Abstract
Introduction
Both smoking and infection adversely impact pregnancy. Previously, our group identified in a rodent model that 6 mg/kg/d nicotine increased the risk of fetal infection at gestation day (GD) 18. Here, we investigate lower nicotine doses.
Methods
Pregnant Sprague-Dawley rats received nicotine infusion at 0, 1, or 3 mg/kg/d (no, low-, and mid-dose nicotine, respectively) from GD 6, with intravenous inoculation with Mycoplasma pulmonis (MP) at 107 CFU (N = 20) or sterile broth (sham) (N = 11) on GD 14. Uterus and fetuses were retrieved on GD 18 for MP culture and histopathologic evaluation of maternal and fetal inflammatory responses (MIR and FIR).
Results
At 1 mg/kg/d nicotine, MP colonization rates were decreased, from 100% (9 of 9) to 40% (2 of 5) of MP-inoculated dams (p = .03), and 59% (66 of 111) to 39% (24 of 62) of fetuses (p = .01), versus no nicotine. Low-dose nicotine resulted in increased MIR and FIR in the sham-inoculated group; in the MP-inoculated group, this resulted in reduced relative risk (RR) for placental colonization (RR, 95% CI with high MIR = 0.14, 0.02 to 0.65; FIR = 0.38, 0.12 to 0.93). In contrast, 3 mg/kg/d nicotine treatment did not alter colonization rates; furthermore, FIR was completely suppressed, even in the face of placental or amniotic fluid colonization.
Conclusion
The 1 mg/kg/d nicotine dose decreased risk of intrauterine infection, with increased MIR and FIR. The 3 mg/kg/d nicotine dose inhibited FIR, and increased risk for intrauterine infection. Nicotine alterations of the intrauterine environment were markedly dose-dependent.
Implications
Nicotine exposure alters intrauterine infection and inflammation in a dose-dependent manner, potentially impacting fetal development and programming. Previous work in a rodent model showed that high-dose nicotine (6 mg/kg/d) exposure exacerbated intrauterine infection during pregnancy. The current study found that low-dose nicotine (1 mg/kg/d) exposure reduced colonization of placenta and amniotic fluid; this decrease was associated with increased intrauterine inflammation. Exposure to mid-dose nicotine (3 mg/kg/d) suppressed fetal inflammation. Elucidation of underlying mechanisms of these phenomena will inform public health and clinical care decisions, particularly in the context of risk assessment of nicotine replacement therapy during pregnancy for smoking cessation.
Introduction
The intrauterine environment during pregnancy influences fetal development and programming,1 altering perinatal and adult postnatal health outcomes, including mental health and chronic illness.2 Further, intrauterine infection and inflammation may trigger preterm birth,3–8 which in turn results in neonatal morbidity and mortality as well as long-term postnatal effects.9–14 Cigarette smoke components, including nicotine, can exacerbate or otherwise modulate such impacts.15–17
In the past, concerns around cigarette smoking related to fetal exposure to carcinogens, toxins, and products of combustion (particulates, carbon monoxide, etc.). The recent rise in use of electronic nicotine delivery systems (ENDS, eg, vape pens and E-cigarettes) by young adults,18 as well as the use of nicotine replacement therapies (eg, nicotine patches or nicotine gum) for smoking cessation during pregnancy necessitates continued investigation of maternal exposure to nicotine and subsequent in utero and postnatal impacts. The proinflammatory response is modulated in part by nicotinic acetylcholine receptors (nAChR)19–21; therefore nicotine exposure during pregnancy potentially alters maternal and fetal responses to microbial invasion as well as the physiologic functions of inflammation in the reproductive tract.
Previously, our group identified that exposure to high-dose nicotine (6 mg/kg/d) during gestation in rodents had a dramatic impact on inflammatory cytokines; histologic lesions; and bacterial colonization in the endometrium, placenta, and fetal tissues; following inoculation with Mycoplasma pulmonis (MP),22 a pathogen of the reproductive tract. This outcome suggests that nicotine may increase the risk for fetal infection. The current study used the same rodent model of nicotine exposure and infection during pregnancy to evaluate impacts of two lower doses of nicotine on intrauterine infection and the inflammatory response to infection. Lower nicotine doses (1 or 3 mg/kg/d) were used for a closer approximation of nicotine exposure in pregnant women using nicotine replacement therapies23–25 or electronic/conventional cigarettes,26 respectively. We hypothesized that lower doses of nicotine would have a similar increase of histologic inflammatory indicators as well as fetal infection risk, but proportionately lower than the previous high-dose nicotine study.
Methods
Animals
All procedures involving animals were performed in accordance with the University of Florida Institutional Animal Care and Use Committee-approved protocols. The University of Florida is an AAALAC-accredited institution, with PHS Policy Assurance with OLAW and USDA registration. Specific pathogen-free (SPF) adult male and primiparous female Sprague Dawley rats (Crl:SD; Charles River Laboratories International, Inc, Wilmington, MA) were used. Animal handling took place within a laminar airflow hood. Animal rooms were maintained on a 12:12 hour light-dark cycle. Rats were initially housed in same-sex pairs in an SPF barrier facility in individually ventilated cages (Microisolator, Lab Products, Inc, Maywood, NJ) and provided ad libitum access to food (LabDiet 5053, Purina Mills International, St Louis, MO) and water. Upon sexual maturity, males were separated and housed singly. For breeding, females were paired with males overnight, and were checked daily for evidence of copulation as determined by either visual observation of a vaginal plug or sperm on vaginal cytology. The morning of plug or sperm detection was considered the beginning of gestation day (GD) 0. Bred females were housed in pairs until surgical implantation of the osmotic pump at GD 6, after which they were housed singly.
Experimental Design and Treatment Groups
After breeding, pregnant dams were randomly assigned to 1 of 6 treatment groups: saline infusion with 0 mg/kg/d nicotine and a sham inoculation of sterile broth injection (Control); 1 mg/kg/d nicotine infusion and sterile broth injection (Low-NIC only); 3 mg/kg/d nicotine infusion and sterile broth injection (Mid-NIC only); saline infusion and 107 colony forming units (CFU) MP inoculation (MP only); 1 mg/kg/d nicotine infusion and 107 CFU MP inoculation (Low-NIC+MP); 3 mg/kg/d nicotine infusion and 107 CFU MP inoculation (Mid-NIC+MP).
