Abstract
Repeated mild traumatic brain injury (TBI) can cause persistent neuropathological effects and is a major risk factor for chronic traumatic encephalopathy. PUFAs (n-3 polyunsaturated fatty acids) were shown to improve acute TBI outcomes in single-injury models in most cases. In this study, we demonstrate positive effects of dietary n-3 PUFA on long-term neuropathological and functional outcome in a clinically relevant model of repeated mild TBI using the Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA). Adult mice, reared on n-3 PUFA adequate (higher n-3 PUFA) or deficient (lower n-3 PUFA) diets, were given a mild CHIMERA daily for 3 consecutive days. At 2 months after injury, visual function and spatial memory were evaluated. Glia cell activation was assessed by immunostaining using antibodies of ionized calcium-binding adaptor molecule 1 and glial fibrillary acidic protein, and axonal damage was examined using silver staining. Repeated CHIMERA (rCHIMERA)-induced gliosis was significantly suppressed in the optic tract, corpus callosum, and hippocampus of mice fed the n-3 PUFA adequate diet compared to the deficient diet group. Considerable axonal damage was detected in the optic tract after rCHIMERA, but the adequate diet group displayed less axonal damage compared to the deficient diet group. rCHIMERA induced a drastic reduction in N1 amplitude of the visual evoked potential in both diet groups and the a-wave amplitude of the electroretinogram in the deficient diet group. However, reduction of N1 and a-wave amplitude were less severe in the adequate diet group. The Morris water maze probe test indicated a significant decrease in the number of platform crossings in the deficient diet group compared to the adequate group. In summary, dietary n-3 PUFA can attenuate persistent glial cell activation and axonal damage and improve deficits in visual function and spatial memory after repeated mild TBI. These data support the neuroprotective potential of a higher n-3 PUFA diet in ameliorating the adverse outcome of repeated mild TBI.
Keywords: CHIMERA, DHA, ERG, gliosis, n-3 PUFA, repeated mild TBI, VEP
Introduction
Mild traumatic brain injury (mTBI) is the most common form of traumatic brain injury (TBI). Certain populations, such as amateur and professional athletes and military personnel, are at a higher risk of repeated mild TBI (rmTBI). Whereas mTBI mostly results in acute deficits that disappear with time, rmTBI has been associated with long-term cognitive and neurobehavioral impairments and considered a major risk factor for chronic traumatic encephalopathy.1–4 rmTBI has been shown to produce neuropathological consequences, such as cortical cell loss5 and ultrastructural changes,6 in the gray and white matter as well as neuroinflammation.7–9 Persistent neuroinflammation accompanied by cognitive8,9 and visual impairment10 has been observed in a rmTBI model using the Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA).11
Extensive pre-clinical research has been focused on the use of natural products and nutrients that are anti-inflammatory to improve the outcome in numerous models of cerebrovascular disease and injury. The n-3 polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA), have been reported to possess both prophylactic as well as therapeutic value in models of brain injury,12–16 including closed TBI.17,18 Administration of DHA protects against lipopolysaccharide-induced neuroinflammation19,20 and modulates microglia/macrophage morphology after TBI.21 To date, few studies have evaluated the effects of the dietary n-3 fatty acids that raise the brain DHA status in repeated TBI models. In this study, we tested the impact of dietary n-3 PUFA on neuropathological and functional outcome, using a mouse model of closed TBI caused by closed TBI by CHIMERA that produces persistent gliosis and behavioral alteration.8 Repeated TBIs were given to mice on an adequate (higher) or deficient (lower) n-3 PUFA diet.
Methods
Experimental animals and diet
All experiments were conducted according to the National Institutes of Health (NIH) Guidelines for the Health and Care of Animals (LMS-HK-13). Pregnant C57BL6/NCr mice at 13 days of gestation (G13) were purchased from Charles River Laboratories (Wilmington, MA) and randomly placed on either the n-3 PUFA adequate or n-3 PUFA deficient diet from G14. Pups were weaned on the same diet as the dams. The diets were purchased from Dyets, Inc. (Bethlehem, PA) and were isocaloric, but contained a different composition of the n-3 PUFA, alpha linolenic acid (ALA), which was 3.4% in the n-3 PUFA adequate diet and 0.05% in the n-3 PUFA deficient diet (Table 1). In addition to ALA, there are small but significant differences in myristic (16.02 ± 0.41 vs. 17.14 ± 0.16) and oleic acid content (5.27 ± 0.20 vs. 4.55 ± 0.04) in the adequate versus deficient diet. All procedures were done using previously established protocols.8,11
Table 1.
Fatty Acid Composition in Diet
| Fatty acid | Higher n-3 PUFA diet (adequate) | Lower n-3 PUFA diet (deficient) |
|---|---|---|
| Lauric acid (12:0) | 33.31 ± 1.84 | 34.21 ± 0.53 |
| Myristic acid (14:0) | 16.02 ± 0.41 | 17.14 ± 0.16* |
| Palmitic acid (16:0) | 10.64 ± 0.44 | 11.26 ± 0.10 |
| Stearic acid (18:0) | 12.11 ± 0.54 | 12.90 ± 0.15 |
| Arachidic acid (20:0) | 0.23 ± 0.01 | 0.25 ± 0.02 |
| Oleic acid (18:1n-9) | 5.27 ± 0.20 | 4.55 ± 0.04† |
| Vaccenic acid (18:1n-7) | 0.22 ± 0.01 | 0.21 ± 0.01 |
| Linoleic acid (18:2n-6) | 16.76 ± 0.69 | 17.42 ± 0.23 |
| α-Linolenic acid (18:3n-3) | 3.37 ± 0.14 | 0.05 ± 0.00‡ |
The weight percent data are expressed as mean ± standard deviation (n = 3).
p < 0.05; †p < 0.01; ‡p < 0.001.
PUFA, polyunsaturated fatty acid.
Total lipid analysis of mouse red blood cells and cortical tissue
Mouse blood collected by cardiac puncture was centrifuged at 500g for 5 min to pellet red blood cells (RBCs). The RBC pellet was washed twice with phosphate-buffered saline (PBS), before lipid extraction. Lipids were extracted from RBCs or cortical tissues according to the method of Bligh and Dyer,22 and the fatty acid profile was determined by gas chromatography after transmethylation as described previously.23 Transmethylated samples were injected onto an Agilent 6890 gas chromatograph with a flame ionization detector by a 15-m DB-FFAP capillary column (Agilent Technologies, Santa Clara, CA). Individual fatty acid methyl esters (FAMEs) were identified by comparing with retention times of known FAME standard GLC-411 (Nu-Chek Prep, Elysian, MN), and the percentage of each FAME relative to total was determined based on peak areas.
