through four distinct stages of maturation [7]. Pmel17 is a 575–668-amino acid protein (depending on the isoform, the canonical sequence is shown in Fig. 1) with a single transmembrane domain and large luminal domain. During melanogenesis, Pmel17 undergoes multiple proteolytic processing events, the first of which occurs in stage I of melanosome maturation and produces a large luminal domain, termed Mα (residues 25–467, Fig. 1). In stage II, Mα undergoes additional proteolysis to produce amyloidogenic fragment(s) (Fig. 1), which make up the fibrillar matrix in melanosomes [8, 9]. Melanin then begins to deposit on the fibrillar matrix in stage III, and fully coats the melanosome in stage IV [7].
Figure 1: Proteolytic processing of Pmel17 during melanogenesis.
In stage I of melanogenesis, Pmel17 (UniProt P40967-1) is proteolytically processed to produce a luminal domain (Mα, residues 25–467) and membrane-associated domain (Mβ, residues 468–661), indicated by scissors. Mα is made up of an N-terminal domain (NTD), polycystic kidney disease-like domain (PKD), and repeat domain (RPT). During stage II of melanogenesis, Mα is further cleaved to produce fragment(s) that make up the amyloid fibrillar matrix observed in melanosomes, indicated by scissors. The exact sites of cleavage during stage II are not known. Known glycosylation sites are marked with an asterisks. The primary amino acid sequence of the RPT construct described herein contains an N-terminal His-tag and TEV protease recognition sequence (R.S.). Blue, red, and purple residues represent basic, acidic, and aromatic residues, respectively.
Mα is made up of an N-terminal domain (NTD), polycystic kidney disease-like domain (PKD), and an imperfect repeat domain (RPT, Fig. 1). Previous work has found that RPT is required for fibril formation in melanosomes [10] and forms amyloid fibrils when expressed and purified in vitro using a C-terminal hexa-histidine tag (RPT-His, residues 315–444) [11, 12]. RPT-His fibril formation is pH-dependent, where aggregation occurs under acidic melanosomal-like conditions but not under cytosolic neutral conditions, and fibrils formed at acidic pH undergo rapid disaggregation upon exposure to neutral pH [8, 9, 11-19]. This occurs via protonation/deprotonation of acidic (D/E) residues, of which RPT has 16 total, with those in the C-terminal repeats 7 and 9 being most important [16]. Given that protonation of acidic residues within the C-terminus of RPT-His is crucial for fibril formation in vitro, we questioned whether the hexa-histidine tag located at the C-terminus influenced the pH-dependent behavior of the protein. Therefore, we developed a new strategy for purifying a RPT construct that recapitulated the native sequence.
In this report, we demonstrate by shifting three residues upstream of the canonical RPT domain, native RPT (nRPT) spanning Pmel17 residues 312–444 can be generated with a His-tagged, tobacco etch virus (TEV) protease-cleavable construct. We further describe the characterization of pH-dependent nRPT aggregation and disaggregation processes, which are mostly similar to those observed with RPT-His. This strategy utilizing native N-terminal glycine or serine residues for TEV cleavage is advantageous for generating tagless sequences and pertinent because aggregation propensities of amyloidogenic proteins have been demonstrated to be highly sequence dependent.
MATERIALS AND METHODS
Materials
Ultrapure urea was purchased from Thermo Fisher Scientific. Unless otherwise noted, all additional chemicals/reagents and equipment were procured from Sigma-Aldrich and VWR International, respectively.
Plasmid design and expression
The nRPT construct was designed with an N-terminal hexa-histidine tag and TEV recognition sequence followed by Pmel17 residues 313–444 (Fig. 1), cloned into a pET-30a(+) vector using NdeI and XhoI restriction sites, and purchased from GeneScript. The nRPT plasmid was then transformed into E. coli BL21(DE3) competent cells (Fisher Scientific) and expressed as previously reported [18].
