Abstract
Introduction
Theory and data suggest that attentional bias (AB) to drug-related cues should be associated with craving when smoking motivation is high, and that AB should be predictive of drug use when immediate use is possible. The current study is the first to test these propositions in smokers in a controlled laboratory environment.
Aims and Methods
Ninety daily smokers were randomly assigned to a high smoking motivation (nicotine-deprived and/or smoking cue exposure) or low smoking motivation (non-deprived and/or control cue exposure) condition. Participants engaged in an AB task in which they viewed smoking and matched control pictures while their eye movements were continuously monitored. Participants were then given the option to smoke, and latency to first puff and number of puffs were coded.
Results
High motivation smokers had significantly higher urges to smoke (p < .001) and shorter latencies to smoke (p = .001) than low motivation smokers, but AB measures (ie, dwell time and initial fixation bias scores) and number of puffs did not differ across groups (ps > .45). As predicted, the association between dwell time bias scores and urge to smoke was stronger in the high (r = .47) than low (r = .18) smoking motivation condition, but this difference failed to reach significance (p = .068). Contrary to predictions, neither AB measure was significantly associated with smoking behavior (SB). Internal reliability was excellent for dwell time bias scores (alpha = .90) but very low for initial fixation bias scores (alpha = .20).
Conclusions
Maintenance of attention on drug-related cues may be a valid index of incentive motivation. Importantly, however, these dwell time bias scores were not predictive of actual SB.
Implications
This study tested key predictions made by theoretical accounts of addiction that emphasize AB to drug-related cues as fundamental components of the development and maintenance of drug use. Namely, this is the first experimental study in smokers to test whether AB to smoking-related cues is associated with craving when smoking motivation is high and whether AB predicts SB assessed immediately after the AB task. As predicted, the association between AB and craving was stronger in smokers randomly assigned to a high rather than a low smoking motivation condition. Contrary to predictions, AB did not predict SB.
Introduction
Smoking-related cues can capture and/or hold smokers’ attention, a phenomenon referred to as attentional bias (AB).1–3 Several theories posit that AB to drug-related cues are fundamental to the development and maintenance of drug use, including smoking behavior (SB) (eg, 4–6). For instance, according to Incentive-Sensitization theory, drug-related cues acquire strong motivational properties through classical conditioning and, as a consequence, such cues “grab attention, become attractive and wanted, and thus guide behavior to the incentive.” 6 Accordingly, the magnitude of AB to smoking-related cues should track closely with smoking craving,7 as both are thought to arise from common underlying appetitive motivational processes.4–9 Further, a causal reciprocal relationship between subjective craving and AB has been proposed,2,6,9 such that an increase in craving increases the attention that is paid to substance cues and vice versa (see 10,11 for experimental studies demonstrating this).
Much research has been conducted to test whether AB reflects an underlying appetitive motivational process and is therefore strongly positively correlated with self-reported craving, and whether individual differences in AB predict future drug use behavior (see 4). A meta-analysis conducted on 68 independent samples indicated a significant association (r = .19) between AB and craving measured in laboratory settings.3 Importantly, the strength of the association was moderated by motivational strength during the AB measurement, with it being significantly stronger when reported craving levels were high (r = .23) versus low (r = .08). Finally, the association was significantly stronger when direct measures of AB (ie, eye-tracking) were used (r = .36), rather than indirect measures (eg, secondary response time) (r = .18).3 Together, findings indicate that AB may become more clinically meaningful during high craving states, and that eye-tracking methods provide a less ambiguous indicator of AB.2,4,12
Several reviews on the potential clinical significance of AB to substance-related cues have been conducted.13–16 These reviews focus on examining whether AB prospectively predicts relapse to substance use and on the effectiveness of AB modification training in preventing relapse. The review conclusions vary13–16 and inspection of the individual studies show mixed evidence regarding the clinical significance of AB. For instance, some studies testing whether AB to smoking cues predicts smoking relapse have found positive results,17–19 one found no association,20 and one found a negative association.21 These conflicting findings have been explained by noting that AB to smoking cues are not stable traits, but are rather indicative of an underlying appetitive motivational state, the strength of which can fluctuate over time.4 Thus, due to mismatches in motivational states across assessment settings, it is perhaps unsurprising that AB measured in clinical or laboratory settings often do not predict behavior outside of these settings.13 Indeed, the predictive validity of AB to smoking cues is likely reduced as the delay between assessment and actual SB increases.4 For instance, Begh et al.22 found that clinic measures of AB were not predictive of noticing and/or attention to smoking cues or craving assessed in real-time, but noticing and/or attention to smoking cues in real-time were associated with craving assessed at the same time.
If AB fluctuates with appetitive motivational states, then it should be most predictive of substance use when craving states are high and immediate drug use is possible.3,4,13,23 In fact, in a sample of heroin-dependent patients undergoing detoxification, AB assessed in real-time was particularly high just before participants relapsed.23 More research is needed to test whether AB to smoking cues correlate more strongly with subjective craving when smoking motivation is high, and whether AB to smoking cues under high craving predicts imminent SB.4,13 To our knowledge, the current study is the first to test these propositions in a controlled laboratory setting.