Repeated mild traumatic brain injury and experimental timeline
The CHIMERA model was used for TBIs as described previously.8 The CHIMERA apparatus consists of a small, hinged platform to support the animal's body and a head plate to support the head. The head plate is provided with a small aperture through which a 50-g, free-floating piston is forced up by controlled air pressure such that it strikes the head.8 Three-month-old mice were anesthetized with isoflurane and mounted in a supine position on the CHIMERA apparatus such that the piston struck the unconstrained head at 0.55 J of energy. After the injury, mice were returned to the cage and allowed to recover. One injury was given each day for 3 consecutive days with 24 h between injuries. The day after the last injury was considered as the first day after injury. Control groups (sham) were treated identically to the experimental injury groups without impact. Brain samples were collected at 2 months after injury after functional tests according to the following timeline: day 0, third injury; days 51–55, water maze; days 58–60, visual evoked potential (VEP)/electroretinogram (ERG); day 60, immunohistochemistry.
Immunofluorescence and silver staining
Mice were perfused with chilled PBS and fixed in fresh 4% paraformaldehyde (PFA). Mouse brains were carefully removed and fixed in 4% PFA solution overnight and transferred into 30% sucrose solution until sinking to the bottom of the tube at 4°C, then embedded with OCT compound medium (Tissue-Tek, 4583; VWR International, Radnor, PA), frozen on dry ice, and stored at −800C in a freezer. Coronal sections (25 μm) were sliced by Leica Cryostat (Leica Biosystems Inc., Buffalo Grove, IL) and stored in cryoprotectant solution at −20°C. Three sections from approximately the same position (−1.68 mm bregma) from each mouse brain were selected for ionized calcium-binding adaptor molecule 1 (Iba-1), glial fibrillary acidic protein (GFAP), and silver staining by an investigator blind to the experimental groups (4 mice per group). Sections were incubated with Iba-1 and GFAP antibodies at 40C overnight and Alexa Fluor-488–conjugated F (ab’)2 fragment goat antirabbit immunoglobulin G (111-546-003; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at room temperature for 1 h. After washing, sections were mounted on slides and covered with mounting medium containing 4′,6-diamidino-2-phenylindole (H-1500; Vector Laboratories, Inc., Burlingame, CA). Immunofluorescence images were captured by an Olympus 1X81 microscope (Olympus Corporation, Tokyo, Japan) for Iba-1 and GFAP in the optic tract (OT), corpus callosum (CC), and hippocampus (HP). GFAP and Iba-1 protein expression in the regions of interest (ROIs) was quantified by measuring the fluorescence intensity per μm2 after subtracting the background intensity using Metamorph software (Molecular Devices Inc., Sunnyvale, CA).
For silver staining, sections from 3 mice per group were placed in 4% PFA solution for at least 7 days at 4°C before processing with NeuroSilver kit II, according to the manufacturer's protocol (PK301A; FD NeuroTechnologies, Inc., Columbia MD). Images of the OT were acquired in bright field by an Olympus 1X81 microscope system. Staining intensity of images was quantified using ImageJ software (NIH, Bethesda, MD).
Visual evoked potential and electroretinogram
Mouse flash VEP and ERG were recorded with an Espion Visual Electrophysiology System from Diagnosys LLC (Lowell, MA) to assess visual functions at 2 months after injury. To prevent bias, experimental groups were coded such that the investigator was not aware of codes. The total animal number used was 36 (9 mice per group, 5 males and 4 females per group).
All procedures were performed in the room under dim red light after mice were acclimated in the room for at least 1 h. Mice were anesthetized with an intraperitoneal injection of ketamine and xylazine solution. Each pupil of the mouse was dilated with a drop of 2.5% phenylephrine hydrochloride solution before being placed on a heated platform of color-dome. A needle electrode placed in the lower lip of the mouse was used as a reference while a needle electrode placed in the tail served as the ground. For ERG recordings, a drop of topical petrolatum ophthalmic ointment was applied to the corneal surface of one eye, and a gold-wire active electrode was placed with the other eye covered. A light-adapted (photopic) protocol was used as previously reported.24 For VEP recordings, the active electrode was subcutaneously inserted in the middle of two ears. Both eyes were stimulated by the white flashlights with a constant intensity of 3.0 cd s/m2, 100 trials three times. After testing, mice were transferred to the home cage and placed on a heating pad until having recovered. The data of three times per mouse were averaged and analyzed.
Morris water maze test
The Morris water maze test was performed over a period of 5 days by the investigator blinded to the identity of individual mice, with the first 4 days consisting of learning trials followed by a probe trial on the fifth day. Mice were gently released into a pool 120 cm in diameter filled with water and mixed with white non-toxic tempera paint. The trial ended when the mouse managed to locate the submerged platform. In case the mouse did not find the platform within 90 sec, it was gently guided to the platform. Four trials were given daily for 4 days for learning. On the fifth day, the platform was removed, and mice were allowed to swim for 60 sec. The total number of mice used for this experiment was 51; 10–15 mice per group with approximately half male and female per group.
Statistical analyses
All data are represented as mean ± standard error of the mean. Statistical analyses were performed using GraphPad Prism software (version 8.01; GraphPad Software Inc., La Jolla, CA). A p value set to <0.05 was considered significant. Statistical significance was determined by an unpaired Student t test or one-way analysis of variance (ANOVA), followed by Tukey's test for multiple comparisons. Repeated-measures two-way ANOVA was used for analysis of data obtained for the learning trials of the Morris water maze test.
