The article by Aetesam‐ur‐Rahman et al in this issue of the Journal of the American Heart Association (JAHA) 1 provides a timely opportunity to delve into the meaning and physiologic mechanisms of coronary steal.
Steal Scenarios
To our knowledge, the first use of the term coronary steal dates to 1967, later synonymous with myocardial steal. It originally described flow reversal in the setting of a coronary arteriovenous fistula.2 However, over the almost 55 years since its initial description, the term has come to encompass various pathophysiologies that we separate here into 5 distinct scenarios.
In keeping with its original description, a literature review for “coronary steal” demonstrates that a large minority (213 of the 514 results [41%]) involves congenital heart disease, within the limitations of a PubMed search. (PubMed search performed on February 8, 2021, using terms (“coronary steal”[tiab] OR “myocardial steal”[tiab]) in general, (“fistula”[tiab] OR “fistulae”[tiab] OR “fistulas”[tiab] OR “congenital”[tiab] OR “anomalous”[tiab] OR “hypoplastic”[tiab] OR “arterial switch”[tiab] OR “Bland‐White‐Garland”[tiab] OR “arteriovenous malformation”[tiab] OR “fetal hydrops”[tiab] OR “tetralogy”[tiab]) for congenital heart disease, (“mammary”[tiab] OR “thoracic artery” OR “bypass”[tiab] OR “subclavian steal”[tiab] OR “IMA graft”[tiab] OR “thoracic branch”[tiab]) for bypass‐related steal, (“myxoma”[tiab] OR “mediastinal mass”[tiab] OR “cardiac hemangioma”[tiab] OR “tumor”[tiab]) for tumors, “isoflurane”[tiab] for isoflurane).
Coronary steal has been described after bypass grafting of an internal mammary artery distal to a subclavian stenosis3 or attributed to unligated side branches of the mammary artery, although the latter mechanism remains controversial because coronary flow occurs during diastole whereas side branches perfuse during systole.4 This group accounts for 86 of the 514 results (17%).
Symptoms associated with cardiac tumors have been attributed to coronary steal in a small number of cases series, albeit without supporting hemodynamics, making up 9 of the 514 results (2%).
Inhaled anesthetic isoflurane underwent intense investigation because of an initial concern for its potential to cause coronary steal that was ultimately disproven.5 This topic consumes 22 of the 514 results (4%).
The remaining 184 of 514 results (36%) from PubMed largely focus on severe coronary artery disease as detailed subsequently.
Physiologic Mechanisms
The preceding classification of coronary steal into 5 scenarios provides a direct link with physiologic mechanisms. The large group of patients with congenital heart disease generally present with shunt physiology and resulting clinical consequences such as high‐output failure, pulmonary hypertension, and endocarditis. The unproven and uncommon scenarios related to inhaled anesthesia or cardiac tumors require no further discussion. Additionally, we defer to prior work arguing against steal via mammary artery side branches.4
Therefore, the scenario we discuss in detail involves native or bypassed coronary atherosclerosis without congenital defects. In this setting, “coronary steal” or “myocardial steal” (a distinction largely based on measurement technique) refers to a fall in absolute myocardial perfusion during pharmacologic or exercise stress, most often vasodilator hyperemia. Coronary flow reserve (CFR) as the unitless ratio of absolute perfusion between stress and baseline conditions is then <1.6, 7 Hence the paradox: falling regional blood flow during global hyperemia, usually manifested as some combination of severe angina, ST‐segment changes, lactate production, or new wall motion abnormality.
The left panel of Figure 1 displays a schematic of the coronary steal phenomenon from 1978, proposed by several investigators during that decade.6, 7, 8 Its key feature is collateral‐dependent myocardium supplied under resting conditions by a diseased donor vessel. High hyperemic flow produces large turbulent or viscous pressure loss at collaterals arising distally with corresponding fall in collateral flow to below resting levels. Collateral steal always involves a totally or subtotally occluded and collateralized vessel, although severe diffuse disease can produce “branch steal” in the distal or apical myocardium.9
Figure 1. Mechanism of coronary steal.