We experimentally manipulated smoking motivational strength (high and/or low) in daily smokers and assessed urge to smoke, AB to smoking cues, and SB moments after the AB task. [Note: Like others,24,25 we use the terms “urge” and “craving” interchangeably.] We used a potent manipulation to induce a heightened smoking motivational state by combining smoking deprivation and in vivo cigarette cue exposure.26–28 To create a relatively weak smoking motivational state, non-deprived smokers were exposed to a roll of tape. We measured AB to smoking cues using an eye-tracker to obtain a direct and sensitive assessment of AB2,3 and to measure both the initial orienting to versus maintenance of attention on smoking cues, which have shown differential relationships with craving based on smoking motivation levels.29,30 Consistent with prior studies3,29,30 and theories of craving,2,4,6,7 we predicted that the association between craving and the maintenance of attention (ie, dwell time scores) on smoking cues would be stronger in smokers randomly assigned to the deprived and/or smoking cue (HIGH) condition compared with those randomly assigned to the non-deprived and/or control cue (LOW) condition. We did not expect this to be the case for initial shifts in gaze, since two prior eye-tracking studies found that only attention maintenance was related to differing smoking motivation levels.29,30 Finally, we predicted that greater AB would be associated with SB assessed moments after the AB task, and that the association would be stronger for participants in the HIGH than the LOW condition.13,23
Methods
Participants
This study was approved by the Carnegie Mellon University IRB, and all participants provided informed consent. G*Power was used to determine the sample size based on an estimated effect size of r = .30 for the associations among direct AB measures, craving, and SB (eg, see 3,31). With a two-tailed alpha of .05, 90 participants yielded sufficient power (.83) for detecting significant effects. While this sample size is 2× larger than previous eye-tracking studies using similar cue exposure methodology (eg, 29,30), power was low (~.52) to detect significant associations when examining conditions separately and to detect differences in associations across conditions. As such, we focus on the strength of these associations (ie, effect sizes) and the magnitude of the differences across conditions.
Daily smokers (N = 90) were recruited through newspaper and/or bus advertisements and local fliers inviting inquiries from smokers willing to refrain from smoking and/or nicotine for part of 1 day. Participants were required to be 18–50 years old, have smoked ≥10 cigarettes/day for ≥12 months, have no current interest in quitting smoking, speak fluent English, have no medical conditions that ethically contraindicated smoking, and be able to read sentences 24 inches away (the distance to the eye-tracking stimuli). To verify smoking status, a breath carbon monoxide (CO) reading of ≥8 ppm was necessary during the screening session.32
Following screening, participants were randomly assigned to the HIGH or LOW smoking motivational strength condition for the experimental session. HIGH participants (N = 45) were asked to abstain from smoking and nicotine-containing products for ≥12 hours and were exposed to a smoking cue; LOW participants (N = 45) smoked within 15 minutes of the session and were exposed to a control cue. All participants were asked to bring a pack of their cigarettes to the session. The two groups did not differ on gender (52.2% male), age (M = 38.4, SD = 9.9), race and/or ethnicity (52.2% African American, 34.4% Caucasian, 13.3% other), marital status (70% single), cigarettes/day (M = 16.2, SD = 5.8), years smoking (M = 19.5, SD = 10.2), score on the Fagerström Test for Nicotine Dependence33 (M = 3.5, SD = 1.4), or screening session CO readings (M = 22.1 ppm, SD = 10.2) (all ps > .06).
Materials
Questionnaires assessing demographic information and smoking patterns and/or history were administered using standard forms.34 During the experimental session, participants rated their urge to smoke and completed a task assessing AB to smoking-related cues (described below).
Urge to Smoke
Self-reported urge to smoke was assessed on a scale from 0 (“absolutely no urge to smoke at all”) to 100 (“strongest urge to smoke I’ve ever experienced”).35
Attention Task and Stimuli
Participants were asked to view a series of smoking and control pictures while their eye movements were monitored by a computerized eye-tracking system (Pan and/or Tilt optics system, Model ETPC-D6, Applied Science Laboratories, Bedford, MA). The eye-tracking task used here was modeled after a procedure used in previous studies.29,30,36
Stimuli were drawn from the International Smoking Image Series (ISIS, V1.237,38) and consisted of 20 pairs of color photographs of smoking-related scenes (eg, image of a smoker inhaling smoke) and control pictures matched as closely as possible for content and stimulus parameters (complexity, color, saturation, and brightness) but lacking any smoking-related cues (eg, image of a woman applying lipstick).37,38 Pictures were 95 mm high × 130 mm wide and separated by a distance of 65 mm. Each picture pair was presented twice (once with the smoking picture on the left and once on the right) in random order for 2000 ms. This presentation time allowed us to assess both initial orienting and attention maintenance (described below).39,40 Before each trial, a central fixation cross appeared on the screen for 2000 ms and then was replaced by the picture pair. Participants were instructed to focus on the cross while it was on the screen. During each trial, eye-movement data were recorded starting immediately before the onset of the cross and terminated immediately after the offset of the picture pair.
