Abstract
Human cyclin A1, a newly discovered cyclin, is expressed in testis and is thought to function in the meiotic cell cycle. Here, we show that the expression of human cyclin A1 and cyclin A1-associated kinase activities was regulated during the mitotic cell cycle. In the osteosarcoma cell line MG63, cyclin A1 mRNA and protein were present at very low levels in cells at the G0 phase. They increased during the progression of the cell cycle and reached the highest levels in the S and G2/M phases. Furthermore, the cyclin A1-associated histone H1 kinase activity peaked at the G2/M phase. We report that cyclin A1 could bind to important cell cycle regulators: the Rb family of proteins, the transcription factor E2F-1, and the p21 family of proteins. The in vitro interaction of cyclin A1 with E2F-1 was greatly enhanced when cyclin A1 was complexed with CDK2. Associations of cyclin A1 with Rb and E2F-1 were observed in vivo in several cell lines. When cyclin A1 was coexpressed with CDK2 in sf9 insect cells, the CDK2-cyclin A1 complex had kinase activities for histone H1, E2F-1, and the Rb family of proteins. Our results suggest that the Rb family of proteins and E2F-1 may be important targets for phosphorylation by the cyclin A1-associated kinase. Cyclin A1 may function in the mitotic cell cycle in certain cells.
The cell cycle is a highly complex process that is regulated by many factors. Cyclin-dependent kinases (CDKs) play important roles in the regulation of the cell cycle. In mammalian cells, several CDKs function at different stages of the cell cycle and the activities of CDKs are regulated by various cofactors and modifying enzymes. The activities of CDKs require the physical association of the positive regulators, cyclins, while the binding of negative regulators, the CDK inhibitors, inhibit kinase activity (14, 31–33). The D cyclin-associated CDK4 and CDK6 are the earliest CDKs, being activated in the G1 phase. CDK2 binding to cyclins E and A is then activated before S phase. CDK1 (also known as CDC2) in association with cyclins A and B functions at the G2/M transition (17, 26, 29, 31, 32).
Human cyclin A forms complexes with both CDK2 and CDK1. The activities of CDK2-cyclin A and CDK1-cyclin A are required for entry into S and M phases, respectively (30). We recently described a second human cyclin A (cyclin A1) whose high expression is restricted to testis in nonleukemic tissues (39). Human cyclin A1 is associated with CDK2 in vitro and in vivo. The recently cloned murine cyclin A1 (the homolog of human cyclin A1) is also expressed specifically in testis, and it binds to both CDK2 and CDK1 (34). In situ hybridization studies showed that the murine cyclin A1 is expressed only in germ cells undergoing meiosis in testis, suggesting that cyclin A1 plays a role in meiotic cell division (34). Similarly, the Xenopus cyclin A1 is expressed only in eggs and early embryos, not in either late embryos or cultured cells (15).
Although human cyclin A1 is expressed at high levels only in the testis among nonleukemic tissues, we observed high expression of cyclin A1 in several leukemia cell lines and low expression in many other human cell lines and in healthy brain (39). On the basis of these results, we explored whether cyclin A1 also plays a role in the regulation of the mitotic cell cycle.
The D-type cyclins are known to bind to the retinoblastoma susceptibility gene product (Rb) protein, and this interaction targets Rb for phosphorylation by CDK4 or CDK6 (6, 7, 19). Cyclin A can bind to the Rb family member proteins which are known as p107 and p130 but cannot bind to Rb directly in vitro (2, 5, 8, 9, 24). The CDK2-cyclin A complex also binds directly to E2F-1 and phosphorylates E2F-1 in vitro and in vivo (21, 38). In addition to the Rb family of proteins, the CDK inhibitors p21 and p27 also bind directly to cyclins A, D, and E (11).
In a previous study (39), we showed that cyclin A1 binds to CDK2, as well as several other unidentified proteins in the ML-1 myeloid leukemia cell line. We now report that cyclin A1 interacts with several important cell cycle regulators including the Rb family of proteins and E2F-1. Furthermore, the CDK2-cyclin A1 complex formed in insect cells could phosphorylate the Rb family of proteins and E2F-1 in vitro. These results suggest that the Rb family of proteins and E2F-1 may be important substrates for phosphorylation by the cyclin A1-associated kinase activities.
MATERIALS AND METHODS
Cell culture.
Human leukemia cells were cultured in RPMI 1640 with l-glutamine and 10% fetal calf serum (FCS). Human osteosarcoma cell lines MG63 and SAOS-2 were cultured in Dulbecco modified Eagle medium with 10% FCS. The HF7c Saccharomyces cerevisiae cells were maintained on YPD medium, and yeast transformants were grown on SD (lacking Trp, Leu, and/or His) medium. Escherichia coli DH5α and HB101 were used for plasmid propagation and the expression of glutathione transferase (GST) fusion proteins, respectively.
Antibody production and affinity purification.
The anti-cyclin A1 C-terminal peptide antibody was produced as briefly described below. A 16-amino-acid peptide unique to the carboxy terminus of cyclin A1 (residues 421 to 437) was synthesized and coupled to the carrier protein keyhole limpet hemacyanin. The conjugate was used to immunize two rabbits by using a standard protocol. The antibodies against the peptide were affinity purified from the rabbit serum by using an affinity column with peptide-coupled Sepharose beads as described previously (12). This antibody, which we named anti-A1C16, could specifically bind to the recombinant cyclin A1 expressed in sf9 insect cells and the GST-cyclin A1 fusion protein expressed in E. coli in our immunoblot and immunoprecipitation experiments (data not shown). Another polyclonal antibody against the full-length cyclin A1 was previously described (39). Other antibodies used in our experiments include anti-CDK2 monoclonal antibody clone 55 (Transduction Laboratory), anti-cyclin A monoclonal antibody clone BF683 (Santa Cruz Biotech), anti-Rb monoclonal antibody clone G3-245 (Pharmingen), anti-p107 monoclonal antibody clone SD9 (Pharmingen), anti-p130 polyclonal antibody (Santa Cruz Biotech), and anti-E2F-1 monoclonal antibody clone KH95 (Pharmingen).
Synchronization of MG63 cells and analysis of cyclin A1 expression.
