Abstract
Females have been understudied in pre-clinical and clinical traumatic brain injury (TBI), despite distinct biology and worse clinical outcomes versus males. Sulfonylurea receptor 1 (SUR1) inhibition has shown promising results in predominantly male TBI. A phase II trial is ongoing. We investigated whether SUR1 inhibition effects on contusional TBI differ by sex given that this may inform clinical trial design and/or interpretation. We studied the moderating effects of sex on post-injury brain tissue loss in 142 male and female ATP-binding cassette transporter subfamily C member 8 (Abcc8) wild-type, heterozygote, and knockout mice (12–15 weeks). Monkey fibroblast-like cells and mouse brain endothelium-derived cells were used for in vitro studies. Mice were injured with controlled cortical impact and euthanized 21 days post-injury to assess contusion, brain, and hemisphere volumes (vs. genotype- and sex-matched naïves). Abcc8 knockout mice had smaller contusion volumes (p = 0.012) and larger normalized contralateral (right) hemisphere volumes (nRHV; p = 0.03) after injury versus wild type. This was moderated by sex: Contusions were smaller (p = 0.020), nRHV was higher (p = 0.001), and there was less global atrophy (p = 0.003) in male, but not female, knockout versus wild-type mice after TBI. Less atrophy occurred in males for each copy of Abcc8 lost (p = 0.023–0.002, all outcomes). In vitro, sex-determining region Y (SRY) stimulated Abcc8 promoter activity and increased Abcc8 expression. Loss of Abcc8 strongly protected against post-traumatic cerebral atrophy in male, but not female, mice. This may partly be mediated by SRY on the Y-chromosome. Sex differences may have important implications for ongoing and future trials of SUR1 blockade.
Keywords: atrophy, brain injuries, central nervous system, genotypic intracranial hemorrhage, sex, mice, traumatic
Introduction
Multiple pre-clinical and early clinical studies suggest that inhibiting sulfonylurea receptor 1 (SUR1) may be neuroprotective after traumatic brain injury (TBI).1–4 Based on encouraging results in preliminary studies demonstrating reduced cerebral edema and post-traumatic contusion growth,1–9 a phase II trial is currently evaluating the efficacy of glyburide, a SUR1 inhibitor, in contusional TBI (ASTRAL, NCT03954041), and a phase III trial is ongoing in stroke (NCT2869453). However, previous TBI therapies have reached similar points of translation only to fail in phase III trials.10–12 Identifying subgroups likely to respond to a novel therapy may both improve the probability of demonstrating efficacy and increase effectiveness if adopted into clinical practice.
Male bias is increasingly recognized in TBI research and neuroscience research as a whole.13 Although epidemiological studies report that females make up 30–40% of hospitalized TBI patients,14–17 they frequently comprise only 20% of patients enrolled into phase III therapeutic trials.11,12,18–21 A recent scoping review of >150 clinical reports by Gupte and colleagues identified that although males are ∼40% more likely to suffer TBI, most studies report worse clinical outcomes in females.22 They further identified a discontinuity between clinical and pre-clinical studies, given that the latter identify poorer outcomes in males.22 Females are additionally grossly under-represented in both pre-clinical and clinical studies—a phenomenon that is only partly attributable to disease epidemiology.22
Sex is also a major source of biological heterogeneity; it influences molecular drivers of secondary injury and functional outcomes after TBI.23,24 Sex-based differences in SUR1 inhibition have been reported for non-TBI neurological diseases.25,26 However, pre-clinical SUR1-TBI reports have been limited to males,1,2,7,8 and females have been under-represented in clinical SUR1-related reports.3,4,27,28 Sex-based differences in response to SUR1 inhibition post-TBI could have important implications for both design and analysis of current and future clinical trials, as well as bedside prognosis and treatment.29
In humans, biological sexual dimorphism is driven largely by the sex-determining region Y (SRY) gene on the Y chromosome.30 A previous study demonstrated that SRY forms a transcriptional complex with specificity protein 1 (Sp1), an activator of ATP-binding cassette transporter subfamily C member 8 (Abcc8; SUR1) transcription,31 which synergistically activates transcription of the monoamine oxidase A gene in neural tissue.32 A similar mechanism could contribute to sex differences in Abcc8 post-traumatic expression.
We hypothesized that sex moderates effects of genetic SUR1 inhibition on contusion volume and brain atrophy after contusional TBI in mice, and that the SRY gene contributes to these differences.
Methods
Mice
The University of Pittsburgh Institutional Animal Care and Use Committee approved the experiments. One hundred forty-two adult (12- to 15-week-old) male and female mice with global Abcc8 knocked out on a C57/BL6J background were used with heterozygote and wild-type littermates.33 Mice were given food and water ad libitum, group housed (12-h day-night cycles) until injury, and single housed thereafter.
Injury
Mice underwent a controlled cortical impact (CCI) model of moderate-severe TBI.2 Briefly, mice were anesthetized with 4% isoflurane in a 2:1 N2O/O2 gas mixture and placed in a stereotactic frame (Kopf, Tujunga, CA). A left scalp incision was made. A dental drill was used to make a 5-mm craniotomy over the left parietal cortex, and the bone flap was removed. CCI was performed with a pneumatic impactor (Bimba, Monee, IL) with a flat 3-mm tipped impounder on the left parietal cortex (5 m/s, depth = 1.2 mm, dwell time = 50–60 ms). Mice recovered in a temperature-controlled chamber before returning to their cage. Naïve mice were euthanized at 12–15 weeks without injury.
Histological volumetric analyses
Contusion volume (CV) and brain hemisphere (right hemisphere volume [RHV] and left hemisphere volume [LHV]) volumes were measured as previously described (Supplementary Methods).34 Total brain volume (TBV) was also calculated (RHV + LHV). Normalized values for each parameter in injured mice were calculated as a percentage of the corresponding mean hemisphere or mean total brain volume for sex- and genotype-matched naïve mice (e.g., normalized CVmale,KO [nCVmale,KO] = CVinjured,male,KO/LHVnaive,male,KO). We normalized to naïve volumes, rather than right (contralateral) hemisphere volume of the same mouse, because right hemisphere atrophy after injury was disproportionate across genotype and sex (Table 1). Volume measurements were performed by technicians blinded to genotype and sex.
Table 1.