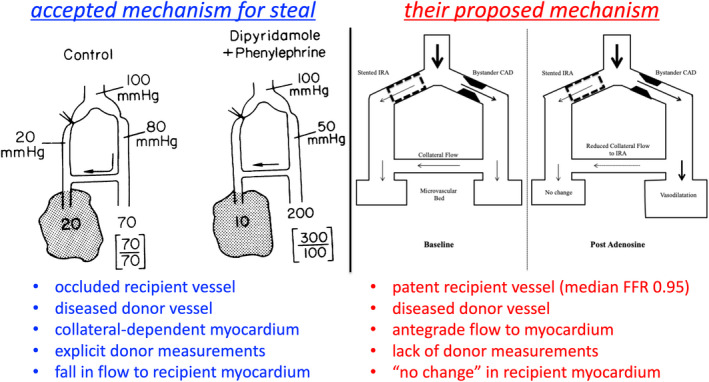
Left, As detailed in the legend to figure 4 of the original publication8 from 1978: “Schematic diagram of coronary circulation showing proposed mechanism for dipyridamole‐induced coronary steal. Coronary artery divides into two branches, one completely occluded, the other stenosed but providing collaterals to the first. In the control situation on the left, distal pressure is low in the occluded arterial bed and there is a small gradient in mean pressure across the stenosis. Flow in the ischemic region (dotted area) is 20 mL/min per 100 g and is determined by the collateral driving pressure, or the difference between distal pressures in the bed supplying collaterals (80 mm Hg) and the ischemic bed (20 mm Hg). Flow in the distribution of the stenotic vessel is normal at 70 mL/min per 100 g and is evenly distributed between subendocardium (lower value in bracket) and subepicardium (upper value). During dipyridamole, with blood pressure maintained constant by phenylephrine, flow increases in the nonischemic bed to 200 mL/min per 100 g but becomes maldistributed between subendocardium and subepicardium. In addition, pressure distal to the stenosis falls to 50 mm Hg, causing a reduction in collateral driving pressure. As a result, flow to the ischemic region decreases to 10 mL/min per 100 g, interpreted as a coronary steal.” Right, Proposed mechanism in Figure 1 of the article by Aetesam‐ur‐Rahman et al.1 Key differences from the left panel include the widely patent recipient vessel (median fractional flow reserve [FFR] of 0.95) with sufficient antegrade flow to its myocardium to produce “no change” as opposed to a fall in flow under conditions of vasodilator hyperemia. The illustration in the left panel was reproduced from Becker8 with permission from the American Heart Association, Inc. © 1978. The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health, Inc., on behalf of the American Heart Association, Inc. The right panel is adapted with permission from Aetesam‐ur‐Rahman et al.1 Copyright © 2021, The Authors. JAHA is published on behalf of the American Heart Association, Inc., by Wiley Periodicals Inc. Open access under CC‐BY license (https://creativecommons.org/licenses/by/4.0/).”
At baseline or under control conditions, the left panel of Figure 1 indicates sufficient chronic perfusion to the occluded artery via the donor vessel, thereby preventing myocardial infarction or limiting its extent. In response to vasodilation, distal coronary pressure in the donor vessel falls as a result of upstream stenosis or diffuse narrowing. Consequently, collateral flow during hyperemic stress drops below resting levels owing to a decrease in collateral driving pressure. CFR can become <1 depending on the aggregate effects of residual antegrade flow, coronary pressure at the origin of collateral(s), and the extent of collateral development and hence their resistance. Additionally, the low pressure at the origin of collaterals causes maldistribution across the myocardial wall as depicted in the left panel of Figure 1 (upper and lower numbers in brackets indicate subepicardial and subendocardial perfusion).
