Abstract
Introduction: Increased peripheral inflammation has been consistently documented in both adult and pediatric depression. However, elevated levels of C-reactive protein (CRP), a nonspecific biomarker for inflammation, have been primarily reported in adults; whether CRP plays a similar role in adolescent depression has not been conclusively established. In our prior work, we identified relationships between CRP and reward neurocircuitry in adolescents with psychiatric symptoms (N = 64) but not with depressive symptoms. Extending this work, we sought to examine CRP across the full range of mood and anxiety symptom severity in a larger, clinically diverse cohort of psychotropic medication-free adolescents and healthy controls (HCs).
Methods: Subjects were adolescents (N = 127, age: 15.17 ± 2.19 years, 78 female) with psychiatric symptoms (n = 96, including previous cohort of 64) and HC (n = 31). All completed a semi-structured psychiatric evaluation and dimensional assessments for depression, anxiety, anhedonia, and suicidality. Group-comparison and correlation analyses utilized nonparametric statistics controlled for body mass index, sex, and age at pFWE < 0.05.
Results: No group differences were identified in CRP levels between the clinical cohort and HCs. In addition, correlations between CRP and clinical symptomatology were not significant in either the whole sample or the psychiatric group.
Conclusions: We found that, unlike in adults, CRP was not associated with depressive symptoms. This suggests that inflammation in pediatric depression is more narrowly delimited at the onset of psychiatric symptoms and may only become systemic with chronicity.
Keywords: CRP, depression, anhedonia, anxiety, inflammation, RDoC
Introduction
Adolescence represents a critical development period when many psychiatric conditions first emerge. Studying young cohorts is therefore crucial to better understand the underlying mechanisms contributing to symptom onset. One pathway frequently implicated across psychiatric conditions has been immune system activation. Work by our group and others has shown increased levels of immune mediators in both adults and children with depression as well as other psychiatric disorders (Gabbay et al. 2009a, 2009b, 2009c, 2010; Miller et al. 2009; Bradley et al. 2015, 2019; Freed et al. 2019). However, previous research has been limited by the heterogeneity of psychiatric conditions, high diagnostic comorbidity, and overlapping symptomatology between categorical psychiatric diagnoses. In response, researchers have increasingly focused on relationships between inflammation and specific psychiatric symptoms. Supporting this notion are recent findings from our laboratory documenting associations between blood cytokine levels and severity of anhedonia (i.e., reduced capacity to experience pleasure), reflecting reward dysfunction, in a clinically diverse cohort of adolescents (Freed et al. 2019). Moreover, a similar set of cytokines was found to correlate with brain activation during a functional magnetic resonance imaging (fMRI) reward task (Bradley et al. 2019).
In addition to cytokines, an immune mediator that has been widely investigated in depression is C-reactive protein (CRP), a nonspecific biomarker for systemic inflammation produced by hepatocytes following tissue injury or inflammatory states. Studies in adults have consistently linked elevated CRP levels with depression (Danner et al. 2003; Ford and Erlinger 2004; Danese et al. 2008; Elovainio et al. 2009; Baumeister et al. 2016; Haroon et al. 2018). However, studies with depressed youth have largely failed to document such an association (Chaiton et al. 2010; Khandaker et al. 2014; Baumeister et al. 2016; Flouri et al. 2020; Jha et al. 2020), although one group reported a positive association between CRP levels and depression severity in adolescent girls (Tabatabaeizadeh et al. 2018). Our group recently carried out an investigation of CRP levels, neural activity during an fMRI reward task, and depression symptomology in a clinical cohort of 64 adolescents with predominantly mood and/or anxiety symptoms (Liu et al. 2020). While we found that CRP was associated with brain function during reward, no relationships were found with depression or anxiety symptom severity (Liu et al. 2020).
To better understand the disparate CRP findings reported in adult versus pediatric depression, we aimed to extend our previous research using a larger adolescent sample. As mood and anxiety symptoms are prevalent across psychiatric conditions, we employed an Research Domain Criteria (RDoC)-based approach and focused on a naturalistic sample of psychotropic medication-free youth with diverse psychiatric profiles, including subthreshold and comorbid diagnoses, as well as healthy controls (HCs). Based on prior findings in adults and our cytokine findings in youth, we hypothesized that blood CRP levels would be elevated in depressed adolescents relative to HCs and would be correlated with depression and anhedonia severity across adolescents.