Sex, Genotype, and Injury Effects on Total Brain and Hemispheric Volumes
| |
Naïve (mm3, mean ± SD) |
Injured (mm3, mean ± SD) |
Normalized (%, mean ± SD) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Wildtype | Knockout | p value | Wild type | Knockout | p value | Wild type | Knockout | p value | |
| Total brain volume | |||||||||
| Stratified by sex | |||||||||
| Male | 262.1 ± 18.1 | 239.7 ± 19.4 | 0.011* | 216.4 ± 15.2 | 218.2 ± 10.9 | 0.70 | 82.4 ± 5.8 | 91.0 ± 4.5 | 0.001* |
| Female | 257.7 ± 17.6 | 250.4 ± 11.7 | 0.25 | 232.1 ± 11.6 | 221.1 ± 9.6 | 0.02 | 90.1 ± 4.6 | 88.3 ± 3.7 | 0.32 |
| Base model (all mice): pgenotype=0.004†, psex = 0.037, pinjury< 0.001† | Base model (all mice): pgenotype = 0.06, psex = 0.32 | ||||||||
| Interaction model (all mice): pinteraction = 0.023† | Interaction model (all mice): pinteraction = 0.001† | ||||||||
| Ipsilateral (left) hemisphere volume | |||||||||
| Stratified by sex | |||||||||
| Male | 130.4 ± 9.1 | 118.5 ± 10.7 | 0.012* | 95.9 ± 6.9 | 96.1 ± 4.1 | 0.53 | 73.5 ± 5.3 | 81.1 ± 3.4 | 0.0004* |
| Female | 127.6 ± 8.7 | 123.6 ± 5.9 | 0.20 | 105.1 ± 4.2 | 99.3 ± 5.3 | 0.008 | 82.4 ± 3.3 | 80.3 ± 4.2 | 0.21 |
| Base model (all mice): pgenotype=0.001†, psex = 0.014†, pinjury< 0.0001† | Base model (all mice): pgenotype = 0.047†, psex = 0.008a† | ||||||||
| Interaction model (all mice): pinteraction = 0.022† | Interaction model (all mice): pinteraction < 0.0001† | ||||||||
| Contralateral (right) hemisphere volume | |||||||||
| Stratified by sex | |||||||||
| Male | 131.7 ± 9.1 | 121.2 ± 9.0 | 0.013 | 120.2 ± 9.8 | 122.0 ± 8.1 | 0.62 | 91.2 ± 7.4 | 100.1 ± 6.7 | 0.004* |
| Female | 130.1 ± 9.1 | 126.8 ± 5.9 | 0.31 | 127.0 ± 5.8 | 121.8 ± 7.2 | 0.07 | 97.6 ± 4.5 | 96.0 ± 5.6 | 0.47 |
| Base model (all mice): pgenotype = 0.019†, psex = 0.11, pinjury = 0.009† | Base model (all mice): pgenotype = 0.046†, psex = 0.70 | ||||||||
| Interaction model (all mice): pinteraction = 0.037† | Interaction model (all mice): pinteraction = 0.004† | ||||||||
Represents statistical significance in sex- and injury-stratified models at p = 0.0125. †Represents statistical significance in base or interaction model at p = 0.05.
SD, standard deviation.
Cell culture
COS-7 cells were cultured as previously described (Supplementary Methods).35 Control cell lines and cell lines expressing SRY were developed by transfecting bEnd.3 cells (Supplementary Methods). Transfections were performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), unless otherwise indicated. Colonies that survived in a medium containing 0.2 mg/mL of hygromycin B were selected, and SRY expression was confirmed by immunoblot analysis.
Luciferase promotor assay
SRY-stimulated activity of the promotor region of Abcc8 was determined using luciferase reporter plasmids (Supplementary Methods).36 COS-7 cells were transfected with luciferase reporter plasmids containing rat Abcc8 or Fra1 promoter, and expression was compared with cells transfected with chicken β-actin promoter-driven luciferase. Photinus luciferase reporter plasmids (pGL3-basic; Promega, Madison, WI), containing the rat Abcc8 promoter (−578 to +80) or rat FOS-like antigen 1 (Fra1) promotor (−878 to +61), were transfected into COS-7 cells using Lipofectamine 2000 (Invitrogen). Fra1 was used as a positive control for SRY-driven expression.37 Cotransfection of pRL-CMV, Renilla luciferase expression plasmid was a control for transfection efficiency. After transfection, cells were maintained in basal conditions for 24 h, then transfected with either rat SRY, human SRY, or empty vector. Photinus and Renilla luciferases activity in extracts of transfected cells were measured using the Dual Luciferase Reporter Assay System (Promega). Values of luciferase activity with SRY were divided by those from empty vector transfection to obtain fold-induction.38
Electrophoretic mobility shift assay
We measured the effect of SRY on the Kd value of binding of Sp1 to the 22-bp-long cis-regulatory element by electrophoretic mobility shift assay (EMSA; Supplementary Methods).32 To measure DNA binding activity of Sp1 to the putative binding sites in the Abcc8 promoter region, we used a biotinylated double-stranded DNA oligonucleotide probe containing the Sp1 binding sites (5’-GTGGGGGCGGGGCGGGGCGGGCCT-3’). Lysates from COS-7 cells transfected with two expression vectors driving expression of SRY and Sp1 or Sp1 and control empty vector were incubated with the biotinylated DNA fragments for 20 min at 25°C. To examine the DNA binding activity of Sp1 in high salt concentration, additional sodium chloride was added to the reaction mixture as indicated. Biotinylated DNA oligonucleotides on the membrane were detected by a chemiluminescent nucleic acid detection module kit (ThermoFisherScientific, Waltham, MA), and the images were processed by using commercial imaging software (FUJIFILM Life Science, Cambridge, MA.
RNA isolation and real-time polymerase chain reaction
Determination of the relative abundance of Abcc8 messenger RNA (mRNA) in bEnd.3 cells transfected with either empty vector or SRY was performed as previously described (Supplementary Methods).31 Total RNA was extracted using TRIzol Reagent (Invitrogen). RNA was further purified with Amplification Grade DNase I (Invitrogen). Complementary DNA was synthesized from 1 μg of total RNA of each sample using SuperScript III Reverse Transcriptase (Invitrogen). Abundance of Abcc8 mRNA in samples was determined by real-time PCR (ABI PRISM 7300; Applied Biosystems, Carlsbad, CA) and normalized to S18. Fra1 and vascular endothelial growth factor (Vegf) were used as positive and negative controls, respectively, of SRY-driven expression.
Statistical analysis
This study was powered (0.8) to detect a difference in CV between wild-type and knockout mice in analyses stratified by sex with n = 12 mice per group for each sex and genotype (Supplementary Methods). An additional 12 heterozygous mice were used for secondary analyses of how the number of alleles knocked out affects outcome. An equal number of naïve mice of the same sex and genotypes and similar age (12–15 weeks) were tested. Data are presented as mean ± standard deviation (SD). Associations between sex, genotype, and histological lesions were assessed with multi-variable linear regression models (Supplementary Methods). Primary models included two-level genotypes (wild-type and knockout), sex, and injury status (for LHV, RHV, and TBV) or only injured mice (for CV). Secondary analyses for the association between number of alleles knocked out and histological outcome (including heterozygous mice) were performed using a Wilcoxon-type non-parametric test of trend across genotypes.39
Use of regression models was a priori selected over multiple independent t-tests. Our large sample size of 142 mice allowed for the use of regression models including sex and genotype as covariates to address the research questions of interest without model overfitting. Alpha was set at 0.05 for initial regression Wald tests for genotype, 0.025 for post hoc analyses stratified by sex, and 0.0125 for post hoc analyses stratified by both sex and injury (Bonferroni). All analysis was performed with Stata software (version 16.1; StataCorp LP, College Station, TX).
Results
One hundred forty mice were included in the final analysis: Of 72 injured mice, 2 died after injury (1 female wild type, 1 male heterozygote), resulting in n = 11–12 per group. For naïve mice, breeding limitations resulted in only 10 obtainable male wild types, for a total of 70 naïve mice analyzed. Males weighed more than females (31.5 ± 3.0 vs. 23.2 ± 2.5 g; p < 0.001). Body weight did not differ by genotype in either sex.
Effect of sex on neuropathology
In wild-type mice, males and females had similar absolute contusion, hemisphere, and brain volumes post-injury (all p > 0.05). However, wild-type females were protected from post-injury atrophy versus wild-type males: They had higher post-injury nTBV (90.1 ± 3.7% vs. 82.4 ± 5.8%; p = 0.001), nRHV (97.6 ± 4.4% vs. 91.2 ± 7.4%; p = 0.02), and nLHV (82.4 ± 3.3% vs. 73.5 ± 5.3%; p = 0.0001).