Nicotine Dose Selection
The selected doses were estimated to represent levels of both nicotine and the more stable nicotine metabolite, cotinine, of pregnant women smoking less than a pack per day or wearing a nicotine patch (1 mg/kg/d)23–25 or pregnant women smoking more than a pack a day (3 mg/kg/d).26 While data for nicotine and cotinine levels in pregnant women using ENDS are not widely available, we estimated that typical ENDS use by pregnant women would result in comparable levels to those of pregnant cigarette smokers. See the methods in the Supplementary Materials for additional discussion on nicotine dose selection.
Infusion Pump Implantation
At GD 6, pregnant dams were weighed, and anesthesia was induced in a chamber with 3%–4% isoflurane in 100% oxygen; a surgical plane of anesthesia was maintained with 1%–3%, isoflurane in 100% oxygen delivered by facemask. Lidocaine diluted to 0.5% in sterile saline was injected subcutaneously near the surgical site. The surgical site was clipped and antiseptically prepared with povidone-iodine scrub and 70% ethanol. A 28-day osmotic minipump (ALZET Osmotic Pump Model 2ML4, DURECT Corp, Cupertino, CA) was inserted subcutaneously via a small incision between the scapulae. The incision was closed in two layers using absorbable subcutaneous intradermal sutures and nylon skin sutures. The minipump was pre-filled with either sterile saline or nicotine tartrate (Sigma-Aldrich, St. Louis, MO) dissolved in sterile saline at a concentration sufficient to deliver 1 or 3 mg/kg/d based on the projected weight at midpoint of gestation. Insulated heating pads (SnuggleSafe, Lenric C21 Ltd. Littlehampton, West Sussex, United Kingdom) provided supplemental heat during anesthesia and recovery, and warmed subcutaneous fluids were administered. Carprofen 5 mg/kg/d and buprenorphine 0.03–0.05 mg/kg/8h were given subcutaneously for peri- and postoperative analgesia.
MP Preparation and Intravenous Injection
To ensure identical inocula for all experiments, MP X-1048 was grown to late logarithmic phase in Frey’s medium, aliquoted, and frozen at −80°C. The stock culture was thawed, and diluted in vehicle to achieve a concentration of 107 CFU per 0.1 mL for inoculation of each rat. Dose concentration was confirmed by both optical density and culture.
At GD 14, dams were re-anesthetized as described above, and placed in dorsal recumbency to allow access to the right subclavian vein. The site was cleaned with 70% ethanol and an IV injection of 107 CFU MP or sterile broth (vehicle) was delivered slowly over 10 to 15 seconds. This infection dose was selected based on previous experiments.27 Pressure was applied to the injection site for 30–60 seconds following withdrawal of the needle.
Necropsy
Pregnant dams were euthanized at GD 18 by intraperitoneal injection of euthanasia solution (1.5 mL Beuthanasia-D Special, Merck Animal Health, Summit, NJ). Tissues and swabs were collected using aseptic technique. Maternal spleen was minced in Frey’s broth culture medium; vaginal and endometrial mucosae were swabbed with calcium alginate swabs which were then inoculated into broth culture medium for serial dilution and culture. The uterus was opened, and fetal units were isolated and collected. Each fetus and associated membranes, amniotic fluid, cord, placenta, and attached uterus (including decidua, mesometrial triangle, and the uterine wall and endometrium associated with that unit) were treated as individuals (referred to herein as units, fetal units, or maternal-fetal units) for the purposes of sample collection and data analysis. For all units, amniotic fluid was swabbed for inoculation of broth medium, and a portion of the placental peripheral margin was minced in broth medium. Samples from MP only and NIC + MP groups were serially diluted tenfold in Frey’s broth to 1010. Inoculated broths were incubated at 37°C and monitored daily for color change. Half of the units of each litter were randomly selected for fixation in 10% buffered formalin for histological analysis. Fixed tissues were transferred to 70% alcohol after 48 hours of fixation.
Histology Scoring of Intrauterine Sites
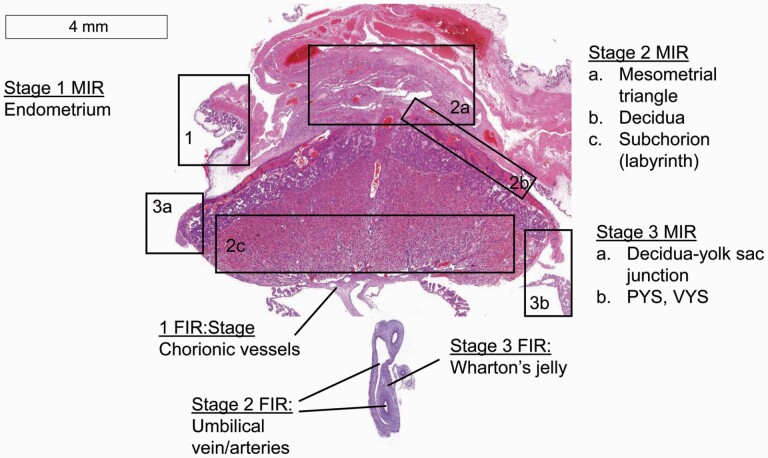
Tissue processing and histologic evaluation is described in detail in the Supplementary Material (Methods, Supplementary Tables S1 and S2 and Figures S1–S7). Briefly, the presence of neutrophil infiltration and of markers of chronic inflammation at certain microanatomical sites were evaluated, and a score was determined for each site for the severity of maternal and fetal inflammatory responses (MIR and FIR, respectively). An overview of the sites evaluated for each stage of MIR and FIR is presented in Figure 1. The scoring methodology is further described in the Supplementary Material. Stage 0 MIR (no inflammation) was defined as the absence of threshold-level lesions at any site. Stage 1 MIR was defined as the presence of lesions in the endometrium (an average score > 0 was the threshold for stage 1). Stage 2 MIR was defined by an average score of > 0.5 among the mesometrial triangle, the decidua, and the labyrinth. Stage 3 MIR was defined as the presence of purulent exudate associated with the decidua-yolk sac junction and/or the luminal aspect of the parietal and visceral membranes of the ruptured yolk sac; a score > 0.5 was the threshold for stage 3. Stage 0 FIR (no inflammation) was defined as the absence of threshold-level lesions at any site. Stage 1 FIR was defined as a chorionic vessel score > 0.75. Stage 2 FIR was defined by an average score > 0 among the umbilical vein and arteries. Stage 3 FIR was defined by a Wharton’s jelly score of 1. The data points analyzed below were the maximum MIR or FIR stage determined for each fetal unit.
