Abstract
In the 1st trimester of human pregnancy, low oxygen tension or hypoxia, is essential for proper placentation and placenta function. Low oxygen levels and activation of signaling pathways have been implicated as critical mediators in the promotion of trophoblast differentiation, migration, and invasion with inappropriate changes in oxygen tension and aberrant Notch signaling both individually reported as causative to abnormal placentation. Despite crosstalk between hypoxia and Notch signaling in multuple cell types, the relationship between hypoxia and Notch in 1st trimester trophoblast function is not understood. To determine how a low oxygen environment impacts Notch signaling and cellular motility, we utilized the human 1st trimester trophoblast cell line, HTR-8/SVneo. Gene set enrichment and ontology analyses identified pathways involved in angiogenesis, Notch and cellular migration as upregulated in HTR-8/SVneo cells exposed to hypoxic conditions. DAPT, a γ-secretase inhibitor that inhibits Notch activation, was used to interrogate the crosstalk between Notch and hypoxia pathways in HTR-8/SVneo cells. We found that hypoxia requires Notch activation to mediate HTR-8/SVneo cell migration, but not invasion. To determine if our in vitro findings were associated with preeclampsia, we analyzed 2nd trimester chorionic villous sampling (CVS) samples and 3rd trimester placentas. We found a significant decrease in expression of migration and invasion genes in CVS from preeclamptic pregnancies, and significantly lower levels of JAG1 in placentas from pregnancies with early-onset preeclampsia with severe features. Our data support a role for Notch in mediating hypoxia-induced trophoblast migration, which may contribute to preeclampsia development.
Keywords: hypoxia, Notch signaling, trophoblast, HTR-8/SVneo, preeclampsia
Introduction
In humans, the initial phase of placentation occurs in a uterine environment with oxygen tension ranging from 1–3% (Red-Horse et al. 2004, James et al. 2006, Huppertz et al. 2009, Pringle et al. 2010, James et al. 2012). Low oxygen levels have been implicated in the promotion of trophoblast differentiation, migration, and invasion. Under these low oxygen, “hypoxic” conditions, undifferentiated cytotrophoblasts differentiate into villous cytotrophoblasts that can either fuse to form syncytiotrophoblasts or differentiate into extravillous trophoblasts (EVTs). EVTs further differentiate into interstitial or endovascular EVTs, which migrate into and invade the maternal decidua and myometrium or the maternal vasculature, respectively (Huppertz and Peeters 2005, James et al. 2006, James et al. 2012). Initially, endovascular EVTs invade and plug the uterine spiral arteries, resulting in minimal maternal blood flow into the placenta and maintenance of a low oxygen uterine environment (Burton et al. 1999, Pringle et al. 2010). In humans between 8 and 12 weeks of gestation, EVT plugs are displaced and physiologic transformation of maternal spiral arteries by endovascular EVTs creates low resistance, high capacity, large-diameter vessels. By week 14 of gestation, oxygen tension rises to above 8% O2 (Rodesch et al. 1992, Burton et al. 1999, Roberts et al. 2017, Burton et al. 2021). EVT-mediated spiral artery remodeling supports adequate placental perfusion critical for proper fetal growth and development (Huppertz et al. 2009, Knofler et al. 2019) Impaired EVT migration and invasion leads to insufficient vascular remodeling, resulting in the poor placental perfusion and ischemia which is linked to adverse pregnancy outcomes such as IUGR and early-onset PE, the more severe form of PE associated with improper placenta formation (Laresgoiti-Servitje and Gomez-Lopez 2012, Yang et al. 2015, Burton and Jauniaux 2018, Burton et al. 2021).
In many cell types, hypoxia induces dimerization of hypoxia-inducible factor (HIF) proteins leading to upregulation of target genes, such as vascular endothelial growth factor (Vegf) (Safran and Kaelin 2003, Pringle et al. 2010, Borggrefe et al. 2016). Hypoxia also induces the Notch signaling pathway in both physiologic and pathologic settings (Gustafsson et al. 2005, Sahlgren et al. 2008, Borggrefe et al. 2016). The Notch pathway is an evolutionarily conserved, signaling mechanism that is essential for mammalian placentation and placental function (Gasperowicz and Otto 2008, Hunkapiller et al. 2011, Cuman et al. 2014, Haider et al. 2014, Velicky et al. 2014, Knofler et al. 2019, Lu et al. 2019a). The mammalian Notch pathway is comprised of four single-pass transmembrane receptors (Notch1–4) that are activated by membrane bound ligands, Jagged (Jag) 1, Jag2, Delta-like (Dll) 1 and Dll4 on adjacent cells (Artavanis-Tsakonas et al. 1999, Shawber and Kitajewski 2004, Kofler et al. 2011). Upon ligand binding, the receptor undergoes two sequential proteolytic cleavages, the latter by the γ-secretase complex. The resulting Notch intracellular domain (NICD) translocates to the nucleus to regulate transcription of downstream effectors of Notch signaling, including members of the Hairy/Enhancer of Split (Hes), the Hairy/Enhancer of Split related with YRPW motif (Hey) families and the Notch-regulated ankyrin repeat-containing protein (Nrarp) (Krebs et al. 2001, Kadesch 2004, Kovall 2007, Kofler et al. 2011). In the endothelium, hypoxia-induced VEGF up-regulates expression of Notch ligand, Dll4 (Mailhos et al. 2001, Min et al. 2016), while direct binding of HIF-1α to the NICD has been shown to promote transcription of Notch effectors (Gustafsson et al. 2005, LaFoya et al. 2016), suggesting crosstalk between hypoxia and Notch signaling.
Pathological Notch signaling and inappropriate changes in oxygen tension are both implicated as causative to the placental insufficiency associated with PE (Hunkapiller et al. 2011, Haider et al. 2014, Velicky et al. 2014, Haider et al. 2017). Studies have investigated Notch signaling (Haider et al. 2014, Plessl et al. 2015, Haider et al. 2016, Haider et al. 2017, Knofler et al. 2019, Wang and Zou 2020) and hypoxia (James et al. 2006, Huppertz et al. 2009, Pringle et al. 2010, James et al. 2012, Chaudhary et al. 2019) as independent mediators of 1st trimester trophoblast function, but the relationship between hypoxia and Notch in trophoblasts has not been elucidated. To determine how a low oxygen environment impacts Notch and cellular motility of human 1st trimester trophoblasts, we utilized HTR-8/SVneo, an immortalized human trophoblast cell line derived from a 1st trimester placenta (Graham et al. 1993, Highet et al. 2012, Luo et al. 2015, Yang et al. 2015, Highet et al. 2016, Luo et al. 2020). mRNA-seq was performed on HTR-8/SVneo cells exposed to hypoxic conditions. Gene set enrichment and gene ontology (GO) analyses identified pathways involved in angiogenesis, Notch signaling and cellular migration to be upregulated. We hypothesized that Notch signaling mediates the effects of hypoxia-induced trophoblast cell migration and invasion. To inhibit Notch activation, DAPT, a γ-secretase inhibitor, was used to interrogate the crosstalk between Notch and hypoxia signaling in HTR-8/SVneo cells and their role in HTR-8/Svneo cell motility. We found that hypoxia induces Notch gene expression and activity and requires Notch activation to mediate cell migration, but not invasion. To determine if our in vitro findings were associated with PE pregnancy outcomes, we analyzed 2nd trimester chorionic villous sampling (CVS) samples and 3rd trimester placentas. CVS were subjected to mRNA-seq, while placentas were analyzed for Notch pathway expression. We found a significant decrease in expression of migration and invasion genes in CVS from PE pregnancies, as well as a significantly lower levels of JAG1 in placentas from PE pregnancies. Together, our data support a role for Notch signaling in mediating hypoxia induced trophoblast migration, which may contribute to the development of PE.
Material and methods
Human subjects and tissue collection
Collection of CVS and placental tissue samples were performed under an Institution Review Board approved protocol (#AAAN4357) at Columbia University Medical Center. Between December 14, 2015 and October 31, 2016, 43 women with singleton gestations between 78 and 96 days of gestation, provided written informed consent and enrolled for participation in the procurement and use of villus tissue (Supplementary Fig. 1, Table 1). 5–20 mg of excess villous tissue obtained for genetic testing was collected, snap frozen on dry ice and stored at −80°C for analyses after pregnancy outcomes became available.
Table 1.
Maternal characteristics and outcomes after chorionic villus sampling
| ID | Maternal age (years) | Race | G P | Hypertension and PE history | GA at CVS (days) | CVS result | GA at delivery (days) | Delivery outcome |
|---|---|---|---|---|---|---|---|---|
| PE | 38 | Asian | G3P0 | None | 83 | 46 XX | 260 | preeclampsia |
| PE-SF | 41 | Hispanic | G3P1 | None | 92 | 46 XY | 263 | preeclampsia |
| H1 | 41 | White | G4P1 | None | 88 | 46 XY | 276 | healthy |
| H2 | 28 | White | G3P2 | None | 78 | 46 XX | 280 | healthy |
| H3 | 40 | White | G6P1 | PE in a prior pregnancy | 96 | 46 XX | 266 | healthy |
| H4 | 42 | White | G1P0 | None | 88 | 46 XY | 295 | healthy |
G: gravida; GA: gestational age; H: healthy; P: para; PE: preeclampsia; PE-SF: preeclampsia with severe features
To compare expression of Notch proteins in 3rd trimester placentas from healthy, uncomplicated pregnancies and PE pregnancies, women with singleton gestations were recruited and provided written informed consent for the procurement and use of placental tissues. Between June 1, 2016 and December 31, 2016, collection of placental tissues was performed within 10 minutes after Cesarean delivery from 4 healthy, uncomplicated pregnancies delivered at term (≥37 weeks of gestation) and 4 pregnancies delivered preterm (<37 weeks of gestation) because of PE with severe features (Table 2). Diagnosis of PE with severe features was made according to the American College of Obstetricians and Gynecologists criteria (doi: 10.1097/01.AOG.0000437382.03963.88). Placental tissue (1 cm × 1 cm × 1 cm) was collected from the maternal side of the placenta, within 1 inch of the umbilical cord. All collected placenta samples were macroscopically normal excluding sites of infarction, hemorrhage and calcification. Specimens were rinsed in phosphate buffered saline (PBS), snap frozen on dry ice and stored at −80°C until use.