Procedure
Participants arrived for experimental sessions between 2 and 5 pm. Compliance with deprivation instructions was assessed via expired CO levels. To reduce the chance of partial deprivation, non-deprived participants needed to smoke within 15 minutes of arrival and produce a CO level >8 ppm. CO levels for the deprived (HIGH) participants had to be either <20 ppm and at least 50% lower than their (non-deprived) baseline CO level, or <10 ppm.27,41 All participants gave their cigarettes to the experimenter, who returned them after the session. They next rated their urge to smoke (ie, the pre-cue-exposure urge rating).
Cue Exposure
To create conditions with different levels of craving, HIGH and LOW participants received the smoking and control cue, respectively.26,42 As done in prior research, our aim was not to disentangle the effects of nicotine deprivation and smoking cue exposure,41 but to examine whether the relationships between urge to smoke, AB to smoking cues, and SB varied as a function of smoking motivation level. The smoking cue exposure protocol used here mirrors our prior work (eg, 27,34,41,42). The experimenter placed a tray with a plastic cover on the desk in front of participants and then went into an adjacent room to give instructions over the intercom. Participants were told to lift the cover, revealing the cue underneath. The LOW participants found a roll of tape, which they were asked to hold in their dominant hand and look at. The HIGH participants found a pack of their cigarettes, a lighter, and an ashtray. They were told to remove a cigarette from the pack and light it without putting it in their mouths. Once the cigarette was lit, they were told to put down the lighter, hold the cigarette in a comfortable manner, and look at it. Thirty seconds after lighting the cigarette or picking up the tape, participants were asked to rate their urge to smoke (ie, the post-cue-exposure urge rating). Due to experimenter error, post-cue-exposure urge was not recorded for one participant. Following this, participants placed their respective cues on the tray and covered them (cigarettes were extinguished).
Attention Task
After cue exposure, the experimenter reentered the room and described the eye-tracking task. Participants were seated 24″ in front of a computer screen (19″) and were asked to view and pay attention to a series of picture pairs. Before starting the task, the eye-tracking equipment was calibrated by displaying nine dots on the screen in a 3 × 3 array and recording gaze directions as participants looked at each dot sequentially. Data were not recorded for two participants due to eye-tracker malfunction. Participants rated their urge to smoke immediately before and after the task (ie, pre- and post-attention urge rating, respectively).
SB Assessment
Following the attention task, the experimenter informed participants that she needed to get the next task ready, that she would return in about 10 minutes, and that there was time for a smoke break. Participants were told that the room had special air clearance so they could smoke “right here in this room” if they wanted. For HIGH participants, the experimenter removed the tray cover revealing the cigarette pack, lighter, and ashtray; for the LOW participants, the experimenter brought these items in and placed them on the table. The experimenter then left the room and unobtrusively videotaped the next 10 minutes while the participant was free to smoke ad libitum. This recording was later coded for smoking latency (in seconds) and frequency of puffs27,43,44 by coders blind to study condition. As is common in the literature, participants who chose not to smoke were assigned the maximum value of 600 s (eg, 27,31,43,45). Five LOW participants and one HIGH participant chose not to smoke. A subset of participants (N = 18; 20%) was coded for reliability; inter-rater reliability was excellent for both latency to smoke and number of puffs (κ = 0.99 and κ = 0.98, respectively). Latency values were log transformed to meet normality assumptions for analyses (see also 27). Latency to smoke and number of puffs values were correlated (r = −.45, p < .001), but not so highly as to preclude their separate examination (see also 44).
At the end of the 10-minute period, participants were informed the study was over. They were debriefed and compensated. The first 74 participants were paid $50, but due to difficulties with recruitment and increased parking rates, the last 16 participants were paid $70. Payment was not related to any variable except pre-cue-exposure urge to smoke (p < .05). Controlling for payment in analyses did not change any results.
Eye Data Preparation
Data were processed using ASL Results Plus analysis software (Applied Science Laboratories) according to procedures outlined by Field et al.29,30,36 The screen for the attention task was divided into three regions (smoking picture, control picture, and central). The central region was a vertical band 1.03° wide, and areas outside this region to the right or left were the smoking or control regions. The gaze direction (in degrees) was measured every 17 ms. Fixations were defined as eye movements that were stable within 1° of visual angle for ≥100 ms. Fixations were classified as directed to the left or right pictures if they were 1° wide of the central region.29 The key measures were initial fixation directions and time spent fixating on smoking pictures (ie, dwell time).
Based on previous studies,30,36,46 initial fixations were calculated if the following criteria were met: (1) participants fixated on the central region before picture onset, (2) the latency to initially fixate on the pictures after onset was ≥100 ms, and (3) fixations were directed at either the left or right picture (ie, outside the central region). For analyses, initial fixation bias scores were calculated for each participant by subtracting the number of trials gazes were directed initially to the control picture from the number of trials gazes were directed initially to the smoking picture (ie, larger initial fixation bias scores = greater AB). Internal reliability was very low for initial fixation bias scores (Cronbach’s alpha = .20; Guttman split-half coefficient = .28). [Note: Initial fixation bias score reliability was calculated using list-wise deletion and a binary variable for each trail, with 1 = initial fixation on the smoking picture and 0 = initial fixation on the control picture.]