Protein purification
A detailed protocol for purifying RPT-His has been described prior [18]. For nRPT, a 10 g cell pellet was resuspended in 60 mL of buffer A (6 M urea, 100 mM NaCl, 100 mM Na2HPO4, 20 mM imidazole, pH 7.4), split into two 50-mL conical tubes, and lysed on ice by two 20-s pulses of sonication using a Branson Sonifier 450 operating at 50 % power. To allow for complete cell lysis, tubes were kept under constant rotation overnight at 4 °C. The cell lysate was then clarified by centrifugation at 38,724 rcf for 30 min at 4 °C, followed by loading the supernatant onto a HisPrep FF 16/10 column (Cytiva Life Sciences) using an ÄKTA PURE fast protein liquid chromatography (FPLC) system (Cytiva Life Sciences). The protein was eluted by increasing the concentration of elution buffer (6 M urea, 100 mM NaCl, 100 mM Na2HPO4, 500 mM imidazole, pH 7.4), where His-TEV-nRPT eluted at ~ 30 % B (Fig. 2a, shaded area). Fractions containing His-TEV-nRPT were then combined and dialyzed against 20 mM tris(hydroxymethyl)aminomethane (Tris, pH 8.0) overnight at 4 °C using a 3.5 kDa molecular weight cut-off (MWCO) (Spectrum SpectraPor 3 dialysis membrane). The resulting amount of protein was quantified using a Cary 300-series UV-Vis instrument (Agilent Technologies) by measuring the absorbance at 280 nm (ε = 6,990 M−1 cm−1 determined by ProtParam (ExPASy)). After adding dithiolthreitol to a final concentration of 1 mM, the protein was cleaved overnight at 4 °C using a His-tagged TEV protease, purified as described previously [20]. For every 50 mg of His-TEV-nRPT, 1 mg of TEV protease was added. The cleaved protein was loaded onto a HisPrep FF 16/10 column pre-equilibrated in 20 mM Tris (pH 8) using an ÄKTA PURE FPLC system (Cytiva Life Sciences), where nRPT was eluted in the unbound fraction (Fig. 2c, shaded area). Any proteins bound to the column were then eluted with 20 mM Tris, 500 mM imidazole (pH 8) (100% Buffer B). nRPT was quantified using the absorbance at 280 nm (ε = 5,500 M−1 cm−1), followed by adding ethylenediaminetetraacetic acid (EDTA) to a final concentration of 1 mM, aliquoting the protein into sterile 1.5 mL low-binding tubes (Eppendorf), and storing at −80 °C. The purity of both His-TEV-nRPT and nRPT was determined to be >90 % using SDS-PAGE and LC-MS (Fig. 2b and Fig. 2d) as described below.
Figure 2: Purification of fully native RPT (Pmel17 312–444).
Nickel affinity purification (a) and mass analysis (b) of the His-TEV-nRPT construct. The inset shows an SDS-PAGE gel of the same sample. The shaded box in (a) represents fractions which were collected for mass analysis and cleaved using TEV protease, followed by an additional round of nickel affinity purification (c) where the unbound fraction (shaded box) represents nRPT (d). The inset shows an SDS-PAGE gel of the same sample, which migrates as a ~37 kDa band.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Protein samples were mixed with NuPAGE LDS sample buffer (Invitrogen), heated at 100 °C for 10 min, then loaded onto NuPAGE 4–12%, Bis-Tris Protein Gels (Invitrogen) and separated at 200 V for 35 min using a PowerEase 500 (Invitrogen) power supply. Gels were then washed with deionized water and stained using SimplyBlue SafeStain (Invitrogen) following the manufacturer’s protocol. Gel images were collected on either a LaserJet Pro MFP scanner (Hewlett-Packard) or a Typhoon imaging scanner using ImageQuant software (Cytiva Life Sciences). Precision Plus Protein dual color protein standards (Bio-Rad) were ran in parallel to evaluate approximate molecular weights.