Results
Adequate n-3 polyunsaturated fatty acid diet significantly increases docosahexaenoic acid content in mouse cortex and red blood cells
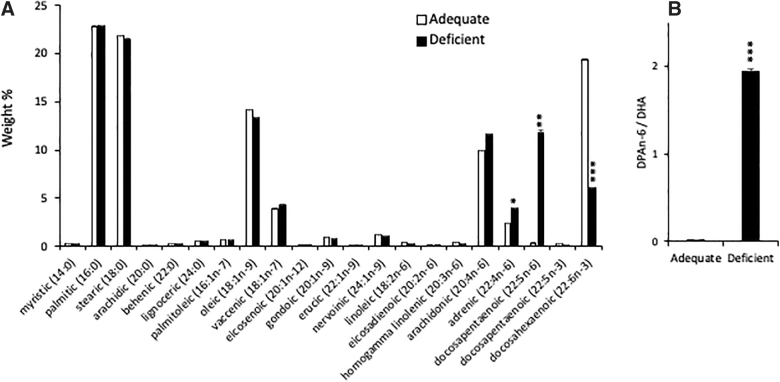
Figure 1 shows the fatty acid profile in the cortex from 3-month-old mice on n-3 PUFA adequate or deficient diets. The higher n-3 PUFA (adequate) diet produced a significantly higher DHA level in the cortex compared to the lower n-3 PUFA (deficient) group (Fig. 1A). Conversely, the docosapentaenoic acid (DPAn-6; 22:5n-6) level was significantly decreased, resulting in the DPAn-6 to DHA ratio, an indicator for n-3 PUFA deficiency,13 reduced by >100 times in the adequate group (Fig. 1B). Similarly, DHA content in RBCs was significantly higher in the adequate diet group compared to the deficient group (6.70 ± 0.06 vs. 0.40 ± 0.02%; p < 0.001), whereas DPAn-6 was elevated in the deficient diet group compared to the adequate group (4.30 ± 0.04 vs. 0.50 ± 0.01%; p < 0.001; Table 2). The small but significant difference in myristic and oleic acid in the diet was not reflected in the fatty acid composition in the brain or RBCs, indicating that the diet effect is unlikely derived from these fatty acids.
FIG. 1.
Effect of n-3 PUFA diet on DHA content in mouse cortex. (A) Total fatty acid profile (weight percent) in brain cortex from 3-month-old mice indicates that mice on an adequate n-3 PUFA (Adequate) diet contain significantly higher DHA (22:6n-3) and lower DPAn-6 (22:5n-6) in cortex than mice on a low n-3 PUFA (Deficient) diet. Data are expressed as mean ± standard error (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001. (B) Ratio of DPAn-6 to DHA in cortex of mice on a high n-3 PUFA diet is 110 times lower compared to the low n-3 PUFA diet group. DHA, docosahexaenoic acid; DPAn-6, docosapentaenoic acid; PUFA, polyunsaturated fatty acid.
Table 2.
Fatty Acid Composition in Red Blood Cells
| Fatty acid | Higher n-3 PUFA diet (adequate) | Lower n-3 PUFA diet (deficient) |
|---|---|---|
| Lauric (12:0) | 0.20 ± 0.02 | 0.20 ± 0.08 |
| Myristic (14:0) | 0.70 ± 0.02 | 0.80 ± 0.08 |
| Palmitic (16:0) | 26.40 ± 0.20 | 26.60 ± 0.27 |
| Stearic (18:0) | 11.80 ± 0.21 | 12.20 ± 0.26 |
| Arachidic (20:0) | 0.20 ± 0.01 | 0.20 ± 0.02 |
| Behenic (22:0) | 0.70 ± 0.01 | 0.60 ± 0.05 |
| Lignoceric (24:0) | 1.40 ± 0.03 | 1.20 ± 0.08 |
| Palmitoleic (16:1n-7) | 1.40 ± 0.04 | 1.50 ± 0.07 |
| Oleic (18:1n-9) | 12.10 ± 0.28 | 12.40 ± 0.16 |
| Vaccenic (18:1n-7) | 3.00 ± 0.10 | 3.20 ± 0.15 |
| Eicosenoic (20:1n-12) | 0.30 ± 0.00 | 0.40 ± 0.01* |
| Gondoic (20:1n-9) | 0.20 ± 0.01 | 0.20 ± 0.01 |
| Erucic (22:1n-9) | 0.10 ± 0.00 | 0.10 ± 0.01 |
| Nervoinic (24:1n-9) | 1.20 ± 0.06 | 0.90 ± 0.07* |
| Linoleic (18:2n-6) | 9.60 ± 0.15 | 9.30 ± 0.45 |
| Eicosadienoic (20:2n-6) | 0.10 ± 0.00 | 0.10 ± 0.01 |
| Docosadienoic acid (22:2n-6) | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Gamma linolenic (18:3n-6) | 0.10 ± 0.00 | 0.10 ± 0.00 |
| Homogamma linolenic (20:3n-6) | 1.80 ± 0.02 | 1.60 ± 0.04* |
| Arachidonic (20:4n-6) | 18.50 ± 0.19 | 21.00 ± 0.53† |
| Adrenic (22:4n-6) | 1.40 ± 0.00 | 2.30 ± 0.06‡ |
| Docosapentaenoic (22:5n-6) | 0.50 ± 0.01 | 4.30 ± 0.04‡ |
| Alpha linolenic (18:3n-3) | 0.10 ± 0.00 | 0.01 ± 0.00‡ |
| Timnodonic (20:5n-3) | 0.50 ± 0.01 | 0.10 ± 0.01‡ |
| Docosapentaenoic (22:5n-3) | 0.80 ± 0.01 | 0.20 ± 0.03‡ |
| Docosahexaenoic (22:6n-3) | 6.70 ± 0.06 | 0.40 ± 0.02‡ |
Weight percent data are expressed as mean ± standard error of the mean (n = 3).
p < 0.05; †p < 0.01; ‡p < 0.001.
PUFA, polyunsaturated fatty acid.