Sex, genotype, and total brain volume
Male, but not female, Abcc8 knockout mice were protected from post-injury atrophy/TBV loss with increased protection for each Abcc8 allele knocked out (Fig. 1A; Table 1).
FIG. 1.
Genotype effects of Abcc8 knockout on total brain and hemispheric volumes differ by sex. (A-i) There was a significantly lower total brain volume for each copy of Abcc8 lost (wild type: dark gray; heterozygote: light gray; knockout: red) in male naïve mice (p < 0.0125), (A-ii) whereas after injury there was less brain volume lost after injury for each copy of Abcc8 knocked out (wild type: dark gray; heterozygote: light gray; knockout: red; p < 0.025). (B-i) There was a significant decrease in left hemisphere volume for each copy of Abcc8 lost in naïve male mice (wild type: dark gray; heterozygote: light gray; knockout: red) in male naïve mice (p < 0.0125), (B-ii) whereas after injury there was less diffuse hemisphere loss after injury for each copy of Abcc8 knocked out (wild type: dark gray; heterozygote: light gray; knockout: red; p < 0.025). (C-i) There was no significant effect of sequential Abcc8 allele knockout on right hemisphere volume in naïve mice (wild type: dark gray; heterozygote: light gray; knockout: red; p > 0.0125), (C-ii) whereas after injury there was less diffuse hemisphere loss after injury for each copy of Abcc8 knocked out in male mice (wild type: dark gray; heterozygote: light gray; knockout: red; p < 0.025). Abcc8, ATP-binding cassette transporter subfamily C member 8 (SUR1); CCI, controlled cortical impact; Het, heterozygote; KO, knockout; WT, wild type.
In all mice, a genotype-by-sex-by-injury interaction suggests that relationships between TBV and these variables are interdependent (pinteraction = 0.023; pinteraction_normalized = 0.001). In uninjured mice, male, but not female, Abcc8 knockouts had smaller brains/lower TBV versus wild types (p = 0.011; Table 1). However, male Abcc8 knockout mice were also protected from post-injury whole-brain atrophy (higher nTBV) versus wild type (p = 0.001; Table 1; Fig. 1A-ii). This protection was almost 2-fold—total atrophy in male wild-type mice was 17.6% of naïve TBV, whereas it was only 8.9% in male knockout mice (100% nTBV). Knockout did not protect females. In the secondary analyses including heterozygotes, there was increased post-injury sparing of nTBV in males for each copy of Abcc8 lost (p = 0.002; Fig. 1A-ii). Taken together, these results suggest that the role of Abcc8 in determining TBV both before and after injury depends on the number of alleles knocked out.
Sex, genotype, and hemisphere volumes
Male, but not female, Abcc8 knockouts were protected from global post-injury atrophy in both left (injured) and right (contralateral) hemispheres (Table 1). Again, the effect was dependent on the number of alleles knocked out (Fig. 1B,C).
In the left (ipsilateral) hemisphere, a genotype-by-sex-by-injury interaction indicates that the association between LHV and these three variables are interdependent (pinteraction = 0.022, pinteraction_normalized < 0.0001; Table 1). As with TBV, the association between genotype, sex, and LHV differed before versus after injury: In naïves, LHV was smaller in male knockouts versus wild types, but similar across genotypes in females (Table 1; Fig. 1B-i). After injury, LHV was lower in female knockouts versus wild types (p = 0.008), but not in males (Table 1). Post-injury benefit of Abcc8 knockout was exclusively observed in males: nLHV was higher in male knockouts versus wild types (p = 0.0004), but similar in females across genotypes, denoting a male-specific protection (Table 1; Fig. 1B-ii). When heterozygotes were included, there was a significant loss of LHV in male naïve mice (p = 0.011) and increased protection against atrophy in males after injury for each Abcc8 allele lost (p = 0.004; Fig. 1B-ii).
In the contralateral (right) hemisphere, a genotype-by-sex-by-injury interaction model again suggested an interdependence between these three variables in their effects on RHV (pinteraction = 0.037; pinteraction_normalized = 0.004). In naïves, male knockouts had lower RHV (p = 0.013), though it was not statistically significant after adjustment for multiple comparisons. This was not noted in females (Fig. 1C-i). nRHV was markedly higher in male Abcc8 knockouts versus male wild types, with essentially no post-injury volume loss (Fig. 1C-ii). nRHV did not differ by genotype in females. The decrease in RHV in naïve males was dependent on the number of alleles knocked out (p = 0.015; Fig. 1C-i), and nRHV loss decreased (i.e., less right hemisphere atrophy) in injured males for each copy of Abcc8 lost (p = 0.005; Fig. 1C-ii).
Sex, genotype, and contusion volume
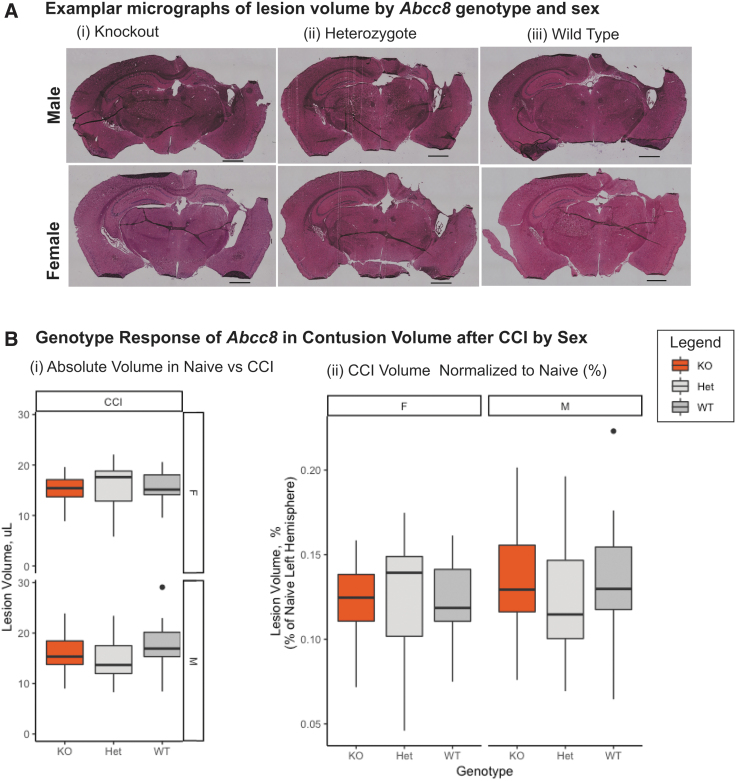
CV was lower in knockout versus wild-type mice (Table 2, base model). Abcc8 knockout was protective in male (17.7 ± 3.7 vs. 15.9 ± 3.6 mm3; p = 0.02), but not female, mice (Fig. 2B-i). There was no genotype-by-sex interaction (Table 2). There was no difference in nCV between wild types and knockouts of either sex (Fig. 2B-ii).
Table 2.