MG63 cells were synchronized as described previously (3). Briefly, cells were arrested at G0 by serum starvation (0.1% serum) for 48 h and then they were collected at either 6 h after refeeding with medium containing 10% FCS (early G1 phase), 24 h after refeeding with medium containing 200 μM mimosine (late G1 phase), 24 h after refeeding with medium containing 2 μg of aphidicolin per ml (G1/S phase), 5 h after washing off aphidicolin (S phase), or 30 h after refeeding with medium containing 0.1 μg of nocodazole per ml (G2/M phase). MG63 cells were also released from serum starvation without any drug treatment, and samples were taken at 0, 4, 8, 12, 16, 20, 24, 28, and 32 h for analysis. The cell cycle distributions were analyzed by fluorescence-activated cell sorting (FACS) analysis (3). The expression of cyclin A1 in these cells was analyzed by Northern blotting and immunoblotting, using standard protocols. Immunoprecipitation (anti-cyclin A1) followed by histone H1 kinase assay was described previously (39).
In vitro binding assay.
Cyclin A1 protein was synthesized in vitro and labeled with [35S]methionine by using the TNT system (Promega). The Rb family of proteins and E2F-1 were produced in E. coli as GST fusion proteins. Deletion mutants of cyclin A1 and the Rb family of proteins were generated by PCR methods with Pfu polymerase (Invitrogen). Four microliters of the in vitro translation reaction mixture was incubated with 1-μg amounts of GST fusion proteins bound to glutathione Sepharose beads in 100 μl of binding buffer (50 mM Tris-Cl [pH 7.5], 1% Nonidet P-40, 400 mM NaCl, 1 mM dithiothreitol) for 1 h. The beads were washed three times in the same buffer. The proteins bound to the beads were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and autoradiography. Cyclin A1 or cyclin A1-CDK2 complexes produced in insect cells (see below) were also tested for binding to various E2F-1 fusion proteins by the same method described above except that the bound proteins were analyzed by immunoblot analyses with the anti-A1C16 antibody.
Yeast two-hybrid assay.
The interaction between cyclin A1 and several CDK inhibitors was studied by using the yeast two-hybrid system (10). Cyclin A1 was fused to the Gal4 DNA-binding domain in plasmid pGBT8, and test proteins were fused to the Gal4 activation domain in plasmid pGAD10. The two plasmids were cotransformed into yeast, and the interactions between cyclin A1 and test proteins lead to cell growth on medium lacking His.
Immunoprecipitation and immunoblotting.
Cells were washed twice with phosphate-buffered saline and lysed on ice with radioimmunoprecipitation buffer (12). Protease inhibitors (0.2 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, and 1 μg of pepstatin per ml) were added just before use. The protein concentrations of the lysates were determined with the Bio-Rad protein assay kit, and the same amounts of total protein were used in either immunoprecipitation or immunoblot experiments. Immunoprecipitations were performed at 4°C for 2 h using protein A- or G-Sepharose (Pharmacia) as the solid phase. In some experiments, antibodies were covalently coupled to the Sepharose beads (12) to prevent the interference of immunoglobulin in subsequent immunoblot analyses. Immunoblot analyses were done with nitrocellulose membranes according to standard protocols using either anti-rabbit or anti-mouse immunoglobulin G secondary antibodies labeled with horseradish peroxidase, and the enhanced chemiluminescence system (Amersham) was used for detection. When immunoprecipitation was coupled with immunoblotting, the precipitated proteins on beads were washed three times with the lysis buffer and eluted with SDS sample buffer for SDS-PAGE.
Expression of cyclin A1 in insect cells and in vitro kinase assay.
The cyclin A1 cDNA was cloned into transfer plasmid pVL1392 (Pharmingen), and baculovirus encoding cyclin A1 was generated by using the BaculoGold system (Pharmingen). The CDK2 baculovirus was a gift from H. Piwnica-Worms (Washington University, St. Louis, Mo.). The cyclin A1 and CDK2 viruses (titers about 108 PFU/ml) were used to infect sf9 cells either alone or together. Wild-type viruses were used as controls. Forty-eight hours after infection, the cells were lysed in buffer containing 0.5% Nonidet P-40, 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, and protease inhibitors as described above. For in vitro kinase assay, 3 μl of the lysate (2 mg of total protein per ml) was used in 30 μl of kinase reaction buffer (50 mM HEPES [pH 7.0], 10 mM MgCl2, 5 mM MnCl2, 1 mM dithiothreitol) containing 10 μM ATP, 10 μCi of [γ-32P]ATP and 2-μg amounts of GST fusion proteins bound to glutathione beads. The reaction mixtures were incubated at 30°C for 20 min, the beads were washed once with kinase buffer, and the phosphorylated proteins were analyzed by SDS-PAGE and autoradiography.
Transient growth suppression assay.
SAOS-2 osteosarcoma cells (5 × 105 cells/60-mm-diameter plate) were transfected with 4.5 μg of Rb expression vector combined with either 4.5 μg of empty vector or 4.5 μg of cyclin A1 expression vector by a standard calcium phosphate precipitation method. One microgram of EGFPF (enhanced green fluorescent protein farnesylated) was also included in each transfection for the identification of transfected cells. The EGFPF construct expresses a fusion protein of EGFP and the last 20 residues of c-Ha-Ras which targets the EGFP to cell membrane, and the modified EGFP remains bound to the membrane throughout the fixation processes for cell cycle analysis (18). Forty-eight hours after transfection, cells were trypsinized and fixed by adding ice-cold methanol (1 ml) in drops to 0.5 ml of the cell suspension. The cells were stained with propidium iodide as previously described (1a), and 10,000 GFP-positive and -negative cells were analyzed for cell cycle distribution as described previously (18).
RESULTS
Cyclin A1 expression is regulated in the mitotic cell cycle in MG63 cells.
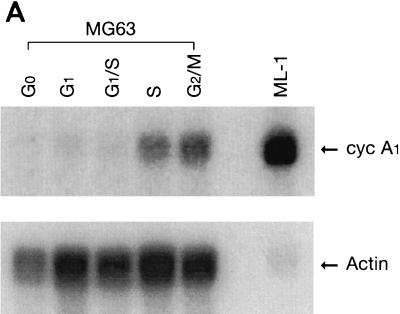
To determine whether cyclin A1 has a function in the mitotic cell cycle, the osteosarcoma cell line MG63 was used to study cyclin A1 expression during the cell cycle. MG63 cells were synchronized as described in Materials and Methods, and FACS analysis was used to confirm that the cells were synchronized at the predicted phases of the cell cycle (data not shown). First, we investigated whether cyclin A1 mRNA levels changed during the cell cycle. Northern blots (Fig. 1A) showed that cyclin A1 mRNA was low before the S phase but increased significantly at the S and G2/M phases. MG63 cells expressed much less cyclin A1 mRNA than the ML-1 myeloid leukemia cells, which were used as a positive control. A more sensitive semiquantitative reverse transcription-PCR–Southern blot method could also detect cyclin A1 mRNA in G0 phase cells but at much lower levels than those of S and G2/M phase cells (data not shown).