Adequate n-3 polyunsaturated fatty acid diet suppresses repeated CHIMERA–induced microglia activation
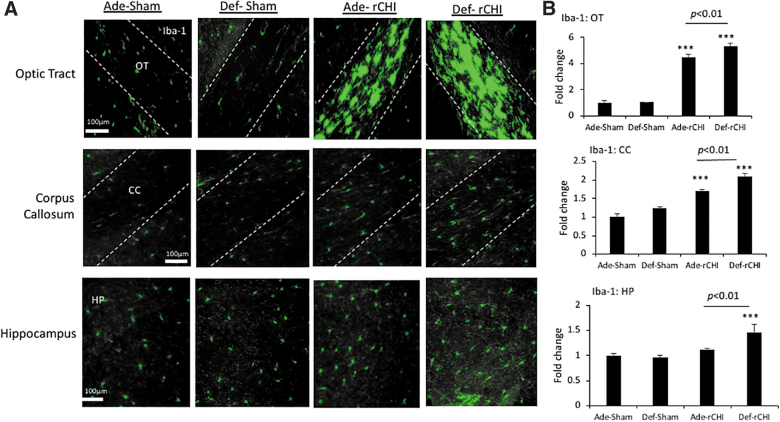
It has been well established that glia activation plays a vital role in the development of neuropathology after injury.25,26 Iba-1 expression was analyzed in the OT, CC, and HP at 2 months post-injury, and the fold changes from the adequate sham value (Ade-Sham) are presented in Figure 2. After injury, there were significant increases in Iba-1 expression in the OT (F = 115.8; p < 0.0001) and CC (F = 43.42; p < 0.0001) in both adequate and deficient diet groups. The increase of the Iba-1 expression was particularly pronounced in the OT area, showing a 4.5 ± 0.25– and 5.3 ± 0.26–fold increase compared to the adequate sham group for the adequate-injured (Ade-rCHI) and deficient-injured group (Def-rCHI), respectively. In the CC, a 1.7 ± 0.06– and 2.1 ± 0.1–fold increase was observed after injury in the adequate and deficient group, respectively. Average Iba-1 expression in the HP also increased after injury, but a statistically significant increase was observed only in the deficient diet group (F = 7.763; p < 0.01). In all three regions examined, injury upregulated Iba-1 expression significantly more in the deficient group compared to the adequate group (p < 0.01), indicating a protective role of the n-3 PUFA adequate diet.
FIG. 2.
High n-3 PUFA diet suppresses Iba-1 expression after rCHIMERA. (A) Representative photomicrographs of activated microglia indicated by Iba-1 expression in optic tract (OT), corpus callosum (CC), and hippocampus (HP) obtained from high (Ade) or low (Def) n-3 PUFA diet groups at 2 months after injury by rCHIMERA (rCHI). Regions of interest are outlined with dashed lines. (B) Quantitative analysis of Iba-1 expression. Data are normalized to Ade-Sham values and expressed as mean ± standard error (n = 4; ***p < 0.001 vs. Ade-Sham). Iba-1, ionized calcium-binding adaptor molecule 1; PUFA, polyunsaturated fatty acid; rCHIMERA, repeated CHIMERA.
Adequate n-3 polyunsaturated fatty acid diet suppresses repeated CHIMERA–induced astrocyte activation
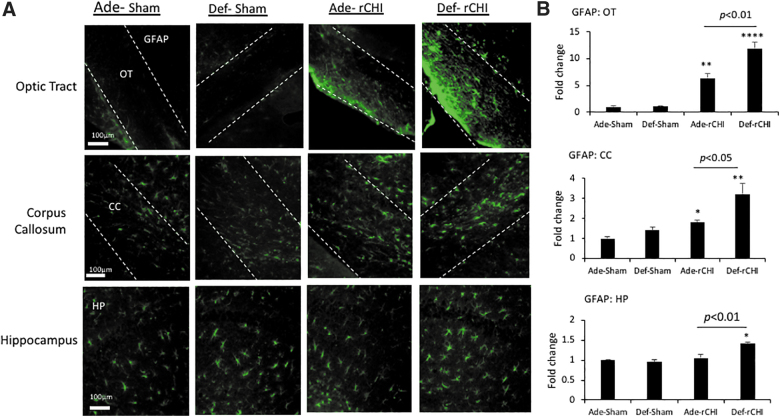
GFAP immunostaining was performed to label astrocytes in the OT, CC, and HP at 2 months after injury (Fig. 3). Similar to the case with Iba-1 staining, injury increased GFAP staining in all three regions examined. The increase was most pronounced in OT given that a 6.3- and 11.9-fold increase was observed for the adequate and deficient group, respectively. Injury significantly increased the GFAP signal only in the deficient group in the CC (F = 9.93; p < 0.01) and HP (F = 11.61; p < 0.001) by 3.2- and 1.4-fold, respectively. The comparison between the two diet groups showed that the adequate diet significantly suppresses injury-induced GFAP expression in these regions (p < 0.001; Fig. 3).
FIG. 3.
High n-3 PUFA diet suppresses GFAP expression after rCHIMERA. (A) Representative photomicrographs of GFAP immunostaining in optic tract (OT), corpus callosum (CC), and hippocampus (HP) obtained from the high (Ade) or low (Def) n-3 PUFA diet group at 2 months after injury by rCHIMERA (rCHI). Regions of interest are outlined with dashed lines. (B) Quantitative analysis. Data are normalized to Ade-Sham values and expressed as mean ± standard error (n = 4; ***p < 0.001 vs. Ade-Sham). GFAP, glial fibrillary acidic protein; PUFA, polyunsaturated fatty acid; rCHIMERA, repeated CHIMERA.
Adequate n-3 polyunsaturated fatty acid diet attenuates repeated CHIMERA–induced axonal damage
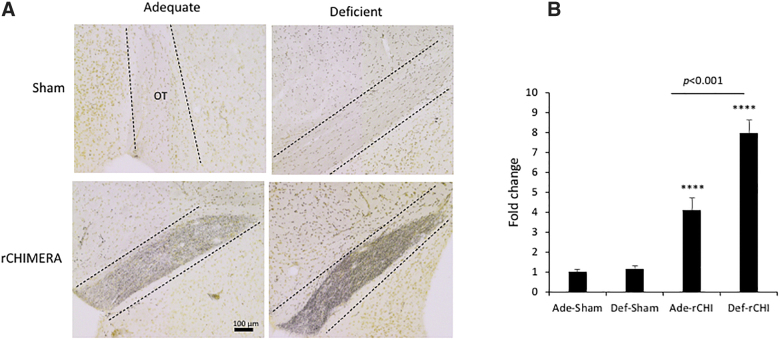
Silver staining was performed for mouse OT at 2 months after injury to detect degenerating axons.27 Regardless of the diet, the intensity of silver staining was massively increased in the injured groups (p < 0.0001 vs. adequate-sham group), as shown in Figure 4. The Ade-rCHI group showed a significantly reduced intensity of staining compared to the Def-rCHI group (F = 13.21; p < 0.01).
FIG. 4.
