Abstract
This study is the first to examine cognitive outcomes after pediatric mild TBI using the National Institutes of Health Toolbox Cognition Battery (NIHTB-CB), a computerized cognitive test battery. The NIHTB-CB includes two complex measures of attention and executive function that allow differentiation of accuracy and response speed. We compared performance on the NIHTB-CB among children 8–16 years of age with mild TBI (n = 143) versus children with orthopedic injuries (OIs; n = 74) recruited in emergency departments and followed for 6 months post-injury. Mixed-model analyses showed that the mild TBI group showed significantly lower Fluid Cognition composite scores than the OI group at 10 days (group intercept, p = 0.018); the magnitude of group differences declined modestly over time (group × time interaction, p = 0.055). Effect sizes were d = 0.34 at 10 days post-injury, d = 0.27 at 3 months, and d = 0.10 at 6 months. No significant effects of group or time were found for the Crystallized Cognition composite. Analyses of Fluid Cognition subtests indicated that children with mild TBI displayed deficits for as long as 3 months on measures of attention and executive function (e.g., cognitive flexibility, inhibitory control), but not on measures of explicit memory, working memory, or processing speed. The poorer performance of the mild TBI group on measures of attention and executive function was attributable largely to slowed reaction time, not decreased accuracy. The findings suggest that children with mild TBI demonstrate persistent deficits in fluid cognition that are most apparent on tasks that combine demands for both speed and executive function.
Keywords: cognitive assessment, crystalized cognition, fluid cognition, pediatric, traumatic brain injury
Introduction
In 2014, >812,000 children visited an emergency department (ED) for a traumatic brain injury (TBI) in the United States, and TBI-related ED visits have continued to increase in recent years.1 Severity of TBI is graded on a continuum ranging from mild to severe, with the degree of cognitive impairment after TBI varying as a function of the severity of the injury.2 Mild TBI accounts for 70–90% of all TBI-related ED visits, and in the United States alone, the cost of TBI-related pediatric ED visits exceeds $1 billion USD annually, suggesting that pediatric mild TBI poses a significant public health burden.1,3,4
The cognitive sequelae of moderate-to-severe TBI are well documented2 and are frequently associated with impairments in children's academic, behavioral, and functional outcomes.5 In particular, children and adolescents who sustain a TBI are at risk for impairments in domains of fluid cognition, which include attention, working memory, episodic memory, executive functioning, and processing speed.5,6 In contrast, domains of crystalized cognition, such as language abilities, are generally spared or less affected after TBI.2
Although moderate-to-severe TBI is frequently associated with persistent cognitive deficits, striking inconsistencies are apparent in research on cognitive outcomes for children and adolescents after mild TBI.2 In general, the existing research suggests that a single, uncomplicated mild TBI (i.e., without accompanying intracranial abnormalities or skull fractures) has minimal long-term consequences for children's cognitive abilities. Several systematic reviews report few, if any, neurocognitive impairments after mild TBI, even post-acutely.2,6,7 Nonetheless, in some studies, children and adolescents with mild TBI exhibit cognitive weaknesses after mild TBI.2,8 Most studies that have reported cognitive deficits attribute them to psychological factors, pre-morbid functioning, a general injury effect, or the specific tests used.9 However, failure to detect differences in cognition beyond the post-acute period may, in fact, reflect a lack of sensitivity in existing cognitive tests.
The use of traditional neuropsychological tests to identify cognitive deficits after mild TBI has been called into question. Most traditional paper-and-pencil tests were designed to identify changes in cognitive abilities in persons with significant neurological and psychiatric disorders and may not be sensitive enough to capture the subtle cognitive changes that may occur after mild TBI.10 Computerized tests have gained in popularity and sophistication in recent years, particularly in the context of assessment of mild TBI.11 One major strength of computerized tests is the ability to accurately record response time in milliseconds. This is important because the neurocognitive deficits that have been reported after mild TBI typically include slower response time and reduced information processing speed, which may not be captured in a sensitive manner by traditional paper-and-pencil measures.11,12 The utility of computerized response-time measures for capturing subtle cognitive changes after mild TBI has been shown in several studies in the adult literature.12–14
The National Institutes of Health Toolbox Cognition Battery (NIHTB-CB) is a computerized cognitive test battery that is a component of the National Institutes of Health Toolbox for Assessment of Neurological and Behavioral Function, which consists of a set of measures used to assess cognitive, emotional, sensory, and motor function across the life span (3–85 years).15,16 The NIHTB-CB consists of seven subtests that measure language, reading, processing speed, episodic memory, working memory, and attention and executive function.17 Three composite measures are derived from combinations of the individual subtests: Overall Cognition, Crystallized Cognition, and Fluid Cognition.18 Administration time for the NIHTB-CB is ∼30 min. Research has established the reliability and validity of the NIHTB-CB in children and across the life span.18–25
One potential advantage of the NIHTB-CB in the context of mild TBI is that two fluid cognition subtests are complex measures of attention and executive function that depend on both speed and accuracy and not only provide overall scores, but also allow for response time and accuracy to be examined separately. Tests of fluid cognition may be especially important for capturing the subtle cognitive changes that emerge after mild TBI because they depend on the flexible application of cognitive skills on novel tasks, whereas tests of crystallized cognition depend on previous learning and knowledge. TBI tends to have a greater impact on fluid cognition than on crystallized cognition.17
Only one other recently published study has focused on the use of the NIHTB-CB with children and adolescents after acquired brain injury (ABI). The researchers determined that the NIHTB-CB is feasible to administer to children 4–18 years of age in inpatient rehabilitation and day-treatment settings and reported deficits in fluid cognition, but not in crystallized cognition, compared to normative expectations among 38 children with heterogenous etiologies of ABI.26 Tulsky and colleagues17 evaluated the use of the NIHTB-CB in distinguishing among adults specifically with complicated mild/moderate or severe TBI, examined on average 5 years post-injury, as compared to a demographically similar comparison group. Both groups of persons with TBI showed significantly lower scores than the comparison group on the fluid, but not crystallized cognition, composite as well as on the individual subtests that make up the fluid composite score. Effect sizes for the mild/moderate TBI versus comparison group were moderate in magnitude.