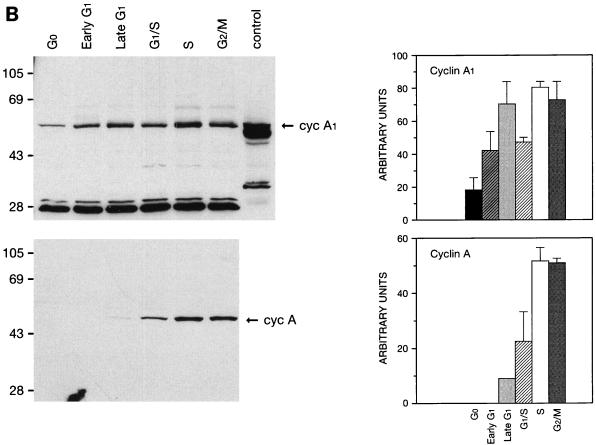
FIG. 1.
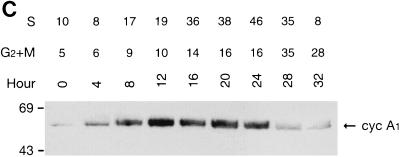
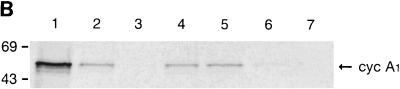
Regulation of the expression of cyclin A1 (cyc A1) during the cell cycle in MG63 cells. The cells were synchronized as described in Materials and Methods (G0, serum starvation for 48 h; early G1, 6 h after refeeding with medium containing 10% FCS; late G1, 24 h after refeeding with medium containing 200 μM mimosine; G1/S, 24 h after refeeding with medium containing 2 μg of aphidicolin per ml; S, 5 h after washing off aphidicolin; G2/M, 30 h after refeeding with medium containing 0.1 μg of nocodazole per ml). (A) Northern blot showing the level of cyclin A1 mRNA in MG63 cells at various stages of the cell cycle. Each lane contains 4 μg of poly(A)-selected RNA, except the positive-control lane which contains 5 μg of total RNA from ML-1 cells. The G1 lane represents late G1 cells arrested by mimosine. (B) Immunoblots comparing the kinetics of cyclin A1 and cyclin A protein during progression of the cell cycle. Twenty micrograms of total proteins was loaded in each lane. The control lane contains recombinant cyclin A1 proteins expressed in insect cells (2 μl of insect cell lysate). The blot was probed with the anti-A1C16 antibody (upper blot) and then anti-cyclin A monoclonal antibody after stripping the blot (lower blot). The positions of the molecular mass markers (in kilodaltons) are shown on the left. The histograms on the right show the intensity of cyclin A1 or cyclin A bands measured by a densitometer (mean ± standard deviation from three independent experiments). (C) Immunoblot showing the cyclin A1 protein levels at various time points after releasing MG63 cells from serum starvation. The time points and cell cycle distribution (as a percentage of the entire cell population) at each time point are indicated above the blot.
Cyclin A1 protein level also fluctuated during the cell cycle as shown by immunoblot analysis (Fig. 1B). Cyclin A1 was present in G0 cells at a low level, started to increase in early G1, and reached the highest levels during the S and G2/M phases. We also probed the same blot with anti-cyclin A antibody which showed an expression profile different from that of cyclin A1 (Fig. 1B). Cyclin A protein was not detectable at either G0 or early G1, started to appear in late G1, and peaked at the S and G2/M phases. The expression patterns for both cyclin A1 and cyclin A were highly repeatable in this series of experiments.
To rule out the possibility that induction of cyclin A1 (top blot in Fig. 1B) was a consequence of treating the cells with mimosine, aphidicolin, or nocodazole, we released MG63 cells from serum starvation without any drug treatment and analyzed the levels of cyclin A1 at various time points. Although this experiment produced less-synchronous cell populations, the results were similar to those of the experiment described above (Fig. 1C). Cyclin A1 increased to high levels at 12 h when most cells were at the G1/S phase, and the level of cyclin A1 remained high until 24 h. The level of cyclin A1 decreased at 28 h, and most cells had completed the cell cycle at 32 h.
Cyclin A1 binds to the transcription factor E2F-1.
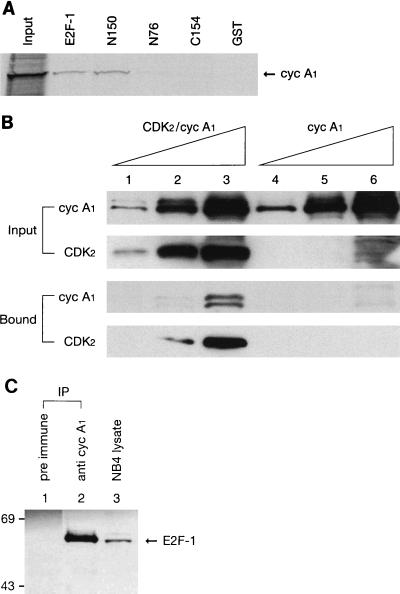
Since E2F-1 has been shown to interact with cyclin A-CDK2 (21, 38), we tested whether E2F-1 could also bind to cyclin A1. GST–E2F-1 and various GST–E2F-1 deletion mutants (38) were tested for binding to cyclin A1 in an in vitro binding assay. In these experiments, in vitro-translated and 35S-labeled cyclin A1 was tested for binding to the various fusion proteins immobilized on glutathione Sepharose beads. GST–E2F-1, but not GST, bound to cyclin A1 (Fig. 2A). Furthermore, the first 150 N-terminal residues of E2F-1 were sufficient to bind to cyclin A1; neither the first 76 N-terminal residues nor the 154 C-terminal residues of E2F-1 significantly bound to cyclin A1 (Fig. 2A). These results indicated that the sequences between positions 76 and 150 of E2F-1 were important for binding of cyclin A1; this is the same region of E2F-1 that binds to cyclin A (21, 38). Probably, cyclin A1 and cyclin A interact with E2F-1 in a similar fashion.
FIG. 2.