Sex and Genotype Effects on Contusion Volume
| |
Injured (mm3, mean ± SD) |
Normalized (%, mean ± SD) |
||||
|---|---|---|---|---|---|---|
| Wildtype | Knockout | p value | Wildtype | Knockout | p value | |
| Contusion volume | ||||||
| Stratified by sex | ||||||
| Male | 17.7 ± 3.7 | 15.9 ± 3.6 | 0.020* | 13.6 ± 4.1 | 13.4 ± 3.4 | 0.91 |
| Female | 15.8 ± 3.5 | 15.2 ± 3.0 | 0.73 | 12.4 ± 2.8 | 12.3 ± 2.4 | 0.96 |
| Base model (all mice): pgenotype = 0.012†, psex = 0.45 Interaction model (all mice): pinteraction = 0.26 |
Base model (all mice): pgenotype = 0.25, psex = 0.64 Interaction model (all mice): pinteraction = 0.95 |
|||||
Represents statistical significance in sex and injury stratified models at p = 0.025.
Represents statistical significance in base or interaction model at p = 0.05.
SD, standard deviation.
FIG. 2.
Genotype effects of Abcc8 knockout on contusion volume after injury differ by genotype and sex. (A) Representative photomicrographs of post-injury hemispheres from median mouse in each group. Top row from left to right: male knockout, male heterozygote, and male wild type. Second row from left to right: female knockout, female heterozygote, and female knockout. Scale bar is 1000 μm. (B) There was no relationship between number of alleles knocked out and contusion volume for either sex, either before (i) or after (ii) normalization. Abcc8, ATP-binding cassette transporter subfamily C member 8; CCI, controlled cortical impact; Het, heterozygote; KO, knockout; WT, wild type.
Sex-determining region Y–mediated ATP-binding cassette transporter subfamily C member 8 expression
In COS-7 cells luciferase activity driven by the Abcc8 promoter was markedly increased (up to 3.5-fold) in response to SRY coexpression (Fig. 3A). Serial deletion of the Abcc8 promotor identified a 22-bp cis-regulatory element (−100/–79) that when deleted caused a marked reduction in basal Abcc8 expression and completely abrogated SRY-mediated stimulation of Abcc8 expression (Fig. 3B). Sequence analysis of this region revealed no binding sites for SRY, but three binding sites for the transcription factor, Sp1. This suggests that SRY likely activates Abcc8 transcription indirectly rather than direct DNA binding. In an EMSA, coexpression of SRY with Sp1 increased the affinity of Sp1 to its binding sites ∼2- to 3-fold and stabilized the Sp1/DNA complex versus Sp1 alone across a wide range of NaCl concentrations (Fig. 3C). This suggests that SRY may increase Sp1-mediated Abcc8 transcription. When the effect of SRY on Abcc8 expression was evaluated in two independent cell lines originating from brain microvascular endothelium (i.e., overexpression of SRY in bEnd.3 cells), an ∼6-fold increase in Abcc8 mRNA was observed versus empty vector (Fig. 3D).
FIG. 3.
Sex-determining region Y (SRY) expression may stabilize Sp1 (specificity protein 1) and increase Abcc8 expression in males. (A) Coexpression of SRY stimulates SUR1 promoter activity. A luciferase reporter construct containing an ∼4-kb promoter region of rat Abcc8 was cotransfected into COS-7 cells along with plasmids directing expression of rat SRY (gray), human SRY (black), or vector alone (white). A luciferase reporter construct containing a promoter region of the rat FOS-like antigen 1 (Fra1) gene, which is regulated by SRY, was used as a positive control. (B) A 22-bp-long cis-regulatory element (−100/–79 plays a critical role in the stimulatory effect of SRY on transcription of Abcc8). A luciferase reporter construct containing −578/+80, −100/+80, or −78/+80 of the promoter region of the rat Abcc8 gene was transfected together with empty vector (Vector) or expression plasmid with SRY (SRY), and luciferase activities were measured. Each luciferase activity is represented as relative to the luciferase activity driven by a β-actin promoter (n = 5). (C) SRY stabilizes the Sp1/DNA complex. The biotinylated 22-bp-long cis-regulatory element (−100/–79 region) was used as a probe in an electrophoretic mobility shift assay. DNA binding activities of lysate from COS7 cells transfected with Sp1 plasmid alone or Sp1 and SRY expression plasmids together were compared at the indicated salt concentrations. (D) bEnd.3 cells were transfected with an empty vector (EV) of Flag-SRY vector (SRY cell lines), and stably transfected cells were selected. (D-i) Stable expression of SRY was validated in two independent cell lines (#1 and #2; PC indicates positive control). (D-ii) Comparison of mRNA abundance for Fra1 (positive control), vascular endothelial growth factor (VEGF; negative control), and SUR1 in control (white) and SRY-overexpressing (gray) bEnd.3 cells. Abcc8, ATP-binding cassette transporter subfamily C member 8.
Discussion
In this study, genetic knockout of Abcc8 (SUR1) yielded smaller contusions and conferred a robust protection from brain tissue atrophy after experimental TBI in male, but not female, mice. Protection appeared dependent on the number of alleles knocked out. Our in vitro studies suggest that this sex difference may partly be related to Y-chromosome gene SRY-mediated stimulation of Abcc8 transcription by Sp1. To our knowledge, this is the first study to identify sex differences associated with SUR1 inhibition/absence after TBI.
Sex and traumatic brain injury
Supported by both clinical and pre-clinical studies, it is widely acknowledged that biological sex may moderate pathophysiology and outcome after TBI.22,40–44 Effects are complex and vary based on mechanism, severity, and measured outcome.22 Nevertheless, females have been understudied in pre-clinical and clinical TBI, despite distinct biology and worse clinical outcomes versus males.22 Previous studies, specifically in the CCI model, have found inconsistent effects of sex on CV23,40,45: Some report smaller lesions in females23,40 whereas others identify no difference.45 Although we found no impact of sex on 21-day CV, total post-traumatic tissue loss was lower in wild-type females versus males.
Sex-based differences after TBI may be mediated by both hormonal and non-hormonal mechanisms.46 Several studies have implicated disruption of normal sex hormone regulation as a key prognostic factor after TBI.47,48 Similarly, pre-clinical data suggest that ovariectomized rats may have phenotypic similarities to male rats in development of secondary injury cascades and response to therapy.49–51 There is also increasing evidence that estrous cycle phase may be associated with outcome after TBI, with better outcomes in the follicular phase (potentially dependent on follicular phase progesterone level).52–55 The sex hormone, progesterone, has been extensively studied as a neuroprotectant, although randomized clinical trials were ultimately negative.10,23,56–58 However, controversy over dosing and other details of those trials remain. Non-hormonal mechanisms that may mediate sex differences after TBI include sex-based differences in the activation of neuronal death pathways,49,59 differences in excitotoxicity,60,61 different antioxidant defense mechanisms,59 differences in autophagy,62 and differential expression of neurotrophic factors.63 There may be additional complex differences related to both sex and gender in mechanism of injury, circumstances surrounding injury, and medical comorbidities.64
Sex, sulfonylurea receptor 1, and traumatic brain injury
Abcc8 knockout in male, but not female, mice reduced CV in our study. A novel ∼2-fold reduction in global brain tissue atrophy was also noted in male, but not female, Abcc8 knockouts after CCI (8.9% vs. 17.6%). This protective effect increased for each additional Abcc8 allele knocked out.