Table 2.
Maternal characteristics and outcomes for placental tissue samples
| ID | Maternal age (years) | Race | G P | Hypertension and PE history | Placental weight (g) | Infant sex | GA at delivery (days) | Delivery outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | White | G7P2 | PE in a prior pregnancy | 624 | F | 266 | healthy |
| 2 | 35 | White | G2P2 | None | 479 | M | 276 | healthy |
| 3 | 34 | Black | G3P3 | None | 496 | M | 273 | healthy |
| 4 | 35 | White | G2P1 | None | 515 | F | 273 | healthy |
| 5 | 37 | Asian | G3P0 | cHTN | 234 | M | 209 | PE-SF |
| 6 | 29 | Hispanic | G6P2 | cHTN | 411 | F | 253 | PE-SF |
| 7 | 34 | Black | G5P2 | cHTN | 433 | M | 240 | PE-SF |
| 8 | 33 | Black | G2P0 | None | 606 | M | 256 | PE-SF |
cHTN: chronic hypertension; G: gravida; GA: gestational age; F: female; M: Male; P: para; PE: preeclampsia; PE-SF: preeclampsia with severe features
Cell culture and treatment.
HTR-8/SVneo cells (Graham et al. 1993) (ATCC), were grown in RPMI-1640 media (ThermoFisher) with 5% fetal bovine serum (FBS; Sigma-Aldrich), 5,000 units/mL penicillin/streptomycin (Sigma-Aldrich) and maintained in a humidified incubator under standard culture conditions which are normoxic (21% O2, 5% CO2). For hypoxia studies, HTR-8/SVneo cells were exposed to hypoxic (2.5% O2, 5% CO2) or normoxic conditions for 6 or 24 hours.
γ-secretase activity was blocked by N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine tert-butyl ester (DAPT; Sigma-Aldrich) dissolved in DMSO and used at a final concentration of 25μM or 50μM DAPT. Vehicle controls were treated with equivalent amounts of DMSO (1:1000), at final concentrations of 11.7 mM and 23.5 mM DMSO, for 25μM or 50μM DAPT, respectively.
RNA Extraction
For next-generation mRNA-sequencing, total RNA was purified with the RNeasy Plus Mini Kit (Qiagen) and RNA integrity number (RIN) determined. For RT-PCR and quantitative (q) RT-PCR, total RNA was extracted with TRIzol reagent (Invitrogen).
Next-generation mRNA sequencing.
6×105 HTR-8/SVneo cells per well were plated on fibronectin (Sigma-Aldrich) coated 12-well dishes. RNA was isolated after 6 hours of exposure to 21% O2 (n=3) or 2.5% O2 (n=3). mRNA-Seq library construction was done using NEBNext UltraRNA Library Prep Kit for Illumina (NEB) from all 6 HTR-8/SVneo samples and 6 CVS samples at Novogene Corporation Inc. The multiplexed library was sequenced on an Illumina Novaseq 6000 platform (Illumina, San Diego, CA) in a 2×150bp configuration to a target depth of 20–30M reads per sample.
Sequence analysis was performed following NF-Core RNA-Seq guidelines (v1.4.2) for rigor and reproducibility (Ewels et al. 2020). The raw reads were aligned to the human genome (GRCh37) using STAR (v2.6.1), followed by transcript abundance and hit counts generation through StringTie (v2.0) and featureCounts (v1.6.4) (Liao et al. 2014, Pertea et al. 2015). Subsequent hit count normalization and pairwise differential gene expression comparisons were performed using the DESeq2 (v1.22.1) method with significance thresholds set at FDR adjusted p <0.05 (Love et al. 2014). Pathway term enrichment was performed via Panther (Gene Ontology) and Ingenuity Pathway Analysis (Canonical Pathways), as well as Gene Set Enrichment Analysis (GSEA) to identify hallmark pathway enrichment (Mi et al. 2019). mRNA-Seq data have been uploaded to the Genome Expression Omnibus/ National Center for Biotechnology Information with accession number GSE15575.
RT-PCR and qRT-PCR.
1μg total RNA was reverse transcribed using qScript cDNA Supermix (Quanta BioSciences). After 35 cycles of amplification, agarose gel visualization was performed for PCR products. Gene expression was determined by qRT-PCR performed in triplicate using the QuantiNova SYBR Green PCR Kit (Qiagen) using gene specific primers (Supplementary Table 1). The relative expression level of each target gene was quantified using (2−ΔΔCt) comparing to 18s rRNA (Livak and Schmittgen 2001). Expression level was determined as a mean of the triplicates.
Immunofluorescence.
HTR-8/SVneo cells were grown on Millicell EZ slides (MilliporeSigma). After reaching confluency, cells were fixed in 4% paraformaldehyde (PFA) at room temperature for 10 minutes and then stained to detect Notch protein expression (Supplementary Methods). Images were captured with a Keyence BZ-X710 fluorescence microscope (Keyence).
Cell viability assay.
The MTT Cell Proliferation Kit (Roche) was used to assess cell viability. HTR-8/SVneo cells were seeded in a 24 well dish at 5×103 cells per well and incubated at 37˚C until confluent. To examine the effect of DAPT in normoxic conditions, HTR-8/SVneo cells were exposed to 25μM DAPT, 50μM DAPT, or DMSO in 21% O2 for 24 hours. To examine the effect of hypoxia, cells were exposed to 2.5% O2 for 24 hours. To examine the effect of 25μM DAPT in hypoxic conditions, HTR-8/SVneo cells were exposed to 25μM DAPT in 2.5% O2 for 24 hours. Following treatment, the MTT assay was initiated as per protocol. Percent cell viability was calculated using the formula (mean absorbance of treatment group/mean absorbance of control group) × 100.
Cell proliferation.
HTR-8/SVneo cells were grown on Millicell EZ slides at 37˚C until 50% confluence. Cells were exposed to either 2.5% O2, 25μM DAPT in 21% O2, or 25μM DAPT in 2.5% O2 for 24 hours. Proliferation was determined by Ki67 staining of HTR-8/SVneo cells. Two independent observers counted the number of Ki67 positive cells and the total number of cells per field in three 40x magnification fields per slide. Percentage of proliferating cells was determined as the number of Ki67 positive cells per total cell count × 100.
Western blot.
For HTR-8/SVneo cells, cold RIPA cell lysis buffer (Boster Bio), containing protease inhibitors (Roche) was added directly to the cells to prepare lysates. For placentas, cold RIPA cell lysis buffer containing protease inhibitors was added and tissues homogenized on ice and centrifuged for 20 min at 12,000 rpm at 4°C. Protein was measured using BCA protocol (Supplementary Methods). Band intensity was determined by densitometric with ImageJ software (http://rsb.info.nih.gov/ij/) and normalized relative to alpha tubulin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Migration assays.
Scratch migration and transwell migration assays were performed as described (Liang et al. 2007, Yue et al. 2010). For the scratch assays, HTR-8/SVneo cells were grown to confluency in 6 well dishes. To assure measurement of the same location in the scratch, dishes were marked and then scratched with a 200 μL pipette tip. After scratching, the wells were rinsed with PBS to remove non-adherent cells. To determine the impact of hypoxia, cells were continuously exposed to normoxia (21% O2) or hypoxia (2.5% O2) for 6 or 24 hours. To determine the impact of γ-secretase inhibition on HTR-8/SVneo cell migration, following a scratch, cells were incubated continuously with 25μM DAPT or equivalent amounts of DMSO for 6 or 24 hours. Cells were imaged at the time of the scratch (0 hours) and at the end of each individual experiment, 6 and 24 hours, using the EVOS FL Auto 2 (Invitrogen). To measure the distance traveled, cells bordering the open area were traced using ImageJ software. The scratch width was calculated from the mean of 10 random horizontal measurements. Percent wound closure was calculated as the difference of the scratch width at 0 hours compared to 6 or 24 hours.
For transwell migration assays, 24-well transwell chambers (Corning) and Falcon transparent cell culture inserts (8 μm pore size, Corning) were used. The upper compartment contained RPMI at 0.5% FBS and the lower compartment of the insert contained RPMI and 5% FBS as a chemoattractant. To determine the effect of hypoxia on cell migration, 1×105 cells were resuspended in hypoxic or normoxic RPMI media with 0.5% FBS and added to the upper compartment of the insert. Transwell chambers were then exposed to normoxia (21% O2) or hypoxia (2.5% O2) for 6 or 24 hours. To determine the effect of inhibiting γ-secretase in normoxic conditions, 1×105 cells were resuspended in RPMI media with 0.5% FBS and 25μM DAPT or equivalent amounts of DMSO and added to the upper compartment. The extent of cell migration was assessed at 6 and 24 hours. To determine the effect of inhibiting γ-secretase in hypoxic conditions, cells were seeded as described above and transwell chambers were then exposed to hypoxia (2.5% O2) for 24 hours. Non-migrated cells in the upper compartments were removed with a cotton swab. Cells on the undersurface of the membrane were fixed with 4% PFA, stained with H&E. Slides were imaged using the Keyence BZ-X710 fluorescence microscope at 10x magnification. The extent of cell migration was determined by counting nuclei of cells that migrated in a 1400 × 1400 pixel area of the insert using ImageJ software.