Interaction of cyclin A1 (cyc A1) with E2F-1. (A) The autoradiography shows cyclin A1 (in vitro-translated and [35S]methionine-labeled) binding to GST fusions of E2F-1 and E2F-1 deletion mutants (E2F-1, GST fusion of full-length E2F-1; N150 and N76, GST fusions of the initial 150 and 76 residues at the N terminus of E2F-1, respectively; C154, GST fusion of the 154 residues at the C terminus of E2F-1). The input lane contains 25% of the total input of cyclin A1. (B) Immunoblots showing the binding of either cyclin A1-CDK2 or cyclin A1 (expressed in insect cells) alone to the GST fusion of E2F-1. Five hundred microliters of diluted insect cell lysate containing either cyclin A1-CDK2 or cyclin A1 alone was incubated with 2-μg amounts of GST-E2F-1 fusion proteins bound to glutathione beads. The cyclin A1 and CDK2 that bound to E2F-1 were analyzed by immunoblotting with either anti-A1C16 or a monoclonal anti-CDK2 antibody, respectively. Lanes 1 and 4, 1/250 dilution of insect cell lysate; lanes 2 and 5, 1/50 dilution of insect cell lysate; lanes 3 and 6, 1/10 dilution of insect cell lysate. The two upper immunoblots show the input cyclin A1 and CDK2. The two lower blots show the cyclin A1 and CDK2 bound to E2F-1. (C) Immunoblot showing the interaction of cyclin A1 with E2F-1 in vivo. Cyclin A1 complexes were precipitated from 500 μl of NB4 cell lysate with either preimmune sera or anti-A1C16 antibody. The precipitated proteins were analyzed with an anti-E2F-1 monoclonal antibody on the immunoblot. Ten microliters of NB4 lysate was used as a positive control in lane 3. The positions of the molecular mass markers (in kilodaltons) are shown on the left. IP, immunoprecipitation.
Cyclin A, when associated with CDK2, has been reported to bind much more efficiently to E2F-1 (38). We investigated whether this was also the case for cyclin A1. Cyclin A1, either alone or together with CDK2, was expressed in sf9 insect cells with baculovirus, and an in vitro binding assay was performed using GST fusions of E2F-1 as described previously (38). As shown in Fig. 2B, the binding of cyclin A1 and CDK2 to E2F-1 was detected at a 1/50 dilution of the insect cell lysate that expressed both cyclin A1 and CDK2 (lane 2) and the binding was very strong at a 1/10 dilution (lane 3). In contrast, cyclin A1 alone bound to E2F-1 weakly at a 1/10 dilution (lane 6), while CDK2 alone could not bind to E2F-1 (data not shown). The cells expressing cyclin A1 alone contained either a similar or larger amount of cyclin A1 than those with both cyclin A1 and CDK2 (cyclin A1 input). A 1/10 dilution of the lysate containing CDK2-cyclin A1 did not show any binding to control GST-Sepharose (data not shown). These results indicated that cyclin A1 bound more strongly to E2F-1 in the presence of CDK2.
The interaction of cyclin A1 and E2F-1 was also detected in NB4 leukemia cells by immunoprecipitation and immunoblot analyses. As shown in Fig. 2C, E2F-1 was detected in anti-cyclin A1 immunoprecipitates and the addition of a competing immunogenic peptide eliminated the coprecipitation (data not shown). A different antibody, the antibody against full-length cyclin A1, also coprecipitated E2F-1 from MG63 and U937 (leukemia) cells (data not shown), suggesting that the precipitation of E2F-1 was probably not due to cross-reaction of the antibodies to E2F-1.
Cyclin A1 binds to the Rb family of proteins.
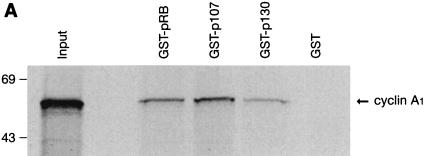
The D cyclins are known to interact with the Rb family of proteins. However, cyclin A does not bind directly to Rb in vitro (8, 9). We have previously observed several cyclin A1-associated proteins with molecular weights similar to those of the Rb family of proteins in immunoprecipitation experiments using 35S-labeled cells (39), so we decided to examine whether cyclin A1 could bind to members of the Rb family of proteins. We employed the same in vitro binding assay described above with in vitro-translated, 35S-labeled cyclin A1 and GST fusions of the large pockets of the Rb family of proteins. Using this assay, we observed interactions between cyclin A1 and GST-Rb (residues 373 to 928), GST-p107 (residues 252 to 1068), and GST-p130 (residues 327 to 1139) fusion proteins but not with GST alone (Fig. 3A).
FIG. 3.