Although these sex-based differences have not been reported, CV reduction with Abcc8 knockout is consistent with earlier literature of pharmacological SUR1 inhibition. An early pre-clinical study of glyburide in TBI reported an ∼50% reduction in 24-h CV.8 A more modest reduction (∼30%) persisted at 21 days in a blinded, rigorous multi-center pre-clinical study in male rat CCI recently reported by the Operation Brain Trauma Therapy (OBTT) consortium.7 Of the 12 drugs tested to date by OBTT, only glyburide decreased 21-day CV after CCI.7 Surprisingly, reductions in male CV observed with glyburide were larger than those noted in our study of genetic SUR1 knockout,1,7 albeit in rats versus mice and at different post-injury time points. Nonetheless, this suggests complex effects of constitutive, complete, and global SUR1 knockout or, potentially, off-target beneficial effects of glyburide. SUR1 overexpression has also been observed in predominantly male (81%) human traumatic contusions, the corresponding injury pattern to CCI.28 Two placebo-controlled randomized trials (with ∼87% and 72% male patients, respectively) have demonstrated reduced contusion expansion with glyburide.3,4 The ongoing multi-center phase II ASTRAL trial of glyburide is specifically enrolling patients with contusional TBI (NCT03954041).
Although the protective effect observed in the contusion was expected, the robust protection against diffuse atrophy has not previously been described. It is consistent with a small randomized clinical trial demonstrating improved functional outcome in diffuse axonal injury27—of note, this study had only 2 females (out of 40 patients). Contralateral damage after CCI has been previously described and is associated with neuroinflammation or Wallerian degeneration, among other mechanisms.65,66 How SUR1 contributes to extracontusional brain atrophy remains to be defined. However, a greater global tissue sparing effect by knockout vs. glyburide therapy might reflect glyburide's limited BBB permeability.
A pre-clinical study of trigeminal neuralgia reported sex-based differences in SUR1-mediated pathology: SUR1 activation by selective-agonist diazoxide attenuated capsaicin-induced mechanical hypersensitivity in male, but not female, rats.26 Despite this, mechanisms underlying effects of sex on SUR1-mediated secondary injury are unknown. Our in vitro findings suggest that the SRY gene may stimulate Sp1-mediated Abcc8 expression. If Abcc8 expression is greater in males, then this could contribute to preferential protection of SUR1 knockout in males. Surprisingly, in naïve male mice, Abcc8 knockouts had lower brain volumes. Although the neurodevelopmental importance of SUR1 remains undefined, our data suggest a teleological role for SUR1 in normal central nervous system (CNS) development (particularly in males) either directly or indirectly by systemic effects on metabolic function and growth.25
Research on SUR1 in TBI has been exclusively (pre-clinical) or predominantly (clinical) conducted in males.1–4,7,8,27,28 This is suboptimal for a variety of reasons, including potential biological sex-based differences in the role of this pathway post-injury with ensuing disparate responses to targeted inhibition. Although the strong neuropathological protection in males is highly encouraging for ongoing and future trials of SUR1 inhibition in contusional TBI, our finding that females were not protected may be important to optimize and inform trial design/analysis and future targeted therapy. Further work evaluating mechanistic differences in this pathway between the sexes, as well as comparing global versus conditional genetic inhibition versus pharmacological inhibition, may valuably identify potential off-target effects as well as the role of dose/timing in males versus females.
Strengths and limitations
This is one of the largest pre-clinical studies of sex-based differences in TBI, with findings that potentially inform ongoing and future clinical trials of SUR1 inhibition. Our large sample size allowed for a detailed analysis of both sex and genotype effects and inclusion of naïve mice of both sexes, and all three genotypes allowed for appropriate normalization of baseline tissue volumes and assessment of non-injury-related effects of SUR1, which have not previously been reported. Our injury model specifically mimics human contusional TBI and is relevant to the population being enrolled in ASTRAL, and both the model and the neuropathological outcomes are well established. Blinding research technicians to sex and genotype decreased risk of bias.
Our study also has several limitations. Although genetic knockout allowed us to assess complete SUR1 inhibition, this does not necessarily reflect pharmacological inhibition, which may produce incomplete inhibition and/or off-target effects. Pharmacological inhibition can also be modulated with regard to dose and timing. Surprisingly, our findings suggest that genetic inhibition may underestimate potential efficacy, at least on CV.1,7 Global Abcc8 knockout has consequences outside the CNS, particularly on glucose metabolism and the cardiovascular system25,67; this study does not parse out CNS versus non-CNS effects. Global Abcc8 knockouts also indiscriminately inhibit all SUR1-regulated channels, including constitutively expressed potassium channels in the CNS.68 Additionally, our study is limited by not having tracked the estrous phase of female mice, with increasing pre-clinical and clinical evidence that the estrous cycle and endogenous female sex hormones may play an important role in modulating secondary injury after TBI.52–55,69,70
Our approach, however, mimicked clinical practice where injury can occur at any point in the cycle, and resultant endocrine dysregulation may impede clinical assessment of cycle phase after severe injury.48 Nevertheless, the impact of estrous phase on response to this pathway should be assessed in future studies, particularly those testing glibenclamide therapy, given its use in current clinical trials. Further, investigating the impact of the estrous phase on this pathway in future work may help identify novel and potentially intervenable targets involved in lesion volume and brain atrophy after TBI. Given the large sample size required to appropriately power the study and normalize appropriately across both sex and genotype, our specific focus on neurohistopathological effects, and a desire to promptly generate findings on this important issue (particularly in the context of the radiographical end-point of the ongoing ASTRAL study), behavioral testing was beyond the current scope and was not performed. However, behavioral performance is a key metric for therapy translatability, and previous studies have demonstrated sex-specific responses to therapy in behavioral outcomes.71–74
Follow-up pre-clinical work investigating effects of sex differences in this pathway on behavioral outcome may importantly guide upcoming phase 3 clinical trial design to evaluate potentially disparate effects of SUR1 inhibition in male and female patients (sample-size calculations, analysis plans, etc.). Similarly, our study is limited by studying mechanisms only in vitro, and not assessing whether Abcc8 expression was increased in vivo in these mice. These studies will be critical for a better understanding of the potential translational significance of the observed sex-based differences and should be performed in the future.
Conclusion
Abcc8 knockout reduced contusion volume and protected against global brain-tissue atrophy after experimental moderate-severe TBI: benefit was restricted to males, with increasing protection for each additional Abcc8 allele knocked out. Studies exploring sex-dependent effects of pharmacological SUR1 inhibition are warranted—these could have important implications for analysis of the ongoing ASTRAL trial evaluating contusion expansion, as well as design of future studies testing SUR1 inhibition in TBI, and beyond.
Supplementary Material
Authors' Contributions
S.T.: Conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript, substantive revision of manuscript, and final approval before submission. B.E.Z.: Conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript, substantive revision of manuscript, and final approval before submission. P.M.K.: Conception and design of the study, drafting a significant portion of the manuscript, substantive revision of the manuscript, and final approval prior to submission. J.M.S.: Conception and design of the study, acquisition and analysis of data, substantive revision of the manuscript, and final approval before submission. V.G.: Conception and design of the study, acquisition and analysis of data, substantive revision of the manuscript, and final approval before submission. M.S.K.: Conception and design of the study, acquisition and analysis of data, substantive revision of the manuscript, and final approval before submission. S.K.W.: Conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript, substantive revision of the manuscript, and final approval before submission. R.S.B.: Drafting a significant portion of the manuscript, substantive revision of the manuscript, and final approval before submission. K.J.F.: Acquisition and analysis of data, substantive revision of the manuscript, and final approval before submission. V.A.V.: Acquisition and analysis of data, substantive revision of the manuscript, and final approval before submission. R.M.J.: Conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript, substantive revision of the manuscript, and final approval before submission.