Invasion assays.
Invasion assays were performed in 24-well transwell chambers using 8 μm pore size cell culture inserts (Corning). Cell culture inserts were coated with RPMI media with 0.3 mg/mL Matrigel™ (phenol red-free, growth factor reduced basement membrane matrix, LOT 8050009, Corning) or RPMI media with 0.1mg/mL collagen I, rat tail (LOT 2103637, Gibco). Experiments were performed in exact same manner as described for the transwell migration assays except 6×104 cells (Matrigel™) or 2×104 cells (collagen I) were resuspended in experimental media and added to the upper compartment of the inserts and then exposed to normoxia (21% O2) or hypoxia (2.5% O2) for 24 hours.
Statistical analysis.
All experiments were performed at least 3 times with at least 3 technical replicates. Data were analyzed to identify outliers. For non-parametric data, medians were compared using the Wilcoxon rank sum or Mann Whitney U tests and data are expressed as medians with interquartile range (IQR). For normally distributed data, means were compared using Student’s t test and data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using Prism Version 8.0 (GraphPad). Statistical significance was defined as p<0.05.
Results
Exposure to hypoxia alters biological pathways
In vitro exposure to low oxygen tension of 1–5% O2 mimics the physiologic hypoxia of early pregnancy, alters gene expression and promotes cell invasion and migration in multiple trophoblast cell subtypes (Highet et al. 2015, Wu et al. 2019). To investigate the impact of hypoxia on HTR-8/SVneo gene expression, HTR-8/SVneo cells were exposed to 2.5% O2 for 6 hours, mimicking the uterine 1st trimester environment. Expression of hypoxia-responsive genes, ANGPTL4 and VEGFA, is increased in HTR-8/SVneo cells grown in hypoxic (2.5% O2) conditions as compared to normoxic (21% O2) conditions (Fig. 1A–C), demonstrating activation of the hypoxia pathway.
Figure 1. Exposure of HTR-8/Svneo cells to hypoxic (2.5% O2) conditions induces expression of genes involved in hypoxia signaling and alters multiple biological pathways.
HTR-8/SVneo cells were exposed to normoxic (21% O2) or hypoxic (2.5% O2) conditions for 6 hours. qRT-PCR was used to determine expression of hypoxia responsive genes, ANGPTL4 (A) and VEGFA (B). The relative level of expression was compared to 18s rRNA. Expression of ANGPTL4 (A) and VEGFA (B) was significantly increased in HTR-8/SVneo cells exposed to hypoxia as compared to cells exposed to normoxia (n = 5 – 6 per condition; **p<0.01). Data are represented as median + IQR. (C-E) mRNA-seq was used to identify differentially expressed genes, altered signaling pathways and altered biological processes. (C) Volcano plot of differentially expressed genes in HTR-8/SVneo cells after 6 hours exposure to hypoxia compared to normoxia. A total of 8353 genes are differently expressed based on an adjusted p<0.05, with 4184 upregulated and 4169 downregulated. ANGPTL4 and VEGFA are labeled. (D) Gene Ontology (GO) enrichment identified biological processes related to placentation. Left panel shows biological processes altered by upregulated genes. Right panel shows biological processes altered by downregulated genes. (E) Top 10 canonical, non-disease specific signaling pathways from Ingenuity Pathway Analysis.
We performed whole-genome transcriptome profiling (mRNA-seq) of HTR-8/SVneo cells exposed to hypoxic or normoxic conditions for 6 hours. Exposure to hypoxia resulted in 8353 differentially expressed genes with 4184 upregulated and 4169 downregulated (Fig. 1C). We used a combination of bioinformatics applications to identify biological processes and signaling pathways associated with differential gene expression. GO analysis shows that hypoxia induces genes that positively regulate processes related to placentation, including hypoxia, Notch signaling, vascular development, and cellular migration (Fig. 1D). Gene set enrichment analysis (GSEA) confirms enrichment of hypoxia, angiogenesis and Notch signaling pathways (Supplementary Fig. 2), while Ingenuity Pathway Analysis (IPA) reveals that HIF1 signaling is induced in HTR-8/SVneo cells exposed to hypoxia (Fig. 1E). These analyses support a relationship between hypoxia and Notch signaling and cell motility.
HTR-8/SVneo cells express Notch pathway transcripts and proteins
As the GO analysis and GSEA showed hypoxia-induced Notch signaling in HTR-8/SVneo, we determined the expression of specific Notch signaling components in HTR-8/SVneo cells grown in normoxic conditions. HTR-8/SVneo cells express NOTCH1, NOTCH2, NOTCH3, NOTCH4, DLL4, JAG1, and JAG2 (Fig. 2A). mRNA-seq showed that expression levels of NOTCH2, NOTCH3 and JAG1 is high, whereas NOTCH4 and DLL4, are expressed at low levels (Fig. 2A lower panels). Consistent with Notch signaling being active, HTR-8/SVneo cells express both HES1 and HEY1. Notch protein expression as detected by IF staining was consistent with mRNA expression. NOTCH1 NOTCH2, NOTCH3 and JAG1 proteins are expressed in all HTR-8/SVneo cells, and HES1 is expressed in a subset of HTR-8/SVneo nuclei (Fig. 1B–F). Together these data show that Notch genes are expressed and signaling active in HTR-8/SVneo cells, supporting a role for Notch signaling in HTR-8/SVneo under normoxic conditions.
Figure 2. Notch signaling is active in HTR-8/SVneo cells in normoxic (21% O2) conditions.
(A) RT-PCR and normalized hit counts from DESeq2 output were utilized to detect Notch family gene expression in untreated HTR-8/SVneo grown under normoxic conditions. Expression of receptors, NOTCH1 (N1), NOTCH2 (N2), NOTCH3 (N3) and NOTCH4 (N4); ligands, DLL4, JAG1 and JAG2; and effectors, HES1, HEY1 and HEY2 is shown. (B, C) Representative images of phase contrast and H&E stained HTR-8/SVneo cells. (D-H) Representative images of immunostained HTR-8/SVneo cells. IF identifies expression of NOTCH1, NOTCH2, NOTCH3, JAG1 and HES1 in HTR-8/SVneo cells. Scale bars = 100 μm in A and 50 μm in B, D-G.
Hypoxia alters expression of Notch signaling genes and proteins in HTR-8/SVneo cells
mRNA-seq analysis shows significant differential expression of 23 Notch pathway genes in HTR-8/SVneo cells exposed to hypoxia for 6 hours as compared to normoxia (Fig. 3A). Expression of Notch genes (NOTCH1, NOTCH2, and NOTCH3), ligands (DLL4 and JAG1) and effectors (HEY2 and NRARP) are significantly increased by hypoxia (Fig. 3A). qRT-PCR confirms increased mRNA expression of NOTCH1, JAG1, HEY2 and NRARP (Fig. 3B–E) and IF demonstrates increased protein expression of HES1 (Fig. 3F–H). These data confirm that hypoxia induces Notch signal activation in HTR-8/SVneo cells.
Figure 3. Exposure to hypoxia alters expression of genes in the Notch signaling pathway in HTR-8/SVneo cells.
HTR-8/SVneo cells were exposed to normoxic (21% O2) or hypoxic (2.5% O2) conditions for 6 hours and mRNA-seq was used to determine differentially expressed genes in the Notch signaling pathway. (A) Heatmap of normalized hit counts shows 36 differentially expressed genes in the Notch signaling pathway in HTR-8/SVneo cells after 6 hours exposure to hypoxia compared to normoxia. Columns represent individual samples (n=3) per condition and rows represent each gene. Color intensity represents gene expression, with blue indicating low expression levels and red indicating high expression levels. Red asterisks indicate the 23 Notch pathway genes that were significantly differentially expressed with FDR p-value < 0.05. (B-E) qRT-PCR was used to confirm hypoxia-induced Notch pathway gene expression changes identified by mRNA-seq. Expression of NOTCH1 (B), JAG1 (C), and effectors, HEY2 (D) and NRARP (E) was significantly increased in HTR-8/SVneo cells exposed to hypoxia (n = 4 – 9 per condition; *p <0.05, **p<0.01). Data are represented as median + IQR. (F, G) Representative IF images of HES1 protein expression in HTR-8/SVneo cells exposed to normoxia and hypoxia. (H) Expression of nuclear HES1 per total number of cells in 3 high powered fields was determined. Exposure to hypoxia for 6 hours significantly increased expression of HES1 protein in HTR-8/SVneo cells (n = 3 per condition; ****p<0.0001). Data are represented as mean ± SD. Scale bars = 50 μm in F and G.