As has been noted before,6, 7 the term “coronary steal” is a misnomer because blood does not flow backwards through the collaterals. Flow follows the downward pressure gradient from donor to recipient that is smaller during vasodilation because of upstream pressure loss in the donor vessel.
Hemodynamics Immediately After Myocardial Infarction
The current study from the Cambridge group enrolled 93 patients who presented with an ST‐segment elevation myocardial infarction.1 Immediately after successful stenting of the infarct‐related artery, coronary physiology was measured at “rest” as well as during intravenous adenosine infusion. In addition to standard aortic and coronary pressure measurements, bolus thermodilution mean transit time was recorded under both conditions to calculate CFR in the culprit, stented artery. Within 1 to 3 days a large subset of 68 subjects underwent cardiac magnetic resonance imaging.
An unexpected finding from their work was that 19 of 93 subjects (20%) had a bolus thermodilution CFR <0.9 in the stented culprit artery attributed by the authors to coronary steal that would imply decreased flow and pressure below resting levels in the patent culprit artery during hyperemia.1 However, the right panel of Figure 1 contrasts their explanation with the classic proven schematic for myocardial steal on the left.7, 8 Three facts in this report do not support the claim of myocardial steal and even suggest its impossibility as follows. First, the infarct‐related artery is widely patent because of successful stenting, unlike a totally or subtotally occluded recipient vessel. Second, although the donor vessel often had upstream coronary disease, no measurements were made in this artery.
Third, the median fractional flow reserve of 0.95 in the stented, widely patent “recipient” artery indicates the absence of any physiologically significant lower pressure than the nonculprit diseased “donor” artery. Although the authors did not make explicit measurements in the nonculprit vessel to prove any pressure gradient, visual >50% lesion in 10 of the 19 subjects indicates nonculprit disease that would cause lower coronary pressure during hyperemia than in the stented patent artery with median fractional flow reserve of 0.95.
Because collateral flow follows the pressure gradient, there can be no collateral flow and no myocardial steal in the patent culprit vessel since its pressure is the same or higher than the diseased nonculprit artery. Indeed, based on higher hyperemic pressure with a fractional flow reserve of 0.95, any collateral flow would be from the stented patent culprit artery to the nonculprit artery with mild to moderate unstented stenosis and lower hyperemic coronary pressure. Therefore, any collateral flow would be in the opposite direction than depicted in the right panel of Figure 1.
In addition to these 3 facts contravening myocardial steal, the data in table 1 reveal physiologic inconsistencies suggesting methodologic variability of flow measured by thermodilution. Basic physiologic principles dictate that flow and epicardial pressure gradients across stenosis or diffuse coronary artery disease track in the same direction: higher flow produces a greater pressure loss and vice versa. In table 1, the group with CFR <0.9 by thermodilution had a pressure gradient of 4.15 mm Hg at baseline (mean aortic pressure minus coronary pressure of 91.47–87.32 mm Hg) compared with 3.79 mm Hg during adenosine hyperemia (79.57–75.78 mm Hg). For the group with CFR from 0.9 to 1.1, the pressure gradient was 3.07 at baseline (82.33–79.26 mm Hg) compared with 3.07 mm Hg during adenosine hyperemia (67.73–64.66). Thus, for these 2 groups, the pressure gradient did not increase during adenosine hyperemia. In contrast, the group with higher flow by thermodilution with CFR >1.1 had the expected increased gradient from 4.17 mm Hg at baseline (99.32–95.15 mm Hg) to 6.32 mm Hg after adenosine (88.84–82.52 mm Hg). These facts suggest that coronary flow by thermodilution is not precise enough to define small flow differences around a CFR of 1.0.