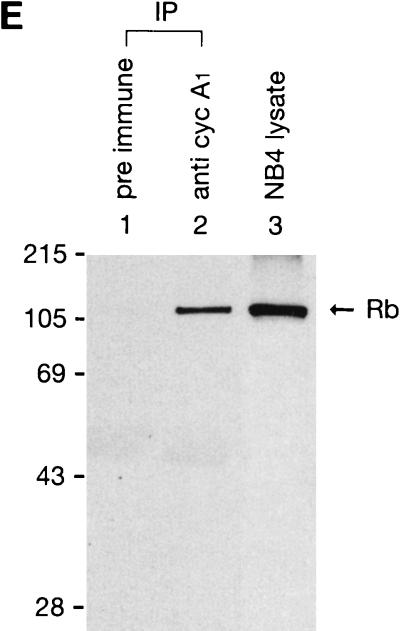
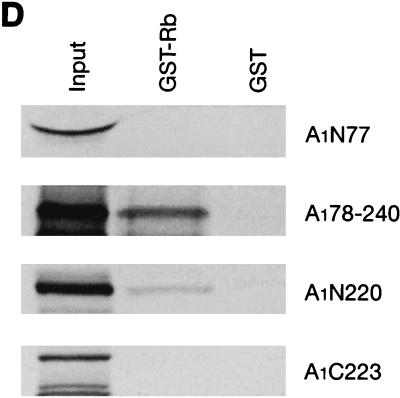
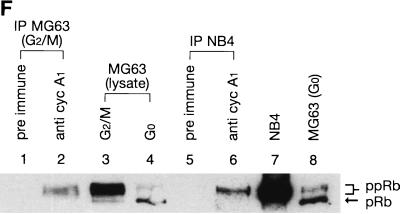
Interaction of cyclin A1 with the Rb family of proteins. (A) An in vitro binding assay showing that cyclin A1 (in vitro translated and [35S]methionine labeled) bound to GST fusions of the large pocket region of Rb, p107, and p130 but not to the GST control. The GST fusions used are indicated above the blot. The input lane represents 50% of total input of the labeled cyclin A1. (B) Binding of cyclin A1 (cyc A1) to GST fusions of various regions of Rb. Lane 1, input; lane 2, Rb large pocket (amino acids 373 to 928); lane 3, Rb A pocket (amino acids 373 to 590); lane 4, Rb B and C pocket (amino acids 623 to 928); lane 5, Rb C pocket (amino acids 773 to 928); lane 6, Rb838-928 (amino acids 838 to 928); lane 7, GST. (C) Binding of cyclin A1 to three regions of p107 (fused to GST) as shown above the gel. The input lane represents 25% of total input of the labeled cyclin A1. (D) Various regions of cyclin A1 were in vitro translated and labeled as described above and analyzed for binding to GST-Rb and GST control. A1N77 and A1N220, the 77 and 220 residues at the N terminus of cyclin A1, respectively; A1C223, the 223 residues at the C terminus of cyclin A1; A178-240: residues from 78 to 240 of cyclin A1. (E) Immunoblot showing the interaction of cyclin A1 with Rb in vivo. Cyclin A1 complexes were precipitated from 500 μl of NB4 cell lysate with either preimmune sera or anti-A1C16 antibody. The precipitated proteins were analyzed by anti-Rb immunoblotting. NB4 lysate (10 μl) was used as a positive control in lane 3. The positions of the molecular mass markers (in kilodaltons) are shown on the left. IP, immunoprecipitation. (F) Immunoprecipitation (IP) and immunoblot analyses show that cyclin A1 mainly associated with hyperphosphorylated forms of Rb. Cyclin A1-containing complexes were precipitated with anti-A1C16, and the Rb proteins in the precipitated complexes were analyzed by anti-Rb immunoblotting. Lanes 1 and 2, immunoprecipitations from lysates of MG63 cells synchronized at the G2/M phase by preimmune serum and anti-cyclin A1, respectively; lanes 3 and 4, cell lysates of MG63 cells synchronized at the G2/M and G0 phases, respectively; lanes 5 and 6, immunoprecipitations from NB4 cell lysates by preimmune serum and anti-cyclin A1, respectively; lanes 7 and 8, lysates of asynchronous NB4 and G0-synchronized MG63 cells, respectively. pRb and ppRb, hypophosphorylated and hyperphosphorylated Rb, respectively.
Further analysis with different GST-Rb deletion mutants indicated that the C-pocket region of Rb (residues 773 to 928) was sufficient for binding to cyclin A1 (Fig. 3B). Further deletion to residue 838 greatly reduced binding to cyclin A1 (Fig. 3B). The region of p107 that confers cyclin A1 binding was mapped to between residues 649 and 816 as shown in Fig. 3C. Interestingly, this region of the p107 contains the SAKRRLFGE sequence that has been shown to contribute to the p107-cyclin A interaction (8, 42). This sequence is conserved in p130 and is also responsible for the ability of p130 to bind to cyclin A (22). Our results suggest that cyclin A1 may also bind to p107 and p130 through this sequence.
Several deletion mutants of cyclin A1 were used in in vitro binding assays to determine the regions of cyclin A1 that binds to Rb. As shown in Fig. 3D, the 77 residues at the N terminus and the 223 residues at the C terminus did not bind to Rb, but the 220 residues at the N terminus as well as residues between 78 and 240 were sufficient for binding of Rb.
To test whether cyclin A1 could also interact with the Rb family of proteins in vivo, we used NB4 cells that express high levels of cyclin A1 and have an intact Rb gene. Immunoprecipitation (anti-A1C16) followed by immunoblot analysis (anti-Rb monoclonal antibody) showed that the Rb protein was coprecipitated by the anti-A1C16 antibody but not by the preimmune antibody (Fig. 3E). The coprecipitation of Rb was greatly reduced if a competing immunogenic peptide was added (data not shown). Another antibody that was raised against the full-length cyclin A1 could also coprecipitate Rb but less efficiently (data not shown). We have not yet detected the association of p107 and p130 with cyclin A1 in similar experiments.
We next examined the phosphorylation state of the Rb protein found in the cyclin A1 complexes. As shown in Fig. 3F, the Rb in the cyclin A1 complexes from both the MG63 cells arrested at the G2/M phase and the asynchronous NB4 cells (lanes 2 and 6) comigrated (as diffused bands) with the slower-migrating Rb bands that were present in MG63 cells at G2/M (lane 3) and asynchronous NB4 cells (lane 7). MG63 cells synchronized at G0 contained mostly the fast-migrating hypophosphorylated Rb (lanes 4 and 8). These results suggest that most of the Rb associated with cyclin A1 was phosphorylated.
The p21 family of CDK inhibitors bind to cyclin A1.
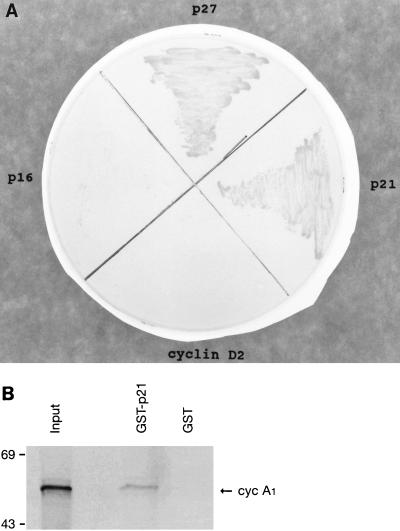
The p21 family of proteins can interact directly with many cyclins, including cyclins A, D, and E (11). Using a yeast two-hybrid assay, we showed that the CDK inhibitors p21 and p27 but neither p16INK4a nor cyclin D2 could interact with cyclin A1 (Fig. 4A). An in vitro binding assay using GST-p21 and 35S-labeled cyclin A1 also confirmed this interaction (Fig. 4B). Although we showed that the p21 family of proteins could interact with cyclin A1, the effect of this interaction on the function of CDK2-cyclin A1 is still not known at this time.
FIG. 4.
Interactions of cyclin A1 with members of the p21 protein family. (A) Binding of cyclin A1 to either p21 or p27 was indicated by the growth of HF7c yeast cells transformed with pGBT-A1 and pGAD-p21 or -p27 on a plate containing medium lacking Trp, Leu, and His. The pGAD-cyclin D2 and -p16INK4a were negative, as expected. (B) In vitro binding assay shows that cyclin A1 (cyc A1) (in vitro translated and [35S]methionine labeled) binds to GST-p21.