Inhibition of γ-secretase downregulates Notch in HTR-8/SVneo cells
To assess the effect of inhibiting Notch signaling in HTR-8/SVneo, we used the γ-secretase inhibitor, DAPT, which prevents release of Notch from the cell surface and inhibits canonical Notch signaling. We first determined the effect of DAPT on cell viability by exposing HTR-8/SVneo cells to increasing concentrations of DAPT in normoxia. Exposure to 25μM DAPT for 24 hours in normoxic conditions (21% O2) does not impact HTR-8/SVneo viability, while it is compromised at 50μM DAPT (Fig. 4A). As 25μM DAPT does not compromise cell viability, it was chosen for future studies. To determine the effect of DAPT on cell growth under normoxic conditions, staining for Ki67 was performed. The percentage of HTR-8/SVneo cells expressing Ki67 is similar after exposure to DMSO (vehicle) or 25μM DAPT in normoxia, indicating that exposure to 25μM DAPT for 24 hours does not impact cellular proliferation (Fig. 4B). We next determined the effect of exposure to 25μM DAPT for 24 hours in normoxic conditions on Notch signal activation. Expression of γ-secretase cleaved-NOTCH1, the active form of NOTCH1, was determined by Western blot and expression of Notch effector, HES1, by qRT-PCR. Exposure to 25μM DAPT significantly decreased expression of cleaved-NOTCH1 (Fig. 4C–D) and HES1 (Fig. 4E), indicating that inhibition of γ-secretase with DAPT in normoxic conditions suppresses Notch signaling in HTR-8/SVneo cells.
Figure 4. Inhibition of γ-secretase in normoxic (21% O2) and hypoxic (2.5% O2) conditions reduces Notch signaling activity in HTR-8/SVneo cells.
(A) HTR-8/SVneo cells exposed to DMSO (vehicle control), 25μM DAPT or 50μM DAPT in normoxia for 24hrs were analyzed for viability by a MTT assay. Relative cell viability, normalized to viability in cells exposed to DMSO, was significantly decreased after exposure to 50μM DAPT, but did not differ after exposure to 25μM DAPT (n = 6 per condition, *p<0.05). (B) HTR-8/SVneo cells exposed to DMSO or 25μM DAPT in normoxia for 24hrs were analyzed for proliferation by Ki67 IF. Expression of nuclear Ki67 per total number of cells in 3 high powered fields was determined. Exposure to DAPT did not impact cellular proliferation, (n = 6 per condition). (C-E) HTR-8/SVneo cells exposed to DMSO or 25μM DAPT in normoxia were analyzed for expression of cleaved NOTCH1 (N1) and alpha tubulin by western blot and expression of HES1 by qRT-PCR. (C) Representative Western blots of cleaved NOTCH1 and alpha tubulin. (D) Expression of cleaved NOTCH1, relative to alpha tubulin, was decreased in HTR-8/SVneo cells treated with 25μM DAPT treated as compared to DMSO, (n = 4 per condition, **p<0.01). (E) HES1 expression was significantly decreased in cells treated with 25μM DAPT as compared to DMSO, (n = 6 per condition, **p<0.01). (F-J) HTR-8/SVneo cells grown in hypoxic conditions (2.5% O2) were exposed to DMSO or 25μM DAPT for 24hrs. (F) Exposure to 25μM DAPT in hypoxia did not impact cell viability. (G) Expression of nuclear Ki67 per total number of cells in 3 high powered fields was determined. Exposure to DAPT in hypoxia did not impact cellular proliferation, (n = 6 per condition). (H) Representative western blots of cleaved NOTCH1 and alpha tubulin. (I) Expression of cleaved NOTCH1, relative to alpha tubulin, was decreased in HTR-8/SVneo cells treated with 25μM DAPT in hypoxic conditions as compared to DMSO (n=6 per condition, **p<0.01). (J) Expression of HES1 was significantly decreased in cells treated with 25μM DAPT in hypoxic conditions as compared to DMSO (n=6 per condition, **p<0.01). All data are represented as median + IQR.
Given our interest in the impact of a hypoxic microenvironment on HTR-8/SVneo cells, we assessed the effect of exposure to DAPT in hypoxia for 24 hours on Notch signaling. Exposure of HTR-8/SVneo cells to hypoxia for 24 hours increases mRNA expression of HES1 (Supplementary Fig. 3A). These data show that hypoxia-mediated induction of Notch activity is maintained at 24 hours. HTR-8/SVneo cells were then exposed to 25μM DAPT in hypoxic conditions for 24 hours. Similar to normoxic conditions, exposure to 25μM DAPT in hypoxia does not impact cell viability (Fig. 4F), nor proliferation relative to vehicle treated cells (Fig. 4G). Exposure to 25μM DAPT in hypoxia significantly decreases expression of cleaved-NOTCH1 (Fig. 4H–I) and HES1 (Fig. 4J), as determined by Western blot and qRT-PCR, respectively. Thus, inhibition of γ-secretase in hypoxia blocks Notch signal activation in HTR-8/SVneo cells without effecting cell viability and growth.
HTR-8/SVneo directed cell migration is increased by hypoxia and decreased by γ-secretase inhibition
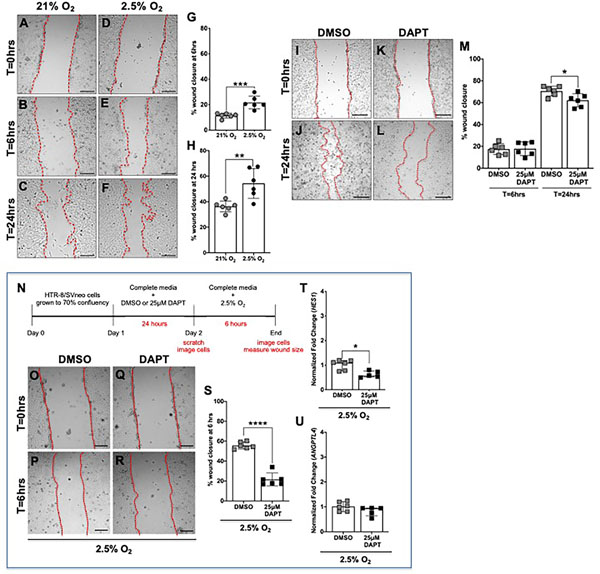
To determine if exposure to hypoxia or inhibiting γ-secretase impacts HTR-8/SVneo cell directed migration, scratch assays were performed (Fig. 5). We first determined that exposure to hypoxia for 24 hours does not impact HTR-8/SVneo cellular viability (Supplementary Fig. 3B) or proliferation (Supplementary Fig. 3C). To assess the impact of hypoxia on directed migration, HTR-8/SVneo cells were grown to confluence in a normoxic environment. A scratch was made and migration into the region monitored after exposure to normoxia or hypoxia (Fig. 5A–F). HTR-8/SVneo cell migration is significantly increased when cells are exposed to hypoxic, as compared to normoxic conditions for 6 and 24 hours (Fig. 5G, H).
Figure 5. Hypoxia increases and inhibition of γ-secretase decreases HTR-8/Svneo migration in both normoxia and hypoxia.
(A-F) Representative images of the scratch migration assay at time (T) = 0 hours, T=6 hours, T=24 hours after exposure to normoxia (21% O2). or hypoxia (2.5% O2). HTR-8/SVneo cell migration was significantly increased with exposure to hypoxia for 6 hours (G) and 24 hours (H), (n = 6 per condition, **p <0.01, *** p<0.001). Data are represented as mean ± SD. (I-L) Representative images of the scratch migration assay at T=0 hours and T=24 hours after exposure to DMSO or 25μM DAPT. (M) HTR-8/SVneo cell migration was unchanged with exposure to 25μM DAPT for 6 hours and significantly decreased with exposure to 25μM DAPT for 24 hours in normoxic conditions (n = 6 per condition, *p <0.05). Data are represented as mean ± SD. (N) Timeline representing experimental design. HTR-8/SVneo cells were grown to 70% confluency and then DMSO or DAPT was added to the culture media. After 24 hours of exposure to 0.1% DMSO or 25μM DAPT, the treated media was removed and replaced with complete media. Scratches were made and cells were imaged at T=0 hours. HTR-8/SVneo cells were exposed to hypoxia (2.5% O2) for 6 hours and then imaged again. (O-R) Representative images of the scratch migration assay at time (T) =0 hours and T=6 hours. (S) Compared to pretreatment with DMSO, pretreatment with DAPT significantly decreased migration of cells exposed to hypoxic conditions (n = 6 per condition, ****p<0.0001). Data are represented as mean ± SD. (T, U) Expression of HES1 and ANGPTL4 in DAPT and DMSO pretreated cells was determined by qRT-PCR. HES1 (T) expression was significantly decreased and ANGPTL4 (U) was similar in HTR-8/SVneo cells that were pretreated with DAPT and then exposed to 2.5% O2 (n = 5–6 per condition, *p <0.05). Data are represented as median + IQR. Scale bars = 250 μm.
To assess the impact of inhibiting γ-secretase on directed migration, HTR-8/SVneo cells were grown to confluence in a normoxic environment. After a scratch was made, cells were exposed to media containing 25μM DAPT or vehicle in normoxic conditions (Fig. 5I–L). HTR-8/SVneo cell migration is significantly decreased at 24 hours with exposure to 25μM DAPT as compared to vehicle in normoxia (Fig. 5M). These data show that hypoxia increases HTR-8/SVneo directional migration, whereas γ-secretase inhibition decreases Notch signaling and retards HTR-8/SVneo directional migration in normoxic conditions.
Inhibition of γ-secretase blocks hypoxia-induced HTR-8/SVneo cell migration
To understand the interaction between Notch and hypoxia in trophoblast migration, HTR-8/SVneo cells were pretreated with 25μM DAPT or vehicle for 24 hours, then a scratch was made and cells were exposed to hypoxic conditions for 6 hours (Fig. 5N). Hypoxia-induced migration of HTR-8/SVneo cells is significantly decreased after pretreatment with DAPT as compared to vehicle (Fig. 5O–S). Similar to HTR-8/SVneo cells exposed to DAPT in hypoxia for 24 hours (Fig. 4J), expression of HES1 is significantly reduced in HTR-8/SVneo pretreated with DAPT and then exposed to hypoxia for 6 hours (Fig. 5T). In contrast, expression of ANGPTL4 is unaffected in HTR-8/SVneo cells exposed to DAPT in hypoxic conditions (Fig. 5U), indicating that DAPT does impact induction of hypoxic responses in HTR-8/SVneo cells. The combination of decreased migration and decreased HES1 expression in HTR-8/SVneo cells exposed to DAPT/hypoxia, suggests that hypoxia requires Notch signal activation to promote directional migration in HTR-8/SVneo.