The current study is the first to examine cognitive outcomes after pediatric mild TBI using the NIHTB-CB. We compared performance on the NIHTB-CB among children with mild TBI versus a comparison group of children with orthopedic injuries (OIs) recruited upon presentation to the emergency department (ED) and followed for the first 6 months post-injury. Based on previous research, we hypothesized that children with mild TBI would show poorer performance on measures of fluid cognition relative to children with OI, but not on measures of crystallized cognition. We expected to find group differences even after controlling for socioeconomic status (SES) and sex, as well as after excluding children who displayed invalid performance on validity testing; however, we anticipated that the magnitude of group differences would diminish as a function of time post-injury, reflecting recovery in the mild TBI group. We also compared groups on accuracy versus speed of response, when possible, to better understand any differences in fluid cognition.
Methods
Study design and procedure
This study drew on data from a larger parent project, the Mild Injury Outcomes Study (MIOS), which involved a prospective cohort design with longitudinal follow-up. Participants were 8:0- to 16:11-year-old children with either mild TBI or OI not involving the head who were recruited during ED visits within 24 h post-injury at two large children's hospitals in the midwestern United States: Nationwide Children's Hospital in Columbus, Ohio and Rainbow Babies & Children's Hospital in Cleveland, Ohio. Children with OI were included because they are comparable to children with mild TBI both demographically and in terms of risk factors that predispose to injury; their inclusion also helps to control for the general effects of trauma. Recruitment occurred over a period of 46 months, from March 2014 to December 2017.
The study was conducted in compliance with institutional review board standards for ethical clinical research at each location. Informed parental consent and child assent were obtained in writing before participation. Injury information was collected in the ED at the time of recruitment. At that time, parents also provided retrospective ratings of their child's pre-injury functioning. Children completed computerized neuropsychological testing using the NIHTB-CB at a post-acute assessment between 3 and 18 days post-injury (mean [M] = 10.31 days; standard deviation [SD] = 2.84) and again at follow-up assessments 3 and 6 months post-injury.
Participants
Children with mild TBI were included if they experienced a blunt TBI resulting in at least one of the following: 1) an observed loss of consciousness no longer than 30 min; 2) Glasgow Coma Scale (GCS) score of 13 or 14; or 3) at least two acute symptoms of concussion as documented by ED medical personnel (e.g., post-traumatic amnesia, vomiting, nausea, headache, diplopia, dizziness, disorientation to time, place, or person, or any other indications of mental status changes). Exclusion criteria for the mild TBI group included any GCS score below 13, delayed neurological deterioration, or any medical contraindication to magnetic resonance imaging (MRI). Children were not excluded if they were hospitalized or demonstrated intracranial lesions or skull fractures on acute computed tomography, which was completed only if deemed clinically indicated by ED physicians. Thus, the mild TBI group included injuries often described as complicated (e.g., those with intracranial abnormalities), but excluded injuries that would typically be defined as moderate in severity. However, only 6 children in the mild TBI group demonstrated trauma-related abnormalities on MRI completed at the post-acute visit.27 Thus, the large majority of children in the mild TBI group had what would be diagnosed as a concussion.
Children with OI were eligible if they sustained upper- or lower-extremity factures associated with an Abbreviated Injury Scale (AIS) score of ≤4. The AIS is a widely used scoring system that assesses severity of injuries to specific anatomical regions on a scale of 1–6.28 Children were excluded from the OI group if they displayed any evidence of TBI or symptoms of concussion. Children with mild TBI were eligible to participate if they had a co-occurring OI.
Both groups were subject to multiple exclusion criteria: any injury that occurred >24 h before admission to the ED; neurosurgical or surgical intervention; any associated injury with an AIS score of >4; any associated injury that interfered with neuropsychological testing; hypoxia, hypotension, or shock; ethanol, drug, or sedative medication ingestion involved with the injury; any previous TBI requiring medical treatment; pre-morbid neurological disorder or intellectual disability, per parent report; any injury resulting from child abuse or assault; pre-morbid severe psychiatric disorder requiring hospitalization; and inability to speak proficient English. Children were not excluded for pre-morbid learning difficulties or attention problems. The mild TBI and OI groups did not differ in the proportion of children whose parents reported a history of learning disability or attention-deficit/hyperactivity disorder diagnoses.
A total of 588 (mild TBI = 307; OI = 281) eligible children were approached in the ED about participating in the study. Of those children, 315 (mild TBI = 195; OI = 120) provided consent and completed an initial assessment in the ED. Children who consented did not differ from those who did not consent in sex (χ2(1) = 0.182; p = 0.669) or age (t(581) = 0.119; p = 0.905). A higher proportion of participants consented in the mild TBI group (63.5%) than in the OI group (42.7%; χ2(1) = 25.552; p < 0.001). Additionally, consent rates were higher among Black (60.9%) and multi-racial (76.3%) children than white (45.3%) children (χ2(3) = 21.261; p < 0.001). Those who consented also lived in census tracts with significantly lower median family incomes and a higher percentage of residents living below the poverty line than those who refused (p = 0.019 and p = 0.030 [Mann-Whitney U tests], respectively). Consent was not related to percent of minorities in children's census tracts (p = 0.216).
Among the 315 children who consented, 217 returned for the post-acute assessment (mild TBI = 143; OI = 74), with 18% of those visits completed within 7 days after injury and 93.5% completed within 14 days after injury. Children who were only seen in the ED did not differ from those who returned for the post-acute assessment in sex (χ2(1) = 0.078; p = 0.781) or age (t(313) = −0.245; p = 0.807). More participants in the mild TBI group (73.3%) returned for the post-acute visit than participants in the OI group (61.7%; χ2(1) = 4.718; p = 0.030). Participants who were multi-racial (96.6%) or white (79.8%) were more likely to return for the post-acute visit than those who were Black (54.3%; χ2(3) = 32.579; p < 0.001). Children who returned for the post-acute assessment also resided in census tracts with higher median family incomes and a lower percentage of minorities and residents living below the poverty line than those who were seen only in the ED (p = 0.003, p < 0.001, and p = 0.028 [Mann-Whitney U tests], respectively).