Dwell times were calculated as the total amount of time (summed across trials) that fixations were directed to the smoking and control picture regions. Consistent with prior studies (eg, 29,30), dwell time data came from trials that met the following criteria: (1) the percentage of fixations tracked during the trial was ≥20%, and (2) the percentage of time spent looking outside the central region for that trial was ≥20%. For analyses, dwell time bias scores were calculated for each participant by subtracting dwell time duration for the control pictures from dwell time duration for the smoking pictures (ie, larger dwell time bias scores = greater AB). Internal reliability was excellent for dwell time bias scores (Cronbach’s alpha = .90; Guttman split-half coefficient = .88). [Note: Dwell time bias score reliability was calculated using list-wise deletion and a continuous measure of the duration of time spent looking at the smoking picture for each trial.]
In previous studies,29,30,36,46,47 participants with excessive missing eye-tracking data (ie, <15% usable data) were excluded from AB analyses. A total of four participants met these exclusion criteria in the current study (two from the HIGH and two from the LOW condition). However, results remained the same regardless of whether they were included versus excluded; thus, we included them.
Data Analytic Strategy
We first examined box plots and histograms to identify outliers. There were no outliers in urge to smoke values, initial fixation bias scores, or log-transformed latency to smoke values. There were three outliers (3+ SDs away from the mean and clearly disconnected from the rest of the distribution based on visual inspection of histograms) in dwell time bias scores and two in the number of puffs data. These values were replaced with the next most extreme score.48
Using analysis of variance, we then examined the effectiveness of the smoking motivation level manipulation by determining whether HIGH participants reported greater urges to smoke, larger AB to smoking cues, shorter smoking latencies, and greater number of puffs than LOW participants. We used one sample t tests to determine whether the sample as a whole showed an AB to smoking cues (by testing whether values for initial fixation bias scores and dwell time bias scores significantly differed from zero). Bivariate correlations were used to examine associations, for the full sample and separately by condition, among the following variables: urge to smoke during cue exposure, dwell time bias and initial fixation bias scores, latency to smoke, and number of puffs. Fisher’s z tests were used to determine whether correlations across conditions were significantly different. We also examined partial correlations among these variables in the full sample controlling for the effect of condition.
Results
Preliminary Analyses—Effectiveness of the Smoking Motivation Level Manipulation
Table 1 shows descriptive statistics for all variables for the full sample and across conditions. As expected, HIGH participants (who were nicotine-deprived) had significantly lower CO values than LOW participants at the start of the experimental session. Also as expected, HIGH participants reported significantly higher urge ratings at all four assessment points. A 2 × 2 repeated measures analysis of variance, with condition as a between-subjects variable and time (pre-cue- and post-cue-exposure urge ratings) as a repeated variable, revealed a condition × time interaction, F(1,87) = 7.85, p = .006, , such that, as expected, the HIGH smokers reported a significant increase in urge following smoking cue exposure, F(1,43) = 10.87, p = .002, , while the LOW smokers’ urge ratings did not change following control cue exposure, F(1,44) = 1.08, p = .30, . Despite significantly lower urge values, the LOW participants reported a significant increase in urge following the AB task, F(1,44) = 15.85, p < .001, , while the HIGH participants’ urge ratings did not change, F(1,44) = 0.47, p = .50, .
Table 1.
Descriptive Statistics for Variables Across Conditions
| Variable | Full sample | HIGH condition | LOW condition | F | p | |
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| CO levels (ppm) | 16.44 (14.21 | 6.71 (4.78) | 26.18 (13.84) | 79.50 | <.001 | 0.48 |
| Pre-cue-exposure urge | 53.47 (33.52) | 78.25 (20.58) | 29.31 (25.44) | 97.35 | <.001 | 0.53 |
| Post-cue-exposure urge | 55.11 (37.31) | 83.95 (21.28) | 26.91 (26.38) | 125.75 | <.001 | 0.59 |
| Pre-attention task urge | 53.04 (36.31) | 76.27 (26.98) | 29.81 (28.89) | 62.14 | <.001 | 0.41 |
| Post-attention task urge | 57.34 (35.39) | 74.38 (30.15) | 40.30 (32.10) | 26.94 | <.001 | 0.23 |
| Latency to smokea | 57.19 (149.46) | 27.26 (94.12) | 87.07 (185.74) | 12.22 | .001 | 0.12 |
| Number of puffs | 11.94 (5.81) | 12.42 (5.71) | 11.47 (5.93) | 0.61 | .439 | 0.01 |
| Initial fixation bias scores | 1.69 (6.33) | 1.88 (5.31) | 1.51 (7.23) | 0.08 | .784 | 0.00 |
| Dwell time bias scores | 1.21 (7.33) | 1.51 (7.09) | 0.93 (7.63) | 0.14 | .714 | 0.00 |
CO = carbon monoxide.
aNon-transformed values are presented here for descriptive purposes. Initial fixation bias scores were calculated for each participant by subtracting the number of trials gazes were directed initially to the control picture from the number of trials gazes were directed initially to the smoking picture. Dwell time bias scores were calculated for each participant by subtracting dwell time duration (in seconds) for the control pictures from dwell time duration for the smoking pictures.