Funding Information
The authors are grateful to funding from the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS; K23NS101036 and 1R01NS115815-01A1 [to R.M.J.] and R01NS102589 [to J.M.S.]). Drs. Jha and Kochanek are supported by the Chuck Noll Foundation. Dr. Kochanek is also supported by the Ake Grenvik Endowment.
Author Disclosure Statement
R.M.J. is a paid consultant for and on the advisory board for Biogen. J.M.S. holds a US patent (7,285,574), “A novel non-selective cation channel in neural cells and methods for treating brain swelling.” J.M.S. is a member of the board of directors for and holds shares in Remedy Pharmaceuticals and is a paid consultant for Biogen.
Supplementary Material
References
- 1.Zweckberger, K., Hackenberg, K., Jung, C.S., Hertle, D.N., Kiening, K.L., Unterberg, A.W., and Sakowitz, O.W. (2014). Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience 272, 199–206 [DOI] [PubMed] [Google Scholar]
- 2.Jha, R.M., Molyneaux, B.J., Jackson, T.C., Wallisch, J.S., Park, S.-Y., Poloyac, S., Vagni, V.A., Janesko-Feldman, K.L., Hoshitsuki, K., Minnigh, M.B., and Kochanek, P.M. (2018). Glibenclamide produces region-dependent effects on cerebral edema in a combined injury model of traumatic brain injury and hemorrhagic shock in mice. J. Neurotrauma 35, 2125–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalili, H., Derakhshan, N., Niakan, A., Ghaffarpasand, F., Salehi, M., Eshraghian, H., Shakibafard, A., and Zahabi, B. (2017). Effects of oral glibenclamide on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injuries: a randomized double-blind placebo-controlled clinical trial. World Neurosurg. 101, 130–136 [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg, H.M., Shenton, M.E., Pasternak, O., Simard, J.M., Okonkwo, D.O., Aldrich, C., He, F., Jain, S., and Hayman, E.G. (2020). Magnetic resonance imaging pilot study of intravenous glyburide in traumatic brain injury. J. Neurotrauma 37, 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimberly, W.T., Bevers, M.B., von Kummer, R., Demchuk, A.M., Romero, J.M., Elm, J.J., Hinson, H.E., Molyneaux, B.J., Simard, J.M., and Sheth, K.N. (2018). Effect of IV glyburide on adjudicated edema endpoints in the GAMES-RP Trial. Neurology 91, e2163–e2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheth, K.N., Elm, J.J., Molyneaux, B.J., Hinson, H., Beslow, L.A., Sze, G.K., Ostwaldt, A.-C., Del Zoppo, G.J., Simard, J.M., Jacobson, S., and Kimberly, W.T. (2016). Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 15, 1160–1169 [DOI] [PubMed] [Google Scholar]
- 7.Jha, R.M., Mondello, S., Bramlett, H.M., Dixon, C.E., Shear, D.A., Dietrich, W.D., Wang, K.K.W., Yang, Z., Hayes, R.L., Poloyac, S.M., Empey, P.E., Lafrenaye, A.D., Yan, H.Q., Carlson, S.W., Povlishock, J.T., Gilsdorf, J.S., and Kochanek, P.M. (2021). Glibenclamide treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma 38, 628–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simard, J.M., Kilbourne, M., Tsymbalyuk, O., Tosun, C., Caridi, J., Ivanova, S., Keledjian, K., Bochicchio, G., and Gerzanich, V. (2009). Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma 26, 2257–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheth, K.N., Kimberly, W.T., Elm, J.J., Kent, T.A., Yoo, A.J., Thomalla, G., Campbell, B., Donnan, G.A., Davis, S.M., Albers, G.W., Jacobson, S., del Zoppo, G., Simard, J.M., Stern, B.J., and Mandava, P. (2014). Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit. Care 21, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, J., Huang, S., Qin, S., You, C., and Zeng, Y. (2016). Progesterone for acute traumatic brain injury. Cochrane Database Syst. Rev. 12, CD008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson, C.S., Hannay, H.J., Yamal, J.-M., Gopinath, S., Goodman, J.C., Tilley, B.C., Epo Severe TBI Trial Investigators, Baldwin, A., Rivera Lara, L., Saucedo-Crespo, H., Ahmed, O., Sadasivan, S., Ponce, L., Cruz-Navarro, J., Shahin, H., Aisiku, I.P., Doshi, P., Valadka, A., Neipert, L., Waguspack, J.M., Rubin, M.L., Benoit, J.S., and Swank, P. (2014). Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 312, 36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper, D.J., Nichol, A.D., Bailey, M., Bernard, S., Cameron, P.A., Pili-Floury, S., Forbes, A., Gantner, D., Higgins, A.M., Huet, O., Kasza, J., Murray, L., Newby, L., Presneill, J.J., Rashford, S., Rosenfeld, J.V., Stephenson, M., Vallance, S., Varma, D., Webb, S.A.R., Trapani, T., and McArthur C., ; POLAR Trial Investigators and the ANZICS Clinical Trials Group. (2018). Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA 320, 2211–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shansky, R.M., and Murphy, A.Z. (2021). Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. 24, 457–464 [DOI] [PubMed] [Google Scholar]
- 14.Andriessen, T.M.J.C., Horn, J., Franschman, G., van der Naalt, J., Haitsma, I., Jacobs, B., Steyerberg, E.W., and Vos, P.E. (2011). Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J. Neurotrauma 28, 2019–2031 [DOI] [PubMed] [Google Scholar]
- 15.Majdan, M., Plancikova, D., Brazinova, A., Rusnak, M., Nieboer, D., Feigin, V., and Maas, A. (2016). Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health 1, e76–e83 [DOI] [PubMed] [Google Scholar]
- 16.Spaite, D.W., Bobrow, B.J., Keim, S.M., Barnhart, B., Chikani, V., Gaither, J.B., Sherrill, D., Denninghoff, K.R., Mullins, T., Adelson, P.D., Rice, A.D., Viscusi, C., and Hu, C. (2019). Association of statewide implementation of the prehospital traumatic brain injury treatment guidelines with patient survival following traumatic brain injury: the Excellence in Prehospital Injury Care (EPIC) study. JAMA Surg 154, e191152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor, C.A., Bell, J.M., Breiding, M.J., and Xu, L. (2017). Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CRASH-3 trial collaborators. (2019). Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 394, 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts, I., Yates, D., Sandercock, P., Farrell, B., Wasserberg, J., Lomas, G., Cottingham, R., Svoboda, P., Brayley, N., Mazairac, G., Laloë, V., Muñoz-Sánchez, A., Arango, M., Hartzenberg, B., Khamis, H., Yutthakasemsunt, S., Komolafe, E., Olldashi, F., Yadav, Y., Murillo-Cabezas, F., Shakur, H., and Edwards P., ; CRASH trial collaborators. (2004). Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 364, 1321–1328 [DOI] [PubMed] [Google Scholar]
- 20.Skolnick, B.E., Maas, A.I., Narayan, R.K., van der Hoop, R.G., MacAllister, T., Ward, J.D., Nelson, N.R., and Stocchetti, N.; SYNAPSE Trial Investigators. (2014). A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476 [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson, P.J., Kolias, A.G., Timofeev, I.S., Corteen, E.A., Czosnyka, M., Timothy, J., Anderson, I., Bulters, D.O., Belli, A., Eynon, C.A., Wadley, J., Mendelow, A.D., Mitchell, P.M., Wilson, M.H., Critchley, G., Sahuquillo, J., Unterberg, A., Servadei, F., Teasdale, G.M., Pickard, J.D., Menon, D.K., Murray, G.D., and Kirkpatrick, P.J.; RESCUEicp Trial Collaborators. (2016). Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 375, 1119–1130 [DOI] [PubMed] [Google Scholar]