Adequate n-3 PUFA diet attenuates axonal damage post-injury. (A) Representative photomicrographs indicating axonal damage in the optic tract (OT) visualized by silver staining of brain sections obtained from high (Ade) or low (Def) n-3 PUFA diet groups at 2 months after rCHIMERA (rCHI). (B) Quantitative analysis. Data are normalized to Ade-Sham values and expressed as mean ± standard error (n = 3; ****p < 0.0001 vs. Ade-Sham). PUFA, polyunsaturated fatty acid; rCHIMERA, repeated CHIMERA.
Adequate n-3 polyunsaturated fatty acid diet improves repeated CHIMERA–induced visual dysfunction
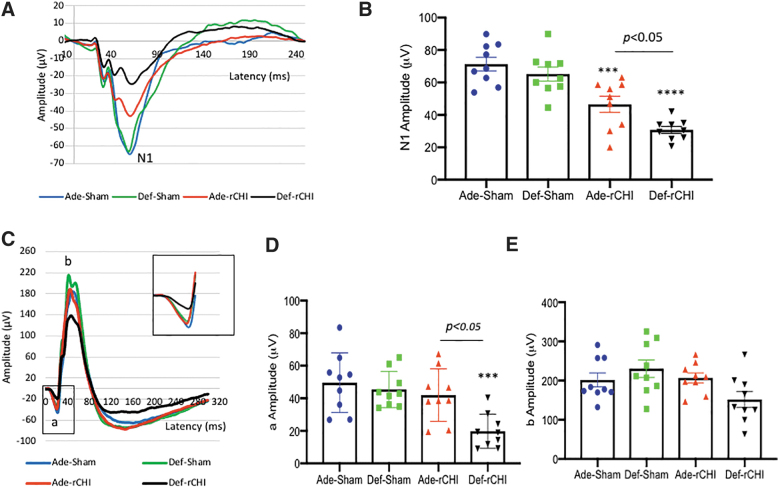
According to our previous report that rCHIMERA results in persistent visual dysfunction,10 we evaluated the effect of dietary n-3 PUFA on visual function at 2 months after rCHIMERA. The N1 amplitude of VEP decreased by injury (F = 20.72; p < 0.0001) was significantly improved in the adequate diet group compared to the deficient group (p < 0.05; Fig. 5A,B). Interestingly, the a-wave amplitude of ERG was also significantly reduced only in the Def-rCHI group, but not in Ade-rCHI mice (F = 7.68, p < 0.001; Fig. 5C,D). A statistically significant difference in a-wave amplitude was observed between Ade-rCHI and Def-rCHI groups (p < 0.05). The b-wave amplitude was not significantly affected by diet or injury (Fig. 5E).
FIG. 5.
Adequate n-3 PUFA diet improves visual deficit. (A,B) Average traces of VEP (A) with quantitative analysis of N1 amplitude (B). (C–E) Average traces of ERG (C) with quantitative analysis of a (D) and b-wave amplitude (E). VEP and ERG were measured for the high (Ade) or low (Def) n-3 PUFA diet group at 2 months after TBI induced by rCHIMERA (rCHI). Inset in (C) shows the magnified view of a-wave (n = 9; ***p < 0.001, ****p < 0.0001 vs. Ade-Sham). ERG, electroretinogram; PUFA, polyunsaturated fatty acid; rCHIMERA, repeated CHIMERA; VEP, visual evoked potential.
Adequate n-3 polyunsaturated fatty acid diet improves repeated CHIMERA–induced impairment of spatial learning and memory
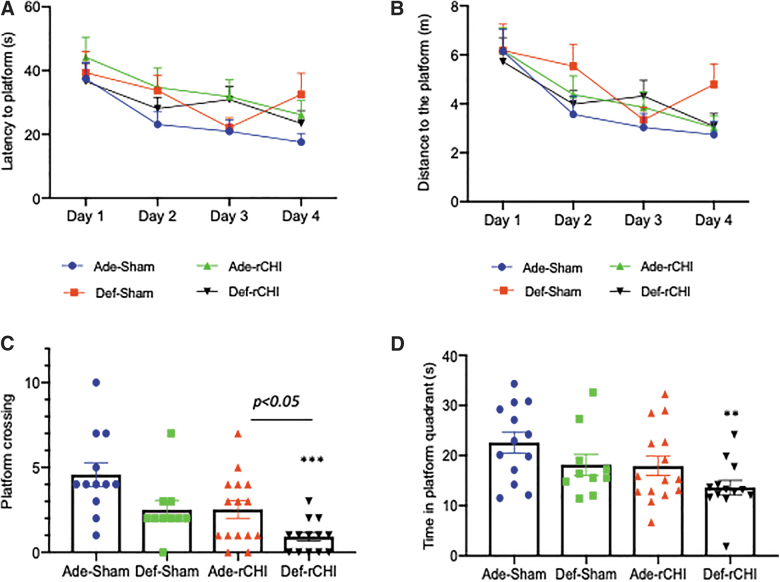
The effect of dietary n-3 PUFA on learning and memory deficit caused by rCHIMERA was assessed using the Morris water maze test. Average latency to platform decreased for all the experimental groups from day 1 to day 4 of the learning trials (Fig. 6A). The decrease in latency was most striking for the adequate sham group with an improvement of >50%, from 37.4 ± 5.2 sec on day 1 to 17.6 ± 2.5 sec on day 4. The similar trend was observed for the distance traveled to find the platform (Fig. 6B). Improvement for the other groups was relatively modest. Repeated-measures two-way ANOVA revealed a significant difference in latency and distance over training time (F = 6.04; p < 0.001), indicating a significant improvement in learning; however, diet and injury did not have any significant effect on learning in the water maze.
FIG. 6.
Adequate n-3 PUFA diet improves the spatial memory impaired by injury. Morris water maze was conducted at 2 months after rCHIMERA (rCHI). (A,B) Learning curves for the high (Ade) or low (Def) n-3 PUFA diet group showing average latency (A) and average distance to the platform on each day of 4 days of the learning trial (B). (C,D) Number of platform crossings (C) and time in platform quadrant (D) during the 60-sec probe trial. **p < 0.001, ***p < 0.001 versus Ade-Sham; n = 10–15. PUFA, polyunsaturated fatty acid; rCHIMERA, repeated CHIMERA.