As predicted, latency to smoke was significantly shorter for HIGH than LOW participants. Although in the expected direction, the number of puffs did not differ across conditions. Across conditions, participants showed a significant AB for smoking cues as indexed by initial fixation bias scores, t(87) = 2.51, p = .014, but not dwell time bias scores, t(87) = 1.55, p = .124. Contrary to prediction, AB measures did not differ across conditions.
Bivariate and Partial Correlational Analyses
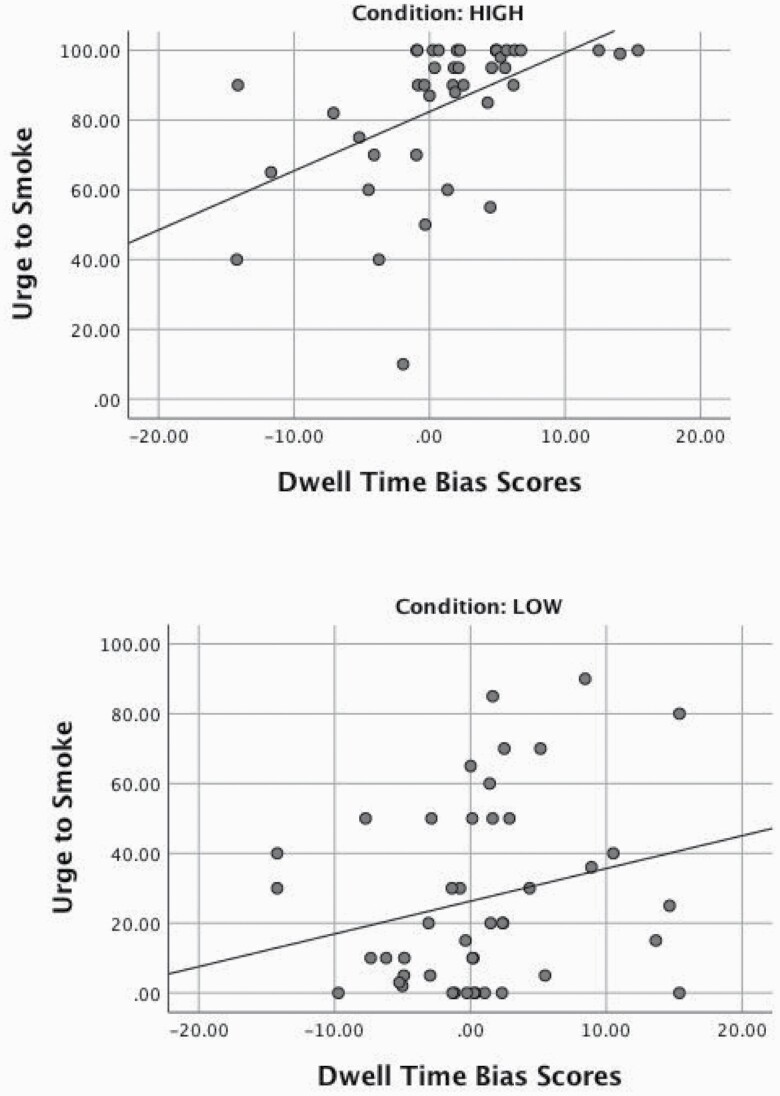
Table 2 shows bivariate correlations for study variables for the full sample and across conditions. As predicted, dwell time bias scores were significantly associated with urge to smoke during cue exposure in the full sample and for HIGH participants. Though the difference in correlations across conditions did not reach significance (p = .068; one-tailed), consistent with our predictions, the magnitude of the association was stronger for HIGH versus LOW participants (see Figure 1 for scatter plots of these associations). Contrary to predictions, dwell time bias scores were not significantly associated with either SB measure. Replicating prior studies, initial fixation bias scores were not associated with urge to smoke during cue exposure in the full sample nor in either condition separately, though they were significantly associated with latency to smoke in the full sample. In the full sample, higher urge to smoke values during cue exposure were significantly associated with shorter smoking latencies and more puffs. There was a significant association between urge to smoke and latency to smoke in the LOW participants, although the strength of the association was not significantly different from that of the HIGH participants. Finally, we also examined partial correlations among these variables controlling for the effect of condition. The results presented for the full sample in Table 2 held (ie, none of the significant p values became nonsignificant, and none of the nonsignificant p values became significant). However, the association between dwell time bias scores and urge to smoke increased to r = .29, and the association between urge to smoke and latency to smoke decreased to r = −.27.
Table 2.