Liquid chromatography mass spectrometry (LC-MS)
Protein samples were analyzed by LC-MS using an Agilent 6224 instrument equipped with an electrospray ionization and time-of-flight detector (NHLBI Biochemistry Core Facility). Protein samples were diluted to 0.1 mg/mL into an LC-MS vial using a solution of 5 % acetonitrile (MeCN)/0.05 % trifluoroacetic acid (TFA), of which 5 μL was loaded onto a Zorbax StableBond 300 C18 column (Agilent Technologies) pre-equilibrated in buffer A (99% H2O/0.05% TFA, v/v). The protein was eluted by increasing the concentration of buffer B (99% MeCN/0.05% TFA, v/v) using a linear gradient, where both His-TEV-nRPT and nRPT eluted at ~ 47 % B. Masses were Native Pmel17 RPT Purification and Characterization extracted from the total ion chromatogram and deconvoluted using MassHunter software (Agilent Technologies). The theoretical masses of His-TEV-nRPT (15,190.8 Da) and nRPT (13,441.90 Da) were determined using ExPASy ProtParam tool.
Aggregation reactions
Aggregation reactions were performed as described before [18]. Briefly, protein stocks were thawed on ice and filtered through a 100 kDa MWCO centrifugal filter (Millipore-Sigma) to remove any pre-formed aggregates. Protein was then buffer-exchanged into either 20 mM sodium acetate, 100 mM NaCl (pH 4, 4.5, 5, and 5.5), 20 mM 2-(N-morpholino)ethanesulfonic acid (MES), 100 mM NaCl (pH 6), or 20 mM sodium phosphate, 100 mM NaCl (pH 7) using PD-10 columns (Cytiva Life Sciences). Protein concentration was then determined by measuring the absorbance at 280 nm (ε = 5,500 M−1 cm−1) before diluting to 30 μM using the appropriate buffer. Thioflavin-T (ThT) was then added to a final concentration of 10 μM before aliquoting (70 μL) into a black, flat-bottom 384-well polypropylene microplate (Greiner Bio-One) and sealing with MicroAmp optical adhesive film (Thermo Fisher Scientific). A sterile 2 mm borosilicate glass bead was added to facilitate aggregation. Measurements were collected every hour by measuring ThT fluorescence emission at 480 nm upon excitation at 415 nm using a SPARK multimode microplate reader (Tecan Life Sciences) maintained at 37 °C under constant linear shaking (6 mm). After 50 h, wells of the same solution condition were pooled together and analyzed.
Circular dichroism (CD) spectroscopy
CD spectra were collected by scanning from 260–200 nm using a J-715 instrument (Jasco) as described previously [17]. Data were processed by buffer subtraction and conversion to mean residue ellipticity (θMRE) using IgorPro 7.08 software (WaveMetrics) as described previously [17, 21].
Intrinsic tryptophan fluorescence
Fluorescence spectra were collected using a Fluorolog FL-3 instrument (Horiba Scientific) by scanning the emission spectrum from 290 to 450 nm (2 nm slit width) upon excitation at 280 nm (1 nm slit width). Data were processed by buffer subtraction and normalized for protein concentration using IgorPro 7.08 software (WaveMetrics).
Transmission electron microscopy (TEM)
Grids were prepared by depositing 5 μL of 30 μM nRPT fibrils onto a 400-mesh copper grid with a formvar/carbon film (Electron Microscopy Sciences) and negatively-stained using a 2% (w/v) solution of uranyl acetate. Images were collected on a JEOL JEM 1200 EXII microscope (NHLBI EM Core) equipped with an XR-60 digital camera (Advanced Microscopy Techniques) operating at 80 kV.