In the probe test, there was a graded decrease in the number of platform crossings in 60 sec from the adequate sham to deficient injured group (Fig. 6C). The adequate sham group had the highest number of platform crossings (4.2 ± 0.7) whereas the deficient injured group had the least crossings (0.9 ± 0.3). A statistically significant difference was observed for platform crossings between adequate sham and deficient injured groups (F = 7.94; p < 0.001). The adequate injured and deficient injured groups also indicated a significant difference (p < 0.05). Similarly, time in the platform quadrant showed a graded decrease from adequate sham (22.6 ± 2.1 sec) to deficient injured groups (13.6 ± 1.7 sec; Fig. 6D), and only the deficient injured group showed a statistically significant decrease compared to the sham adequate group (p < 0.01). The deficient injured group also showed a lower platform quadrant time than the adequate injured group, although statistical significance was not reached (p = 0.09).
Discussion
It has been reported that n-3 PUFA reduces neuroinflammation and improves functional outcome in brain injury models where injury is caused by a single cortical impact or pulse of fluid percussion.10,12–14 In this study, we investigated the effect of dietary n-3 PUFA on TBI outcome using rCHIMERA, a clinically relevant model of mild brain injury, which results in persistent gliosis in the brain and impaired memory in mice.8 We demonstrate that higher dietary n-3 PUFA effectively increases brain DHA content, reduces axon degeneration in the OT, suppresses gliosis in multiple brain regions, and ameliorates visual dysfunction and memory deficit caused by rCHIMERA.
Activated astrocytes and microglial cells are heterogeneous populations that respond to brain injury.28,29 Reactive astrocytes and activated microglia affect neuroinflammation after TBI. They can ameliorate or exacerbate TBI pathology based on injury, phenotype, local conditions, and time after injury.30 Upon stimulation, microglia produce a variety of proinflammatory molecules, such as IL-1 beta, tumor necrosis alpha, C-C chemokine ligand 2, and cyclooxygenase-2, as well as anti-inflammatory factors like IL-4, IL-10, IL-13, and peroxisome proliferator-activated receptor gamma.31 Though astrocytes produce neurotrophic factors, they can also upregulate immune components and exacerbate injury whereas microglia can activate neurotoxic astrocytes.32 Given that persistent activation of glial cells after TBI is associated with and may contribute to chronic injury,33 controlling glial cell activation likely improves TBI outcome. It has been shown that n-3 PUFA suppress lipopolysaccharide-induced inflammation in microglial cell lines20 and in hypoxic astrocytes.34
Numerous reports also indicate an n-3 PUFA– or DHA-induced decrease in microglial and astrocytic markers in various models of TBI and cerebral ischemia, along with a concomitant improvement in functional recovery.13,21,35,36 Similarly, in our model of repeated mild TBI where persistent activation of both microglia and astrocytes was observed, dietary n-3 PUFA that increased DHA in the brain led to significant suppression of rCHIMERA-induced gliosis in multiple brain areas (Figs. 2 and 3).
It has been shown that the white matter area, especially the OT, is particularly vulnerable to TBI induced by the accelerating/decelerating force.8,37,38 As reported earlier,10 rCHIMERA produced a significant reduction in N1 amplitude of VEP (Fig. 5A,B), with pronounced glial activation (Figs. 2 and 3) and axonal damage in the OT (Fig. 4). The rCHIMERA-induced gliosis and axon degeneration in the OT as well as visual dysfunction were more severe with the lower n-3 PUFA diet, suggesting a preventive role of dietary n-3 PUFA that increases brain DHA level. The fact that the a-wave amplitude of ERG was significantly decreased only in the lower n-3 diet group (Fig. 5C,D) indicates that retinal photoreceptor function39 can also be compromised by rCHIMERA when n-3 PUFA supply is not sufficient.
Similar improvement by the higher n-3 PUFA diet was also observed in spatial memory test (Fig. 6B) as reported earlier in other TBI models.40,41 Given that sustained inflammation compromises cognitive ability,42–44 a decrease in glial activation by n-3 PUFA may be partly responsible for attenuation of spatial memory after repeated TBI. Interestingly, white matter injury can also produce cognitive deficits.45,46 OT damage, in particular, is associated with visual dysfunction, which, in turn, can influence behavioral test outcome. Although a recent study using quantitative MRI showed no discernable effect of the n-3 PUFA diet on white matter damage after TBI,47 the difference in water maze performance in this study may be attributable to protective effects of increased n-3 PUFA on neuronal integrity of axons in the OT in addition to that on the HP. Indeed, the injury-induced VEP reduction was significantly ameliorated by the n-3 adequate diet, suggesting that the difference in water maze performance may not be specific for memory function. Further studies can reveal whether these protective effects are attributable to differences in the enrichment of DHA in cell membranes or attributable to the production of anti-inflammatory DHA metabolites48,49 after repeated TBI.
During n-3 PUFA deficiency, DPAn-6 replaces DHA in the brain and increases the ratio of DPAn-6/DHA.50 As depicted in Figure 1, brains of mice on the n-3 PUFA adequate diet have barely detectable DPAn-6 whereas those on the n-3 PUFA deficient diet have a substantial level of this PUFA. Previous studies51 indicated the presence of DPAn-6 in human brains, suggesting that humans may have suboptimal DHA in the brain. Given that the DHA level in the central nervous system correlates well with that of blood cells, including RBCs, in humans52,53 and rodents,54 the fatty acid compositional changes in RBCs can reflect changes in the brain. The global survey of omega-3 fatty acids in human blood revealed that the average value of DHA in RBCs reported for individual studies ranges from 2.71% to 7.84% with the low level of DHA generally observed in populations on Western diets.55 Despite difficulties in the direct comparison between humans and experimental animals, the DHA level in mouse RBCs (0.4–6.7%) observed in this study (Table 2) is likely to be in the range in humans with a diverse dietary background. Omega-3 PUFA supplementation, which significantly increases n-3 fatty acids in human RBCs,56 can also enrich DHA in the brain, presumably resulting in protective effects in humans.
Conclusion
The current study demonstrated a significant impact of dietary n-3 PUFA on microglial/astrocyte activation and axonal damage and improved visual function and spatial memory months after injury in a clinically relevant model of rmTBI. We concluded that having higher n-3 PUFA in the diet helps to alleviate chronic gliosis and functional deficits associated with mTBI caused by rCHIMERA.