The cyclin A1-associated histone H1 kinase activity is regulated during the cell cycle, and the cyclin A1-CDK2 complex formed in insect cells has kinase activity to the Rb family of proteins and E2F-1.
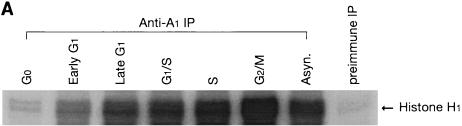
To investigate whether the cyclin A1-associated histone H1 kinase activity fluctuated during the mitotic cell cycle, immunoprecipitation with antibodies against the full-length cyclin A1 and in vitro kinase assay were performed with histone H1 as the substrate. The cyclin A1-associated activity increased as the cell cycle progressed and reached a peak level at the G2/M phase (Fig. 5A).
FIG. 5.
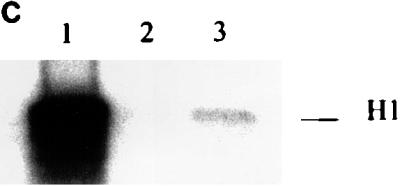
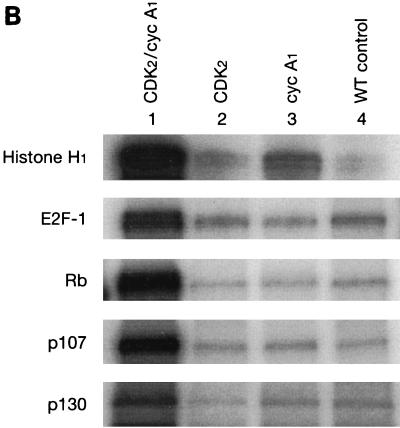
Cyclin A1-associated kinase activities. (A) The cyclin A1-associated histone H1 kinase activities in MG63 cells at different stages of the cell cycle. For the in vitro kinase assay, the anti-full-length cyclin A1 antibody was used for immunoprecipitation (IP) and histone H1 was used as the substrate. The preimmune serum (IP from asynchronous cells) was used as a negative control. Asyn., asynchronous MG63 cells. (B) Lysates from insect cells infected with cyclin A1 (cyc A1) and CDK2-containing viruses either alone or together were tested for kinase activity using as targets either histone H1 or GST fusions of either E2F-1 or the large pockets of Rb, p107, and p130 as the substrates. Cells infected with the wild-type virus (WT) were used as a control in lane 4. The autoradiography shows the incorporation of 32P into the substrates by the kinase activity. The viruses used for infections are shown above the gel. (C) Immunoprecipitation (anti-A1C16) followed by kinase assay using histone H1 shows that the precipitated cyclin A1 complex has kinase activity. Cyclin A1 was precipitated from lysates of insect cells infected with either CDK2-cyclin A1 (lane 1), CDK2 (lane 2), or cyclin A1 (lane 3), and the kinase assay was performed as described in Materials and Methods.
To determine whether CDK2-cyclin A1 complexes also have kinase activity against E2F-1 and the Rb family of proteins, we expressed CDK2-cyclin A1 in insect cells and used the insect cell lysate in an in vitro kinase assay with histone H1, GST fusion of E2F-1, and GST fusions of the Rb family of proteins as substrates. The lysate containing CDK2-cyclin A1 showed strong kinase activity to histone H1 (Fig. 5B), which is consistent with the previous immunoprecipitation and in vitro kinase assay results (Fig. 5A). The same lysate also phosphorylated GST-Rb, GST-p107, GST-p130, and GST–E2F-1 (Fig. 5B). Lysates containing either cyclin A1 or CDK2 alone, which expressed either equal or more recombinant proteins than the coinfections (data not shown), showed only background activities (Fig. 5B). To ensure that the kinase activities in these assays were contributed by the CDK2-cyclin A1 complex and not by other endogenous kinases activated by CDK2-cyclin A1, we performed immunoprecipitation with anti-cyclin A1 antibodies followed by kinase assay. As shown in Fig. 5C, the immunoprecipitated cyclin A1 complexes showed kinase activity to histone H1. Similar results were also observed for GST-Rb (data not shown). These results indicated that the CDK2-cyclin A1 expressed in the insect cells contributed directly to the kinase activities.
Cyclin A1 can reverse the Rb-induced G1 arrest in SAOS-2 osteosarcoma cells.
Studies have shown that the expression of Rb in SAOS-2 cells can induce a G1 arrest which can be reversed by the expression of either cyclin A, cyclin E, or the D cyclins (6, 7, 13). We studied whether cyclin A1 also has this function by cotransfecting SAOS-2 cells with a Rb expression vector and either a control or a cyclin A1 expression vector. A vector expressing a membrane-bound GFP was also included for identification of transfected cells, and cell cycle analysis was done for both GFP-positive (transfected) and GFP-negative (nontransfected) cells. As shown in Table 1, the expression of Rb increased the G1 population by 15 to 20% and the coexpression of cyclin A1 reversed this increase. Transfection of a control empty vector alone did not change significantly the cell cycle distribution (data not shown).
TABLE 1.
Effect of cyclin A1 on Rb-induced G1 arrest in SAOS-2 cells
| Expt and transfection plasmidsa | GFPb | Cell cycle distribution (%)
|
||
|---|---|---|---|---|
| G1 | S | G2/M | ||
| Expt 1 | ||||
| Rb-vector | + | 67 | 15 | 18 |
| − | 52 | 24 | 24 | |
| Rb-cycA1 | + | 49 | 24 | 27 |
| − | 51 | 19 | 30 | |
| Expt 2 | ||||
| Rb-vector | + | 65 | 17 | 18 |
| − | 47 | 29 | 24 | |
| Rb-cycA1 | + | 52 | 26 | 22 |
| − | 47 | 30 | 23 | |
| Expt 3 | ||||
| Rb-vector | + | 71 | 14 | 15 |
| − | 52 | 24 | 24 | |
| Rb-cycA1 | + | 54 | 29 | 17 |
| − | 44 | 30 | 26 | |
Rb, Rb expression plasmid; vector, empty vector; cycA1, cyclin A1 expression plasmid.
GFP-positive (+) cells are those that show at least 40-fold-more green fluorescence than the GFP-negative (−) background.