Disaggregation
To estimate fibril amounts prior to performing disaggregation, aggregated RPT samples were pelleted via centrifugation followed by resuspending in a fixed volume of pH 4, 5, or 5.5 buffer supplemented with 0.01 % NaN3. An aliquot of the stock was then diluted 1:1 with 8 M GuHCl to denature the fibrils prior to estimating the concentration using UV-Vis spectroscopy. For Trp fluorescence analysis of disaggregation, experiments were performed by diluting a 20 μM solution of quantified nRPT fibrils 6.25-fold (80 μL of nRPT into 420 μL of buffer) into either its corresponding buffer (i.e., fibrils formed at pH 4 diluted into pH 4 buffer) or into pH 7 buffer. After 5 min of incubation in a cuvette, Trp measurements were collected as described above. For analysis by centrifugation and SDS-PAGE, self-diluted or pH 7-diluted samples were centrifuged at 16,000 rcf for 30 min followed by carefully removing the soluble fraction and resuspending the insoluble pellet in the same volume of the same buffer. Soluble and insoluble fractions were then analyzed by SDS-PAGE as described above.
RESULTS AND DISCUSSION
Purification of native RPT (Pmel17 312–444)
TEV protease is a site-specific cysteine protease that cleaves after the glutamine residue in the ENLYFQ(G/S) recognition sequence, leaving either a glycine or serine overhang [22]. In our construct, we utilized this to our advantage by inserting a TEV recognition sequence three residues upstream of the traditional RPT start sequence (315), where a native glycine residue is located (Fig. 1). The exact sites of proteolytic cleavage within the Mα domain of Pmel17 during stage II of melanosome maturation are unknown [10], therefore we chose this site because it allowed us to generate a native Pmel17 fragment that was most similar to RPT-His, which has been characterized extensively by our group as well as others [8, 9, 13, 15-19, 23-27]. We also included a hexa-histidine tag on the N-terminus to allow for nickel affinity purification.
The His-tagged, TEV-cleavable nRPT (His-TEV-nRPT) plasmid was transformed into BL21(DE3) E. coli cells and expressed following standard procedures described previously [18]. From a 3-L culture, a 10 g cell pellet was obtained and lysed under denaturing conditions as described above. The lysate was clarified by centrifugation, loaded onto a nickel-chelating column and purified following standard procedures (Fig. 2a). This produced highly homogenous His-TEV-nRPT, as analyzed by SDS-PAGE and LC-MS (Fig. 2b). Upon cleavage of His-TEV-nRPT with a His-tagged TEV protease, the sample was passed back over a nickel-chelating column and the unbound fraction was collected (Fig. 2c, boxed region) which contained 150 mg of pure nRPT (Fig. 2d). Using SDS-PAGE, RPT-His has been shown to migrate with an apparent molecular weight much higher than its actual molecular weight [11], which was also observed here for nRPT (Fig. 2b and Fig. 2d, inset). Overall, this represents a fast and efficient method of generating a native Pmel17 RPT fragment.
nRPT undergoes pH-dependent aggregation and fibril maturation
To determine whether a pH-dependent aggregation behavior similar to RPT-His is also observed for nRPT, protein stocks were buffer-exchanged into 20 mM sodium acetate (pH 4, 4.5, 5, and 5.5), 20 mM MES (pH 6), or 20 mM sodium phosphate (pH 7) buffers, each supplemented with 100 mM NaCl. Immediately after buffer exchange and prior to aggregation, the soluble state of nRPT was characterized by circular dichroism (CD) spectroscopy and intrinsic Trp fluorescence spectroscopy (Fig. 3). A similar CD spectrum was observed for all pH conditions, where a minimum at ~200 nm suggested a random coil conformation (Fig. 3a). Trp fluorescence was also collected to determine whether the single Trp at residue 423 (423W) is a site-specific reporter of nRPT aggregation, as it is for RPT-His [13]. In the soluble, disordered state, a similar Trp fluorescence was observed for all conditions with a λmax of ~350 nm, indicative that 423W is solvent-exposed (Fig. 3b). A slight blue-shift and fluorescence quenching was observed at pH 4.5 (Fig. 3b, orange), which was more apparent at pH 4 (Fig. 3b, red). This suggests that the local environment of the Trp residue is slightly different under highly acidic conditions, although the protein remains disordered, as determined by CD (Fig. 3a).
Figure 3: Characterization of soluble nRPT.