Funding Information
This research was supported by the intramural program of the National Institute on Alcohol Abuse and Alcoholism and Center for Neuroscience and Regenerative Medicine/Henry M. Jackson Foundation for the Advancement of Military Medicine. The funding agencies did not have any role in the design and implementation of this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Schretlen, D.J., and Shapiro, A.M. (2003). A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int. Rev. Psychiatry 15, 341–349 [DOI] [PubMed] [Google Scholar]
- 2.Luo, J., Nguyen, A., Villeda, S., Zhang, H., Ding, Z., Lindsey, D., Bieri, G., Castellano, J.M., Beaupre, G.S., and Wyss-Coray, T. (2014). Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front. Neurol. 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shultz, S.R., Bao, F., Omana, V., Chiu, C., Brown, A., and Cain, D.P. (2012). Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma 29, 281–294 [DOI] [PubMed] [Google Scholar]
- 4.Mouzon, B.C., Bachmeier, C., Ferro, A., Ojo, J.O., Crynen, G., Acker, C.M., Davies, P., Mullan, M., Stewart, W., and Crawford, F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 5.Ojo, J.O., Bachmeier, C., Mouzon, B.C., Tzekov, R., Mullan, M., Davies, H., Stewart, M.G., and Crawford, F. (2015). Ultrastructural changes in the white and gray matter of mice at chronic time points after repeated concussive head injury. J. Neuropathol. Exp. Neurol. 74, 1012–1035 [DOI] [PubMed] [Google Scholar]
- 6.Petraglia, A.L., Plog, B.A., Dayawansa, S., Chen, M., Dashnaw, M.L., Czerniecka, K., Walker, C.T., Viterise, T., Hyrien, O., Iliff, J.J., Deane, R., Nedergaard, M., and Huang, J.H. (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma 31, 1211–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corps, K.N., Roth, T.L., and McGavern, D.B. (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, H., Desai, A., and Kim, H.Y. (2017). Repetitive closed-head impact model of engineered rotational acceleration induces long-term cognitive impairments with persistent astrogliosis and microgliosis in mice. J. Neurotrauma 34, 2291–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donat, C.K., Scott, G., Gentleman, S.M., and Sastre, M. (2017). Microglial activation in traumatic brain injury. Front. Aging Neurosci. 9, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai, A., Chen, H., and Kim, H.Y. (2020). Multiple mild traumatic brain injuries lead to visual dysfunction in a mouse model. J. Neurotrauma 37, 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namjoshi, D.R., Cheng, W.H., McInnes, K.A., Martens, K.M., Carr, M., Wilkinson, A., Fan, J., Robert, J., Hayat, A., Cripton, P.A., and Wellington, C.L. (2014). Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Mol. Neurodegener. 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai, A., Kevala, K., and Kim, H.Y. (2014). Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One 9, e86472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, A., Park, T., Barnes, J., Kevala, K., Chen, H., and Kim, H.Y. (2016). Reduced acute neuroinflammation and improved functional recovery after traumatic brain injury by α-linolenic acid supplementation in mice. J. Neuroinflammation 13, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu, A., Ying, Z., and Gomez-Pinilla, F. (2004). Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 21, 1457–1467 [DOI] [PubMed] [Google Scholar]
- 15.Wu, A., Ying, Z., and Gomez-Pinilla, F. (2011). The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J. Neurotrauma 28, 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begum, G., Yan, H.Q., Li, L., Singh, A., Dixon, C.E., and Sun, D. (2014). Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J. Neurosci. 34, 3743–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailes, J.E., and Mills, J.D. (2010). Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J. Neurotrauma 27, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 18.Mills, J.D., Hadley, K., and Bailes, J.E. (2011). Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery 68, 474–481; discussion, 481. [DOI] [PubMed] [Google Scholar]
- 19.Orr, S.K., Palumbo, S., Bosetti, F., Mount, H.T., Kang, J.X., Greenwood, C.E., Ma, D.W., Serhan, C.N., and Bazinet, R.P. (2013). Unesterified docosahexaenoic acid is protective in neuroinflammation. J. Neurochem. 127, 378–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang, P.K., Khatchadourian, A., McKinney, R.A., and Maysinger, D. (2015). Docosahexaenoic acid (DHA): a modulator of microglia activity and dendritic spine morphology. J. Neuroinflammation 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey, L.D., Yin, Y., Attarwala, I.Y., Begum, G., Deng, J., Yan, H.Q., Dixon, C.E., and Sun, D. (2015). Administration of DHA reduces endoplasmic reticulum stress-associated inflammation and alters microglial or macrophage activation in traumatic brain injury. ASN Neuro 7, 1759091415618969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bligh, E.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 23.Kevala, K., Lagarde, M., Spector, A.A., and Kim, H.Y. (2020). Biosynthesis of N-docosahexanoylethanolamine from unesterified docosahexaenoic acid and docosahexaenoyl-lysophosphatidylcholine in neuronal cells. Int. J. Mol. Sci. 21, 8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benchorin, G., Calton, M.A., Beaulieu, M.O., and Vollrath, D. (2017). Assessment of murine retinal function by electroretinography. Bio Protoc. 7, e2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loane, D.J., and Kumar, A. (2016). Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp. Neurol. 275, Pt. 3, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajja, V.S., Hlavac, N., and VandeVord, P.J. (2016). Role of glia in memory deficits following traumatic brain injury: biomarkers of glia dysfunction. Front. Integr. Neurosci. 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Olmos, J.S., Beltramino, C.A., and de Olmos de Lorenzo, S. (1994). Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol. Teratol. 16, 545–561 [DOI] [PubMed] [Google Scholar]
- 28.Karve, I.P., Taylor, J.M., and Crack, P.J. (2016). The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 173, 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson, M.A., Ao, Y., and Sofroniew, M.V. (2014). Heterogeneity of reactive astrocytes. Neurosci. Lett. 565, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pekny, M., Wilhelmsson, U., and Pekna, M. (2014). The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 565, 30–38 [DOI] [PubMed] [Google Scholar]