Bivariate Correlations Among Study Variables for the Full Sample and Across Conditions
| Variables | Full sample | HIGH condition | LOW condition | Fisher’s z |
|---|---|---|---|---|
| Dwell time and urge to smoke | .22* | .47** | .18 | 1.49 |
| Initial fixation and urge to smoke | .05 | .08 | .03 | 0.23 |
| Dwell time and latency to smoke | −.14 | −.04 | −.23 | 0.88 |
| Initial fixation and latency to smoke | −.23* | −.22 | −.25 | 0.14 |
| Dwell time and number of puffs | .17 | .19 | .14 | 0.23 |
| Initial fixation and number of puffs | .03 | −.05 | .08 | −0.59 |
| Urge to smoke and latency to smoke | −.43** | −.18 | −.34* | 0.78 |
| Urge to smoke and number of puffs | .23* | .24 | .25 | −0.05 |
*p < .05.
**p < .01.
Figure 1.
Relationship between dwell time bias scores and post-cue-exposure urge to smoke ratings across HIGH and LOW conditions.
Discussion
Theory and data suggest that AB to drug-related cues should be associated with craving when craving levels are high, and that AB should be predictive of drug use when immediate use is possible (eg,3,4,6,13,22,23). The current study aimed to test these propositions in daily smokers in a controlled laboratory environment. We created HIGH and LOW smoking motivation conditions by exposing nicotine-deprived smokers to a potent smoking cue and non-deprived smokers to a control cue. This manipulation was effective in creating conditions that had significantly different craving levels (across all timepoints) and smoking latencies, in the expected directions. However, the conditions did not differ on initial fixation and dwell time bias scores nor on the number of puffs taken. While these initial fixation data are in accord with prior work by Field et al.,29 the dwell time bias findings are inconsistent with this prior study which found that 23 nicotine-deprived smokers maintained their gaze for significantly longer on smoking (vs. control) cues, than when these same smokers were non-deprived. It is unclear why we failed to observe an effect of smoking motivation level on dwell time bias scores, as our task was based on the Field et al.29 task (except that we did not use a concurrent dot probe task). One possibility is that the Field et al.29 study may have been underpowered (the current sample size is twice as large), and the results may not have been reliable. Given highly significant differences between the conditions on urge ratings and smoking latencies found here, though, we assume the smoking level motivation manipulation was successful, thus creating ideal conditions to test our hypotheses.
We hypothesized that AB to smoking-related cues would be associated more strongly with craving when smoking motivation was high,3 and this would be true only for dwell time bias scores.29 As predicted, dwell time bias scores were significantly associated with urge to smoke in the HIGH smoking motivation participants, with a large effect size.49 In contrast, dwell time bias scores were not significantly associated with urge to smoke in the LOW condition, with a small effect size and a correlation value 2.5 times weaker than that found in the HIGH condition. While the difference between these two values failed to reach significance at p < .05, the findings support theoretical models proposing that AB to smoking-related cues and craving are outputs of a common underlying appetitive motivational process and should be correlated, strongly so with high smoking motivational strength.3–6,8,12 Our results add to a growing body of evidence suggesting that measures of attention maintenance on drug-related cues (ie, dwell times) may tap into fundamental mechanisms hypothesized to underlie addiction (eg, incentive motivation) (see also 29).
Internal reliability for initial fixation bias scores was very low, and results for this variable should be interpreted with caution. Although our null findings for the association between initial fixation bias scores and craving are consistent with two prior studies,29,30 it is unclear whether initial fixation bias scores in those studies also had unacceptable internal reliability (these statistics were not reported). Notably, though, two recent eye-tracking studies using similar methodology (but different cue types, see 50,51) found the same pattern of findings reported here (ie, internal reliability was excellent for dwell time scores and very low for initial fixation scores). Field et al.29 explained null findings for the association between initial fixation bias scores and smoking craving by noting that cognitive scientists draw an important distinction between the mechanisms involved in the initial orienting versus maintenance of attention,52 and that motivational states may be more likely to influence the latter, rather than the former.53 Serious concerns with the reliability of initial fixation bias scores may alternatively be the reason for these null findings. Future smoking studies should routinely report reliability estimates for AB measures to aid in the interpretation of results.
We also examined the extent to which AB measures predicted immediate SB, as previous data23 and theories (eg, 4) suggest that AB indices should predict drug use when immediate use is possible. Initial fixation bias scores were associated with latency to smoke, but it is unclear what to make of this finding given the very low internal reliability of the initial fixation bias scores. Contrary to prediction, dwell time bias scores were not significantly associated with smoking latency or number of puffs. These findings contrast with a prior study that showed that AB to drug-related cues assessed on mobile devices in the real-world was particularly high just before participants relapsed to heroin.23 Our results are consistent, however, with the only other lab study (that we are aware of) that examined associations between individual differences in AB to drug-related cues and actual drug use in the lab. Specifically, Field et al.54 examined the associations between cognitive biases for alcohol cues (including an indirect measure of AB) and operant responding to earn sips of beer. The correlation values for these associations were less than .15 and none were significant. They speculated that these null findings might have been due to methodological shortcomings, in that they used a specific reinforcer (beer) during the operant task but used a range of alcohol-related cues to assess cognitive biases. In general, AB to drug-related cues may be more predictive of drug use outside of artificial lab environments, but more research is needed to test this proposition.