Figure 1.
Overview of maternal and fetal inflammatory response (MIR and FIR) stages. The figure depicts the tissues of the maternal-fetal unit including the uterus, placenta, umbilical cord, and fetal membranes. Black boxes (MIR sites) and lines (FIR sites) point to the various sites evaluated for stage of neutrophil infiltration (sites affected) and number of markers of chronicity for scoring purposes. Stage 1 MIR: endometrial epithelium surrounding the attachment site of the placenta (1). Stage 2 MIR: mesometrial triangle (2a) decidua (2b) and/or the labyrinth zone (2c). Stage 3 MIR: decidua-yolk sac junction and/or the luminal surface of the parietal and visceral yolk sac (PYS and VYS) membranes. Stage 1 FIR: chorionic vessels at the level of the chorionic plate. Stage 2 FIR: umbilical vein and/or arteries. Stage 3 FIR: Wharton’s jelly. H&E stain. Photomicrograph captured by Image Scope software viewing an Aperio-scanned slide. Scale bar is 4 mm.
Data Analysis
Litter size data were analyzed with two-way analysis of variance (ANOVA) with nicotine dose and infection as factors. Fetal weight differences among groups were determined by Kruskal-Wallis nonparametric and Dunn’s multiple comparison tests. Differences in the percentage of microbial colonization in maternal and fetal sites were determined by χ 2 and Fisher exact tests, and relative risk (RR) and 95% confidence interval (CI) calculations using the Koopman asymptotic score method. Differences in the microbial load recovered from maternal and fetal sites were determined by Kruskal-Wallis and Dunn tests. MIR and FIR among both noninfected and infected groups were determined by Kruskal-Wallis and Dunn tests. The relative proportion of high-stage and low-stage MIR and FIR among (1) fetuses culture-negative for both placental and amniotic fluid sites (PL-neg/AF-neg); (2) fetuses culture-positive in placenta and culture-negative in amniotic fluid (PL-pos/AF-neg); and (3) fetuses culture-positive in both sites (PL-pos/AF-pos), within each nicotine dose was determined by the χ 2 test. Principle component and factor analyses of histologic site scores are described in detail in the Supplementary Material. JMP version 15.0.0 (SAS Institute, Inc, Cary, NC) and GraphPad Prism version 8.3.0 for Windows (GraphPad Software, San Diego, CA) were used for statistical analyses and graph production.
Results
Litter size was not impacted by nicotine dose or infection (two-way ANOVA, p > .36, Table 1). Nicotine alone did not impact fetal body weights in the Low-NIC only group (Table 1, p = .28); however, a decrease in fetal body weights in the Mid-NIC only group approached, but did not attain, statistical significance (Table 1, p = .08). Infection, alone (MP only, p = .004) or in concert with mid-dose nicotine (Mid-NIC + MP, p < .001), significantly decreased fetal body weight compared to the Control group (Table 1, Kruskal-Wallis and Dunn’s multiple comparison). Fetal body weight was not decreased in the Low-NIC + MP group (Table 1; p = .70). However, the Low-NIC + MP group was somewhat anomalous, due in part to a single litter of very large fetuses (see Supplementary Figure S8, Dam 6G litter), resulting in a wider range of fetal weights (Table 1) in the Low-NIC + MP group. This litter, in which all fetal body weights exceeded two grams, was from a dam with higher microbial load in spleen and uterus compared to other dams in the group (p = .056, approaching significance by Kruskal–Wallis test). The fetuses did not have any distinguishing differences from their counterparts in other litters from the same group with respect to placental and amniotic fluid cultures or maternal and fetal inflammatory responses. Therefore, the litter was included in all analyses. However, if this single litter was excluded from the fetal body weight data, the Low-NIC + MP group, similar to the other MP-treated groups, had significantly lower fetal body weights (p = .006; in grams, median = 1.55; 1st and 3rd quartiles = 1.38 and 1.75) than Controls.
Table 1.
Litter size and fetal weight
| Treatment group | Median litter size (min–max) | N | Median fetal weight (Q1–Q3) | N | p |
|---|---|---|---|---|---|
| Control | 12 (9–14) | 5 | 1.69 (1.55–1.81) | 35 | NA |
| Low-NIC only | 14 (11–14) | 3 | 1.59 (1.50–1.67) | 39 | .28 |
| Mid-NIC only | 11 (10–13) | 3 | 1.56 (1.47–1.67) | 34 | .08 |
| MP only | 12 (10–16) | 9 | 1.56 (1.48–1.64) | 110 | .004 |
| Low-NIC + MP | 13 (11–13) | 5 | 1.69 (1.41–1.85) | 62 | .70 |
| Mid-NIC + MP | 12 (4–14) | 6 | 1.52 (1.44–1.59) | 65 | <.001 |
Litter size and fetal body weights for each treatment group are shown. Litter size is the count of fetuses in the litter at necropsy on gestation day 18. Resorptions (early fetal losses) were not counted. Median fetal weight is the median fetal body weight in grams of fetuses pooled across litters in the same treatment group. A two-way ANOVA of nicotine dose and infection demonstrated that there were no significant contributions of these factors to the variation of litter size (p > .36). The Kruskal-Wallis nonparametric test found significant variation in pooled fetal body weights among treatment groups (p < .001). The Dunn’s test compared each treatment group to the Control group. Values which were significantly different from Controls and their p values appear in bold. Min = minimum of range; max = maximum of range. Q1 = 1st quartile/25th percentile; Q3 = 3rd quartile/75th percentile. Bodyweight data was not available for some fetuses.