- 22.Gupte, R., Brooks, W., Vukas, R., Pierce, J., and Harris, J. (2019). Sex differences in traumatic brain injury: what we know and what we should know. J. Neurotrauma 36, 3063–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clevenger, A.C., Kim, H., Salcedo, E., Yonchek, J.C., Rodgers, K.M., Orfila, J.E., Dietz, R.M., Quillinan, N., Traystman, R.J., and Herson, P.S. (2018). Endogenous sex steroids dampen neuroinflammation and improve outcome of traumatic brain injury in mice. J. Mol. Neurosci. 64, 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker, L.B., Velosky, A.G., Fu, A.H., and McCabe, J.T. (2019). Chronic neurobehavioral sex differences in a murine model of repetitive concussive brain injury. Front. Neurol. 10, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou, X., Xu, C., Zou, Z., Shen, X., Xie, T., Zhang, R., Liao, L., and Dong, J. (2019). The characteristics of glucose metabolism in the sulfonylurea receptor 1 knockout rat model. Mol. Med. 25, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu, K., Saloman, J.L., Zhang, Y., and Ro, J.Y. (2011). Sex differences in the contribution of ATP-sensitive K+ channels in trigeminal ganglia under an acute muscle pain condition. Neuroscience 180, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafardoost, P., Ghasemi, A.A., Salehpour, F., Piroti, C., and Ziaeii, E. (2016). Evaluation of the effect of glibenclamide in patients with diffuse axonal injury due to moderate to severe head trauma. Trauma Mon. 21, e25113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerzanich, V., Stokum, J.A., Ivanova, S., Woo, S.K., Tsymbalyuk, O., Sharma, A., Akkentli, F., Imran, Z., Aarabi, B., Sahuquillo, J., and Simard, J.M. (2019). Sulfonylurea receptor 1, transient receptor potential cation channel subfamily M member 4, and kir6.2:role in hemorrhagic progression of contusion. J. Neurotrauma 36, 1060–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jha, R.M., Puccio, A.M., Chou, S.H.-Y., Chang, C.-C.H., Wallisch, J.S., Molyneaux, B.J., Zusman, B.E., Shutter, L.A., Poloyac, S.M., Janesko-Feldman, K.L., Okonkwo, D.O., and Kochanek, P.M. (2017). Sulfonylurea receptor-1: a novel biomarker for cerebral edema in severe traumatic brain injury. Crit. Care Med. 45, e255–e264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berta, P., Hawkins, J.R., Sinclair, A.H., Taylor, A., Griffiths, B.L., Goodfellow, P.N., and Fellous, M. (1990). Genetic evidence equating SRY and the testis-determining factor. Nature 348, 448–450 [DOI] [PubMed] [Google Scholar]
- 31.Woo, S.K., Kwon, M.S., Geng, Z., Chen, Z., Ivanov, A., Bhatta, S., Gerzanich, V., and Simard, J.M. (2012). Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J. Cereb. Blood Flow Metab. 32, 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, J.B., Chen, K., Li, Y., Lau, Y.-F.C., and Shih, J.C. (2009). Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J. 23, 4029–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seghers, V., Nakazaki, M., DeMayo, F., Aguilar-Bryan, L., and Bryan, J. (2000). Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J. Biol. Chem. 275, 9270–9277 [DOI] [PubMed] [Google Scholar]
- 34.Hemerka, J.N., Wu, X., Dixon, C.E., Garman, R.H., Exo, J.L., Shellington, D.K., Blasiole, B., Vagni, V.A., Janesko-Feldman, K., Xu, M., Wisniewski, S.R., Bayır, H., Jenkins, L.W., Clark, R.S.B., Tisherman, S.A., and Kochanek, P.M. (2012). Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J. Neurotrauma 29, 2192–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokum, J.A., Kwon, M.S., Woo, S.K., Tsymbalyuk, O., Vennekens, R., Gerzanich, V., and Simard, J.M. (2018). SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 66, 108–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerzanich, V., Kwon, M.S., Woo, S.K., Ivanov, A., and Simard, J.M. (2018). SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS One 13, e0195526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen, D.R., Sinclair, A.H., and McGovern, J.D. (1994). SRY protein enhances transcription of Fos-related antigen 1 promoter constructs. Proc. Natl. Acad. Sci. U. S. A. 91, 4372–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo, S.K., Lee, S.D., Na, K.Y., Park, W.K., and Kwon, H.M. (2002). TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol. Cell. Biol. 22, 5753–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuzick, J. (1985). A Wilcoxon-type test for trend. Stat. Med. 4, 87–90 [DOI] [PubMed] [Google Scholar]
- 40.Villapol, S., Loane, D.J., and Burns, M.P. (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connor, C.A., Cernak, I., and Vink, R. (2006). The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir. Suppl. 96, 121–124 [DOI] [PubMed] [Google Scholar]
- 42.Wagner, A.K., Ren, D., Conley, Y.P., Ma, X., Kerr, M.E., Zafonte, R.D., Puccio, A.M., Marion, D.W., and Dixon, C.E. (2007). Sex and genetic associations with cerebrospinal fluid dopamine and metabolite production after severe traumatic brain injury. J. Neurosurg. 106, 538–547 [DOI] [PubMed] [Google Scholar]
- 43.Jullienne, A., Salehi, A., Affeldt, B., Baghchechi, M., Haddad, E., Avitua, A., Walsworth, M., Enjalric, I., Hamer, M., Bhakta, S., Tang, J., Zhang, J.H., Pearce, W.J., and Obenaus, A. (2018). Male and female mice exhibit divergent responses of the cortical vasculature to traumatic brain injury. J. Neurotrauma 35, 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connor, C.A., Cernak, I., and Vink, R. (2005). Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 1062, 171–174 [DOI] [PubMed] [Google Scholar]
- 45.Hall, E.D., Gibson, T.R., and Pavel, K.M. (2005). Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J. Neurotrauma 22, 669–679 [DOI] [PubMed] [Google Scholar]
- 46.Rubin, T.G., and Lipton, M.L. (2019). Sex differences in animal models of traumatic brain injury. J. Exp. Neurosci. 13, 1179069519844020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakholia, M.V., Kumar, R.G., Oh, B.-M., Ranganathan, P.R., Berga, S.L., Kochanek, P.M., and Wagner, A.K. (2019). Systemic estrone production and injury-induced sex hormone steroidogenesis after severe traumatic brain injury: a prognostic indicator of traumatic brain injury-related mortality. J. Neurotrauma 36, 1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner, A.K., McCullough, E.H., Niyonkuru, C., Ozawa, H., Loucks, T.L., Dobos, J.A., Brett, C.A., Santarsieri, M., Dixon, C.E., Berga, S.L., and Fabio, A. (2011). Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J. Neurotrauma 28, 871–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bramlett, H.M., Furones-Alonso, O., Lotocki, G., Rodriguez-Paez, A., Sanchez-Molano, J., and Keane, R.W. (2009). Sex differences in XIAP cleavage after traumatic brain injury in the rat. Neurosci. Lett. 461, 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki, T., Bramlett, H.M., and Dietrich, W.D. (2003). The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp. Neurol. 184, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 51.Bramlett, H.M., and Dietrich, W.D. (2001). Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma 18, 891–900 [DOI] [PubMed] [Google Scholar]