The final sample for this analysis included the 143 children with mild TBI and 74 children with OI who participated in the post-acute assessment. Participants who attended the post-acute visit did not differ significantly from the combined group of children who did not consent to participate in the study or were seen only in the ED in age (t(581) = −0.011; p = 0.991) or sex (χ2(1) = 0.248; p = .618), or on census tract measures of median family income and percentage of minorities and residents living below the poverty line (all p > 0.451, Mann-Whitney U tests). They did differ in race (χ2(3) = 23.594; p < 0.001), largely because multi-racial children were more likely to attend the post-acute visit (73.7%) than white (36.1%) or Black (33.1%) children.
Participant demographic and clinical characteristics at the time of study entry are presented in Table 1. The mild TBI and OI groups did not differ in sex (χ2(1) = 0.053; p = 0.817) or age (t(215) = 0.565; p = 0.573). The mild TBI group had a higher proportion of white participants (52.4%) and lower proportions of multi-racial (9.8%) participants than the OI group (37.8% and 18.9%, respectively; χ2(3) = 8.297; p = 0.040). Additionally, the mild TBI group had higher SES than the OI group (t(215) = 2.794; p = 0.006), as measured by a z-score composite of standardized measures of total years of maternal education, median family income for the family census tract, and occupational status.28 Of the 217 children completing the post-acute visit, 159 completed the 3-month assessment (mild TBI = 102 [71% of those completing the post-acute visit]; OI = 57 [77%]), and 143 completed the 6-month assessment (mild TBI = 97 [68%]; OI = 46 [62%]). The mild TBI and OI groups did not differ in attrition rates at 3 or 6 months. Children who returned for follow-up assessments were similar to those who did not return for follow-up in terms of injury group, sex, race, age at injury, and median household income.
Table 1.
Sample Demographics and Clinical Characteristics at Study Entry
| |
Mild TBI |
OI |
Significance test |
|
|---|---|---|---|---|
| n = 143 | n = 74 | t/χ2 | p value | |
| Age at injury | 0.565 | 0.573 | ||
| M (SD) | 12.56 (2.61) | 12.35 (2.37) | ||
| Range | 8.04–16.95 | 8.17–16.93 | ||
| Sex | 0.053 | 0.817 | ||
| % Male | 66.4 | 64.9 | ||
| Race | 8.297* | 0.040 | ||
| % White | 52.4 | 37.8 | ||
| % Black | 35.0 | 43.2 | ||
| % Multi-racial | 9.8 | 18.9 | ||
| % Other | 2.8 | 0.0 | ||
| Median household | 1.844 | 0.067 | ||
| income1 | ||||
| M (SD) | 63K ± 34K | 54K ± 33K | ||
| Range | 7K–244K | 11K–177K | ||
| Years of maternal education | 2.354* | 0.020 | ||
| M (SD) | 13.97 (2.37) | 13.19 (2.21) | ||
| Range | 9–18 | 9–18 | ||
| Days post-injury baseline follow-up | –1.372 | 0.172 | ||
| M (SD) | 10.12 (2.79) | 10.68 (2.91) | ||
| Range | 3–15 | 4–18 | ||
| 3-month follow-up | –1.440 | 0.152 | ||
| M (SD) | 98.59 (12.66) | 101.58 (12.36) | ||
| Range | 76–132 | 79–131 | ||
| 6-month follow-up | 1.575 | 0.117 | ||
| M (SD) | 191.56 (13.20) | 188.04 (10.70) | ||
| Range | 167–229 | 171–215 | ||
| Injury setting | 0.149 | 0.699 | ||
| % Sport-related | 78.6 | 80.9 | ||
| Injury mechanism | 8.941* | 0.030 | ||
| % Fall | 33.3 | 43.8 | ||
| % Struck object | 26.2 | 24.7 | ||
| % Struck person | 25.5 | 9.6 | ||
| % Other | 14.9 | 21.9 | ||
| Injury Characteristics2b | ||||
| % experienced LOC | 27.1% | |||
| % experienced PTA | 38.8% | — | — | — |
| % GCS <15 | 11.7% | |||
Based on 2010 Census tract.
Mild TBI group only.
Group difference significant, p < 0.05.
M, mean; SD, standard deviation; LOC, loss of consciousness; PTA, post-traumatic amnesia.
Measures
National Institutes of Health Toolbox for Assessment of Neurological and Behavioural Function
The NIHTB-CB consists of seven subtests to assess language, reading, episodic memory, processing speed, working memory, attention, and executive function that provide age-corrected standard scores, as well as Fluid Cognition and Crystallized Cognition composite scores.15 Table 2 provides a list of each subtest and the corresponding cognitive domains and composite.
Table 2.
NIH Toolbox Subtests and Corresponding Cognitive Domains
| NIH Toolbox subtest | Cognitive domain | Composite |
|---|---|---|
| Flanker Inhibitory Control & Attention | Executive Function & Attention | Fluid |
| Dimensional Change Card Sort | Executive Function | Fluid |
| Picture Sequence Memory | Episodic Memory | Fluid |
| List Sorting Working Memory | Working Memory | Fluid |
| Pattern Comparison Processing Speed | Processing Speed | Fluid |
| Oral Reading Recognition | Language | Crystallized |
| Picture Vocabulary | Language | Crystallized |
NIH, National Institutes of Health.