Low-Dose Nicotine (1 mg/kg/d) Reduced the Colonization Rate, Colonization Risk, and Microbial Load of MP in Intrauterine Sites
Success of infection in MP groups was confirmed by culture of MP in at least one maternal site. Table 2 presents the MP colonization rate (percentage of culture-positive fetal units for each site) among MP infection treatment groups. The percentage of culture-positive intrauterine sites including endometrium, placenta, and amniotic fluid all were lower in Low-NIC + MP dams compared to MP only dams (p < .03). There were no significant differences in the colonization rate of spleen, vagina, or intrauterine sites in the Mid-NIC + MP treatment group as compared to the MP only group. The RR analyses are reported with 95% CIs in Supplementary Table S3. The Low-NIC + MP group had significantly reduced endometrial, placental, and amniotic fluid colonization risks compared to both MP only (RR = 0.00, 95% CI = 0 to 0.73; RR = 0.74, 95% CI = 0.58 to 0.93; and RR = 0.69, 95% CI = 0.56 to 0.86; for respective sites) and Mid-NIC + MP (RR = 0.25, 95% CI = 0.08 to 0.83; RR = 0.62, 95% CI = 0.42 to 0.89; and RR = 0.41, 95% CI = 0.21 to 0.72; for respective sites).
Table 2.
Culture results for maternal systemic and intrauterine sites
| Site | MP only | Low-NIC + MP | Mid-NIC + MP | p |
|---|---|---|---|---|
| Colonization rate, % culture-positive | ||||
| Spleen | 7 of 9 (78%) | 5 of 5 (100%) | 6 of 6 (100%) | .49a |
| Vagina | 6 of 9 (67%) | 2 of 5 (40%) | 4 of 6 (67%) | .57 |
| Endometrium | 9 of 9 (100%) | ↓ 2 of 5 (40%) | 6 of 6 (100%) | .03 a |
| Placenta | 66 of 111 (59%) | ↓ 24 of 62 (39%) | 40 of 65 (62%) | .01 |
| Amniotic Fluid | 38 of 109 (35%) | ↓ 8 of 62 (13%) | 25 of 63 (40%) | <.01 |
| Microbial load mean ± SD log CCU | ||||
| Spleen | 1.6 ± 1.0 | 2.0 ± 0.7 | 2 ± 1.1 | .71 |
| Vagina | 2.8 ± 1.8 | 3.0 ± 0.0 | 2.8 ± 2.0 | .94 |
| Endometrium | 3.4 ± 2.4 | 2.0 ± 1.4 | 3.1 ± 2.7 | .88 |
| Placenta | 3.8 ± 2.0 | ↓ 2.3 ± 2.0 | 4.2 ± 2.3 | <.01 |
| Amniotic Fluid | 1.7 ± 1.2 | 1.4 ± 1.2 | ↑ 2.6 ± 1.4 | .01 |
Colonization rate (% culture-positive) for maternal systemic sites (spleen, vagina) and intrauterine sites (endometrium, placenta, and amniotic fluids). Arrows and bold text indicate a significant difference as compared to at least one other group. P values for the χ 2 test are reported; where conditions for the χ 2 test were not met, a Fisher’s exact test was performed between each group (lowest p values are reported and indicated by superscripted letter a). For spleen colonization rate, MP only vs. Low-NIC + MP p = .50; MP only vs. Mid-NIC + MP p = .49; Low-NIC + MP vs. Mid-NIC + MP p > .999. For endometrium colonization rate, MP only vs. Low-NIC + MP p = .03; MP only vs. Mid-NIC + MP p > .999; Low-NIC + MP vs. Mid-NIC + MP p = .06. Microbial load (mean ± standard deviation [SD] log color changing units [CCU]) for culture-positive fetal units for each site. P values in the table were derived from Kruskal-Wallis test. Where p < .05, the Dunn’s multiple comparison test was performed between all groups and p values are as follows. For placental microbial load, MP only vs. Low-NIC + MP p = .01; MP only vs. Mid-NIC + MP p > .999; Low-NIC + MP vs. Mid-NIC + MP p < .01. For amniotic fluid microbial load, MP only vs. Low-NIC + MP p > .999; MP only vs. Mid-NIC + MP p = .03; Low-NIC + MP vs. Mid-NIC + MP p = .08). Sample sizes are indicated by the numerator value in the percentage culture-positive portion of the table.
Table 2 also provides the mean and standard deviation (SD) of microbial load (log color-changing units, [CCU]) of sites among culture-positive fetal units. There was a marked effect of nicotine dose on the microbial load of culture-positive fetal units. Specifically, low-dose nicotine significantly decreased the mean microbial load in the placenta (p < .01); microbial load in the amniotic fluid was not impacted by this dose (p ≥ .08). In contrast, microbial load in the amniotic fluid was significantly increased in the Mid-NIC + MP group (p = .03).