Growth factor induced migration of HTR-8/SVneo cells is increased by exposure to hypoxia and reduced by inhibition of γ-secretase in normoxia and hypoxia
To determine if migration towards a chemoattractant is impacted by exposure to hypoxia or inhibition of γ-secretase, transwell migration assays were performed. Exposure to hypoxic conditions during 24 hour transwell migration assays significantly increases HTR-8/SVneo cell migration, as compared to exposure to normoxia (Fig. 6A–C). Similar to directed migration, exposure to 25μM DAPT in normoxia for 24 hours (Figs. 6D–F), significantly decreases HTR-8/SVneo cell transwell migration relative to vehicle. These data show that exposure to hypoxia and inhibition of γ-secretase in a normoxic microenvironment have opposite effects on directional and growth factor induced migration of HTR-8/SVneo cells.
Figure 6. Growth factor induced migration of HTR-8/SVneo cells is increased by exposure to hypoxia and decreased by inhibition of γ-secretase in normoxia and hypoxia.
(A, B) Representative images of H&E-stained cells that migrated at T=24 hours after exposure to normoxic (21% O2) or hypoxic (2.5% O2) conditions. (C) HTR-8/SVneo cell transwell migration was unchanged with exposure to hypoxia for 6 hours. After 24 hours of exposure to hypoxia, HTR-8/SVneo cell transwell migration was significantly increased (n = 6 per condition, *p <0.05). (D, E) Representative images of H&E-stained cells that migrated at T=24 hours after exposure to DMSO or 25μM DAPT in normoxic conditions. (F) HTR-8/SVneo cell transwell migration was unchanged with exposure to 25μM DAPT for 6 hours and significantly decreased with exposure to 25μM DAPT for 24 hours (n = 6 per condition, ****p <0.0001). (G, H) Representative images of H&E-stained cells that migrated at T=24 hours after exposure DMSO or 25μM DAPT in hypoxic conditions. (I) HTR-8/SVneo cell transwell migration was significantly decreased with exposure to 25μM DAPT in hypoxic for 24 hours (n = 6 per condition, **** p<0.0001). All data are represented as mean ± SD. Scale bars = 250 μm.
To query the relationship between Notch and hypoxia in mediating trophoblast migration, we performed transwell migration assays in which HTR-8/SVneo cells were exposed to both 25μM DAPT and hypoxia for 24 hours. Exposure to DAPT in a hypoxic microenvironment significantly decreases HTR-8/SVneo cell migration as compared to exposure to vehicle (Figs. 6G–I). These data are consistent with a reduction of Notch signaling activity (Figs. 4H–J, 5T) and reduced directional migration with exposure to DAPT in hypoxia (Fig. 5O–S). The inability of hypoxia to induce cellular migration when γ-secretase is inhibited, suggests that hypoxia uses Notch activation to promote HTR-8/SVneo cell migration.
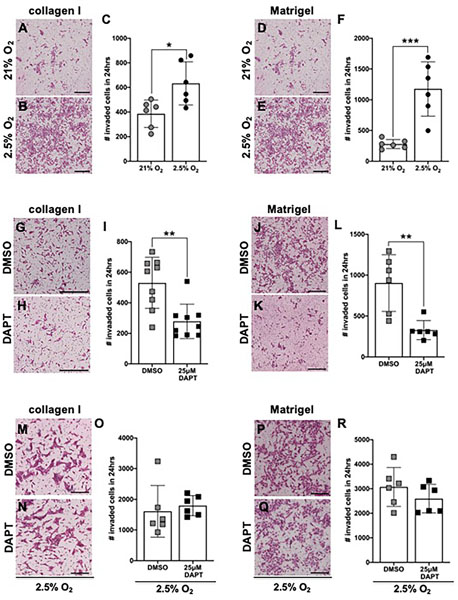
Inhibition of γ-secretase blocks HTR-8/SVneo cell invasion in normoxic conditions
During placentation, trophoblast invasion into the surrounding extracellular matrix is integral to and distinct from migration (Silva and Serakides 2016). Analysis of our mRNA-seq data for changes in expression of genes which are associated with trophoblast invasion (Peng et al. 2016) revealed that hypoxia significantly alters expression of MMP/TIMP genes (Supplementary Fig. 4). We sought to determine if exposure to hypoxia or inhibition of γ-secretase impacts HTR-8/SVneo cellular invasion through both collagen I and Matrigel matrices. Exposure to hypoxia, as compared to normoxia, for 24 hours significantly increases HTR-8/SVneo cell invasion through collagen I (Fig. 7A–C) and Matrigel™ (Fig. 7D–F). Exposure of HTR-8/SVneo cells to 25μM DAPT in normoxia for 24 hours significantly decreases HTR-8/SVneo cell invasion through collagen I (Fig. 7G–I) and Matrigel™ (Fig. 7J–L) matrices, as compared to vehicle. In contrast, invasion through both collagen I (Fig. 7M–O) and Matrigel (Fig. 7P–R) is similar in HTR-8/SVneo cells exposed to 25μM DAPT or vehicle in hypoxic conditions for 24 hours. Although inhibiting γ-secretase in both normoxic and hypoxic conditions significantly decreases Notch signaling activity (Fig. 4C–E, H–J), inhibiting γ-secretase did not perturb hypoxia-induced cell invasion.
Figure 7. Inhibition of γ-secretase blocks HTR-8/SVneo cell invasion in normoxic, but not hypoxic, conditions.
(A, B) Representative images of the H&E-stained cells that invaded through collagen I at T=24hrs after exposure to normoxic (21% O2) or hypoxic (2.5% O2) conditions. (C) HTR-8/SVneo cell invasion through collagen I was increased by exposure to hypoxia for 24 hours (n = 6 per condition, *p <0.05). (D, E) Representative images of the H&E-stained cells that invaded through Matrigel at T=24 hours after exposure to normoxic or hypoxic conditions. (F) HTR-8/SVneo cell invasion through Matrigel was increased by exposure to hypoxia for 24 hours (n = 6 per condition, ***p <0.001). (G, H) Representative images of the H&E-stained cells that invaded through collagen I at T=24 hours after exposure to DMSO or 25μM DAPT in normoxic conditions. (I) HTR-8/SVneo cell invasion through collagen I was decreased by exposure to 25μM DAPT in normoxia for 24 hours (n = 6 per condition, **p <0.01). (J, K) Representative images of the H&E-stained cells that invaded through Matrigel at T=24 hours after exposure to DMSO or 25μM DAPT in normoxic conditions. (L) HTR-8/SVneo cell invasion through Matrigel was decreased by exposure to 25μM DAPT in normoxia for 24 hours (n = 6 per condition, **p <0.01). (M, N) Representative images of the H&E-stained cells that invaded through collagen I at T=24 hours after exposure to DMSO or 25μM DAPT in hypoxic conditions. (O) HTR-8/SVneo cell invasion through collagen I was similar after exposure to 25μM DAPT and DMSO in hypoxia for 24 hours (n = 6 per condition). (P, Q) Representative images of the H&E-stained cells that invaded through Matrigel at T=24 hours after exposure to DMSO or 25μM DAPT in hypoxic conditions. (R) HTR-8/SVneo cell invasion through Matrigel was similar after exposure to 25μM DAPT and DMSO in hypoxia for 24 hours (n = 6 per condition). Scale bars = 500 μm. All data are represented as mean ± SD.
Expression of migration and invasion genes is decreased in CVS samples from preeclamptic pregnancies.
To correlate our in vitro studies with clinical pregnancy outcomes, we prospectively collected CVS tissues with the goal of comparing gene expression in primary placental tissue PE pregnancies to those from healthy, uncomplicated pregnancies. Six CVS samples, 2 from PE pregnancies (PE and PE-severe features (SF)) and 4 from healthy pregnancies (H1-H4) were of sufficient quality to analyze by mRNA-seq (Table 1).