Although not a primary aim of this study, we also examined the extent to which urge to smoke during cue exposure predicted SB. Urge to smoke ratings were associated with smoking latency and number of puffs in the expected directions for both conditions, but the only significant association was with smoking latency in the LOW condition with a medium effect size. The magnitudes of the associations between urge to smoke and SB in the HIGH condition are similar to those reported in previous studies that have used smoking deprivation and/or smoking cue exposure paradigms (eg, 27,31). The significant association found in the LOW condition between urge to smoke and smoking latency was unexpected, but may be a result of greater variation in both urge and latency to smoke values in this condition than the HIGH condition (see also 27).
In sum, this study tested key predictions made by theoretical accounts of addiction that emphasize the role of AB in driving drug use behavior (eg, 3,4,6). We experimentally manipulated smoking motivation level (high versus low) and tested whether AB measures were more strongly associated with craving and SB when smoking motivation was high. Findings were mixed. In line with our predictions and past studies,29,30 dwell time bias scores were more strongly related to urge to smoke when smoking motivation was high (versus low), suggesting that the maintenance of attention on smoking-related cues is associated with transient changes in nicotine deprivation and craving levels. Dwell time bias scores were not significantly associated with SB in either condition, however. Despite the sample size being twice as large as previous smoking studies using eye-tracking methods,29,30 a limitation of this study is that it was not powered to find significant associations when examining conditions separately or to detect significant differences across conditions. Further, the reliability for initial fixation bias scores was very low (see also 50,51), highlighting the need to routinely report reliability estimates for AB measures to facilitate interpretation of findings. In general, future well-powered studies using direct measures of AB (ie, eye-tracking) are indicated to further explore the effects of smoking motivation level on associations between craving, AB for smoking-related cues, and actual SB.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We thank the staff and students of the Behavioral Health Research Laboratory at Carnegie Mellon University.
Funding
This study was supported by a National Institute of Alcohol Abuse and Alcoholism grant to Kasey G. Creswell (L30 AA022509).
Declaration of Interests
None declared.
References
- 1.Drobes DJ, Oliver JA, Correa JB, Evans DE. Attentional bias and smoking. In: Preedy VR, ed. Neuroscience of Nicotine. Academic Press; 2019:145– 150. [Google Scholar]
- 2.Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20. [DOI] [PubMed] [Google Scholar]
- 3.Field M, Munafò MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135(4):589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field M, Werthmann J, Franken I, Hofmann W, Hogarth L, Roefs A. The role of attentional bias in obesity and addiction. Health Psychol. 2016;35(8):767–780. [DOI] [PubMed] [Google Scholar]
- 5.Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):563–579. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. [DOI] [PubMed] [Google Scholar]
- 7.Sayette MA. The role of craving in substance use disorders: theoretical and methodological issues. Annu Rev Clin Psychol. 2016;12(1):407–433. [DOI] [PubMed] [Google Scholar]
- 8.Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychol Rev. 2005;112(2):446–467. [DOI] [PubMed] [Google Scholar]
- 9.Ryan F. Detected, selected, and sometimes neglected: cognitive processing of cues in addiction. Exp Clin Psychopharmacol. 2002;10(2):67–76. [DOI] [PubMed] [Google Scholar]
- 10.Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl). 2005;183(3):350–357. [DOI] [PubMed] [Google Scholar]
- 11.Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol. 2002;16(4):385–392. [DOI] [PubMed] [Google Scholar]
- 12.Sayette MA, Martin CS, Hull JG, Wertz JM, Perrott MA. Effects of nicotine deprivation on craving response covariation in smokers. J Abnorm Psychol. 2003;112(1):110–118. [PMC free article] [PubMed] [Google Scholar]
- 13.Christiansen P, Schoenmakers TM, Field M. Less than meets the eye: reappraising the clinical relevance of attentional bias in addiction. Addict Behav. 2015;44:43–50. [DOI] [PubMed] [Google Scholar]
- 14.Cox WM, Fadardi JS, Intriligator JM, Klinger E. Attentional bias modification for addictive behaviors: clinical implications. CNS Spectr. 2014;19(3):215–224. [DOI] [PubMed] [Google Scholar]
- 15.Field M, Marhe R, Franken IH. The clinical relevance of attentional bias in substance use disorders. CNS Spectr. 2014;19(3):225–230. [DOI] [PubMed] [Google Scholar]
- 16.Marhe R, Luijten M, Franken IH. The clinical relevance of neurocognitive measures in addiction. Front Psychiatry. 2014;4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janes AC, Pizzagalli DA, Richardt S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl). 2010;212(4):537–549. [DOI] [PubMed] [Google Scholar]