Protein stocks were desalted into buffers with a pH of 4 (red), 4.5 (orange), 5 (gold), 5.5 (green), 6 (cyan), and 7 (blue) followed by diluting to 30 μM and collecting circular dichroism (a) and intrinsic tryptophan fluorescence (b) spectra.
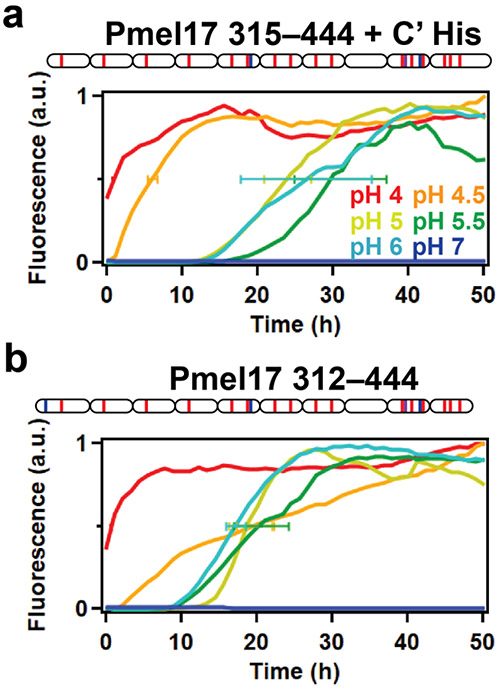
Aggregation kinetics of both RPT-His (Fig. 4a) and nRPT (Fig. 4b) were then collected by the addition of an extrinsic fluorescent dye, thioflavin-T (ThT), which upon binding to β-sheet-rich amyloid fibrils, displays enhanced fluorescence intensity [28]. Samples were aliquoted into a 384-well microplate supplemented with a 2-mm glass bead, and measurements were collected at 37 °C under constant agitation. Similar trends were observed for both RPT-His and nRPT, where aggregation at pH 4 (red curve) occurred immediately without a lag phase, followed by pH 4.5 (orange curve) after a short (~1–2 h) lag phase (Fig. 4a-b). It is noted that while the lag phase of nRPT at pH 4.5 was short, the growth phase was significantly slower as compared to RPT-His. This was evident by determining the time at which the fluorescence intensity reached one-half its maximum value, termed t½. The t½ value of RPT-His at pH 4.5 was 6.1 ± 0.7 h, while it was 19.9 ± 2.5 h for nRPT. No significant differences were observed between RPT-His at pH 5 (gold curve), pH 5.5 (green curve), or pH 6 (cyan curve), as evidenced by comparable t½ values (Fig. 4a-b). When comparing RPT-His to nRPT at pH 5, 5.5, and 6, t½ values were overall shorter (i.e., faster aggregation) for nRPT, although only at pH 5.5 did the differences reach statistical significance (P = 0.03). For both RPT-His and nRPT, aggregation did not occur at pH 7 (blue curve). Overall, this reveals that like RPT-His, nRPT only aggregates at acidic pH, where rates decrease from pH 4 to 5 and are similar from pH 5 to 6.
Figure 4: Aggregation kinetics of native RPT.
A schematic representation of RPT-His (a) and nPRT (b) are shown, where ovals represent individual repeats and red, blue, and purple bars represent acidic, basic, and aromatic residues, respectively. (a-b) Aggregation of RPT-His (30 μM, a) or nRPT (30 μM, b) at pH 4 (red), 4.5 (orange), 5 (gold), 5.5 (green), 6 (cyan), and 7 (blue), monitored by ThT fluorescence (10 μM) at 37 °C under constant linear shaking (6 mm). Curves represent the average of 4 replicates, normalized by dividing each replicate by its maximum ThT intensity. The standard deviation at t½ is also shown. The average and standard deviation for RPT-His at pH 4, 4.5, 5, 5.5, and 6 are 1 ± 0.1, 6.1 ± 0.7, 24 ± 3.1, 31 ± 6.1, and 26.5 ± 8.7 h, respectively. The average and standard deviation for nRPT at pH 4, 4.5, 5, 5.5, and 6 are 0.8 ± 0.1, 19.9 ± 2.5, 19.2 ± 2.9, 20.7 ± 3.6, and 17.3 ± 1.3 h, respectively.