Medical Symptom Validity Test
Children's response validity was measured using the MSVT, which is a computerized, forced-choice test designed to detect invalid performance.29 The MSVT presents 10 word pairs twice on the computer screen. Children are asked to choose the correct word from pairs consisting of a target and foil, both immediately and after a delay. Children are also asked to recall the words during paired-associate and free recall conditions. Children ≥8 years of age are able to perform at very high levels of recognition accuracy, commensurate with adults.29 Performance on the MSVT accounts for substantial variance in cognitive test performance among children with mild TBI.30,31 The traditional MSVT cutoff was used to classify performance as invalid (i.e., a score of ≤85% on all three easy subtests). Of the cases meeting that criteria, some displayed a pattern of substantially worse performance on the difficult subtests than on the easy ones (difference of >20%), suggestive of cognitive impairment or memory deficit rather than performance invalidity.29
Statistical analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Nationwide Children's Hospital.32,33 Linear mixed-model analyses were conducted using SAS Version 9.434 to examine group and time post-injury as predictors of age-adjusted standard scores on the Fluid Cognition and Crystallized Cognition composite scores and on each of the seven subtests of the NIHTB-CB; time post-injury was centered so that the model intercept was at 10 days post-injury. Mixed models are advantageous with longitudinal data because they use all available data for estimation, so that all participants who completed at least one administration of the NIHTB-CB were included in analyses. Before analysis, Little's Missing Completely at Random (MCAR) test was completed using the IBM SPSS35 Missing Value Analysis procedure and was not significant (χ2(54) = 63.357; p = 0.180), indicating that the pattern of missing data in the final sample did not differ significantly from data missing completely at random. SES and sex were included as covariates in analyses. Significant group differences at the intercept or group by time interactions were followed by tests of group differences at each occasion post-injury.
We derived effect sizes (d) using model estimates of group means and pooled standard deviations at each time. Sensitivity analyses were conducted by repeating analyses after excluding children who demonstrated invalid performance on the MSVT. One analysis omitted only those children who failed the traditional MSVT cutoff on any of the three assessments but did not display a profile suggestive of cognitive or memory deficit, and the second omitted all children who failed the traditional MSVT cutoff on any of the three assessments. A total of 10 children were omitted from the first analysis (9 mild TBI, 1 OI), and an additional 9 were omitted from the second analysis (7 mild TBI, 2 OI).
Results
Fluid and crystallized cognition composites
The mild TBI group showed significantly lower Fluid Cognition scores than the OI group at 10 days (F(1,216) = 5.73; p = 0.018), although the magnitude of group differences declined modestly over time (F(1,167) = 3.75; p = 0.055; see Fig. 1). Group differences were significant at the baseline assessment (t(216) = −2.39; p = .018), but not at the 3-month (t(212) = −1.61; p = 0.110) or 6-month (t(191) = −0.56; p = 0.574) assessments. Group differences were small to medium at baseline (d = 0.34) and 3 months (d = 0.27) and small at 6 months (d = 0.10). Children in both groups showed increasing Fluid Cognition scores over time (t(164) = 7.22, p < 0.001 for mild TBI; t(168) = 2.72, p = 0.007 for OI), likely representing practice effects in both groups, as have been reported in normative studies.18
FIG. 1.

Model estimates (M, 95% CI) of performance on the NIH Toolbox Cognitive Battery fluid cognition composite for children with mild TBI and OI over time. CI, confidence interval; M, mean; mTBI, mild traumatic brain injury; OI, orthopedic injuries.
Across the 6 months, the OI group's Fluid Cognition mean score increased by 5.4 points, representing approximately one third of a standard deviation. The mild TBI group's mean score improved by 10 points, or around two thirds of a standard deviation. No significant effects of group (F(1,218) = 0.10; p = 0.758), time (F(1,168) = 2.10; p = 0.150), or their interaction (F(1,168) = 0.02; p = 0.890) were found for Crystallized Cognition (see Fig. 2). SES was significantly and positively related to both the Fluid (F(1,210) = 14.75; p < 0.001) and Crystallized (F(1,216) = 73.75; p < 0.001) composites.
FIG. 2.
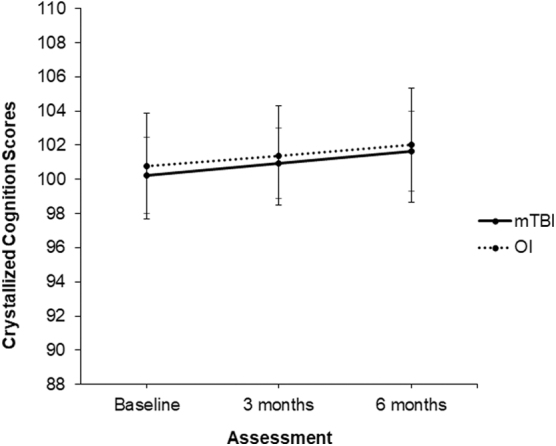
Model estimates (M, 95% CI) of performance on the NIH Toolbox Cognitive Battery crystalized cognition composite for children with mild TBI and OI over time. CI, confidence interval; mTBI, mild traumatic brain injury; OI, orthopedic injuries.
The next analysis excluded participants who displayed invalid MSVT performance at any of the three assessments without indications of memory deficits. The pattern of results was essentially the same. The group intercept at 10 days (F(1,205) = 4.52; p = 0.035) and overall effect of time (F(1,157) = 38.70; p < 0.001) remained significant, and the group × time interaction approached significance (F(1,157) = 3.78; p = 0.054). Group differences were significant at the baseline assessment (t(205) = −2.13; p = 0.035), but not at the 3-month (t(200) = −1.28; p = 0.203) or 6-month (t(181) = −0.26; p = 0.795) assessments. No significant effects of group (F(1,207) = 0.05; p = 0.823), time (F(1,157) = 2.10; p = 0.149), or their interaction (F(1,157) = 0.05; p = 0.820) were found for Crystallized Cognition. SES remained significantly positively related to both the Fluid (F(1,199) = 15.04; p < 0.001) and Crystallized (F(1,206) = 69.94; p < 0.001) composites.
The next analysis omitted all participants who failed the MSVT cutoff, including those who displayed a profile indicative of memory problems. After omitting those children, the overall effect of time remained significant, reflecting general improvement in Fluid Cognition (F(1,151) = 36.16; p < 0.001); however, the group intercept at 10 days (F(1,196) = 3.20; p = 0.075) and the group × time interaction (F(1,151) = 1.96; p = 0.164) were no longer significant. No significant effects of group (F(1,198) = 0.00; p = 0.953), time (F(1,154) = 1.85; p = 0.176), or their interaction (F(1,154) = 0.05; p = 0.818) were found for Crystallized Cognition. SES remained significantly positively related to both the Fluid (F(1,189) = 11.88; p < 0.001) and Crystallized (F(1,196) = 62.36; p < 0.001) composites.