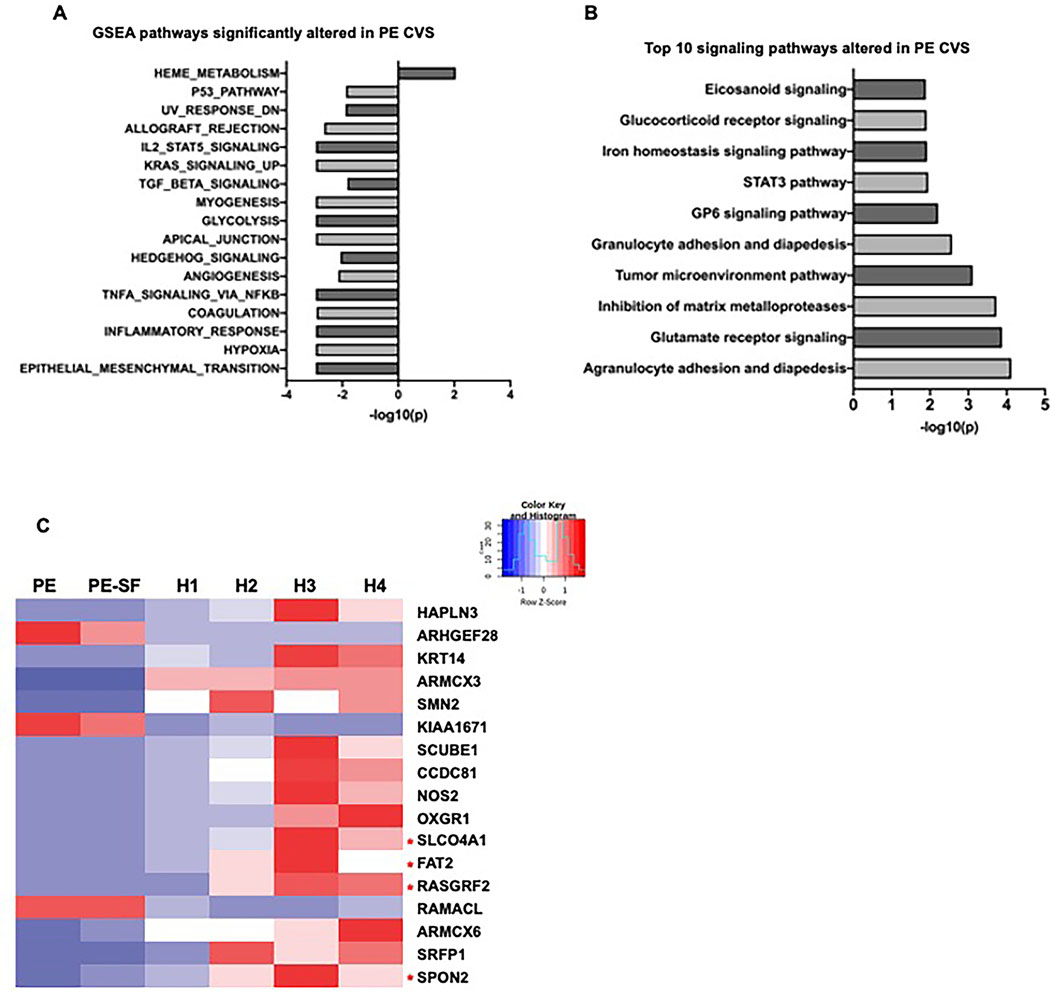
Comparison of the transcriptome profiles in CVS samples from PE to healthy pregnancies identified 396 significant DEGs, with p-value <0.05 and 17 significant DEGs, with FDR p-value < 0.05. To identify enriched pathways, the 396 DEGs were analyzed with GSEA and IPA. Genes in hypoxia and angiogenesis pathways are downregulated (Fig. 8A and Supplementary Fig. 5), and genes in pathways related to inhibition of MMPs are upregulated (Fig. 8B) in CVS from PE pregnancies as compared to healthy pregnancies. We next assessed for altered expression of migration and invasion genes, as well as Notch. We found that Notch signaling components are expressed in CVS samples from healthy pregnancies (Supplementary Fig. 6A). NOTCH2, NOTCH3 and JAG1 are expressed at high levels, whereas NOTCH4 and DLL4 are expressed at low levels. Consistent with Notch signaling being active, HES1 is expressed and expression of Notch signaling components in CVS samples is similar to that in HTR-8/Svneo cells (Fig. 2A). Comparison of normalized hit count in CVS from PE pregnancies relative to healthy pregnancies, demonstrated lower expression of NOTCH1, NOTCH2, and JAG1 in both PE pregnancies, but did not reveal significant differential expression for Notch pathway genes (Supplementary Fig. 6B). Of the 17 differentially expressed genes, 4 genes, FAT2, RASGRF2, and SCLO4A1, and SPON2 are involved in cellular migration and invasion in many cell types (Matsui et al. 2008, Xu et al. 2015, Yu et al. 2018, Lu et al. 2019b, Kang et al. 2020, Lu et al. 2020). Expression of all 4 genes is decreased in CVS samples from PE pregnancies, as compared to healthy pregnancies. These data suggest a link between decreased expression of cellular motility genes in PE CVS samples with development of PE later in pregnancy.
Figure 8. Cellular motility gene expression is altered in CVS samples from preeclamptic pregnancies.
mRNA-seq analysis of CVS samples from PE pregnancies (n=2, PE and PE-SF) and healthy pregnancies (n=4, H1-H4) identified 396 differentially expressed genes with p < 0.05. Gene set enrichment analysis hallmark pathways (A) and significant canonical non-disease specific signaling pathways from Ingenuity Pathway Analysis (B) from differentially expressed genes with p < 0.05. mRNA-seq analysis of the CVS samples was used to determine differentially expressed genes, with FDR p-value < 0.05. (B) Heatmap of normalized hit counts shows the 17 significant differentially expressed genes in 2nd trimester CVS samples from PE versus healthy pregnancies, 4 of 17 differentially expressed genes (red asterisks) are involved in cellular migration and invasion. Expression of FAT2, SPON2, RASGRF2, and SCLO4A1 is decreased in CVS samples from PE pregnancies.
Expression of JAG1 is decreased in placentas from preeclamptic pregnancies
We sought to compare Notch protein expression in 3rd trimester primary placental tissue samples from healthy, uncomplicated term pregnancies to those from preterm PE pregnancies. We collected placental tissues within 10 minutes after Caesarean delivery from 4 healthy, uncomplicated pregnancies delivered at term (≥ 37 weeks of gestation) and 4 pregnancies delivered preterm (<37 weeks of gestation) because of PE with severe features (PE-SF; Table 2). Western blot shows similar expression of NOTCH1, NOTCH2, and NOTCH3 in preterm, PE-SF and term, healthy placentas (Fig. 9A–C, E–G). In contrast, a significant decrease in the expression of JAG1 was observed in preterm, PE-SF as compared to term, healthy placentas (Fig. 9D, H). Our data are in concert with previous studies that link reduced JAG1 expression and failed vascular remodeling in PE (Hunkapiller et al. 2011).
Figure 9. Expression of Notch proteins is decreased in placentas from pregnancies with preterm, preeclampsia with severe features.
Expression of Notch proteins was determined by western blot. Representative western blots of NOTCH1 and GAPDH (A), NOTCH2 and GAPDH (B), NOTCH3 and GAPDH (C), and JAG1 and GAPDH (D) from placenta samples from healthy pregnancies delivered at term, ≥37 weeks of gestation (n=4) and pregnancies complicated by preeclampsia with severe features (PE-SF) delivered preterm, < 37 weeks of gestation (n=4). (E-G) Expression of NOTCH1, NOTCH2 and NOTCH3 was not statistically different in healthy and PE-SF placentas (n=4 per condition). (H) Expression of JAG1 is significantly lower in placenta samples from PE-SF pregnancies (n=4 per condition, *p =0.03). Data are represented as median + IQR.
Discussion
Abnormal expression of Notch proteins has been described in PE placentas from 3rd trimester pregnancies (Cobellis et al. 2007, Zhao et al. 2013, Lacko et al. 2014, Fragkiadaki et al. 2015, Haider et al. 2017, Wang and Zou 2020). Consistent with these reports, we found significantly lower levels of JAG1 in 3rd trimester placental tissues from pregnancies delivered preterm due to early onset PE with severe features. Though these data implicate Notch signaling in the pathogenesis of PE, it is unclear if and how Notch functions in the early 1st trimester when the placenta is forming. Our mRNA-seq analysis of 2nd trimester CVS samples shows a decrease in genes involved in migration and invasion in PE CVS, suggesting defects in trophoblast motility in the first half of pregnancy contribute to the development of PE. To address the outstanding question as to whether altered Notch is a consequence or a cause of PE with severe features, we examined the role of Notch signaling in hypoxia-induced motility of HTR-8/SVneo 1st trimester trophoblast cells. Using DAPT, an inhibitor of Notch signal activation, we found that Notch signaling mediates hypoxia-induced HTR8/SVneo migration, but not invasion, suggesting that hypoxia-induced trophoblast migration is mediated via Notch signaling during human placenta formation. Together, these data suggest that defective Notch signaling that initiates in 1st trimester trophoblasts impairs hypoxia-induced migration and thus, contributes to the development of PE.
In mice, we previously found the expression of multiple Notch proteins and Notch activation in trophoblasts that invade maternal spiral arteries (Levin et al. 2017). Consistent with the murine data, we found that the human trophoblast cell line, HTR-8/SVneo and CVS samples from healthy pregnancies express NOTCH2, NOTCH3, JAG1, and the effector, HES1. Similarly, Notch signaling in multiple trophoblast cell subtypes has been described for placental tissues obtained from 1st and 2nd trimester pregnancies (Herr et al. 2011, Haider et al. 2014, Haider et al. 2016, Haider et al. 2017). Together these data suggest a role for trophoblast Notch signaling in early physiological placentation. Supporting the hypothesis that altered Notch signaling contributes to placentation defects, mice with Notch2 deletion in trophoblasts have poor invasion of the maternal spiral arteries which later leads to a narrowing of the maternal arteries, poor perfusion to the embryo and embryonic death; all hallmarks of PE (Hunkapiller et al. 2011).
Our IPA and GO analysis indicate that exposure of HTR-8/SVneo cells to hypoxia (2.5% O2) activates pathways associated with HIF1α and cellular motility. In culture, exposure of HTR-8/SVneo cells to hypoxic conditions promoted both directional and growth factor-induced cellular migration, as well as cellular invasion into two different matrices, which is consistent with a prior report (Chaudhary et al. 2019). However, hypoxia has also been shown to suppress invasion (Kilburn et al. 2000, Tamaru et al. 2015). The lack of consistency as to how hypoxia impacts HTR-8/SVneo invasion likely stems from the methods used, which includes variable Matrigel concentrations, oxygen tension, and length of hypoxia exposure.
