To the Editor:
The Food and Drug Administration (FDA) recently approved pembrolizumab, a programmed death 1 (PD-1) blocking antibody, for patients who have treatment-refractory cancers with a tumor mutational burden greater than 10 mutations per megabase.1 This approval, for treatment of any type of solid tumor, was based on retrospective evidence from a study showing that high tumor mutational burden was predictive of a favorable radiographic response to treatment with immune checkpoint inhibitors in patients with 10 different rare cancers.2 Of note, high tumor mutational burden did not predict improved overall survival after treatment with immune checkpoint inhibitors, nor did the study evaluate other major tumor types or the cause of the high-mutation phenotype.
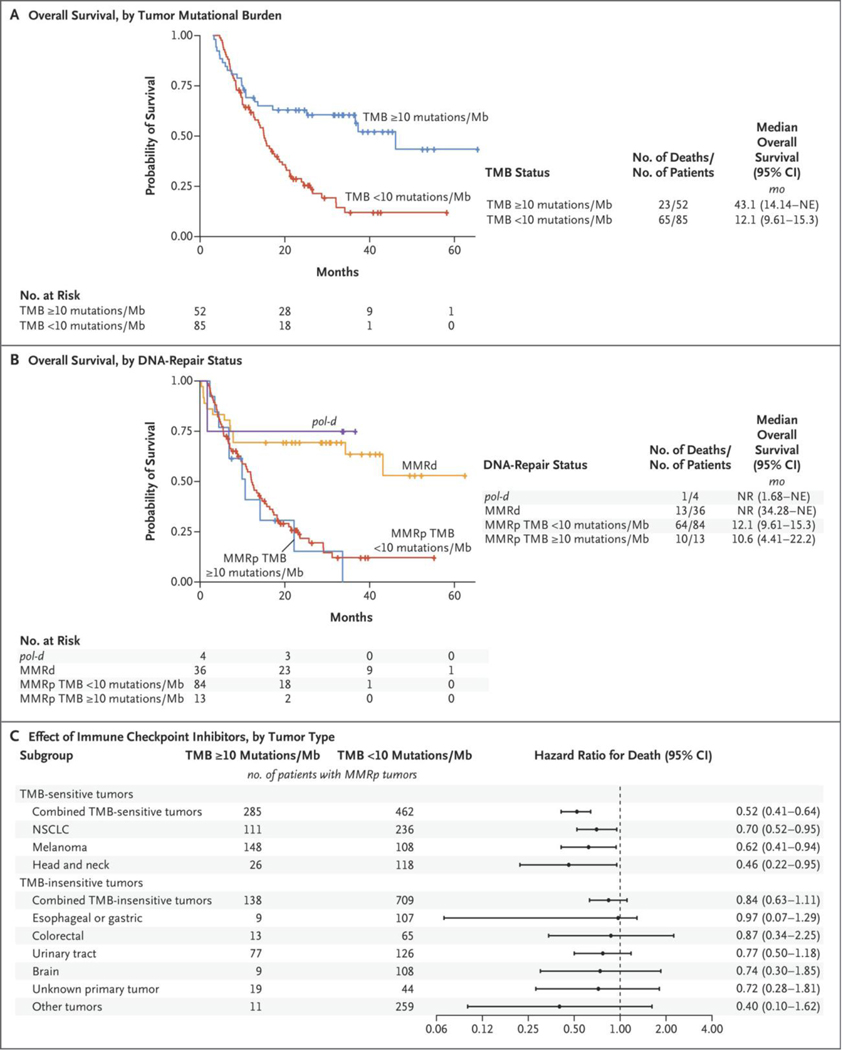
Since this approval would affect approximately 18% of cases of advanced colorectal cancer (Table S1 in Supplementary Appendix 2 [legend in Supplementary Appendix 1]), we evaluated 137 patients with advanced colorectal cancer who were treated with immune checkpoint inhibitors at our institution. Median overall survival was longer in patients with high tumor mutational burden (≥10 mutations per megabase) than in patients with low tumor mutational burden (hazard ratio for death, 0.40; 95% confidence interval [CI], 0.24 to 0.65) after treatment with immune checkpoint inhibitors (Fig. 1A). However, after cohort stratification by mismatch-repair–deficiency status or by status with respect to pathogenic mutations in polymerase ε (POLE) or polymerase δ1 (POLD1) (collectively referred to as pol-d), the difference in overall survival among patients with mismatch-repair–proficient tumors with high tumor mutational burden as compared with low tumor mutational burden disappeared (hazard ratio for death, 1.17; 95% CI, 0.59 to 2.32) (Fig. 1B).
Figure 1. Effect of Immune Checkpoint Inhibitors on Survival in Patients with Tumors with High Tumor Mutational Burden.