Fluid and crystallized cognition subtests
The mild TBI group showed significantly lower scores than the OI group on individual Fluid Cognition subtests measuring attention and executive function (e.g., cognitive flexibility, inhibitory control), but not on subtests measuring working memory, explicit memory, or processing speed. Specifically, the mild TBI group scored significantly lower than the OI group at the 10-day intercept on both the Dimensional Change Card Sort Test (DCCS; F(1,219) = 4.07; p = 0.045) and the Flanker Inhibitory Control and Attention Test (F(1,221) = 3.94; p = 0.048). The group × time interaction for the DCCS subtest was not significant (F(1,186) = 0.27; p = 0.601). Group differences on the DCCS subtest were significant at the post-acute (t(219) = −2.02; p = 0.045) and 3-month (t(208) = −2.06; p = 0.041) assessments, but not at the 6-month assessment (t(179) = −1.45; p = 0.149).
Group differences were small to medium at baseline (d = 0.29), 3 months (d = 0.34), and 6 months (d = 0.26). The group × time interaction for the Flanker subtest was also not significant (F(1,177) = 2.51; p = 0.115). Group differences on the Flanker subtest were significant at the post-acute assessment (t(221) = −1.99; p = .048), but not at the 3-month (t(218) = −1.42; p = 0.159) or 6-month (t(190) = −0.43; p = 0.666) assessments. Group differences were small to medium at baseline (d = 0.28) and small at 3 months (d = 0.23) and 6 months (d = 0.08). No group effects or group × time interactions were significant on subtests assessing working memory, explicit memory, or processing speed (i.e., Picture Sequence Memory, List Sorting Working Memory, and Pattern Comparison) or crystallized cognition (i.e., Oral Reading Recognition, Picture Vocabulary).
After omitting children who demonstrated invalid MSVT performance without evidence of memory deficits, the group intercept approached significance for the DCCS (F(1,208) = 3.77; p = 0.053) and the Flanker (F(1,210) = 3.78; p = 0.053) subtests. No group differences were observed on other fluid cognition subtests or on those assessing crystallized cognition.
After omitting all children who failed the MSVT cutoff, the group difference at the 10-day intercept was no longer significant for the DCCS (F(1,199) = 1.69; p = 0.195) or the Flanker (F(1,201) = 3.18; p = 0.076) subtests. No group differences were observed on any other subtests.
Accuracy and reaction time
The DCCS and Flanker subtests, which displayed significant group differences, provide separate measures of reaction time and accuracy. We analyzed reaction time using linear mixed-model analyses as before, but with age at injury added as an additional covariate. However, accuracy was very skewed, especially on the Flanker subtest, on which at least 80% of all participants achieved 100% correct at all assessments. We compared group accuracy at each occasion using a non-parametric Mann-Whitney U test for the DCCS and chi-square (100% accuracy vs. <100% accuracy) for the Flanker.
The mild TBI group showed significantly slower reaction time than the OI group on the DCCS at the 10-day intercept (F(1,219) = 10.88; p = 0.001). The group × time interaction for DCCS reaction time was not significant (F(1,184) = 2.57; p = 0.111). Group differences in reaction time on the DCCS were significant at the post-acute (t(219) = 3.30; p = 0.001) and 3-month assessments (t(204) = 2.84; p = 0.005), but not at the 6-month assessment (t(158) = 1.38; p = 0.171). Group differences were medium at baseline (d = 0.41) and 3 months (d = 0.33) and small at 6 months (d = 0.19). Neither group showed any significant change in reaction time across assessments (t(180) = −1.65, p = 0.101 for mild TBI; t(185) = 0.81, p = 0.421 for OI). Age and SES were both significantly negatively associated with reaction time on the DCCS (F(1,206) = 96.50, p < 0.001, and F(1,198) = 9.27, p = 0.003, respectively). The results were unchanged when children with invalid MSVT performance of any sort were excluded.
The group difference at 10 days on reaction time on the Flanker subtest approached significance (F(1,220) = 3.42; p = 0.067), as did the group × time interaction (F(1,194) = 3.41; p = 0.067). Group differences in reaction time on the Flanker approached significance at the post-acute assessment (t(220) = 1.85; p = 0.067), but were not significant at the 3-month (t(217) = 1.13; p = 0.261) or 6-month (t(177) = −0.06; p = .951) assessments. Group differences were small at baseline (d = 0.22), 3 months (d = 0.12), and 6 months (d = 0.01). The mild TBI group showed a significant decline in reaction time across assessments (t(190) = −5.04; p < .001), but the OI group did not (t(195) = −1.26; p = .208). Age and SES were both significantly negatively associated with reaction time on the Flanker (F(1,212) = 94.06, p < 0.001, and F(1,202) = 15.26, p < 0.001, respectively). The results were unchanged when children with invalid MSVT performance of any sort were excluded.
In contrast to the group differences in reaction time, no significant group differences were found for accuracy at any assessment on either the DCCS (Mann-Whitney U test = 4878 at post-acute, 2816 at 3 months, and 2187 at 6 months; all p ≥ 0.425) or Flanker subtest (χ2(1) = 0.165 at post-acute, 0.249 at 3 months, and 1.624 at 6 months; all p ≥ 0.203). Overall, group differences in performance on the DCCS and Flanker subtests appeared to be attributable to reaction time, not accuracy, with the mild TBI group displaying slower responses than the OI group.
Discussion
The current study provides evidence that children with mild TBI demonstrate persistent deficits in cognitive abilities after mild TBI. The study further delineates the nature of these cognitive difficulties, by showing that children with mild TBI demonstrate persistent deficits in fluid cognition, but not in crystallized cognition. Even more specifically, children with mild TBI display persisting deficits on measures of attention and executive function (e.g., cognitive flexibility, inhibitory control), but not on other aspects of fluid cognition (e.g., explicit memory, working memory, or processing speed), and deficits are most apparent for reaction time, and not accuracy of responses.