After 50 h of incubation, nRPT samples were collected and analyzed by CD and Trp fluorescence spectroscopies as well as negatively-stained transmission electron microscopy (TEM) to visualize fibril morphology (Fig. 5). At pH 7 (blue curves), no spectral differences from the soluble (t0) were observed, where the protein remains a random coil (Fig. 5a) with a water-exposed 423W (Fig. 5b), corroborating that nRPT does not aggregate at pH 7. The CD spectra of nRPT at pH 4 (red curve) and 4.5 (orange curve) showed a mixture of disordered and structured conformations, whereas canonical β-sheet spectra are seen at pH 5 (gold curve), 5.5 (green curve), and 6 (cyan curve; Fig. 5a). A similar pH-dependent transition was observed with Trp fluorescence, where an initial increase in signal intensity and blue-shift in λmax was observed at pH 4, followed by fluorescence signal quenching from pH 4.5 to 5.5 (Fig. 5b). Characterization of fibril morphologies by TEM showed a similar pH-dependent trend (Fig. 5c). At pH 4, predominantly short and highly curved aggregates were observed, which have been previously observed for RPT-His and described as “squiggles” [11, 13]. Similar aggregates were also present at pH 4.5, in addition to longer and straighter fibrils, which were the predominant species present at pH 5. At pH 5.5 and 6 (cyan box), pairs of straight, rod-like filaments were observed, reminiscent to those observed for RPT-His [18, 25]. Taken this altogether, nRPT clearly undergoes a pH-dependent fibril maturation process from immature aggregates at pH 4–4.5 to mature, rod-like fibrils at pH 5.5–6. This observed transition mirrors in vivo conditions, where the intraluminal pH of melanosomes increases as maturation proceeds from stages I-IV [29].
Figure 5: Characterization of native RPT aggregates.
At the end of aggregation reactions shown in Fig. 3b, nRPT samples at pH 4 (red), 4.5 (orange), 5 (gold), 5.5 (green), 6 (cyan), and 7 (blue) were analyzed by circular dichroism (a) and intrinsic tryptophan fluorescence (b) as well as negatively-stained TEM images (c), pH 7 was not collected because no aggregation was observed.
Pre-formed nRPT fibrils disaggregate at neutral pH
It has been established that pre-formed RPT-His fibrils undergo rapid disaggregation upon dilution into buffers with a pH ≥ 6.5 [11, 15, 16, 18], a unique feature of RPT that is not observed with pathological amyloid proteins. To investigate whether the His-tag influences this process, disaggregation of nRPT fibrils was investigated (Fig. 6). nRPT fibrils formed at pH 4 (red curve), 4.5 (orange curve), 5 (gold curve), and 5.5 (green curve) were diluted ~6-fold with either its corresponding buffer (i.e., fibrils formed at pH 4 diluted into pH 4 buffer) or pH 7 buffer and evaluated by Trp fluorescence (Fig. 6a-b). Compared to self-dilution (Fig. 6a), a red-shift in the λmax was observed for 423W fluorescence upon dilution into pH 7 buffer (Fig. 6b), indicative of disaggregation. To support this observation, the solubility of samples was also evaluated upon centrifugation at 16,000 rcf (Fig. 6c). Upon self-dilution, nRPT fibrils partition into the insoluble (I) pellet (Fig. 6c, left), indicating that nRPT remains in a high molecular weight, amyloid state. However, upon dilution into pH 7 buffer, samples were found in the soluble (S) fraction (Fig. 6c, right), again suggesting fibril dissolution.
Figure 6: Disaggregation of native RPT fibrils at neutral pH.