Indeed, bolus thermodilution to assess CFR displays substantial imprecision compared with an implanted flow meter experimentally: bolus thermodilution CFR had 95% CIs for coronary flow from −1.23 to +0.88 in Bland‐Altman analysis, and inspection of that experimental scatterplot confirms 3 points with flowmeter CFR ≥1 yet bolus thermodilution CFR <1.10 In other words, a bolus thermodilution CFR=0.7 (classified as “coronary steal”) could easily represent a true CFR =1 (no change in flow) because of the proven methodologic imprecision of flow by thermodilution. Figure 2 proposes a potential explanation for the observations in this report due to imprecision from bolus thermodilution that distorts the true CFR distribution by producing an apparent but artifactual group of vessels with CFR <1.
Figure 2. Apparent steal arising from measurement noise.
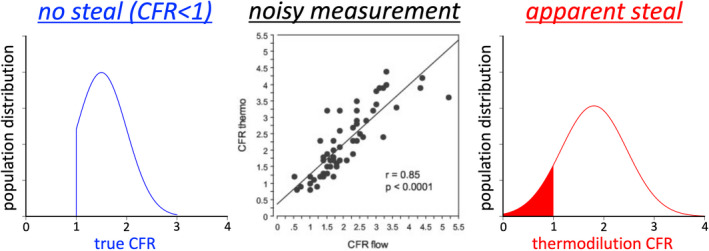
Assume a population with reduced coronary flow reserve (CFR) but no steal (CFR <1), here simulated with mean 1.5 and SD 0.50 but constrained to the range 1 to 3 as might be encountered in the immediate postinfarct population. This true CFR distribution is measured using bolus thermodilution, which has known scatter (bias −0.17 and 95% CI from −1.23 to +0.88 by Bland‐Altman analysis) when compared in paired fashion against an implanted flow meter in an animal model, reproduced from figure 1A of a publication from 2003.10 The resulting population artifactually appears to have a subset of vessels with coronary steal (CFR <1), here simulated by Monte Carlo techniques resulting in a mean of 1.80 and SD 0.65. The center panel was reproduced from Fearon et al 10 with permission from the American Heart Association, Inc. © 2003. The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health, Inc., on behalf of the American Heart Association, Inc.
Physiologically, making an immediate post‐ST‐segment–elevation myocardial infarction measurement of “rest” flow to calculate CFR reduces flow reserve compared with its later value because of elevated baseline flow, as shown by serial assessment between days 0 and 1 after primary stenting for acute infarction.11 In the current article, additional measurements of immediate test/retest repeatability using bolus thermodilution CFR would have quantified its imprecision in the postinfarct clinical setting that is essential for quantifying small changes in thermodilution CFR.
Unresolved Issues in Steal
In reviewing the literature on coronary steal (within the limitations of a PubMed search), we note 2 recurring and unresolved clinical scenarios paralleling our analysis of this report and hence of general interest. First, percutaneous treatment of mammary side branches after bypass grafting to treat “coronary steal” generates recurrent case reports or series but almost always without sufficient physiologic demonstration of need or benefit or plausible mechanism.4 Second, proximal coronary fistulae continue to receive percutaneous or surgical treatment in the absence of a significant left‐to‐right shunt, pulmonary hypertension, or prior endocarditis. Rarely does the physiologic scenario match the left panel of Figure 1. Fractional flow reserve assessed before and its normalization after fistula treatment, as occasionally reported in the literature,12 requires a more sophisticated analysis to account for epicardial side branches to a low resistance circuit and an awareness of the distinction between relative and absolute flow reductions required to produce ischemia.
Because we cannot easily change the names of the past,2 for now we must live with the misnomer of “coronary steal.” Its suboptimal terminology must not distract or confuse readers from the true proven underlying mechanism outlined in the left panel of Figure 1 for severe coronary disease. Solid physiologic principles and quantification of myocardial steal as proven experimentally and in patients must be applied and buttressed by statistical analysis accounting for methodologic imprecision to avoid misinterpretation of data with their potential erroneous clinical application for high‐risk interventions having no physiologically demonstrable gain.