Tests of fluid cognition rely on the flexible application of cognitive skills on novel tasks, whereas tests of crystalized cognition depend on previous learning and knowledge. TBI has been found to have a greater impact on fluid cognition compared to crystallized cognition.2,17 The DCCS and Flanker subtests of the NIHTB-CB combine demands for both speed and complex executive functions, whereas the other fluid cognition subtests of the NIHTB-CB do not. The other subtests either do not depend on speed (e.g., tests that measure explicit and working memory) or do not require executive functions (e.g., tests that measure processing speed), unlike the DCCS and Flanker, which require both. Because the NIH-TBI is computerized, it provides precise measurement of reaction time and hence may be especially sensitive to deficits elicited by complex cognitive tasks that require rapid responses. Other measures that involve competing demands (i.e., dual task performance) also have been used to objectively monitor concussion recovery. For example, deficits in gait and balance have been found in adolescents with concussion as compared to uninjured controls, and the deficits are most pronounced when persons are required to concurrently engage in complex mental tasks.36–38
Previous systematic reviews and meta-analyses have concluded that few, if any, cognitive deficits exist in children and adolescents after mild TBI, including in attention or executive function.2,7 However, most existing studies have used traditional paper-and-pencil neuropsychological tests, and very few have focused on attention and executive functions using tasks that allow response speed and accuracy to be parsed. The current study is the first to examine cognitive outcomes using the NIHTB-CB, which may be advantageous for detecting cognitive deficits after mild TBI compared to traditional tests. Our results showed that children with mild TBI displayed deficits for as long as 3 months on measures of attention and executive function, largely attributable to slowed reaction time rather than decreased accuracy.
The comparisons of our findings to those of previous systematic reviews and meta-analyses raise the possibility that cognitive deficits after mild TBI are subtler than previously recognized, becoming apparent only on speeded tasks that involve executive function. In other words, the lack of persistent cognitive effects in pediatric mild TBI patients, as reported in previous research, may be attributable to the use of tests that are not sensitive enough to capture these effects, rather than to a true lack of differences. Notably, some children report persistent symptoms after mild TBI (i.e., lasting longer than 4 weeks).39–41 The persistence of cognitive difficulties identified in this study is consistent with the report of persistent symptoms in a subgroup of children after mild TBI.
Importantly, the cognitive deficits identified in this study do not appear to be a function of performance validity. The overall pattern of results remained essentially the same when children who fell below the MSVT cutoff were excluded from analysis, particularly if children who displayed evidence of a potential cognitive or memory deficit were retained despite falling below the cutoff. Notably, many clinical populations show an elevated rate of “failure” on performance validity testing,42 suggesting that falling below cutoffs on performance validity tests may, in some cases, reflect the associated neurological or neuropsychiatric disorder and may not necessarily be attributable to non-credible effort and, in most cases, would certainly not reflect intentional malingering. Further research on performance validity testing in children is undoubtedly needed to insure the appropriate use and interpretation of such testing in both research and clinical practice.43,44
Limitations
The results of this study should be interpreted in light of two key limitations. First, the study recruited participants from EDs. The participants may have had somewhat more severe injuries than children presenting to other healthcare settings or who do not seek medical care after injury, so the findings should only be generalized to children seen in ED settings. Second, only 54% of eligible children consented to participate and attrition over time was significant, particularly across the transition from the ED to the post-acute assessment. Although consent rates were consistent with other longitudinal cohort studies of children with mild TBI,45 and attrition was expected given the study's longitudinal design, these factors may have introduced unmeasured bias into the results. Notably, however, comparisons of children who were and were not included in analyses, as well as analysis of the pattern of missing data, suggest that consent and attrition were unlikely to introduce any significant bias. Moreover, mixed-model analyses also helped reduce any attrition bias.
Conclusion
This study adds to the small research literature examining the utility of the NIHTB-CB in TBI and provides evidence that the NIHTB-CB is sensitive to the cognitive effects of mild TBI in children and can help delineate the nature of those effects. The results were consistent with our hypotheses that children with mild TBI would score lower on fluid cognition subtests compared to children with OI, but that the groups would not differ on subtests assessing crystallized cognition, and that the deficits in fluid cognition would lessen over time given that children with mild TBI recovered from their injuries. Future research should examine the relation of the cognitive deficits identified here to post-concussive symptoms and other outcomes after pediatric mild TBI, particularly headache and sleep problems, to more fully characterize recovery from these common injuries. Future research could also examine the neuroimaging correlates of cognitive deficits in pediatric mild TBI, to better understand their neural substrates. Clinically, the findings suggest that computerized tests should be considered as a more sensitive means of documenting the subtle cognitive effects of mild TBI than traditional paper-and-pencil tests, because of their ability to measure response speed more precisely on complex tasks of executive function.
Acknowledgments
We acknowledge Rainbow Babies & Children's Hospital, University Hospitals Cleveland Medical Center, Nationwide Children's Hospital, and Case Western Reserve University for the assistance provided with recruitment. We acknowledge the contributions of Dana Coleman, Emma Lissemore, Anne Birnbaum, and Taylor Wong from Case Western Reserve University and Rainbow Babies & Children's Hospital, as well as Melissa Ginn and Cindy Lin from Nationwide Children's Hospital. We also acknowledge all of the collaborators on the MIOS project and all of the families who participated in the study.
Authors' Contributions
Leah Chadwick: conceptualization; writing-original draft/editing. Elizabeth Roth: conceptualization; writing-review/editing. Nori M. Minich: formal analyses; writing-review/editing.
H. Gerry Taylor: conceptualization; funding acquisition; investigation; writing-review/editing.
Erin D. Bigler: conceptualization; funding acquisition; investigation; writing-review/editing.
Daniel M. Cohen: conceptualization; funding acquisition; investigation; writing-review/editing.
Ann Bacevice: conceptualization; funding acquisition; investigation. Leslie K. Mihalov: conceptualization; funding acquisition; investigation. Barbara A. Bangert: conceptualization; funding acquisition; investigation. Nicholas A. Zumberge: conceptualization; funding acquisition; investigation. Keith Owen Yeates: conceptualization; supervision; funding acquisition; investigation; writing-review/editing.