- 31.Orihuela, R., McPherson, C.A., and Harry, G.J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liddelow, S.A., Guttenplan, K.A., Clarke, L.E., Bennett, F.C., Bohlen, C.J., Schirmer, L., Bennett, M.L., Münch, A.E., Chung, W.S., Peterson, T.C., Wilton, D.K., Frouin, A., Napier, B.A., Panicker, N., Kumar, M., Buckwalter, M.S., Rowitch, D.H., Dawson, V.L., Dawson, T.M., Stevens, B., and Barres, B.A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehily, B., and Fitzgerald, M. (2017). Repeated mild traumatic brain injury: potential mechanisms of damage. Cell Transplant 26, 1131–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu, H., Guo, Y., Zhang, W., Huang, L., Wang, G., Liou, A.K., Zhang, J., Zhang, P., Leak, R.K., Wang, Y., Chen, J., and Gao, Y. (2013). Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zendedel, A., Habib, P., Dang, J., Lammerding, L., Hoffmann, S., Beyer, C., and Slowik, A. (2015). Omega-3 polyunsaturated fatty acids ameliorate neuroinflammation and mitigate ischemic stroke damage through interactions with astrocytes and microglia. J. Neuroimmunol. 278, 200–211 [DOI] [PubMed] [Google Scholar]
- 36.Mallick, R., Basak, S., and Duttaroy, A.K. (2019). Docosahexaenoic acid,22:6n-3: its roles in the structure and function of the brain. Int. J. Dev. Neurosci. 79, 21–31 [DOI] [PubMed] [Google Scholar]
- 37.Evanson, N.K., Guilhaume-Correa, F., Herman, J.P., and Goodman, M.D. (2018). Optic tract injury after closed head traumatic brain injury in mice: a model of indirect traumatic optic neuropathy. PLoS One 13, e0197346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonder Haar, C., Martens, K.M., Bashir, A., McInnes, K.A., Cheng, W.H., Cheung, H., Stukas, S., Barron, C., Ladner, T., Welch, K.A., Cripton, P.A., Winstanley, C.A., and Wellington, C.L. (2019). Repetitive closed-head impact model of engineered rotational acceleration (CHIMERA) injury in rats increases impulsivity, decreases dopaminergic innervation in the olfactory tubercle and generates white matter inflammation, tau phosphorylation and degeneration. Exp. Neurol. 317, 87–99 [DOI] [PubMed] [Google Scholar]
- 39.Perlman, I. (1983). Relationship between the amplitudes of the b wave and the a wave as a useful index for evaluating the electroretinogram. Br. J. Ophthalmol. 67, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schober, M.E., Requena, D.F., Abdullah, O.M., Casper, T.C., Beachy, J., Malleske, D., and Pauly, J.R. (2016). Dietary docosahexaenoic acid improves cognitive function, tissue sparing, and magnetic resonance imaging indices of edema and white matter injury in the immature rat after traumatic brain injury. J. Neurotrauma 33, 390–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueiredo, T.H., Harbert, C.L., Pidoplichko, V., Almeida-Suhett, C.P., Pan, H., Rossetti, K., Braga, M.F.M., and Marini, A.M. (2018). Alpha-linolenic acid treatment reduces the contusion and prevents the development of anxiety-like behavior induced by a mild traumatic brain injury in rats. Mol. Neurobiol. 55, 187–200 [DOI] [PubMed] [Google Scholar]
- 42.Heyser, C.J., Masliah, E., Samimi, A., Campbell, I.L., and Gold, L.H. (1997). Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. U. S. A. 94, 1500–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma, J., Choi, B.R., Chung, C., Min, S.S., Jeon, W.K., and Han, J.S. (2014). Chronic brain inflammation causes a reduction in GluN2A and GluN2B subunits of NMDA receptors and an increase in the phosphorylation of mitogen-activated protein kinases in the hippocampus. Mol. Brain 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, A.H., Wu, M., Shaftel, S.S., Graham, K.A., and O'Banion, M.K. (2009). Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience 164, 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coltman, R., Spain, A., Tsenkina, Y., Fowler, J.H., Smith, J., Scullion, G., Allerhand, M., Scott, F., Kalaria, R.N., Ihara, M., Daumas, S., Deary, I.J., Wood, E., McCulloch, J., and Horsburgh, K. (2011). Selective white matter pathology induces a specific impairment in spatial working memory. Neurobiol. Aging 32, 2324.e7–e12 [DOI] [PubMed] [Google Scholar]
- 46.Suenaga, J., Hu, X., Pu, H., Shi, Y., Hassan, S.H., Xu, M., Leak, R.K., Stetler, R.A., Gao, Y., and Chen, J. (2015). White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp. Neurol. 272, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reyes, L.D., Haight, T., Desai, A., Chen, H., Bosomtwi, A., Korotcov, A., Dardzinski, B., Kim, H.Y., and Pierpaoli, C. (2020). Investigation of the effect of dietary intake of omega-3 polyunsaturated fatty acids on trauma-induced white matter injury with quantitative diffusion MRI in mice. J. Neurosci. Res. 98, 2232–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuda, O. (2017). Bioactive metabolites of docosahexaenoic acid. Biochimie 136, 12–20 [DOI] [PubMed] [Google Scholar]
- 49.Park, T., Chen, H., Kevala, K., and Kim, H. (2016). N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J. Neuroinflammation 13, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim, H.Y., and Spector, A. (2018). N- Docosahexaenoylethanolamine: a neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Aspects Med. 64, 33–44 [DOI] [PubMed] [Google Scholar]
- 51.Hamazaki, K., Choi, K.H., and Kim, H.Y. (2010). Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J. Psychiatr. Res. 44, 688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guest, J., Garg, M., Bilgin, A., and Grant R. (2013). Relationship between central and peripheral fatty acids in humans. Lipids Health Dis. 12, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makrides M., Neumann, M.A., Byard, R.W., Simmer, K., and Gibson, R.A. (1994). Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 60, 189–194 [DOI] [PubMed] [Google Scholar]
- 54.Wrad, G.R., .Huang, Y.S., Bobik, E., Xing, H.C., Mutsaers, L., Auestad, N., and Wainwright, P. (1998). Long-chain polyunsaturated fatty acid levels in formulae influence deposition of docosahexaenoic acid and arachidonic acid in brain and red blood cells of artificially reared neonatal rats. J. Nutr. 128, 2473–2487 [DOI] [PubMed] [Google Scholar]
- 55.Stark, K.D., Van Elswyk, M.E., Higgins, M.R., Weatherford, C.R., and Salem, N.Jr. (2016). Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 63, 132–152 [DOI] [PubMed] [Google Scholar]
- 56.Witte, T.S., A.J., Ballester, O.F., and Hardman, W.E. (2010). RBC and WBC fatty acid composition following consumption of an omega 3 supplement: lessons for future clinical trials. Lipids Health Dis. 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]