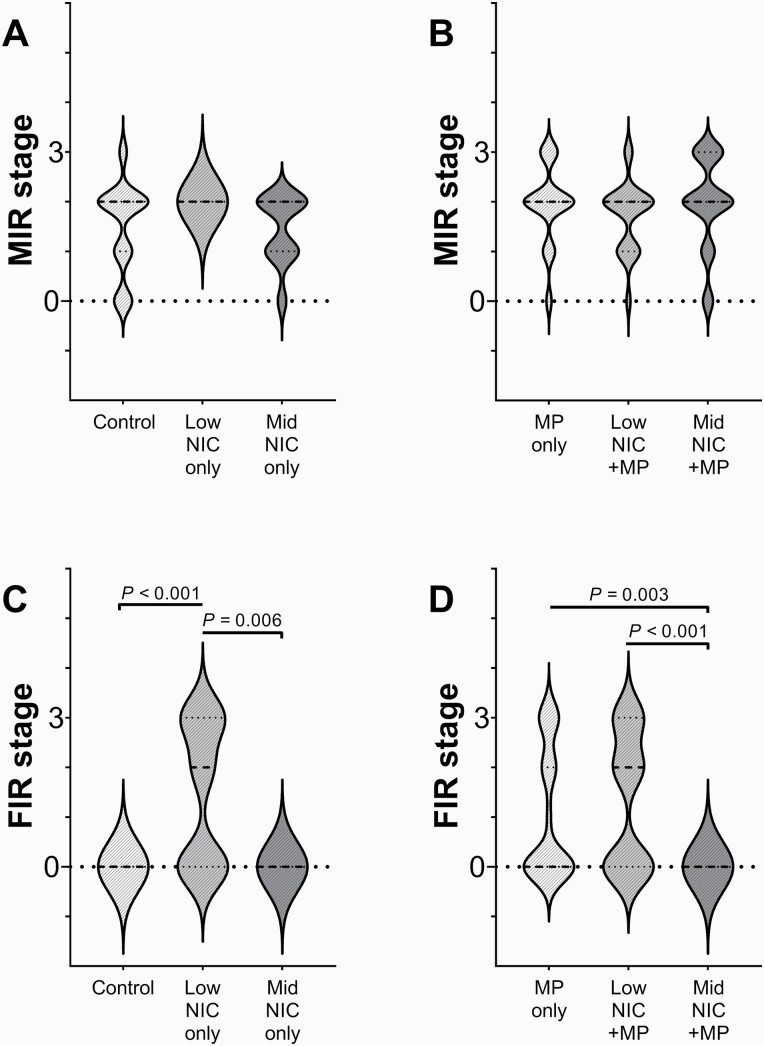
Impact of Nicotine Dose and Infection on Maternal and Fetal Inflammation
The maternal and fetal inflammatory response stages (MIR and FIR, respectively) were defined as none (stage 0), mild (stage 1), moderate (stage 2), and severe (stage 3). Stages are shown for Control, Low-NIC only, Mid-NIC only (Figure 2A and C); and for MP only, Low-NIC + MP, and Mid-NIC + MP (Figure 2B and D) treatment groups. The median MIR stage was not significantly different among nicotine doses in the sham-inoculated (p = .074) nor MP-infected groups (p = .33, Figure 2A and B). However, it is notable that the distribution of MIR scores in the Low-NIC only group are clustered at stage 2 MIR (Figure 2A), without earlier stages represented. Unlike the MIR response, the median FIR stage (Figure 2C and D) was significantly impacted by both nicotine dose and infection. Among sham-inoculated groups (Figure 2C), a subset of fetuses in the Low-NIC only group showed moderate to severe FIR, with median stage significantly higher than either the Control (p < .001) or Mid-NIC only (p = .006) groups, in which no fetal units had a fetal inflammatory response. When animals were infected with MP (Figure 2D), no fetal units in the Mid-NIC + MP group developed moderate to severe FIR, which was significantly different from the FIR response in both the MP only (p < .003) and Low-NIC+MP (p < .001) groups.
Figure 2.
Maternal and fetal inflammatory responses (MIR: panels A and B; and FIR: panels C and D, respectively) for sham inoculated (A, C) and infected (B, D) treatment groups, respectively). The medians and quartiles are denoted by thick and thin dashed lines, respectively. Outliers were identified by the ROUT method (Q = 1%) and eliminated prior to analyses. The Kruskal-Wallis and Dunn’s tests were performed and significant differences and p values are noted. The number of maternal-fetal samples included in the MIR analysis were Control: n = 26; Low NIC only: n = 13 with 3 outliers removed; Mid NIC only: n = 11; MP only: n = 49; Low NIC + MP: n = 22; Mid NIC + MP: n = 31. The number of maternal-fetal samples included in the FIR analysis were Control: n = 18 with 8 outliers removed; Low NIC only: n = 13 with 3 outliers removed; Mid NIC only: n = 8 with 3 outliers removed; MP only: n = 49; Low NIC + MP: n = 22; Mid NIC + MP: n = 21 with 9 outliers removed.
MIR and FIR data were divided by high (moderate to severe/stage 2–3), and low (none to mild/stage 0–1) fetal units. The RR analyses with 95% confidence intervals (CI) for high MIR and FIR are provided in Supplementary Table S4. Among the sham-inoculated groups, risks for high MIR and FIR were increased in the Low-NIC only group relative to Control (MIR RR = 1.81, 95% CI = 1.24 to 2.40; FIR RR ≥ 2.00, 95% CI = 2.00 to infinity) and Mid-NIC only (MIR RR ≥ 1.50, 95% CI = 1.50 to infinity; FIR RR = 2.14, 95% CI = 1.33 to 4.03). Among the MP-infected groups, the risk for high FIR was decreased in the Mid-NIC + MP group fetal relative to both MP only (RR = 0.59, 95% CI = 0.45 to 0.73) and Low-NIC + MP (RR = 0.32, 95% CI = 0.25 to 0.75).
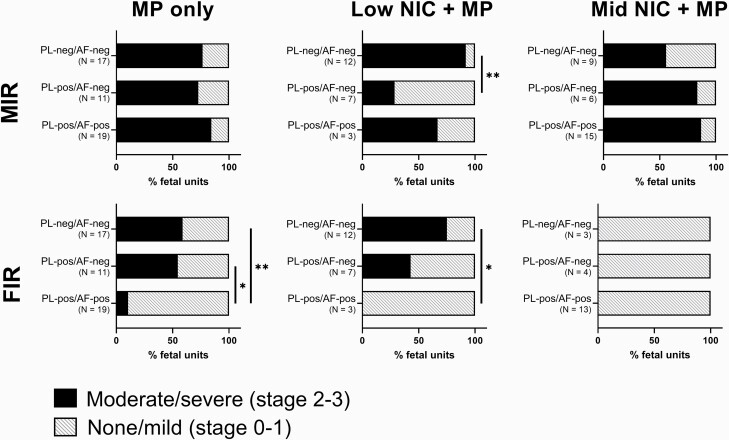
Impact of Nicotine Dose and Maternal and Fetal Inflammation on MP Colonization of Placenta and Amniotic Fluid
Figure 3 compares maternal and fetal inflammatory response severity among treatment groups and colonization subgroups as defined by presence of MP in placenta and amniotic fluid. In the MP only as well as in the Mid-NIC + MP groups, moderate to severe MIR was observed in >50% of all fetal units from the infected animals, regardless of placental infection status. However, in the Low-NIC + MP group, placentas with moderate to severe MIR were more likely (p < .01) to have culture-negative placentas. A strikingly different pattern among treatment groups was observed for FIR. In the Mid-NIC + MP group, no fetal units showed any evidence of FIR regardless of colonization of the placental and amniotic fluid. In both the MP only and Low-NIC + MP groups, a lower FIR (p < .01) was found in fetal units where both the placenta and amniotic fluid were colonized by MP. The treatment groups also differed with respect to overall placental colonization (Table 2), with MP isolated from the placenta of 85% of the Mid-NIC + MP group, 64% of the MP only group, and only 45% of the Low-NIC + MP group.