Methods
Participants
Subjects from throughout the greater New York City region were recruited via community advertisements and referrals from physicians and affiliated Child and Adolescent Psychiatry Outpatient Clinics. Adult participants (aged 18+ years) provided informed consent in writing. A parent/legal guardian provided signed consent on behalf of participants younger than 18 years, who also signed an assent form agreeing to participate. The institutional review board (IRB) at Icahn School of Medicine at Mount Sinai approved all study procedures.
Inclusion and exclusion criteria
Clinical cohort
The clinical cohort included 64 adolescents who were reported in a prior fMRI study (Liu et al. 2020). Inclusion criteria: presence of psychiatric symptoms either meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994) diagnostic criteria or subthreshold, based on diagnostic psychiatric assessment (detailed below). Exclusion criteria comprised: (1) any current or history of neurological or physical health conditions; (2) composite IQ below 80, estimated using the Kaufman Brief Intelligence Test (KBIT) (Kaufman 1990); (3) drug use, detected via a urine toxicology test; (4) pregnancy, detected via a urine hormone test; (5) current substance abuse disorder, psychosis, or pervasive developmental disorder; (6) psychotropic medication use in the past 1–3 months, depending on drug half-life.
Healthy controls
HCs were psychotropic medication naive and did not meet diagnostic criteria for current or have history of any psychiatric disorder.
All participants
Acute illness that might induce inflammation as well as anti-inflammatory medication use in the past 2 weeks were excluded.
Clinical assessments
Psychiatric diagnoses
Psychiatric diagnoses were evaluated for all subjects using the semi-structured Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (KSADS-PL) (Kaufman et al. 1997). Diagnostic interviews were administered by a licensed clinical psychologist or child/adolescent psychiatrist trained in using the KSADS-PL. For participants younger than 18 years, a parent/guardian was also interviewed as an informant using the KSADS-PL.
Anhedonia severity
Anhedonia severity was indexed using two self-rated questionnaires: the Snaith–Hamilton Pleasure Scale (SHAPS, score range 14–56) (Snaith et al. 1995) and the Temporal Experience of Pleasure Scale (TEPS) (Gard et al. 2006). The TEPS measures both consummatory (TEPS-CP, score range 8–48) and anticipatory (TEPS-AP, score range 10–60) aspects of anhedonia. Note that higher levels of anhedonia are indicated by lower TEPS scores.
Depression severity
Depression severity was assessed using both the self-rated Beck Depression Inventory–Second Edition (BDI, score range 0–63) (Beck et al. 1996) and the clinician-rated Children's Depression Rating Scale–Revised (CDRS-R, score range 17–113) (Poznanski et al. 1985). For participants younger than 18 years, input from a parent/guardian was also obtained to help inform the CDRS-R.
Anxiety severity
Anxiety severity was measured using the 39-item Multidimensional Anxiety Scale for Children (MASC, score range 0–117) (March et al. 1997) self-report.
Suicidality severity
Suicidality severity was measured using the 19-item Beck Scale for Suicide Ideation (BSSI, score range 0–38) (Beck et al. 1979) self-report. Unlike other assessments, the BSSI was only completed by clinical participants.
CRP quantification
A peripheral blood draw was performed between 8 and 9AM after an overnight fast. Immunochemiluminometric assays were used to quantify blood CRP concentrations through LabCorp's high-sensitivity CRP test. Subjects were divided into three categories based on high (>3 mg/L), intermediate (1–3 mg/L), and low (<1 mg/L) levels of CRP, categorizations that correspond to high, moderate, and low levels of cardiovascular risk, respectively (Pearson et al. 2003). CRP concentrations for a minority of participants were reported as bounded ranges instead of exact values (two as “<0.1 mg/L,” three as “<0.2 mg/L,” 26 as “<0.3 mg/L,” and one as “<1 mg/L”). These were re-coded as equal to the bounding value (e.g., CRP <0.1 mg/L was re-coded to CRP = 0.1 mg/L) for the purpose of group comparison and symptom correlation analyses in the main text. Findings remained similar when analyses were repeated excluding the re-coded CRP data (Supplementary Results section).