Consistent with our in vitro motility assays, HTR-8/SVneo cells exposed to hypoxia induce genes involved in cellular invasion, including the induction of MMP2 and MMP9 which are involved in extracellular matrix restructuring during vascular remodeling in early pregnancy (Isaka et al. 2003, Chen and Khalil 2017). In mice, loss of MMP9 impairs trophoblast differentiation and invasion, resulting in pregnancies with IUGR and maternal features of PE (Plaks et al. 2013). In humans, aberrant placental MMP expression is associated with PE (Lockwood et al. 2008, Luizon et al. 2013, Sahay et al. 2018). Finally, induction of MMP2 and MMP9 increases HTR-8/SVneo migration and invasion in vitro (Peng et al. 2016). As Notch induces MMP2 and MMP9 expression in human endothelial cells (Funahashi et al. 2011), Notch may mediate hypoxia-induced MMP2 and MMP9 upregulation in HTR-8/SVneo.
Our data demonstrate that hypoxia activates Notch signaling to promote HTR-8/SVneo cell motility. We found that exposure to hypoxia and inhibiting Notch signaling with addition of a γ-secretase inhibitor in a normoxic microenvironment have opposite effects on HTR-8/SVneo motility; hypoxia increases, while inhibition of γ-secretase decreases migration and invasion. In a hypoxic microenvironment, inhibition of γ-secretase blunts HTR-8/SVneo migration but has no impact on hypoxia-induced invasion. Our findings are consistent with Luo et al. who recently reported that DAPT decreases, while DLL4-NOTCH signaling increases HTR-8/SVneo migration and invasion (Luo et al. 2020). In contrast, DAPT treatment of SGHPL-5, another placental cell line with features of invasive trophoblasts, increased their migration in transwell migration assays (Bilban et al. 2010, Haider et al. 2014). Both SGHPL-5 and HTR-8/SVneo were generated by SV40 Large T Antigen transfection of first trimester placental tissue and similar gene expression signatures have been reported (Bilban et al. 2010), thus the origin of these contrasting results is unclear.
Defective EVT invasion and inappropriate remodeling of uterine spiral arteries are thought to have the “ripple effect” of impaired placental perfusion, resulting in placental ischemia that is associated with complications of pregnancy (Burton et al. 2010, Pringle et al. 2010, Burton et al. 2021). IPA to assess biological significance of differentially expressed transcripts in CVS samples from 4 healthy pregnancies and 2 PE pregnancies revealed decreased expression of genes in hypoxia and angiogenic pathways and increased expression of genes in pathways related to inhibition of matrix metalloproteinases in CVS samples from PE pregnancies. mRNA-seq analysis of our limited sample size revealed that genes, including SCLO4A1, involved in cellular migration and invasion in multiple cell types are decreased in CVS samples from PE pregnancies. Our findings were consistent with Founds et al who observed a significant decrease in the expression of SCLO4A1 in CVS samples from PE pregnancies relative to uncomplicated pregnancies (Founds et al. 2009).
Herein, we show that hypoxia induces Notch signaling activation required for 1st trimester HTR-8/SVneo cell migration, but not invasion, suggesting separate roles for Notch in migration and invasion in the hypoxic microenvironment of early pregnancy. Future longitudinal studies are needed to determine if Notch expression is altered in early placental tissues from pregnancies that go on to develop early onset PE that necessitates preterm delivery.
Supplementary Material
Acknowledgements
We thank Dr. Sara Morelli and Dr. Pranela Rameshwar for their insightful comments and assistance with design of the project.
Funding
This work was supported by the National Institutes of Health, National Heart Lung and Blood Institute [grant no. R01HL127013 to N.C.D.].
Footnotes
Declaration of interest
The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Artavanis-Tsakonas S, Rand MD & Lake RJ 1999. Notch signaling: cell fate control and signal integration in development. Science, 284, 770–776. [DOI] [PubMed] [Google Scholar]
- Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, Wagner O & Knofler M 2010. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta, 31, 989–996. [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Lauth M, Zwijsen A, Huylebroeck D, Oswald F & Giaimo BD 2016. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFbeta/BMP and hypoxia pathways. Biochimica et Biophysica Acta, 1863, 303–313. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Cindrova-Davies T, Yung HW & Jauniaux E 2021. Hypoxia and Reproductive Health: Oxygen and development of the human placenta. Reproduction, 161, F53–F65. [DOI] [PubMed] [Google Scholar]
- Burton GJ & Jauniaux E 2018. Pathophysiology of placental-derived fetal growth restriction. American Journal of Obstetrics and Gynecology, 218, S745–S761. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E & Charnock-Jones DS 2010. The influence of the intrauterine environment on human placental development. The International Journal of Developmental Biology, 54, 303–312. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E & Watson AL 1999. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. American Journal of Obstetrics and Gynecology, 181, 718–724. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Babu GS, Sobti RC & Gupta SK 2019. HGF regulate HTR-8/SVneo trophoblastic cells migration/invasion under hypoxic conditions through increased HIF-1alpha expression via MAPK and PI3K pathways. Journal of Cell Communication and Signaling, 13, 503–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J & Khalil RA 2017. Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Progress in Molecular Biology and Translational Science, 148, 87–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobellis L, Mastrogiacomo A, Federico E, Schettino MT, De Falco M, Manente L, Coppola G, Torella M, Colacurci N & De Luca A 2007. Distribution of Notch protein members in normal and preeclampsia-complicated placentas. Cell and Tissue Research, 330, 527–534. [DOI] [PubMed] [Google Scholar]
- Cuman C, Menkhorst E, Winship A, Van Sinderen M, Osianlis T, Rombauts LJ & Dimitriadis E 2014. Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction, 147, R75–86. [DOI] [PubMed] [Google Scholar]
- Ewels PA, Peltzer A, Fillinger S, Patel H, Alneberg J, Wilm A, Garcia MU, Di Tommaso P & Nahnsen S 2020. The nf-core framework for community-curated bioinformatics pipelines. Nature Biotechnology, 38, 276–278. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA & Conrad KP 2009. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta, 30, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkiadaki P, Soulitzis N, Sifakis S, Koutroulakis D, Gourvas V, Vrachnis N & Spandidos DA 2015. Downregulation of notch signaling pathway in late preterm and term placentas from pregnancies complicated by preeclampsia. PLoS One, 10, e0126163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi Y, Shawber CJ, Sharma A, Kanamaru E, Choi YK & Kitajewski J 2011. Notch modulates VEGF action in endothelial cells by inducing Matrix Metalloprotease activity. Vascular Cell, 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperowicz M & Otto F 2008. The notch signalling pathway in the development of the mouse placenta. Placenta, 29, 651–659. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, Macdougall JR, Kerbel RS, Khoo N & Lala PK 1993. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Experimental Cell Research, 206, 204–211. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U & Bondesson M 2005. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Developmental Cell, 9, 617–628. [DOI] [PubMed] [Google Scholar]
- Haider S, Meinhardt G, Saleh L, Fiala C, Pollheimer J & Knofler M 2016. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proceedings of the National Academy of Sciences of the United States of America, 113, E7710-E7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Meinhardt G, Velicky P, Otti GR, Whitley G, Fiala C, Pollheimer J & Knofler M 2014. Notch signaling plays a critical role in motility and differentiation of human first-trimester cytotrophoblasts. Endocrinology, 155, 263–274. [DOI] [PubMed] [Google Scholar]
- Haider S, Pollheimer J & Knofler M 2017. Notch signalling in placental development and gestational diseases. Placenta, 56, 65–72. [DOI] [PubMed] [Google Scholar]
- Herr F, Schreiner I, Baal N, Pfarrer C & Zygmunt M 2011. Expression patterns of Notch receptors and their ligands Jagged and Delta in human placenta. Placenta, 32, 554–563. [DOI] [PubMed] [Google Scholar]
- Highet AR, Buckberry S, Mayne BT, Khoda SM, Bianco-Miotto T & Roberts CT 2016. First trimester trophoblasts forming endothelial-like tubes in vitro emulate a ‘blood vessel development’ gene expression profile. Gene Expression Patterns, 21, 103–110. [DOI] [PubMed] [Google Scholar]
- Highet AR, Khoda SM, Buckberry S, Leemaqz S, Bianco-Miotto T, Harrington E, Ricciardelli C & Roberts CT 2015. Hypoxia induced HIF-1/HIF-2 activity alters trophoblast transcriptional regulation and promotes invasion. European Journal of Cell Biology, 94, 589–602. [DOI] [PubMed] [Google Scholar]
- Highet AR, Zhang VJ, Heinemann GK & Roberts CT 2012. Use of Matrigel in culture affects cell phenotype and gene expression in the first trimester trophoblast cell line HTR8/SVneo. Placenta, 33, 586–588. [DOI] [PubMed] [Google Scholar]
- Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC & Fisher SJ 2011. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development, 138, 2987–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Gauster M, Orendi K, Konig J & Moser G 2009. Oxygen as modulator of trophoblast invasion. Journal of Anatomy, 215, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B & Peeters LL 2005. Vascular biology in implantation and placentation. Angiogenesis, 8, 157–167. [DOI] [PubMed] [Google Scholar]
- Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF & Takayama M 2003. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta, 24, 53–64. [DOI] [PubMed] [Google Scholar]
- James JL, Carter AM & Chamley LW 2012. Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation? Placenta, 33, 327–334. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR & Chamley LW 2006. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Human Reproduction Update, 12, 137–144. [DOI] [PubMed] [Google Scholar]