Funding Information
This work was supported by the National Institutes of Health grant R01HD076885.
Author Disclosure Statement
Erin D. Bigler reports forensic consultation.
References
- 1.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. (2019). Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2014. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Babikian, T., and Asarnow, R. (2009). Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 23, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy, J.D., Carroll, L.J., Peloso, P.M., Borg, J., von Holst, H., Holm, L., Kraus, J., and Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 36, 28–60 [DOI] [PubMed] [Google Scholar]
- 4.Schneier, A.J., Shields, B.J., Hostetler, S.G., Xiang, H., and Smith, G.A. (2006). Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics 118, 483–492 [DOI] [PubMed] [Google Scholar]
- 5.Fay, G.C., Jaffe, K.M., Polissar, N.L., Liao, S., Rivara, J.B., and Martin, K.M. (1994). Outcome of pediatric traumatic brain injury at three years: a cohort study. Arch. Phys. Med. Rehabil. 75, 733–741 [PubMed] [Google Scholar]
- 6.Lloyd, J., Wilson, M.L., Tenovuo, O., and Saarijarvi, S. (2014). Outcomes from mild and moderate traumatic brain injuries among children and adolescents: a systematic review of studies from 2008–2013. Brain Inj. 29, 539–549 [DOI] [PubMed] [Google Scholar]
- 7.Satz, P. (2001). Mild head injury in children and adolescents. Curr. Dir. Psychol. Sci. 10, 106–109 [Google Scholar]
- 8.Catale, C., Marique, P., Closset, A., and Meulemans, T. (2009). Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. J. Clin. Exp. Neuropsychol. 31, 331–338 [DOI] [PubMed] [Google Scholar]
- 9.Babikian, T., Satz, P., Zaucha, K., Light, R., Lewis, R.S., and Asarnow, R.F. (2011). The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 17, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collie, A., Darby, D., and Maruff, P. (2001). Computerised cognitive assessment of athletes with sports related head injury. Br. J. Sports. Med. 35, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schatz, P., and Zillmer, E.A. (2003). Computer-based assessment of sports-related concussion. Appl. Neuropsychol. 10, 42–47 [DOI] [PubMed] [Google Scholar]
- 12.Stuss, D.T., Stethem, L.L., Hugenholtz, H., Picton, T., Pivik, J., and Richard, M.T. (1989). Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J. Neurol. Neurosurg. Psychiatry 52, 742–748. 10.1136/jnnp.52.6.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleiberg J., Garmoe, W.S., Halpern, E.L., Reeves, D.L., and Nadler, J.D. (1997). Consistency of within-day and across-day performance after mild brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 10, 247–253 [PubMed] [Google Scholar]
- 14.Hugenholtz, H., Stuss, D.T., Stethem, L.L., and Richard, M.T. (1988). How long does it take to recover from a mild concussion? Neurosurgery 22, 853–858 [PubMed] [Google Scholar]
- 15.Weintraub, S., Dikmen, S.S., Heaton, R.K., Tulsky, D.S., Zelazo, P.D., Slotkin, J., Carlozzi, N.E., Bauer, P.J., Wallner-Allen, K., Fox, N., Havlik, R., Beaumont, J.L., Mungas, D., Manly, J.J., Moy, C., Conway, K., Edwards, E., Nowinski, C.J., and Gershon, R. (2014). The Cognition Battery of the NIH Toolbox for assessment of neurological and behavioural function: validation in an adult sample. J. Int. Neuropsychol. Soc. 20, 567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HealthMeasures. (2021). NIH Toolbox. www.healthmeasures.net/explore-measurement-systems/nih-toolbox (Last accessed June2, 2021)
- 17.Tulsky, D.S., Carlozzi, N.E., Holdnack, J., Heaton, R.K., Wong, A., Goldsmith, A., Heinemann, A.W., Wegener, S.T., and Ehde, D.M. (2017). Using the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with traumatic brain injury. Rehabil. Psychol. 62, 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akshoomoff, N., Beaumont, J.L., Bauer, P.J., Dikmen, S.S., Gershon, R.C., Mungas, D., Slotkin, J., Tulsky, D., Weintraub, S., Zelazo, P.D., and Heaton, R.K. (2013). VIII. NIH Toolbox Cognition Battery (CB): Composite scores of crystallized, fluid, and overall cognition. Monogr. Soc. Res. Child Dev. 78, 119–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer, P.J., Dikmen, S.S., Heaton, R.K., Mungas, D. Slotkin, J., and Beaumont, J.L. (2013). III. NIH Toolbox Cognition Battery (CB): measuring episodic memory. Monogr. Soc. Res. Child Dev. 78, 34–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershon, R.C., Slotkin, J., Manly, J.J., Blitz, D.L., Beaumont, J.L., Schnipke, D., Wallner-Allen, K., Golinkoff, R.M., Gleason, J.B., Hirsh-Pasek, K., Adams, M.J., and Weintraub, S. (2013). IV. NIH Toolbox Cognition Battery (CB): measuring language (vocabulary comprehension and reading decoding). Monogr. Soc. Res. Child Dev. 78, 49–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlozzi, N.E., Tulsky, D.S., Kail, R.V., and Beaumont, J.L. (2013). VI. NIH Toolbox Cognition Battery (CB): measuring processing speed. Monogr. Soc. Res. Child Dev. 78, 88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon, R.C., Cook, K.F., Mungas, D., Manly, J.J., Slotkin, J., Beaumont, J.L., and Weintraub, S. (2014). Language measures of the NIH Toolbox Cognition Battery. J. Int. Neuropsychol. Soc. 20, 642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tulsky, D.S., Carlozzi, N.E., Chevalier, N., Espy, K.A., Beaumont, J.L., and Mungas, D. (2013). NIH Toolbox Cognitive Function Battery (CFB): measuring working memory. Monogr. Soc. Res. Child Dev. 78, 70–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelazo, P.D., Andersen, J., Richler, J., Wallner-Allen, K., Beaumont, J., and Weintraub, S. (2013). NIH Toolbox Cognitive Function Battery (CFB): measuring executive function and attention. Monogr. Soc. Res. Child Dev. 78, 16–33 [DOI] [PubMed] [Google Scholar]