Body mass index
As a standard measure of obesity, body mass index (BMI) was calculated as the participant's mass (kg) divided by the square of their height (m2).
Statistical analyses
Analyses were performed in Matlab 2018b (The MathWorks, Inc.). As the distribution of CRP levels in our sample was skewed (+4.45), nonparametric analyses were used in all the statistics, with significance defined as two-tailed pFWE < 0.05 across different assessments. Wilcoxon's signed rank tests were used to evaluate group differences in CRP levels between the psychiatric and HC cohorts. Group comparisons were repeated excluding psychiatric subjects who did not present with mood or anxiety symptoms. As immune activation has been specifically linked to depression and reward dysfunction, we also examined CRP differences between HC and depressed participants (defined as CDRS-R ≥ 35) as well as between HC and anhedonic participants (defined as SHAPS ≥26 to match subject number of HCs). Additionally, symptom severity within categorical high and low CRP groups was also examined with Wilcoxon's signed rank test. Differences in categorical (i.e., low, intermediate, and high) CRP distribution between psychiatric and HC groups were assessed using cross-table χ2 tests, which were also repeated excluding the intermediate CRP group. Finally, we used Spearman's rank correlation to assess associations between CRP levels and the severity of clinical symptoms in the whole sample, in the full psychiatric sample excluding HCs, and in the psychiatric subgroup with mood and/or anxiety symptoms. In addition, we carried out a secondary linear regression analysis to assess associations between CRP levels and clinical symptom severity. The sample size varied slightly among different clinical measurements because of data missing (detailed in Table 1). BMI, sex, and age were controlled for in all analyses. All confounds were regressed out from CRP levels using general linear model before group comparison analyses.
Table 1.
Demographic and Clinical Characteristics
| Demographics of whole sample | |||
|---|---|---|---|
| Age, years | 15.17 ± 2.19 (12–20) | Sex, F/M | 78/49 (61.4/38.6) |
| Medication, naive/free | 107/20 (84.3/15.8) | Race, Caucasian/African American/other | 57/45/25 (44.9/35.4/19.7) |
| Group, clinical/HC | 96/31 (75.6/24.4) | BMI, kg/m2 | 25.0 ± 6.6 (15.9–46.5) |
| Psychiatric profile of whole sample (current/past) | |||
|---|---|---|---|
| MDD |
47/5 (37.0/3.94) |
|
|
| Dysthymia |
10/0 (7.87/0) |
ODD |
12/1 (9.45/1.57) |
| DD NOS |
5/0 (3.94/0) |
ADHD |
30/5 (23.6/3.94) |
| Other mood disorders |
6/2 (4.72/1.57) |
OCD |
2/1 (1.57/0.79) |
| Anxiety | 55/3 (43.3/2.36) | Other disorders | 3/1 (2.36/0.79) |
| Symptom severity in whole sample | |||
|---|---|---|---|
| SHAPSa |
22.68 ± 6.19 (14–43) |
BDIa |
11.95 ± 12.35 (0–47) |
| TEPS-APb |
46.70 ± 8.19 (20–60) |
CDRS-Ra |
32.48 ± 15.05 (17–79) |
| TEPS-CPb | 34.42 ± 8.05 (11–48) | MASCc | 41.18 ± 18.59 (2–99) |
| Symptom severity in psychiatric group | |||
|---|---|---|---|
| SHAPS |
23.74 ± 6.09 (14–43) |
BDIa |
15.17 ± 12.58 (0–47) |
| TEPS-APd |
45.59 ± 8.51 (20–60) |
CDRS-Ra |
37.20 ± 14.47 (17–79) |
| TEPS-CPd |
33.35 ± 7.73 (11–48) |
MASCe |
45.19 ± 18.13 (11–99) |
| BSSI | 2.68 ± 5.73 (0–35) | ||
Values are reported as M ± SD (range) or n (%), as appropriate. Diagnoses and assessments were based on the DSM-IV to keep consistency across all participants over time. As participants could meet criteria for more than one disorder, totals do not sum to 100%.
Data missing from: a1 participant; b25 participants; c5 participants; d22 participants; e2 participants.