DISCUSSION
In healthy tissues, both murine and human cyclin A1 show high level of expression only in testis. In situ hybridization studies found that the murine cyclin A1 is expressed exclusively in meiotic germ cells of the testis (34). These observations suggest that cyclin A1 functions in the meiotic cell cycle. Previously, we observed low-level expression of human cyclin A1 in the brain and in many somatic human cell lines. Several leukemia cell lines also expressed high levels of cyclin A1 (39). These results led us to investigate whether cyclin A1 may also have a function in the mitotic cell cycle.
Using the MG63 osteosarcoma cells as a model system, we showed that cyclin A1 mRNA and protein levels as well as the cyclin A1-associated histone H1 kinase activities are regulated during the cell cycle. Both the mRNA and the protein levels of cyclin A1 are the highest during the S and G2/M phases, and the cyclin A1-associated histone H1 kinase activity peaks at the G2/M phase. These results suggest that in addition to its function in the meiotic cell cycle, cyclin A1 may play a role in the mitotic cell cycle. The kinetics of cyclin A1 at the protein level are different from that of cyclin A. Cyclin A appears later in the cell cycle than cyclin A1 but increases rapidly, reaching high levels at the S and G2/M phases (Fig. 1B); cyclin A1, on the other hand, is detectable at the G0 phase and rises more gradually compared to cyclin A. However, like cyclin A-associated histone H1 kinase activity, cyclin A1-associated activity peaks at the G2/M phase. The expression of cyclin A1 is not restricted to tumor cell lines. We found that cyclin A1 expression is relatively high in certain healthy hematopoietic progenitor cells in addition to testis (40). In certain healthy cells, perhaps cyclin A1 has functions that are involved in growth and differentiation. Although we do not yet have definitive evidence to prove that cyclin A1 has a role in the mitotic cell cycle in certain cells, our results encourage future studies in the direction of elucidating the exact role(s) of cyclin A1 in the cells that express relatively high levels of it.
Cyclin A has been shown to bind to E2F-1, and the CDK2-cyclin A complex can phosphorylate E2F-1 which inactivates DNA binding and transactivation by E2F-1 (21, 38). In this study, we also observed the interaction between cyclin A1 and E2F-1, and the same region of E2F-1 that binds to cyclin A was also required for binding to cyclin A1. Like cyclin A, cyclin A1 bound to E2F-1 much more efficiently when complexed with CDK2. Furthermore, the CDK2-cyclin A1 expressed in insect cells had kinase activity against E2F-1 in vitro. The in vivo interaction of cyclin A1 and E2F-1 was observed in several cell lines. These results suggest that E2F-1 may be one of the substrates of cyclin A1-associated kinases in vivo.
Cyclin A is known to bind to p107 and p130 (2, 5, 8, 9, 24). The interaction is through a short sequence in the spacer region of p107 and p130 that is not present in Rb (22, 42). Since cyclin A1 interacts with a fragment of p107 that contains this sequence, cyclin A1 very likely also binds to p107 or p130 through this same sequence. Although cyclin A does not bind directly to Rb in vitro, we showed that cyclin A1 was able to bind to the C-pocket region of Rb in vitro. The D-type cyclins are known to bind to Rb through the LXCXE sequence which is also used by several viral proteins for binding of Rb (6, 35). Cyclin A1 does not have this motif, and we showed that the interaction of cyclin A1 with Rb requires a region between residues 78 and 220 of cyclin A1. This region does not have significant homology with other cyclins.
We observed Rb in association with cyclin A1 in vivo by immunoprecipitation coupled with immunoblot analysis. The coprecipitation of Rb by anti-cyclin A1 antibodies was probably not due to nonspecific cross-reaction, because the anti-cyclin A1 antibodies did not recognize Rb in immunoblot analyses and two different anti-cyclin A1 antibodies could precipitate Rb. Although we have not excluded the possibility that other proteins, such as CDK2 which has been shown to bind to Rb in vitro (1), could mediate the Rb-cyclin A1 interaction in vivo, our in vitro results suggested that cyclin A1 may interact directly with Rb in vivo. Cyclin A and CDK1 have also been shown to associate with Rb in vivo (16, 37), and both CDK1 and CDK2 kinase activities can be coprecipitated by anti-Rb antibodies (1, 16). However, cyclin A does not bind directly to Rb in vitro (8). The mechanism of CDK1-cyclin A-Rb complex formation in vivo is still not known. A complex containing cyclin A, CDK2, p107, and E2F-1 has been observed in cells during the S phase (5). Although we observed both E2F-1 and Rb in cyclin A1 complexes, we do not know if quaternary complexes of cyclin A1, CDK2, Rb, and E2F-1 exist.
We observed that cyclin A1 bound to bands of Rb that migrated slower than the hypophosphorylated species found in G0 cells, suggesting that cyclin A1 mainly bound to phosphorylated forms of Rb. This is surprising because most of the Rb-binding proteins (except the adenoviral E1A) bind to the unphosphorylated or underphosphorylated forms of Rb (for reviews, see references 28, 35, 36). One possible explanation for this observation is that the phosphorylation of Rb by CDK4 or CDK6 bound to cyclin D and CDK2-cyclin E, which occurs before S phase, induces a conformational change of Rb to make it a better target for binding by cyclin A1. The cyclin A1-associated kinase activity may function either to maintain the phosphorylation state of Rb or to phosphorylate new sites in Rb. Recent studies have shown that cyclin D1-CDK4 and cyclin A- or E bound to CDK2 phosphorylate different sites on Rb in vivo (4, 20, 25, 41). One study also showed that the phosphorylation of Rb by CDK4 or CDK6 bound to cyclin D is required for Rb to be further phosphorylated by CDK2-cyclin E (25). Both CDK1 and CDK2 have been shown to phosphorylate Rb in vitro on physiologically relevant sites (1, 16, 23). The cyclin A1-associated kinase activities may also phosphorylate the Rb family of proteins in vivo.