- 19.Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22(4):378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98(10):1409–1417. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalder K, Jähne A, Kyle SD, et al. Is smoking-related attentional bias a useful marker for treatment effects? Behav Med. 2011;37(1):26–34. [DOI] [PubMed] [Google Scholar]
- 22.Begh R, Smith M, Ferguson SG, Shiffman S, Munafò MR, Aveyard P. Association between smoking-related attentional bias and craving measured in the clinic and in the natural environment. Psychol Addict Behav. 2016;30(8):868–875. [DOI] [PubMed] [Google Scholar]
- 23.Marhe R, Waters AJ, van de Wetering BJ, Franken IH. Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J Consult Clin Psychol. 2013;81(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(suppl 2):S189–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2012;1248:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108(6):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creswell KG, Sayette MA, Skrzynski CJ, Wright AGC, Schooler JW, Sehic E. Assessing cigarette craving with a squeeze. Clin Psychol Sci. 2019;7(3):597–611. [Google Scholar]
- 28.Sayette MA, Creswell KG. Self-regulatory failure and addiction. In: Vohs KD, Baumeister RF, eds. Handbook of Self-Regulation: Research, Theory, and Applications. 3rd ed. New York, NY: Guilford Press; 2016. [Google Scholar]
- 29.Field M, Mogg K, Bradley BP. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacology (Berl). 2004;173(1–2):116–123. [DOI] [PubMed] [Google Scholar]
- 30.Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98(6):825–836. [DOI] [PubMed] [Google Scholar]
- 31.Heckman BW, MacQueen DA, Marquinez NS, MacKillop J, Bickel WK, Brandon TH. Self-control depletion and nicotine deprivation as precipitants of smoking cessation failure: a human laboratory model. J Consult Clin Psychol. 2017;85(4):381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111(1–2):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 34.Griffin KM, Sayette MA. Facial reactions to smoking cues relate to ambivalence about smoking. Psychol Addict Behav. 2008;22(4):551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SJ, Creswell KG, Sayette MA, Fiez JA. Ambivalence about smoking and cue-elicited neural activity in quitting-motivated smokers faced with an opportunity to smoke. Addict Behav. 2013;38(2):1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology (Berl). 2005;180(2):333–341. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert DG, Rabinovich NE.. International Smoking Image Series (with Neutral Counterparts), Version 1.2. Carbondale, IL: Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- 38.Gilbert DG, Rabinovich NE.. Manual of Norms for the International Smoking Image Series (with Neutral Counterparts), Version 1.2. Carbondale, IL: Department of Psychology, Southern Illinois University at Carbondale; 2005. [Google Scholar]
- 39.Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl). 2008;197(1):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85(1):75–82. [DOI] [PubMed] [Google Scholar]
- 41.Sayette MA, Loewenstein G, Griffin KM, Black JJ. Exploring the cold-to-hot empathy gap in smokers. Psychol Sci. 2008;19(9):926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donny EC, Griffin KM, Shiffman S, Sayette MA. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine Tob Res. 2008;10(3):447–455. [DOI] [PubMed] [Google Scholar]
- 43.Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. J Abnorm Psychol. 2005;114(1):153–164. [DOI] [PubMed] [Google Scholar]
- 44.Shiffman S, Dunbar M, Kirchner T, et al. Smoker reactivity to cues: effects on craving and on smoking behavior. J Abnorm Psychol. 2013;122(1):264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14(11):1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradley BP, Garner M, Hudson L, Mogg K. Influence of negative affect on selective attention to smoking-related cues and urge to smoke in cigarette smokers. Behav Pharmacol. 2007;18(4):255–263. [DOI] [PubMed] [Google Scholar]
- 47.Bradley BP, Mogg K, Millar N. Biases in overt and covert orienting to emotional facial expressions. Cogn Emot. 2000;14:14–789. [Google Scholar]
- 48.Tabachnick BG, Fidell LS, Ullman JB.. Using Multivariate Statistics. Boston, MA: Pearson; 2007. [Google Scholar]
- 49.Hemphill JF. Interpreting the magnitudes of correlation coefficients. Am Psychol. 2003;58(1):78–79. [DOI] [PubMed] [Google Scholar]
- 50.Soleymani A, Ivanov Y, Mathot S, de Jong PJ. Free-viewing multi-stimulus eye tracking task to index attention bias for alcohol versus soda cues: satisfactory reliability and criterion validity. Addict Behav. 2020;100:106117. [DOI] [PubMed] [Google Scholar]
- 51.van Ens W, Schmidt U, Campbell IC, Roefs A, Werthmann J. Test-retest reliability of attention bias for food: robust eye-tracking and reaction time indices. Appetite. 2019;136:86–92. [DOI] [PubMed] [Google Scholar]
- 52.Allport A. Visual attention. In: Foundations of Cognitive Science. Cambridge, MA: The MIT Press; 1989:631–682. [Google Scholar]
- 53.LaBerge D.Attentional Processing: The Brain’s Art of Mindfulness. Harvard University Press; 1995:286. [Google Scholar]
- 54.Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcohol. 2005;40(6):504–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.