ADHD, attention-deficit/hyperactivity disorder; Anxiety, includes generalized anxiety, social anxiety, phobia, posttraumatic stress, and panic disorders as well anxiety disorder not otherwise specified; BDI, Beck Depression Inventory; BMI, body mass index; BSSI, Beck Scale for Suicide Ideation. BSSI was only reported in psychiatric group as no suicidal ideation in HCs; CDRS-R, Children's Depression Rating Scale–Revised; DD NOS, depressive disorder not otherwise specified; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HC, healthy control; MASC, Multidimensional Anxiety Scale for Children; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; ODD, oppositional defiant disorder; SD, standard deviation; SHAPS, Snaith–Hamilton Pleasure Scale; TEPS-AP, Temporal Experience of Pleasure Scale–anticipatory; TEPS-CP, Temporal Experience of Pleasure Scale–consummatory.
Results
Participants
A total of 127 adolescents (age, M ± SD: 15.17 ± 2.19 years, range: 12–20 years; 78 female) were included. Of these, 87 participants presented with mood and/or anxiety symptoms, 9 presented with other externalizing behavior symptoms but no mood or anxiety symptoms, and 31 presented as HCs with no significant psychiatric presentation or history. All participants were psychotropic medication-free. As noted above, 64 adolescents in the clinical cohort (age: 15.17 ± 2.10 years, 44 female) were previously described in Liu et al. (2020). Demographic and clinical data are compiled in Table 1.
Descriptive statistics
Consistent with our RDoC-style recruitment approach with a diverse psychiatric profile, subjects exhibited a wide range of severity scores on all clinical measurements (detailed in Supplementary Fig. S1). One-sample Kolmogorov–Smirnov tests indicated that clinical assessment scores were not normally distributed (all pKS < 1 × 10−21).
In our sample, CRP concentrations ranged from 0.1 to 26.2 mg/L (M ± SD = 1.72 ± 3.40) and did not follow a normal distribution (pKS = 3.19 × 10−33). In the categorical assessment of CRP levels, 87 participants (68.5%) had low CRP (<1 mg/L), 19 participants (14.96%) had intermediate CRP (1–3 mg/L), and 21 participants (16.54%) had high CRP (>3 mg/L).
Relationship between CRP level and BMI
The levels of CRP were significantly correlated with BMI in the whole group (r = 0.55, p = 1.64 × 10−11) and the psychiatric group (r = 0.62, p = 1.17 × 10−11). Therefore, BMI was controlled for as a covariate in this study.
CRP level in the psychiatric and HC groups
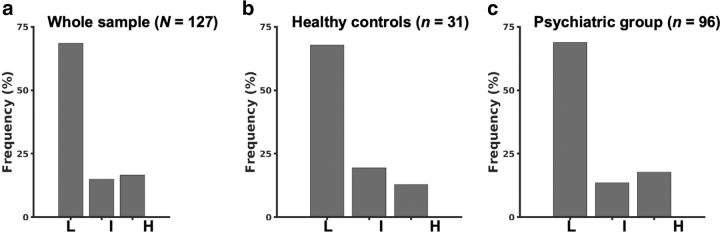
No group differences in CRP levels were detected between the full psychiatric sample and HCs (rank-sum Z = 0.43, p = 0.67). Group differences remained nonsignificant when subjects without mood or anxiety symptoms (n = 9) were excluded from the psychiatric group (rank-sum Z = 0.54, p = 0.59). CRP levels (M ± SD) in each group are presented in Table 2. Similarly, no significant differences were found in the distribution of CRP categorizations between psychiatric and HC subjects [Table 2 and Fig. 1, χ2(2) = 0.86, p = 0.65]. These results also remained nonsignificant when psychiatric patients without mood or anxiety symptoms were excluded [χ2(2) = 0.69, p = 0.71], as well as when only comparing high versus low CRP distributions between the psychiatric and HC groups [χ2(1) = 0.25, p = 0.62].
Table 2.
Concentration Level and Distribution of C-Reactive Protein Across Clinical Groups
| Group | CRP level, M ± SD (Range) | Low, n | Intermediate, n | High, n |
|---|---|---|---|---|
| Whole | 1.71 ± 3.34 (0.1–26.2) | 87 | 19 | 21 |
| HC | 1.45 ± 2.47 (0.1–11.2) | 21 | 6 | 4 |
| Psychiatric | 1.79 ± 3.58 (0.1–26.2) | 66 | 13 | 17 |
CRP, C-reactive protein; HC, healthy control; SD, standard deviation.