- Kadesch T 2004Notch signaling: the demise of elegant simplicity. Current Opinion in Genetics and Development, 14, 506–512. [DOI] [PubMed] [Google Scholar]
- Kang HG, Kim WJ, Noh MG, Chun KH & Kim SJ 2020. SPON2 Is Upregulated through Notch Signaling Pathway and Promotes Tumor Progression in Gastric Cancer. Cancers, 12, 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R & Armant DR 2000. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biology of Reproduction, 62, 739–747. [DOI] [PubMed] [Google Scholar]
- Knofler M, Haider S, Saleh L, Pollheimer J, Gamage T & James J 2019. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cellular and Molecular Life Sciences, 76, 3479–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J & Kitajewski J 2011. Notch signaling in developmental and tumor angiogenesis. Genes & Cancer, 2, 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovall RA 2007Structures of CSL, Notch and Mastermind proteins: piecing together an active transcription complex. Current Opinion in Structual Biology, 17, 117–127. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Deftos ML, Bevan MJ & Gridley T 2001. The Nrarp gene encodes an ankyrin-repeat protein that is transcriptionally regulated by the notch signaling pathway. Developmental Biology, 238, 110–119. [DOI] [PubMed] [Google Scholar]
- Lacko LA, Massimiani M, Sones JL, Hurtado R, Salvi S, Ferrazzani S, Davisson RL, Campagnolo L & Stuhlmann H 2014. Novel expression of EGFL7 in placental trophoblast and endothelial cells and its implication in preeclampsia. Mechanisms of Development, 133, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafoya B, Munroe JA, Mia MM, Detweiler MA, Crow JJ, Wood T, Roth S, Sharma B & Albig AR 2016. Notch: A multi-functional integrating system of microenvironmental signals. Developmental Biology, 418, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laresgoiti-Servitje E & Gomez-Lopez N 2012. The pathophysiology of preeclampsia involves altered levels of angiogenic factors promoted by hypoxia and autoantibody-mediated mechanisms. Biology of Reproduction, 87, 36. [DOI] [PubMed] [Google Scholar]
- Levin HI, Sullivan-Pyke CS, Papaioannou VE, Wapner RJ, Kitajewski JK, Shawber CJ & Douglas NC 2017. Dynamic maternal and fetal Notch activity and expression in placentation. Placenta, 55, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CC, Park AY & Guan JL 2007. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols, 2, 329–333. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK & Shi W 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics, 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF & Schatz F 2008. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biology of Reproduction, 78, 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W & Anders S 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Feng Y, Hu Y, Guo Y, Liu Y, Mao Q & Xue W 2020. Spondin 2 promotes the proliferation, migration and invasion of gastric cancer cells. Journal of Cellular and Molecular Medicine, 24, 98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu W, Xin Q, Zhou C, Wang J, Ni Z, Liu D, Xu Y, Yu Y, Yang N, et al. 2019aSpatiotemporal coordination of trophoblast and allantoic Rbpj signaling directs normal placental morphogenesis. Cell Death & Disease, 10, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Chen J, Yan L, Yang L, Zhang L, Dai J, Hao Z, Bai T, Xi Y, Li Y, et al. 2019bRasGRF2 promotes migration and invasion of colorectal cancer cells by modulating expression of MMP9 through Src/Akt/NF-kappaB pathway. Cancer Biology Therapy, 20, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luizon MR, Amaral LM & Palei AC 2013. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs genetic polymorphisms and plasma levels in hypertensive disorders of pregnancy. Journal of Human Hypertension, 27, 278–279. [DOI] [PubMed] [Google Scholar]
- Luo Q, Liu X, Zheng Y, Zhao Y, Zhu J & Zou L 2015. Ephrin-B2 mediates trophoblast-dependent maternal spiral artery remodeling in first trimester. Placenta, 36, 567–574. [DOI] [PubMed] [Google Scholar]
- Luo Q, Zhang W, Liu X, Zheng Y, Gao H, Zhao Y & Zou L 2020. Delta-Like 4-Notch signaling regulates trophoblast migration and invasion by targeting EphrinB2. Biochemical and Biophysical Research Communications, 527, 915–921. [DOI] [PubMed] [Google Scholar]
- Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R & Ish-Horowicz D 2001. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation, 69, 135–144. [DOI] [PubMed] [Google Scholar]
- Matsui S, Utani A, Takahashi K, Mukoyama Y, Miyachi Y & Matsuyoshi N 2008. Knockdown of Fat2 by siRNA inhibits the migration of human squamous carcinoma cells. Journal of Dermatological Science, 51, 207–210. [DOI] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Ebert D, Huang X & Thomas PD 2019. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Research, 47, D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JH, Lee CH, Ji YW, Yeo A, Noh H, Song I, Kim EK & Lee HK 2016. Activation of Dll4/Notch Signaling and Hypoxia-Inducible Factor-1 Alpha Facilitates Lymphangiogenesis in Lacrimal Glands in Dry Eye. PLoS One, 11, e0147846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Zhu H, Klausen C, Ma L, Wang YL & Leung PC 2016. GnRH regulates trophoblast invasion via RUNX2-mediated MMP2/9 expression. Molecular Human Reproduction, 22, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT & Salzberg SL 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature Biotechnology, 33, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R & Werb Z 2013. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proceedings of the National Academy of Sciences of the United States of America, 110, 11109–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessl K, Haider S, Fiala C, Pollheimer J & Knofler M 2015. Expression pattern and function of Notch2 in different subtypes of first trimester cytotrophoblast. Placenta, 36, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG & Roberts CT 2010. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Human Reproduction Update, 16, 415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, Mcmaster M & Fisher SJ 2004. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. Journal of Clinical Investigation, 114, 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts VHJ, Morgan TK, Bednarek P, Morita M, Burton GJ, Lo JO & Frias AE 2017. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: new insights from contrast-enhanced ultrasound and tissue histopathology. Human Reproduction, 32, 2382–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C & Jauniaux E 1992. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstetrics & Gynecology, 80, 283–285. [PubMed] [Google Scholar]
- Safran M & Kaelin WG Jr. 2003. HIF hydroxylation and the mammalian oxygen-sensing pathway. Journal of Clinical Investigation, 111, 779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay AS, Jadhav AT, Sundrani DP, Wagh GN, Mehendale SS & Joshi SR 2018. Matrix metalloproteinases-2 (MMP-2) and matrix metalloproteinases −9 (MMP-9) are differentially expressed in different regions of normal and preeclampsia placentae. Journal of Cellular Biochemistry, 119, 6657–6664. [DOI] [PubMed] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L & Lendahl U 2008. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proceedings of the National Academy of Sciences of the United States of America, 105, 6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawber CJ & Kitajewski J 2004. Notch function in the vasculature: insights from zebrafish, mouse and man. BioEssays, 26, 225–234. [DOI] [PubMed] [Google Scholar]
- Silva JF & Serakides R 2016. Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adhesion & Migration, 10, 88–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru S, Mizuno Y, Tochigi H, Kajihara T, Okazaki Y, Okagaki R, Kamei Y, Ishihara O & Itakura A 2015. MicroRNA-135b suppresses extravillous trophoblast-derived HTR-8/SVneo cell invasion by directly down regulating CXCL12 under low oxygen conditions. Biochemical and Biophysical Research Communications, 461, 421–426. [DOI] [PubMed] [Google Scholar]
- Velicky P, Haider S, Otti GR, Fiala C, Pollheimer J & Knofler M 2014. Notch-dependent RBPJkappa inhibits proliferation of human cytotrophoblasts and their differentiation into extravillous trophoblasts. Molecular Human Reproduction, 20, 756–766. [DOI] [PubMed] [Google Scholar]
- Wang R & Zou L 2020. Downregulation of LncRNA-MEG3 promotes HTR8/SVneo cells apoptosis and attenuates its migration by repressing Notch1 signal in preeclampsia. Reproduction, 160, 21–29. [DOI] [PubMed] [Google Scholar]
- Wu D, Chen X, Wang L, Chen F, Cen H & Shi L 2019. Hypoxia-induced microRNA-141 regulates trophoblast apoptosis, invasion, and vascularization by blocking CXCL12beta/CXCR2/4 signal transduction. Biomedicine & Pharmacotherapy, 116, 108836. [DOI] [PubMed] [Google Scholar]
- Xu J, Chi F, Guo T, Punj V, Lee WN, French SW & Tsukamoto H 2015. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. Journal of Clinical Investigation, 125, 1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang J, Gong Y, Liu X, Bai Y, Xu W & Zhou R 2015. Increased expression of prostasin contributes to early-onset severe preeclampsia through inhibiting trophoblast invasion. Journal of Perinatology, 35, 16–22. [DOI] [PubMed] [Google Scholar]
- Yu J, Han Z, Sun Z, Wang Y, Zheng M & Song C 2018. LncRNA SLCO4A1-AS1 facilitates growth and metastasis of colorectal cancer through beta-catenin-dependent Wnt pathway. Journal of Experimental & Clinical Cancer Research, 37, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue PY, Leung EP, Mak NK & Wong RN 2010. A simplified method for quantifying cell migration/wound healing in 96-well plates. Journal of Biomolecular Screening, 15, 427–433. [DOI] [PubMed] [Google Scholar]
- Zhao WX, Huang TT, Jiang M, Feng R & Lin JH 2013. Expression of Notch Family Proteins in Placentas From Patients With Early-Onset Severe Preeclampsia. Reproductive Sciences, 21,716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.