Shown are Kaplan–Meier plots of overall survival in 137 patients with advanced colorectal cancer according to tumor mutational burden (TMB; Panel A) and the same cohort of patients with advanced colorectal cancer stratified according to tumor mutational burden, mismatch-repair–deficient (MMRd) and mismatch-repair–proficient (MMRp) status, and pol-deficient (pol-d) status (Panel B). A subgroup analysis (Panel C) shows hazard ratios for death according to tumor type in a separate cohort of 1661 patients with MMRp tumors treated with immune checkpoint inhibitors. Patients with various tumor types were combined into two separate models according to whether tumor mutational burden of 10 or more mutations per megabase significantly predicted benefit from immune checkpoint inhibitors when accounting for mismatch-repair–deficient and pol-d status. Other tumors include kidney, breast, and neuroendocrine tumors, uveal melanoma, and mucosal melanoma (see the Methods Section, Table S4 in Supplementary Appendix 2 [legend in Supplementary Appendix 1]). All hazard ratios were calculated with the use of Cox proportional-hazards univariate regression. CI denotes confidence interval, Mb megabase, NE could not be estimated, NR not reached, and NSCLC non–small-cell lung cancer.
Extension of this analysis to 1661 patients with various tumors treated with immune checkpoint inhibitors3 showed that tumor mutational burden of 10 or more mutations per megabase was associated with improved overall survival in a limited subgroup of patients with mismatch-repair–proficient tumors (Fig. 1C and Table S4) when stratified according to tumor type. Only patients with metastatic head and neck cancer, non–small-cell lung cancer, and melanoma had improved overall survival (hazard ratio for death, 0.52; 95% CI, 0.31 to 0.64). No benefit was seen in patients with 10 other tumor types in which mismatch repair or pol-d was intact (hazard ratio for death, 0.84; 95% CI, 0.63 to 1.11) (Fig. 1C).
Mismatch-repair deficiency is a well-established biomarker of improved overall survival after treatment with immune checkpoint inhibitors,4 and pol-d status may also predict benefit from immune checkpoint inhibitors.5 We observed that other than patients with these two genetic subtypes, the only patients with hyper-mutated tumors who benefited from immune checkpoint inhibitors had cancers strongly associated with environmental carcinogens — chronic exposure to ultraviolet radiation or tobacco.
The current FDA approval granted on the basis of tumor mutational burden may be too broad, and immune checkpoint inhibitors should be considered in the context of the cause of the high tumor mutational burden and not based solely on an absolute threshold. This approval, given purely on the basis of response rate, also neglects more meaningful clinical end points, including survival and quality of life, and slows the development of more effective therapies for this patient population.
Supplementary Material
This week’s letters.
| 1168 | Checkpoint Blockade in Hypermutated Tumors |
| 1170 | Monoclonal Antibody for Patients with Covid-19 |
| 1171 | Drug-Coated Balloons for Dysfunctional Dialysis Arteriovenous Fistulas |
| 1174 | Olaparib in Metastatic Castration-Resistant Prostate Cancer |
| 1176 | Systemic Therapy for Estrogen Receptor–Positive, HER2-Negative Breast Cancer |
| 1177 | Correcting “Stolen Breaths” |
Acknowledgments
Supported by Nuovo Soldati, by grants (T32-CA009512, CA252519, P30 CA008748, and K12CA184746) from the National Institutes of Health, by Swim Across America, by a grant (SU2C-AACR-DT22-17) from the Stand Up to Cancer Colorectal Cancer Dream Team, by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, and by a grant (89/2017) from the Comprehensive Program of Cancer Immunotherapy and Immunology (CAIMI) BBVA Foundation. Stand Up to Cancer is administered by the American Association for Cancer Research.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Benoit Rousseau, Memorial Sloan Kettering Cancer Center, New York, NY
Michael B. Foote, Memorial Sloan Kettering Cancer Center, New York, NY
Steven B. Maron, Memorial Sloan Kettering Cancer Center, New York, NY
Bill H. Diplas, Memorial Sloan Kettering Cancer Center, New York, NY
Steve Lu, Johns Hopkins Medical Institutes Baltimore, MD
Guillem Argilés, Memorial Sloan Kettering Cancer Center, New York, NY
Andrea Cercek, Memorial Sloan Kettering Cancer Center, New York, NY
Luis A. Diaz, Jr., Memorial Sloan Kettering Cancer Center, New York, NY
References
- 1.Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. June 17, 2020. (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors).
- 2.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21: 1353–65. [DOI] [PubMed] [Google Scholar]
- 3.Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019; 51: 202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousseau B, Bieche I, Pasmant T, et al. High activity of Nivolumab in patients with pathogenic exonucleasic domain POLE mutated mismatch repair proficient advanced tumors. Ann Oncol 2020;31: Suppl 4: 5260. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.