FIG. 1.
Distribution of low (L), intermediate (I), and high (H) CRP categorization in the whole sample (a), healthy controls (b), and psychiatric group (c). CRP, C-reactive protein.
CRP levels in adolescents with depressive and anhedonic symptoms
Similarly, no differences in CRP levels were detected in depressive (CDRS-R score ≥35; n = 52; M ± SD = 2.19 ± 4.49; rank-sum Z = 0.72, p = 0.47) or anhedonic (SHAPS score ≥26; n = 30; M ± SD = 2.48 ± 5.38; rank-sum Z = 0.05, p = 0.96) adolescents relative to HCs.
Blood CRP levels and psychiatric symptom severity
Correlation findings are presented in Table 3.
Table 3.
Correlations Between C-Reactive Protein and Clinical Symptom Severity
| SHAPS | TEPS-AP | TEPS-CP | BDI | CDRS-R | MASC | BSSI | |
|---|---|---|---|---|---|---|---|
| Whole sample | |||||||
| N | 126 | 102 | 102 | 126 | 126 | 122 | — |
| Rho | 0.07 | 0.01 | 0.08 | 0.01 | 0.02 | −0.02 | — |
| punc | 0.45 | 0.91 | 0.41 | 0.88 | 0.82 | 0.83 | — |
| Psychiatric subgroup | |||||||
| n | 96 | 74 | 74 | 95 | 95 | 94 | 96 |
| Rho | 0.06 | 0.00 | 0.05 | −0.02 | −0.04 | 0.00 | −0.01 |
| punc | 0.58 | 0.97 | 0.67 | 0.85 | 0.71 | 0.98 | 0.92 |
| Subgroup with mood and/or anxiety symptoms | |||||||
| n | 87 | 70 | 70 | 86 | 86 | 86 | 87 |
| Rho | 0.07 | −0.01 | 0.05 | −0.01 | −0.05 | 0.02 | −0.02 |
| punc | 0.54 | 0.92 | 0.66 | 0.96 | 0.67 | 0.86 | 0.84 |
BDI, Beck Depression Inventory; BSSI, Beck Scale for Suicide Ideation; CDRS-R, Children's Depression Rating Scale–Revised; MASC, Multidimensional Anxiety Scale for Children; SHAPS, Snaith–Hamilton Pleasure Scale; TEPS-AP, Temporal Experience of Pleasure Scale–anticipatory; TEPS-CP, Temporal Experience of Pleasure Scale–consummatory.
Anhedonia and depression
Contrary to our hypotheses, no associations were identified between blood CRP levels and measures of anhedonia (SHAPS, TEPS-CP, TEPS-AP) or overall depression (BDI, CDRS-R) severity in the whole sample (all |rho| < 0.08, p > 0.41), full psychiatric subgroup (all |rho| < 0.06, p > 0.58), or psychiatric subgroup with mood and/or anxiety symptoms (all |rho| < 0.07, p > 0.54).
Anxiety and suicidality
The correlation between CRP level and anxiety (MASC) severity in the whole sample was not significant (rho = −0.02, p = 0.83). Anxiety and suicidality (BSSI) were likewise not significantly correlated with CRP levels in the full psychiatric subgroup (all |rho| < 0.01, p > 0.98) or in the psychiatric subgroup with mood and/or anxiety symptoms (all |rho| < 0.02, p > 0.84).
Post hoc analyses
To exclude the effect of potential confounds, the following analyses were also carried out: (1) In light of a previous study documenting a positive correlation between CRP concentration and depression severity only in adolescent girls (Tabatabaeizadeh et al. 2018), correlation analyses were repeated separately using only female and only male participants. (2) Relationships between CRP levels and clinical measurements were assessed in unadjusted models, excluding cofounds. (3) Correlation analyses were repeated using only participants in the high CRP category to determine if these represented a potential biological subtype of adolescent depression, as has been suggested in adults (Bekhbat et al. 2020). (4) To evaluate the possible effects of outliers on CRP analyses, correlations were repeated after excluding five subjects with CRP measurements >10 mg/L. (5) We repeated all symptom correlation analyses excluding the 32 subjects with re-coded CRP levels (see the Methods section) to avoid quantification inaccuracy. (6) Finally, we performed regression models including CRP levels and confounds as independent variables and clinical measurements as dependent variables. Consistent with our main results, CRP levels were not associated with clinical symptomatology in any of these secondary analyses. Additional details are provided in the Supplementary Results section.