- 52.Maghool, F., Khaksari, M., and Siahposht Khachki, A. (2013). Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 1497, 61–72 [DOI] [PubMed] [Google Scholar]
- 53.Wunderle, K., Hoeger, K.M., Wasserman, E., and Bazarian, J.J. (2014). Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J. Head Trauma Rehabil. 29, E1-E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Fountaine, M.F., Hill-Lombardi, V., Hohn, A.N., Leahy, C.L., and Testa, A.J. (2019). Preliminary evidence for a window of increased vulnerability to sustain a concussion in females: abrief report. Front. Neurol. 10, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen, Y., Herrold, A.A., Gallagher, V., Martinovich, Z., Bari, S., Vike, N.L., Vesci, B., Mjaanes, J., McCloskey, L.R., Reilly, J.L., and Breiter, H.C. (2021). Preliminary report: localized cerebral blood flow mediates the relationship between progesterone and perceived stress symptoms among female collegiate club athletes after mild traumatic brain injury. J. Neurotrauma. 38, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korley, F.K., Pauls, Q., Yeatts, S.D., Jones, C., Corbett-Valade, E., Silbergleit, R., Frankel, M., Barsan, W.G., Cahill, N., Bazarian, J., and Wright, D. (2021). Progesterone treatment does not decrease serum levels of biomarkers of glial and neuronal cell injury in moderate and severe TBI subjects: a secondary analysis of the Progesterone for Traumatic Brain Injury, Experimental Clinical Treatment (ProTECT) III trial. J. Neurotrauma. 38, 1953–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang, H., Hua, F., Wang, J., Sayeed, I., Wang, X., Chen, Z., Yousuf, S., Atif, F., and Stein, D.G. (2013). Progesterone and vitamin D: improvement after traumatic brain injury in middle-aged rats. Horm. Behav. 64, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright, D.W., Yeatts, S.D., Silbergleit, R., Palesch, Y.Y., Hertzberg, V.S., Frankel, M., Goldstein, F.C., Caveney, A.F., Howlett-Smith, H., Bengelink, E.M., Manley, G.T., Merck, L.H., Janis, L.S., and Barsan, W.G.; NETT Investigators. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du, L., Bayir, H., Lai, Y., Zhang, X., Kochanek, P.M., Watkins, S.C., Graham, S.H., and Clark, R.S.B. (2004). Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 279, 38563–38570 [DOI] [PubMed] [Google Scholar]
- 60.Wagner, A.K., Bayir, H., Ren, D., Puccio, A., Zafonte, R.D., and Kochanek, P.M. (2004). Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J. Neurotrauma 21, 125–136 [DOI] [PubMed] [Google Scholar]
- 61.Wagner, A.K., Fabio, A., Puccio, A.M., Hirschberg, R., Li, W., Zafonte, R.D., and Marion, D.W. (2005). Gender associations with cerebrospinal fluid glutamate and lactate/pyruvate levels after severe traumatic brain injury. Crit. Care Med. 33, 407–413 [DOI] [PubMed] [Google Scholar]
- 62.Du, L., Hickey, R.W., Bayir, H., Watkins, S.C., Tyurin, V.A., Guo, F., Kochanek, P.M., Jenkins, L.W., Ren, J., Gibson, G., Chu, C.T., Kagan, V.E., and Clark, R.S.B. (2009). Starving neurons show sex difference in autophagy. J. Biol. Chem. 284, 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen, X., Li, Y., Kline, A.E., Dixon, C.E., Zafonte, R.D., and Wagner, A.K. (2005). Gender and environmental effects on regional brain-derived neurotrophic factor expression after experimental traumatic brain injury. Neuroscience 135, 11–17 [DOI] [PubMed] [Google Scholar]
- 64.Mollayeva, T., Mollayeva, S., and Colantonio, A. (2018). Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 14, 711–722 [DOI] [PubMed] [Google Scholar]
- 65.Hall, E.D., Sullivan, P.G., Gibson, T.R., Pavel, K.M., Thompson, B.M., and Scheff, S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 66.Wiley, C.A., Bissel, S.J., Lesniak, A., Dixon, C.E., Franks, J., Beer Stolz, D., Sun, M., Wang, G., Switzer, R., Kochanek, P.M., and Murdoch, G. (2016). Ultrastructure of Diaschisis Lesions after Traumatic Brain Injury. J. Neurotrauma 33, 1866–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohnen, M.S., Ma, L., Zhu, N., Qi, H., McClenaghan, C., Gonzaga-Jauregui, C., Dewey, F.E., Overton, J.D., Reid, J.G., Shuldiner, A.R., Baras, A., Sampson, K.J., Bleda, M., Hadinnapola, C., Haimel, M., Bogaard, H.J., Church, C., Coghlan, G., Corris, P.A., Eyries, M., Gibbs, J.S.R., Girerd, B., Houweling, A.C., Humbert, M., Guignabert, C., Kiely, D.G., Lawrie, A., MacKenzie Ross, R.V., Martin, J.M., Montani, D., Peacock, A.J., Pepke-Zaba, J., Soubrier, F., Suntharalingam, J., Toshner, M., Treacy, C.M., Trembath, R.C., Vonk Noordegraaf, A., Wharton, J., Wilkins, M.R., Wort, S.J., Yates, K., Gräf, S., Morrell, N.W., Krishnan, U., Rosenzweig, E.B., Shen, Y., Nichols, C.G., Kass, R.S., and Chung, W.K. (2018). Loss-of-function ABCC8 mutations in pulmonary arterial hypertension. Circ. Genom. Precis. Med. 11, e002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia, Y., and Haddad, G.G. (1991). Major differences in CNS sulfonylurea receptor distribution between the rat (newborn, adult) and turtle. J. Comp. Neurol. 314, 278–289 [DOI] [PubMed] [Google Scholar]
- 69.Bruce-Keller, A.J., Dimayuga, F.O., Reed, J.L., Wang, C., Angers, R., Wilson, M.E., Dimayuga, V.M., and Scheff, S.W. (2007). Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma 24, 203–215 [DOI] [PubMed] [Google Scholar]
- 70.Suzuki, T., Bramlett, H.M., Ruenes, G., and Dietrich, W.D. (2004). The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma 21, 842–853 [DOI] [PubMed] [Google Scholar]
- 71.Wagner, A.K., Willard, L.A., Kline, A.E., Wenger, M.K., Bolinger, B.D., Ren, D., Zafonte, R.D., and Dixon, C.E. (2004). Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 998, 113–121 [DOI] [PubMed] [Google Scholar]
- 72.Wagner, A.K., Kline, A.E., Sokoloski, J., Zafonte, R.D., Capulong, E., and Dixon, C.E. (2002). Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci. Lett. 334, 165–168 [DOI] [PubMed] [Google Scholar]
- 73.Free, K.E., Greene, A.M., Bondi, C.O., Lajud, N., de la Tremblaye, P.B., and Kline, A.E. (2017). Comparable impediment of cognitive function in female and male rats subsequent to daily administration of haloperidol after traumatic brain injury. Exp. Neurol. 296, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner, A.K., Kline, A.E., Ren, D., Willard, L.A., Wenger, M.K., Zafonte, R.D., and Dixon, C.E. (2007). Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav. Brain Res. 181, 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.