(a-b) Intrinsic fluorescence spectra of nRPT fibrils formed at pH 4 (red), 4.5 (orange), 5 (gold), and 5.5 (green) and diluted 6.25-fold into its corresponding buffer (a) or into pH 7 buffer (b). (c) SDS-PAGE analysis of the soluble (S) and insoluble (I) fractions from self-diluted (left) or pH 7-diluted (right) samples upon centrifugation at 16,000 rcf for 30 min.
CONCLUSIONS
In this report, we have described the purification of a RPT domain spanning Pmel17 residues 312–444, achieved by introducing a TEV cleavage site immediately upstream of a native glycine residue. Similar to the previously characterized RPT-His (315–444His6), nRPT exhibited pH-dependent aggregation, forming fibrillar aggregates under acidic conditions (≤ 6) but not at neutral pH. At highly acidic pH (4–4.5), nRPT rapidly formed immature aggregates with short, curved morphological features, characterized by a mixed random coil/β-sheet CD signature and a blue-shifted 423W with increased fluorescence intensity. Under milder acidic pH regime (5–6), nRPT aggregation was slower but formed mature fibrils exhibiting a paired, rod-like architecture. They are also characterized by canonical β-sheet CD profiles and a blue-shifted and quenched 423W fluorescence. Both immature and mature nRPT fibrils undergo disaggregation at neutral pH, as indicated by 423W fluorescence and SDS-PAGE analysis upon centrifugation. We anticipate that this approach can be broadly utilized to purify and characterize amyloidogenic domains from larger proteins, such as the prion-like C-terminal domain of TDP-43 (utilizing the native Ser residue at position 266) [30], the core domain of p53 (utilizing one of the 13 native G/S residues located within residues 90–127) [31], and the low-complexity region of RBM14 (utilizing the native G reside located at position 349) [32].
Highlights:
Pmel17 RPT was made by introducing an N-terminal His-tag and TEV cleavage site.
Cleavage with TEV protease yields tagless Pmel17 RPT spanning residues 312–444.
Unique features of pH-dependent (dis)aggregation are intrinsic properties of RPT.
Reversibility of amyloid formation of RPT is of biological significance.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program at the National Institutes of Health, National Heart, Lung, and Blood Institute. Parts of this research were performed on instruments maintained by the NHLBI Electron Microscopy (TEM) and Biochemistry (LC-MS) Core Facilities. We thank Yi He (NHLBI Protein Expression Facility), Ryan McGlinchey, and Sydney Shuster for kindly providing His-tagged TEV protease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement: The authors declare no competing interests.
REFERENCES
- [1].Eisenberg DS, Sawaya MR, Structural studies of amyloid proteins at the molecular level, Annu. Rev. Biochem 86 (2017) 69–95. [DOI] [PubMed] [Google Scholar]
- [2].Knowles TP, et al. , The amyloid state and its association with protein misfolding diseases, Nat. Rev. Mol. Cell Biol 15 (2014) 384–396. [DOI] [PubMed] [Google Scholar]
- [3].Fowler DM, et al. , Functional amyloid – from bacteria to humans, Trends Biochem. Sci 32 (2007)217–224. [DOI] [PubMed] [Google Scholar]
- [4].Hewetson A, et al. , Functional amyloids in reproduction, Biomolecules. 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maji SK, et al. , Functional amyloids as natural storage of peptide hormones in pituitary secretory granules, Science. 325 (2009) 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fowler DM, et al. , Functional amyloid formation within mammalian tissue, PLOS Biol. 4 (2005) e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hurbain I, et al. , Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets, Proc. Natl. Acad. Sci. U.S.A 105 (2008) 19726–19731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McGlinchey RP, Lee JC, Reversing the amyloid trend: Mechanism of fibril assembly and dissolution of the repeat domain from a human functional amyloid, Isr. J. Chem 57 (2017) 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McGlinchey RP, Lee JC, Why study functional amyloids? Lessons from the repeat domain of Pmel17, J. Mol. Biol 430 (2018) 3696–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoashi T, et al. , The repeat domain of the melanosomal matrix protein PMEL17/GP100 is required for the formation of organellar fibers, J. Biol. Chem 281 (2006) 21198–21208. [DOI] [PubMed] [Google Scholar]