- 25.Heaton, R.K., Akshoomoff, N., Tulsky, D., Mungas, D., Weintraub, S., Dikmen, S., Beaumont, J., Casaletto, K.B., Conway, K., Slotkin, J., and Gershon, R. (2014). Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J. Int. Neuropsychol. Soc. 20, 588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson, W., Pedowitz, A., Nowak, S., Neumayer, C., Kaplan, E., and Shah, S. (2020). Feasibility of National Institutes of Health Toolbox Cognition Battery in pediatric brain injury rehabilitation settings. Rehabil. Psychol. 65, 22–30 [DOI] [PubMed] [Google Scholar]
- 27.Mayer, A.R., Cohen, D.M., Wertz, C.J., Dodd, A.B., Shoemaker, J., Pluto, C., Zumberge, N.A., Park, G., Bangert, B.A., Lin, C., Minich, N.M., Bacevice, A.M., Bigler, E.D., Campbell, R.A., Hanlon, F.M., Meier, T.B., Oglesbee, S.J., Phillips, J.P., Pottenger, A., Shaff, N.A., Taylor, H.G., Yeo, R.A., Arbogast, K.B., Leddy, J.J., Master, C.L., Mannix, R., Zemek R.L., and Yeates, K.O. (2020). Radiologic common data elements rates in pediatric mild traumatic brain injury. Neurology 94, e241–e253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Association for Automotive Medicine. (1990). The Abbreviated Injury Scale (AIS): 1990 Revision. American Association for Automotive Medicine: Des Plaines, IL [Google Scholar]
- 29.Green, P. (2004). Green's Medical Symptom Validity Test. Green's: Edmonton, Alberta, Canada [Google Scholar]
- 30.Kirkwood, M.W., and Kirk, J.W. (2010). The base rate of suboptimal effort in a pediatric mild TBI sample: performance on the medical symptom validity test. Clin. Neuropsychol. 24, 860–872 [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood, M.W., Yeates, K.O., Randolph, C., and Kirk, J.W. (2012). The implications of symptom validity test failure for ability-based test performance in a pediatric sample. Psychol. Assess. 24, 36–45 [DOI] [PubMed] [Google Scholar]
- 32.Harris, P.A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J.G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris, P.A., Taylor, R., Minor, B.L., Elliott, V., Fernandez, M., O'Neal, L., McLeod, L., Delacqua, G., Delacqua, F., Kirby, J., and Duda, S.N.; REDCap Consortium. (2019). The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 95, 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SAS Institute Inc. (n.d.). SAS 9.4. SAS Institute Inc.: Cary, NC [Google Scholar]
- 35.IBM Corp. (2019). IBM SPSS Statistics for Windows, Version 26.0. IBM Corp: Armonk, NY [Google Scholar]
- 36.Howell, D.R., Osternig, L.R., and Chou, L.S. (2013). Dual-task effect on gait balance control in adolescents with concussion. Arch. Phys. Med. Rehabil. 94, 1513–1520 [DOI] [PubMed] [Google Scholar]
- 37.Howell, D.R., Osternig, L.R., Koester, M.C., and Chou, L.S. (2014). The effect of cognitive task complexity on gait stability in adolescents following concussion. Exp. Brain. Res. 232, 1773–1782 [DOI] [PubMed] [Google Scholar]
- 38.Lee, H., Sullivan, S.J., and Schneiders, A.G. (2013). The use of the dual-task paradigm in detecting gait performance deficits following a sports-related concussion: a systematic review and meta-analysis. J. Sci. Med. Sport 16, 2–7 [DOI] [PubMed] [Google Scholar]
- 39.Barlow, K.M., Crawford, S., Stevenson, A., Sandhu, S.S., Belanger, F., and Dewey, D. (2010). Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 126, 374–381 [DOI] [PubMed] [Google Scholar]
- 40.Zemek, R., Barrowman, N., Freedman, S.B., Gravel, J., Gagnon, I., McGahern, C., Aglipay, M., Sangha, G., Boutis, K., Beer, D., Craig, W., Burns, E., Farion, K.J., Mikrogianakis, A., Barlow, K.M., Dubrovsky, A.S., Meeuwisse, W., Gioia, G., Meehan, W.P., and Beauchamp, M.H. (2016). Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025 [DOI] [PubMed] [Google Scholar]
- 41.Yeates, K.O., Taylor, H.G., Rusin, J., Bangert, B., Dietrich, A., Nuss, K., Wright, M., Nagin, D.S., and Jones, B.L. (2009). Longitudinal trajectories of postconcussive symptoms in children with traumatic brain injuries and their relationship to acute clinical status. Pediatrics 123, 735–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McWhirter, L., Ritchie, C.W., Stone, J., and Carson, A. (2020). Performance validity test failure in clinical populations—a systematic review. J. Neurol. Neurosurg. Psychiatry 91, 945–952 [DOI] [PubMed] [Google Scholar]
- 43.Bigler, E.D. (2012). Symptom validity testing, effort, and neuropsychological assessment. J. Int. Neuropsychol. Soc. 18, 632–642 [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood, M.W. (2015). A rationale for performance validity testing in child and adolescent assessment, in: Validity Testing in Child and Adolescent Assessment: Evaluating Exaggeration, Feigning, and Noncredible Effort. Kirkwood M.W. (ed). Guilford: New York, pps. 3–21 [Google Scholar]
- 45.Anderson, V., Davis, G.A., Takagi, M., Dunne, K., Clarke, C., Anderson, N., Rausa, V.C., Doyle, M., Parkin, G., Truss, K., Thompson, E., Bressan, S., Hearps, S., and Babl, F.E. (2020). Trajectories and predictors of clinician-determined recovery after child concussion. J. Neurotrauma 37, 1392–1400 [DOI] [PubMed] [Google Scholar]