Clinical symptom severity in subjects with high versus low CRP levels
In light of the skewed distribution of CRP levels, differences in clinical symptom severity between subjects with high versus low CRP levels were also examined. As shown in Table 4, no significant differences in anhedonia, depression, anxiety, or suicidality were found based on Wilcoxon's signed rank tests, either within the whole sample (all |Z| < 0.90, p > 0.37), the full psychiatric subgroup (all |Z| < 1.5, p > 0.14), or the psychiatric subgroup with mood and/or anxiety symptoms (all |Z| < 1.4, p > 0.19).
Table 4.
Clinical Symptom Severity Differences Between Subjects with High and Low C-Reactive Protein Levels
| SHAPS | TEPS-AP | TEPS-CP | BDI | CDRS-R | MASC | BSSI | |
|---|---|---|---|---|---|---|---|
| Whole sample | |||||||
| Z | −0.74 | 0.11 | −0.81 | −0.9 | −0.66 | −0.9 | — |
| punc | 0.46 | 0.91 | 0.42 | 0.37 | 0.51 | 0.37 | — |
| Psychiatric subgroup | |||||||
| Z | −0.29 | −0.2 | −1.46 | −0.33 | −0.48 | −0.7 | 0.2 |
| punc | 0.77 | 0.84 | 0.14 | 0.74 | 0.63 | 0.48 | 0.84 |
| Subgroup with mood and/or anxiety symptoms | |||||||
| Z | −0.18 | 0.06 | −1.31 | −0.08 | −0.09 | −0.61 | 0.29 |
| punc | 0.86 | 0.95 | 0.19 | 0.94 | 0.93 | 0.54 | 0.77 |
BDI, Beck Depression Inventory; BSSI, Beck Scale for Suicide Ideation; CDRS-R, Children's Depression Rating Scale–Revised; MASC, Multidimensional Anxiety Scale for Children; SHAPS, Snaith–Hamilton Pleasure Scale; TEPS-AP, Temporal Experience of Pleasure Scale–anticipatory; TEPS-CP, Temporal Experience of Pleasure Scale–consummatory.
Discussion
The current study sought to examine whether blood concentrations of CRP are increased in adolescents with psychiatric conditions or are related to the severity of clinical symptoms. To answer these questions, we obtained quantitative CRP measurements and detailed psychiatric evaluations from a large cohort of clinically and demographically diverse adolescents. Hypotheses were tested using an extensive array of a priori and post hoc analyses, including group comparisons and correlations within different clinical cohorts. Contrary to our expectations, CRP levels did not differ significantly between groups or correlate with clinical symptomatology.
Our negative findings are in agreement with several pediatric studies focused on mood and anxiety conditions (Chaiton et al. 2010; Khandaker et al. 2014; Baumeister et al. 2016; Flouri et al. 2020; Jha et al. 2020), including our recent neuroimaging study (Liu et al. 2020). To date, only one study has reported an association between CRP levels and depressive symptoms in adolescents, which was observed exclusively among female participants (Tabatabaeizadeh et al. 2018). However, secondary analyses within female- and male-only subsets of our cohort (Supplementary Results section) found no evidence of gender-specific clinical relationships with CRP. Our study design recruited adolescents with a diversity of clinical profiles and a wide severity range, including subthreshold diagnoses and HCs. The unique sample allows for robust dimensional analyses as well as group comparisons between clinical cohorts and HCs in the same study, providing strong evidence against a relationship between CRP levels and adolescent mood and/or anxiety disorders.