Figure 3.
High vs. low maternal and fetal inflammatory responses (MIR and FIR) observed with progressive Mycoplasma pulmonis (MP) colonization of fetal sites. MIR and FIR data are represented as percent of fetal units with moderate to severe response (“high,” stage 2-3, darker fill), or none to mild stage response (“low,” stage 0–1, lighter fill). Colonization is presumed to progress from maternal to placental to amniotic fluid sites. Fetal units within each nicotine dose group were divided into subgroups based on MP culture result of placental (PL) and amniotic fluid (AF) sites, either MP culture-positive (-pos) or MP culture-negative (-neg). The N indicates the number of fetuses having a given colonization profile that also had a tissue sample available for MIR and FIR staging. The χ2 analysis was not valid for differences among subgroups due to some having few numbers in each high vs. low or colonization category. The Fisher exact test was used to compare the high vs. low stage of inflammatory response between two subgroups within each nicotine dose. The asterisks and bars represent significant differences (α = 0.05; one * for p-values < .05, and two ** for p < .01.
RR analyses and χ2p values are provided in Supplementary Table S5. The risk of placental infection was decreased in Low-NIC+MP fetal units with high MIR (RR = 0.14, 95% CI = 0.02 to 0.69). The risk for placental infection with high FIR was decreased in both MP only (RR = 0.56, 95% CI = 0.29 to 0.95) and Low-NIC+MP (RR = 0.38, 95% CI = 0.12 to 0.93) groups. Although not statistically significant, there appeared to be a trend towards increased risk of placental colonization with high MIR in the Mid-NIC + MP group (RR = 3.11, 95% CI = 0.90 to 10.43). The risk of amniotic fluid infection among placenta-positive fetal units with high FIR was decreased in the MP only group (RR = 0.51, 95% CI = 0.24 to 0.86).
Discussion
Here we report a previously unrecognized effect of low-dose (1 mg/kg/d) nicotine: protection against intrauterine colonization by MP during pregnancy at sites including endometrium, placenta, and amniotic fluid. Further, maternal and fetal inflammatory responses (MIR and FIR, respectively) were altered by low-dose nicotine. In our previous study,22 we used a higher dose of nicotine (6 mg/kg/d), which resulted in increased infection accompanied by severe inflammation at GD 18. This inflammation, characterized by a copious mucopurulent exudate, was not observed with the lower doses of nicotine used in the present study. In the previous study, the exacerbated maternal inflammatory response likely contributed to necrosis and placental barrier tissue damage, facilitating microbial invasion into the fetal compartment. In the current study, we expected to see incremental increases in infection in a dose responsive manner. While there was no difference in colonization rates between the MP only and Mid-NIC + MP groups, there was a dramatic and unexpected decrease in both maternal and fetal colonization in the Low-NIC + MP group (Table 2). Notably, all fetal units in the low-dose nicotine group had increased MIR as well as decreased uterine colonization, suggesting that these animals may have been better prepared in general to prevent colonization from an infectious agent. Increased fetal inflammation was not observed in any Control nor mid-dose nicotine fetal units, but an increased FIR was present in a subset of fetal units from the low dose nicotine group. Thus, dams and fetal units receiving low dose nicotine appeared to have higher MIR and FIR, even in the absence of an infectious stimulus, than did other treatment groups.
In the face of MP infection, the overall maternal response pattern was similar with all groups, regardless of nicotine dose. However, the FIR response was quite different. Both the MP only and Low-NIC + MP groups had a subset of fetal units with elevated FIR. In marked contrast, none of the fetal units in the Mid-NIC + MP group had a fetal response. The ability of MP to breach the placental barrier and colonize the amniotic fluid was different across treatment groups (Table 2), and virtually all fetal units where this had occurred were characterized by no to mild FIR. Strikingly, the Mid-NIC + MP group did not show any FIR, even in the presence of colonization of 65% of the placentas and a seemingly appropriate MIR response. This suggests that in the Mid-NIC + MP group, the activation of the MIR response was either not adequate to direct the activation of FIR or the fetal tissues were not responsive.
Our findings are consistent with recent studies of transcriptomic changes in innate immune cell types in women with maternal and/or fetal inflammatory responses.28,29 Maternal and fetal innate immune cells in the fetal membranes and amniotic fluid appear to drive pro-inflammatory responses in both placental and fetal tissues, and these responses are further layered with complexity if infection is present in the fetal compartment.28,29 Within that framework, we suggest that the response to nicotine is not a simple dose continuum as we had originally believed. In the absence of nicotine, no fetal inflammation is present, yet maternal cells can respond effectively to an infectious challenge and direct an appropriate fetal response; the collective response can control or limit the ability of the pathogen to colonize the fetal compartment. At the low dose of nicotine, both the maternal and a subset of individual fetal responses are elevated before the infectious insult, thus permitting a more rapid response and more effective clearance of the pathogen. At the mid-dose of nicotine, however, the maternal response does not appear to drive the fetal response, and the fetal response is not activated even in the face of colonization of the fetal compartment. Because the pattern of maternal inflammatory response coupled with the colonization rate of the placentas is similar between MP only and Mid-NIC + MP dams, we suggest that the failure to activate a fetal response may be due to an effect of nicotine at the level of the fetal compartment, though future work will be needed to explore this hypothesis. At the high dose of nicotine,22 the maternal response is so profound that the tissue damage to the placental barrier facilitates the ability of the pathogen to breach the barrier and gain access to the amniotic fluid and fetus.
There are some caveats with our current study. Unfortunately, a standard histologic scoring system has not been established for rodent maternal and fetal inflammatory responses. Because of the extensive tissue damage in the high-dose nicotine study, our approach in the current study differed slightly with respect to scales and cutoffs used to define severity. Our histologic scoring system also differs from the well-established human criteria in order to accommodate the anatomic differences in rodents, including the prominent mesometrial triangle and the apposition of the endometrium to the yolk sac membranes.30 In our experience, the assessment of intrauterine inflammation at the multiple distinct sites included in our analyses was necessary to fully characterize the host responses.