- [11].McGlinchey RP, et al. , The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis, Proc. Natl. Acad. Sci. U.S.A 106 (2009) 13731–13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu KN, et al. , Segmental polymorphism in a functional amyloid, Biophys. J 101 (2011) 2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pfefferkorn CM, et al. , Effects of pH on aggregation kinetics of the repeat domain of a functional amyloid, Pmel17, Proc. Natl. Acad. Sci. U.S.A 107 (2010) 21447–21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McGlinchey RP, et al. , Repeat domains of melanosome matrix protein Pmel17 orthologs form amyloid fibrils at the acidic melanosomal pH, J. Biol. Chem 286 (2011) 8385–8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McGlinchey RP, et al. , Probing fibril dissolution of the repeat domain of a functional amyloid, Pmel17, on the microscopic and residue level, Biochemistry. 50 (2011) 10567–10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McGlinchey RP, et al. , Molecular origin of pH-dependent fibril formation of a functional amyloid, ChemBioChem. 15 (2014) 1569–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dean DN, Lee JC, pH-Dependent fibril maturation of a Pmel17 repeat domain isoform revealed by tryptophan fluorescence, Biochim. Biophys. Acta Proteins Proteom 1867 (2019) 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dean DN, Lee JC, Modulating functional amyloid formation via alternative splicing of the premelanosomal protein PMEL17, J. Biol. Chem 295 (2020) 7544–7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Flynn JD, Lee JC, Raman fingerprints of amyloid structures, Chem. Commun 54 (2018) 6983–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shuster SO, Lee JC, Tryptophan probes of TDP-43 C-terminal domain amyloid formation, J. Phys. Chem. B 125 (2021) 3781–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kelly SM, et al. , How to study proteins by circular dichroism, Biochim. Biophys. Acta 1751 (2005) 119–139. [DOI] [PubMed] [Google Scholar]
- [22].Raran-Kurussi S, et al. , Removal of affinity tags with TEV protease, Methods Mol. Biol 1586 (2017) 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jiang Z, Lee JC, Lysophospholipid-containing membranes modulate the fibril formation of the repeat domain of a human functional amyloid, Pmel17, J. Mol. Biol 426 (2014) 4074–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pedersen JN, et al. , Lysophospholipids induce fibrillation of the repeat domain of Pmel17 through intermediate core-shell structures, Biochim. Biophys. Acta Proteins Proteom 1867 (2019) 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dean DN, Lee JC, Defining an amyloid link between Parkinson's disease and melanoma, Proc. Natl. Acad. Sci. U.S.A 117 (2020) 22671–22673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dogra P, et al. , pH-responsive mechanistic switch regulates the formation of dendritic and fibrillar nanostructures of a functional amyloid, J. Phys. Chem. B 121 (2017) 412–419. [DOI] [PubMed] [Google Scholar]
- [27].Dogra P, et al. , Hofmeister ions modulate the autocatalytic amyloidogenesis of an intrinsically disordered functional amyloid domain via unusual biphasic kinetics, J. Mol. Biol 432 (2020) 6173–6186. [DOI] [PubMed] [Google Scholar]
- [28].LeVine H, Quantification of β-sheet amyloid fibril structures with thioflavin T, Methods Enzymol. 309 (1999) 274–284. [DOI] [PubMed] [Google Scholar]
- [29].Raposo G, et al. , Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells, J. Cell Biol 152 (2001) 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li Q, et al. , Cryo-EM structure of amyloid fibrils formed by the entire low complexity domain of TDP-43, Nat. Comm 12 (2021) 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ishimaru D, et al. , Fibrillar aggregates of the tumor suppressor p53 core domain, Biochemistry. 42 (2003) 9022–9027. [DOI] [PubMed] [Google Scholar]
- [32].Hennig S, et al. , Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles, J. Cell Biol 210 (2015) 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]