As CRP is a generic marker of nonspecific inflammatory processes, the observed null results suggest that depressed adolescents may not yet exhibit the pattern of generalized immune system activation observed in depressed adults. Indeed, as reported in studies with adults, we also identified a relationship between BMI and CRP levels. This finding further suggests that CRP may be associated with chronicity of medical conditions. Importantly, no associations were found between CRP and depression symptomology regardless of whether we did or did not control for BMI. Despite the apparent decoupling of CRP levels from psychiatric symptoms early in the course of illness, several studies in adolescents have identified relationships between blood CRP and brain function. Recent longitudinal studies in adolescents have found that elevated CRP levels were linked to decreased executive functioning (Mac Giollabhui et al. 2021) as well as increased depression severity (Moriarity et al. 2019) at later time points. Opposite evidence suggested that CRP could not predict later depression status (Copeland et al. 2012), but that depression severity was associated with subsequent CRP levels (Copeland et al. 2012; Duivis et al. 2015). Another study documented that stressful life events in conjunction with increased inflammatory response to these events in adolescents predicted worse outcomes at 1-year follow-up (Kautz et al. 2020). These studies suggest that the relationship between CRP and psychiatric symptomatology in adulthood may be a later manifestation of abnormal brain–immune interactions that first appear in youth.
This study has several limitations. First, although our sample size (N = 127) was relatively large and participants predominantly presented with mood and/or anxiety symptoms, a potential limitation was the diversity of clinical profiles. However, scores on specific symptom scales were highly variable across the cohort, enabling us to perform robust dimensional analyses across the full range of clinical and subclinical severity. A second concern was the large spread in ages (12–20) included in our adolescent cohort. To limit variability related to puberty, participants were restricted to Tanner stage ≥4. Analyses were also adjusted for age as a nuisance covariate to mitigate this concern. Finally, although BMI was included as a confound due to its association with CRP, additional variables such as exercise, diet, stress, sleep, and menstrual cycle might also be relevant. Thus, future studies should examine these health and lifestyle factors along with additional indices of immune activity to fully characterize the relationship between inflammation and psychiatric symptomatology in adolescents.
Conclusions
In summary, we did not find evidence of a relationship between blood CRP levels and any psychiatric features in adolescents, including overall diagnostic status and the severity of several common symptoms. While these results do not preclude the role of the immune system in adolescent mood and/or anxiety disorders, they suggest that CRP alone may not be an adequate biomarker to predict depression symptomatology or to identify specific biological phenotypes based on immune system activation in youth. Future research should utilize longitudinal frameworks to examine whether CRP can serve as a biomarker for clinical outcomes in adolescents.
Clinical Significance
CRP is a widely used nonspecific biomarker for peripheral inflammation and consistently implicated in adult depression. In adolescents, relationships between blood CRP levels and mood and anxiety disorders were not detected through group comparison and dimensional analyses. Our negative findings suggest that CRP is not a proper marker to identify the subgroup of depressed youth who exhibit increased inflammation.
Ethical Statement
This study was approved by the institutional review boards (IRBs) of Albert Einstein College of Medicine and Icahn School of Medicine at Mount Sinai. Signed informed consent was obtained from adult participants or from a parent/guardian for those younger than 18 years, who provided signed assent.
Supplementary Material
Disclosures
No competing financial interests exist.