The present study also differed from other animal studies of nicotine and infection or inflammation. In pregnant rat models, 1 mg/kg/d nicotine administered acutely within 48 h prior to, or 72 h after, lipopolysaccharide (LPS) toxin exposure reduced proinflammatory cytokines in placental tissue31,32 and plasma33; decreased leukocyte infiltration in placental tissue31; and restored normal litter size and fetal weight.33,34 Our model deviates from the acute nicotine LPS model described above in two major aspects. First, we used chronic and continuous administration of nicotine beginning 8 days prior to insult. Second, our use of a live microbe may have resulted in different immune modulatory effects than that of the toll-like receptor (TLR)-4-specific binding in the LPS model. For example, Ureaplasma spp. surface lipoproteins and whole bacterium are detected by binding of TLR-2/6 and 9, respectively35; additionally, recognition of various Mycoplasma spp. relies on TLR-2 in combination with either TLRs 1 or 6.36 This creates an additional layer of complexity, as chronic nicotine exposure may differentially alter receptor expression or functionality.37
Our model takes advantage of the similar reproductive effects of the human reproductive pathogen Ureaplasma parvum and the rat reproductive pathogen MP. Because the rat is the natural host to MP, we believe the tropism, colonization, and disease pathogenesis is more organic to the human host-pathogen relationship which we are modeling. While experimental infections using human pathogens are valuable, a rodent-adapted human pathogen may not capture the full host-pathogen relationship. Routes of infection, including both intravaginal and intravenous inoculation, have been defined for this model previously.27,38–41 Since the microbial load in maternal and fetal tissues was a critical outcome to permit assessment of treatment effects, it was imperative to ensure that each dam in each treatment group received the same infectious dose at the same gestational time. In order to establish consistent placental/fetal infections by the vaginal route, dams have to be infected 10 days prior to breeding.39 Therefore, using intravaginal inoculation to model an ascending route of infection would not allow us to know the exact timing of exposure to MP or infectious dose to which the placenta and fetal unit are exposed. Similarly, using direct instillation of MP into the amniotic fluid would bypass the placental barrier and potentially abrogate our ability to assess the impacts on maternal sites. Based on our previous studies,22,27,38–40 intravenous administration of MP at GD 14 ensures that all dams and fetal units have an equal chance of becoming infected. The integrity of the cervical barrier is a key factor in limiting ascending infections in mouse models,42,43 and a caveat to our approach is that it does not allow us to assess the impact of nicotine on the cervix. However, once the cervical barrier is breached, the uterus must be colonized and the placental barrier also must be breached. Our model was designed to permit us to assess the impact of nicotine on colonization of both the uterus and the placental, but not the cervical, barrier.
In summary, we demonstrated an unexpected protective effect of low-dose nicotine on intrauterine infection. At higher nicotine doses, the ability to generate a strong fetal inflammatory response was impacted, which may in turn impact pregnancy outcome, fetal and neonatal development and programming, and adult postnatal outcomes. While we believe studies of animal models and their natural pathogens are essential to discovery, we also recognize animal models of human pathogen infection, in vitro study of human tissues and primary cells, clinical case studies, and epidemiologic analyses are necessary to put our findings in context. The low-dose nicotine (1 mg/kg/d dose) used in the present study was chosen to model clinically relevant exposure levels comparable to nicotine replacement therapies. While the safety and efficacy of nicotine replacement therapy used in smoking cessation for pregnant women is still an issue of debate, a recent review suggests evidence is accumulating which would argue for a more favorable cost-benefit ratio.44 At this time, the current recommendations from the American College of Obstetricians and Gynecologists include conditional use of nicotine replacement therapy to aid in smoking cessation in pregnant women.45 The results of our study suggest that the implications of intrauterine infections and inflammation should be considered when studying nicotine replacement therapy use during pregnancy. Continued research on the mechanisms by which nicotine alters the response to infection as well as the in utero and postnatal impacts of nicotine exposure is vital for navigating the growing epidemic of E-cigarette use among youth and for making informed decisions regarding nicotine replacement therapy use for smoking cessation during pregnancy.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We would like to thank Alexandra Burne, Mariah Watson, and Marissa Valentine-King for their invaluable help with the high volumes of culture media production and necropsy processing.
Funding
This work was supported by the Florida Department of Health James and Esther King Biomedical Research Foundation (to LFH and MBB), grant number 4KB11.
Declaration of Interests
None declared.