Disclosures
Johnson, Kirkeeide, and Gould received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis and have patents pending on diagnostic methods for quantifying aortic stenosis and transcatheter aortic valve implantation physiology and on methods to correct pressure tracings from fluid‐filled catheters. Johnson has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips Volcano Corporation (DEFINE‐FLOW, NCT02328820) for studies using intracoronary pressure and flow sensors; and has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum fractional flow reserve algorithm commercialized under 510(k) K191008. Kirkeeide reports no additional support or industry relationships. Gould is the 510(k) applicant for CFR Quant (K113754) and HeartSee (K143664 and K171303), software packages for cardiac positron emission tomography image processing, analysis, and absolute flow quantification.
(J Am Heart Assoc. 2021;10:e021000. DOI: 10.1161/JAHA.121.021000.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 5.
See Article by Aetesam‐ur‐Rahman et al.
REFERENCES
- 1.Aetesam‐ur‐Rahman M, Brown AJ, Jaworski C, Giblett JP, Zhao TX, Braganza DM, Clarke SC, Agrawal BSK, Bennett MR, West NEJ, et al. Adenosine induced coronary steal is observed in patients presenting with ST‐elevation myocardial infarction. J Am Heart Assoc. 2021;10:e019899. DOI: 10.1161/JAHA.120.019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effler DB, Sheldon WC, Turner JJ, Groves LK. Coronary arteriovenous fistulas: diagnosis and surgical management. Report of fifteen cases. Surgery. 1967;61:41–50. [PubMed] [Google Scholar]
- 3.Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73:690–693. DOI: 10.1016/S0022-5223(19)41465-7. [DOI] [PubMed] [Google Scholar]
- 4.Kern MJ. Is stealing still a crime? Comment on left internal mammary artery side branch intervention in the management of coronary steal syndrome following coronary artery bypass grafting. Catheter Cardiovasc Interv. 2021;97:105–107. DOI: 10.1002/ccd.29446. [DOI] [PubMed] [Google Scholar]
- 5.Crystal GJ. Isoflurane and the coronary steal controversy of the 1980s: origin, resolution, and legacy. J Anesth Hist. 2017;3:56–62. DOI: 10.1016/j.janh.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Gould KL. Coronary steal. Is it clinically important? Chest. 1989;96:227–228. DOI: 10.1378/chest.96.2.227. [DOI] [PubMed] [Google Scholar]
- 7.Demer LL, Gould KL, Goldstein RA, Kirkeeide RL. Noninvasive assessment of coronary collaterals in man by PET perfusion imaging. J Nucl Med. 1990;31:259–270. [PubMed] [Google Scholar]
- 8.Becker LC. Conditions for vasodilator‐induced coronary steal in experimental myocardial ischemia. Circulation. 1978;57:1103–1110. DOI: 10.1161/01.CIR.57.6.1103. [DOI] [PubMed] [Google Scholar]
- 9.Gould KL, Kirkeeide R, Johnson NP. Coronary branch steal: experimental validation and clinical implications of interacting stenosis in branching coronary arteries. Circ Cardiovasc Imaging. 2010;3:701–709. DOI: 10.1161/CIRCIMAGING.110.937656. [DOI] [PubMed] [Google Scholar]
- 10.Fearon WF, Farouque HM, Balsam LB, Caffarelli AD, Cooke DT, Robbins RC, Fitzgerald PJ, Yeung AC, Yock PG. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. 2003;108:2198–2200. DOI: 10.1161/01.CIR.0000099521.31396.9D. [DOI] [PubMed] [Google Scholar]
- 11.Cuculi F, De Maria GL, Meier P, Dall'Armellina E, de Caterina AR, Channon KM, Prendergast BD, Choudhury RC, Forfar JC, Kharbanda RK, et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894–1904. DOI: 10.1016/j.jacc.2014.07.987. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Liu Z, Ye S. The role of the fractional flow reserve in the coronary steal phenomenon evaluation caused by the coronary‐pulmonary fistulas: case report and review of the literature. J Cardiothorac Surg. 2020;15:32. DOI: 10.1186/s13019-020-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