Supplementary Material
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V: Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 21:642–649, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A: Assessment of suicidal intention: The Scale for Suicide Ideation. J Consult Clin Psychol 47:343, 1979 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown G: Beck Depression Inventory, 2nd ed. Manual San Antonio, TX: The Psychological Corporation, 1996 [Google Scholar]
- Bekhbat M, Treadway MT, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, Felger JC: Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immunity 88:161–165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, Gabbay V: The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res 227:206–212, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Stern ER, Alonso CM, Xie H, Kim-Schulze S, Gabbay V: Relationships between neural activation during a reward task and peripheral cytokine levels in youth with diverse psychiatric symptoms. Brain Behav Immunity 80:374–383, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton M, O'Loughlin J, Karp I, Lambert M: Depressive symptoms and C-reactive protein are not associated in a population-based sample of adolescents. Int J Behav Med 17:216–222, 2010 [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ: Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biol Psychiatry 71:15–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A: Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry 65:409–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner M, Kasl SV, Abramson JL, Vaccarino V: Association between depression and elevated C-reactive protein. Psychosom Med 65:347–356, 2003 [DOI] [PubMed] [Google Scholar]
- Duivis HE, Kupper N, Vermunt JK, Penninx BW, Bosch NM, Riese H, Oldehinkel AJ, de Jonge P: Depression trajectories, inflammation, and lifestyle factors in adolescence: The TRacking Adolescents' Individual Lives Survey. Health Psychol 34:1047–1057, 2015 [DOI] [PubMed] [Google Scholar]
- Elovainio M, Aalto A-M, Kivimäki M, Pirkola S, Sundvall J, Lönnqvist J, Reunanen A: Depression and C-reactive protein: Population-based health 2000 study. Psychosom Med 71:423–430, 2009 [DOI] [PubMed] [Google Scholar]
- Flouri E, Francesconi M, Midouhas E, Lewis G: Prenatal and childhood adverse life events, inflammation and depressive symptoms across adolescence. J Affect Disord 260:577–582, 2020 [DOI] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP: Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 164:1010–1014, 2004 [DOI] [PubMed] [Google Scholar]
- Freed RD, Mehra LM, Laor D, Patel M, Alonso CM, Kim-Schulze S, Gabbay V: Anhedonia as a clinical correlate of inflammation in adolescents across psychiatric conditions. World J Biol Psychiatry 20:712–722, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Coffey BJ, Guttman LE, Gottlieb L, Katz Y, Babb JS, Hamamoto MM, Gonzalez CJ: A cytokine study in children and adolescents with Tourette's disorder. Progr Neuropsychopharmacol Biol Psychiatry 33:967–971, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ: Immune system dysregulation in adolescent major depressive disorder. J Affect Disord 115:177–182, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ: A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol 19:423–430, 2009c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O: The kynurenine pathway in adolescent depression: Preliminary findings from a proton MR spectroscopy study. Progr Neuropsychopharmacol Biol Psychiatry 34:37–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP: Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers 40:1086–1102, 2006 [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC, Miller AH: Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95:43–49, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Cai L, Minhajuddin A, Fatt CC, Furman JL, Gadad BS, Mason BL, Greer TL, Hughes JL, Xiao G, Emslie G, Kennard B, Mayes T, Trivedi MH: Dysfunctional adaptive immune response in adolescents and young adults with suicide behavior. Psychoneuroendocrinology 111:104487, 2020 [DOI] [PubMed] [Google Scholar]
- Kaufman AS: Kaufman Brief Intelligence Test: KBIT. Circle Pines, MN: AGS, American Guidance Service, 1990 [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Williamson D: and Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kautz MM, Coe CL, McArthur BA, Mac Giollabhui N, Ellman LM, Abramson LY, Alloy LB: Longitudinal changes of inflammatory biomarkers moderate the relationship between recent stressful life events and prospective symptoms of depression in a diverse sample of urban adolescents. Brain Behav Immunity 86:43–52, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB: Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry 71:1121–1128, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ely BA, Simkovic SJ, Tao A, Wolchok R, Alonso CM, Gabbay V: Correlates of C-reactive protein with neural reward circuitry in adolescents with psychiatric symptoms. Brain Behav Immun Health 9:100153, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N, Alloy LB, Hartman CA: Investigating whether depressed youth exhibiting elevated C reactive protein perform worse on measures of executive functioning, verbal fluency and episodic memory in a large, population based sample of Dutch adolescents. Brain Behav Immunity 94:369–380, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK: The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36:554–565, 1997 [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL: Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Giollabhui NM, Ellman LM, Klugman J, Coe CL, Abramson LY, Alloy LB: Inflammatory proteins predict change in depressive symptoms in male and female adolescents. Clin Psychol Sci 7:754–767, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr., Taubert K, Tracy RP, Vinicor F: Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511, 2003 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Freeman LN, Mokros HB: Children's depression rating scale-revised. Psychopharmacol Bull 21:979–989, 1985 [Google Scholar]
- Snaith R, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P: A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. Br J Psychiatry 167:99–103, 1995 [DOI] [PubMed] [Google Scholar]
- Tabatabaeizadeh S-A, Abdizadeh MF, Meshkat Z, Khodashenas E, Darroudi S, Fazeli M, Ferns GA, Avan A, Ghayour-Mobarhan M: There is an association between serum high-sensitivity C-reactive protein (hs-CRP) concentrations and depression score in adolescent girls. Psychoneuroendocrinology 88:102–104, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.