References
- 1.Sedaghat K, Zahediasl S, Ghasemi A. Intrauterine programming. Iran J Basic Med Sci. 2015;18(3):212–220. [PMC free article] [PubMed] [Google Scholar]
- 2.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96(4):1509–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Lopez N, Romero R, Xu Y, et al. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci. 2017;24(10):1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. [DOI] [PubMed] [Google Scholar]
- 7.Helmo FR, Alves EAR, Moreira RAA, et al. Intrauterine infection, immune system and premature birth. J Matern Fetal Neonatal Med. 2018;31(9):1227–1233. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter FA, Msall ME. Health disparities and child development after prematurity. Pediatr Ann. 2017;46(10):e360–e364. [DOI] [PubMed] [Google Scholar]
- 10.Kajantie E, Strang-Karlsson S, Evensen KAI, Haaramo P. Adult outcomes of being born late preterm or early term - What do we know? Semin Fetal Neonatal Med. 2019;24(1):66–83. [DOI] [PubMed] [Google Scholar]
- 11.Bilgin A, Mendonca M, Wolke D. Preterm birth/low birth weight and markers reflective of wealth in adulthood: a meta-analysis. Pediatrics. 2018;142(1):e20173625. [DOI] [PubMed] [Google Scholar]
- 12.Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendonça M, Bilgin A, Wolke D. Association of preterm birth and low birth weight with romantic partnership, sexual intercourse, and parenthood in adulthood: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(7):e196961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol. 2016;33(3):318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Liu F, Yu L, et al. nAChRs-ERK1/2-Egr-1 signaling participates in the developmental toxicity of nicotine by epigenetically down-regulating placental 11β-HSD2. Toxicol Appl Pharmacol. 2018;344(April):1–12. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Wu L, Wang Y, et al. Nicotine promotes vascular endothelial growth factor secretion by human trophoblast cells under hypoxic conditions and improves the proliferation and tube formation capacity of human umbilical endothelial cells. Reprod Biomed Online. 2017;34(4):406–413. [DOI] [PubMed] [Google Scholar]
- 17.Stone WL, Bailey B, Khraisha N. The pathophysiology of smoking during pregnancy: a systems biology approach. Front Biosci (Elite Ed). 2014;6:318–328. [DOI] [PubMed] [Google Scholar]
- 18.Fadus MC, Smith TT, Squeglia LM. The rise of e-cigarettes, pod mod devices, and JUUL among youth: factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019;201(August):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii T, Mashimo M, Moriwaki Y, et al. Expression and function of the cholinergic system in immune cells. Front Immunol. 2017;8:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii T, Mashimo M, Moriwaki Y, et al. Physiological functions of the cholinergic system in immune cells. J Pharmacol Sci. 2017;134(1):1–21. [DOI] [PubMed] [Google Scholar]
- 21.Patel H, McIntire J, Ryan S, Dunah A, Loring R. Anti-inflammatory effects of astroglial alpha 7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-kappa B pathway and activation of the Nrf2 pathway. J Neuroinflamm. 2017;14(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Chamier M, Reyes L, Hayward LF, Brown MB. Impact of gestational nicotine exposure on intrauterine and fetal infection in a rodent model. Biol Reprod. 2017;96(5):1071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fewell JE, Smith FG, Ng VK. Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J Appl Physiol (1985). 2001;90(5):1968–1976. [DOI] [PubMed] [Google Scholar]
- 24.Fung YK, Lau YS. Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacol Biochem Behav. 1989;33(1):1–6. [DOI] [PubMed] [Google Scholar]
- 25.Polli FS, Kohlmeier KA. Prenatal nicotine exposure in rodents: why are there so many variations in behavioral outcomes? Nicotine Tob Res. 2020;22(10):1694–1710. [DOI] [PubMed] [Google Scholar]
- 26.Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47(2):331–337. [DOI] [PubMed] [Google Scholar]
- 27.Riggs MA, Maunsell FP, Reyes L, Brown MB. Hematogenous infection of Sprague-Dawley rats with Mycoplasma pulmonis: development of a model for maternal and fetal infection. Am J Obstet Gynecol. 2008;198(3):318.e1–318.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Lopez N, Romero R, Varrey A, et al. RNA sequencing reveals diverse functions of amniotic fluid neutrophils and monocytes/macrophages in intra-amniotic infection. J Innate Immun. 2021;13(2):63–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motomura K, Romero R, Galaz J, et al. RNA sequencing reveals distinct immune responses in the chorioamniotic membranes of women with preterm labor and microbial or sterile intra-amniotic inflammation. Infect Immun. 2021;89(5):e00819–e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter AM, Enders AC. Placentation in mammals: definitive placenta, yolk sac, and paraplacenta. Theriogenology. 2016;86(1):278–287. [DOI] [PubMed] [Google Scholar]
- 31.Bao J, Liu Y, Yang J, et al. Nicotine inhibits LPS-induced cytokine production and leukocyte infiltration in rat placenta. Placenta. 2016;39(March):77–83. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Yang J, Bao J, et al. Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta. 2017;49(December):23–32. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Shi SQ, Shi L, Fang D, Liu H, Garfield RE. Nicotine, an α7 nAChR agonist, reduces lipopolysaccharide-induced inflammatory responses and protects fetuses in pregnant rats. Am J Obstet Gynecol. 2014;211(5):538.e1–538.e7. [DOI] [PubMed] [Google Scholar]
- 34.Bao J, Zou Y, Liu Y, Yuan L, Garfield RE, Liu H. Nicotine protects fetus against LPS-induced fetal growth restriction through ameliorating placental inflammation and vascular development in late pregnancy in rats. Biosci Rep. 2019;39(7):BSR20190386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triantafilou M, De Glanville B, Aboklaish AF, Spiller OB, Kotecha S, Triantafilou K. Synergic activation of toll-like receptor (TLR) 2/6 and 9 in response to Ureaplasma parvum & urealyticum in human amniotic epithelial cells. PLoS One. 2013;8(4):e61199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo LL, Wu YM, You XX. Mycoplasma lipoproteins and toll-like receptors. J Zhejiang Univ Sci B. 2009;10(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julian MW, Shao G, Schlesinger LS, et al. Nicotine treatment improves toll-like receptor 2 and toll-like receptor 9 responsiveness in active pulmonary sarcoidosis. Chest. 2013;143(2):461–470. [DOI] [PubMed] [Google Scholar]
- 38.Brown MB, Peltier M, Hillier M, Crenshaw B, Reyes L. Genital mycoplasmosis in rats: a model for intrauterine infection. Am J Reprod Immunol. 2001;46(3):232–241. [DOI] [PubMed] [Google Scholar]
- 39.Brown MB, Steiner DA. Experimental genital mycoplasmosis: time of infection influences pregnancy outcome. Infect Immun. 1996;64(6):2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltier MR, Richey LJ, Brown MB. Placental lesions caused by experimental infection of Sprague-Dawley rats with Mycoplasma pulmonis. Am J Reprod Immunol. 2003;50(3):254–262. [DOI] [PubMed] [Google Scholar]
- 41.Reyes L, Shelton M, Riggs M, Brown MB. Rat strains differ in susceptibility to maternal and fetal infection with Mycoplasma pulmonis. Am J Reprod Immunol. 2004;51(3):211–219. [DOI] [PubMed] [Google Scholar]
- 42.Pavlidis I, Spiller OB, Sammut Demarco G, et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun. 2020;11(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Racicot K, Cardenas I, Wünsche V, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191(2):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diamanti A, Papadakis S, Schoretsaniti S, et al. Smoking cessation in pregnancy: an update for maternity care practitioners. Tob Induc Dis. 2019;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobacco and nicotine cessation during pregnancy: ACOG committee opinion, number 807. Obstet Gynecol. 2020;135(5):e221–e229. https://pubmed.ncbi.nlm.nih.gov/32332417/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.