Our in vitro results indicated that some of the functions of cyclin A1 are similar to those of cyclin A. Both cyclins interact with E2F-1, p107, and p130 in an analogous manner. Like cyclin A, cyclin A1 was also able to reverse a G1 arrest induced by Rb in SAOS-2 cells. However, the kinetics of cyclin A1 are different from those of cyclin A during the cell cycle in the MG63 cell line. Cyclin A1 could also bind directly to Rb in vitro, while cyclin A cannot. Whether cyclin A1 and cyclin A have similar functions in vivo is still unknown. Genetic evidence suggest that they may have different functions. Deletion of the murine cyclin A2 (homolog of human cyclin A) in mice results in early embryonic lethality at a time when maternal stores of cyclin A2 become depleted (27), suggesting that cyclin A1 cannot compensate for the loss of cyclin A2. However, this conclusion requires the demonstration of cyclin A1 expression in the early murine embryo; this has been demonstrated for Xenopus (15), but expression has not yet been examined in the mouse. Cyclin A1 deletional murine models should provide further understanding of the biological functions of cyclin A1. In addition, since human cyclin A is the homolog of the murine and Xenopus cyclin A2, we suggest that human cyclin A be renamed human cyclin A2.
ACKNOWLEDGMENTS
We thank H. Piwnica-Worms (Washington University, St. Louis, Mo.) for providing the CDK2 baculovirus. We thank W. Jiang and T. Hunter (Salk Institute, La Jolla, Calif.) for providing the EGFPF vector. We thank Lisa Beck-Von-Peccoz for technical assistance.
This research is supported by NIH grants (CA 26038, CA 70675-01, and CA42710), U.S. Army grants, the C. and H. Koeffler Fund, and the Parker Hughes Trust. H.P.K. is a member of the Jonsson Cancer Center and holds the Mark Goodson Chair in Oncology Research. C.M. is supported by a fellowship from the German Research Foundation (DFG).
REFERENCES
- 1.Akiyama T, Ohuchi T, Sumida S, Matsumoto K, Toyoshima K. Phosphorylation of the retinoblastoma protein by CDK2. Proc Natl Acad Sci USA. 1992;89:7900–7904. doi: 10.1073/pnas.89.17.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Becton Dickinson and Co. Becton Dickinson manual. Paramus, N.J: Becton Dickinson and Co.; 1996. [Google Scholar]
- 2.Cao L, Faha B, Dembski M, Tsai L-H, Harlow E, Dyson N. Independent binding of the retinoblastoma protein and p107 to the transcription factor E2F. Nature. 1992;355:176–179. doi: 10.1038/355176a0. [DOI] [PubMed] [Google Scholar]
- 3.Carbonaro-Hall D, Williams R, Wu L, Warburton D, Zeichner-David M, MacDougall M, Tolo V, Hall F. G1 expression and multistage dynamics of cyclin A in human osteosarcoma cells. Oncogene. 1993;8:1649–1659. [PubMed] [Google Scholar]
- 4.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devoto S H, Mudryj M, Pines J, Hunter T, Nevins J R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992;68:167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- 6.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 7.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 8.Ewen M E, Faha B, Harlow E, Livingston D M. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992;255:85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- 9.Faha B, Ewen M E, Tsai L-H, Livingston D M, Harlow E. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science. 1992;255:87–90. doi: 10.1126/science.1532458. [DOI] [PubMed] [Google Scholar]
- 10.Fields S, Song O-K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 11.Hall M, Bates S, Peters G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene. 1995;11:1581–1588. [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 13.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 14.Hirama T, Koeffler H P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995;86:841–854. [PubMed] [Google Scholar]
- 15.Howe J A, Howell M, Hunt T, Newport J W. Identification of a developmental timer regulating the stability of embryonic cyclin A and a new somatic A-type cyclin at gastrulation. Genes Dev. 1995;9:1164–1176. doi: 10.1101/gad.9.10.1164. [DOI] [PubMed] [Google Scholar]
- 16.Hu Q, Lees J A, Buchkovich K, Harlow E. The retinoblastoma protein physically associates with the human cdc2 kinase. Mol Cell Biol. 1992;12:971–980. doi: 10.1128/mcb.12.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackman M R, Pines J N. Cyclins and the G2/M transition. Cancer Surv. 1997;29:47–73. [PubMed] [Google Scholar]
- 18.Jiang W, Hunter T. Analysis of cell-cycle profiles in transfected cells using a membrane-targeted GFP. BioTechniques. 1998;24:349–350. [PubMed] [Google Scholar]
- 19.Kato J, Matsushime H, Hiebert S, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa M, Higashi H, Jung H-K, Suzuki-Takahashi S, Ikeda M, Tamai K, Kato J-Y, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-cdk4 is different from that for phosphorylation by cyclin A/E-cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 21.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G, Livingston D. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 22.Lacy S, Whyte P. Identification of a p130 domain mediating interactions with cyclin A/cdk2 and cyclin E/cdk2 complexes. Oncogene. 1997;14:2395–2406. doi: 10.1038/sj.onc.1201085. [DOI] [PubMed] [Google Scholar]
- 23.Lees J A, Buchkovich K J, Marshak D R, Anderson C W, Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991;10:4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Graham C, Lacy S, Duncan A M V, Whyte P. The adenovirus E1A-associated 130-KD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1996;7:2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 27.Murphy M, Stinnakre M, Senamaud-Beaufort C, Winston N J, Sweeney C, Kubelka M, Carrington M, Brechot C, Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15:83–86. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- 28.Nevins J R, Leone G, DeGregori J, Jakoi L. Role of the Rb/E2F pathway in cell growth control. J Cell Physiol. 1997;173:233–236. doi: 10.1002/(SICI)1097-4652(199711)173:2<233::AID-JCP27>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 30.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed S I. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 32.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 33.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney C, Murphy M, Kubelka M, Ravnik S E, Hawkins C F, Wolgemuth D J, Carrington M. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- 35.Wang J Y J, Knudsen E S, Welch P J. The retinoblastoma tumor suppressor protein. Adv Cancer Res. 1994;64:25–85. doi: 10.1016/s0065-230x(08)60834-9. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 37.Williams R T, Carbonaro-Hall D A, Hall F L. Co-purification of p34cdc2/p58 cyclin A proline-directed protein kinase and the retinoblastoma tumor susceptibility gene product: interaction of an oncogenic serine/threonine protein kinase with a tumor-suppressor protein. Oncogene. 1992;7:423–432. [PubMed] [Google Scholar]
- 38.Xu M, Sheppard K-A, Peng C-Y, Yee A S, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang R, Morosetti R, Koeffler H P. Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines. Cancer Res. 1997;57:913–920. [PubMed] [Google Scholar]
- 40.Yang, R., and H. P. Koeffler. Unpublished data.
- 41.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 42.Zhu L, Harlow E, Dynlacht D. p107 uses a p21cip1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev. 1995;9:1740–1752. doi: 10.1101/gad.9.14.1740. [DOI] [PubMed] [Google Scholar]