Abstract
Cangrelor is the only currently available intravenous platelet P2Y12 receptor inhibitor. It is characterized by potent, predictable, and rapidly reversible antiplatelet effects. Cangrelor has been tested in the large CHAMPION (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) program, where it was compared with different clopidogrel regimens, and it is currently indicated for use in patients with coronary artery disease undergoing percutaneous coronary intervention. However, the uptake of cangrelor use varies across the globe and may also include patients with profiles different from those enrolled in the registration trials. These observations underscore the need to fully examine the safety and efficacy of cangrelor in postregistration studies. There are several ongoing and planned studies evaluating the use of cangrelor in real‐world practice which will provide important insights to this extent. The current article provides a review on the pharmacology, clinical studies, contemporary use of cangrelor in real‐world practice, a description of ongoing studies, and futuristic insights on potential strategies on how to improve outcomes of patients undergoing percutaneous coronary intervention.
Keywords: acute coronary syndromes, cangrelor, percutaneous coronary intervention
Subject Categories: Percutaneous Coronary Intervention, Pharmacology, Treatment
Nonstandard Abbreviations and Acronyms
- ARCANGELO
Italian Prospective Study on Cangrelor
- BRIDGE
The Bridging Antiplatelet Therapy With Cangrelor in Patients Undergoing Cardiac Surgery
- CAMEO
Cangrelor in Acute Myocardial Infarction: Effectiveness and Outcomes
- CANTIC
Platelet Inhibition With Cangrelor and Crushed Ticagrelor in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention
- CHAMPION
Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition
- DAPT‐SHOCK‐AMI
Dual Antiplatelet Therapy for Shock Patients With Acute Myocardial Infarction
- FABOLUS‐FASTER
Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open‐Label Trial in Patients With ST‐Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention
- GPI
glycoprotein IIb/IIIa inhibitor
- IDR
ischemia‐driven revascularization
- MARS
Management of Antiplatelet Regimen During Surgical Procedures
- MONET BRIDGE
Maintenance of Antiplatelet Therapy in Patients With Coronary Stenting Undergoing Surgery
- ST
stent thrombosis
- SWAP
Switching Anti Platelet
The ADP P2Y12 receptor subtype plays a key role in platelet activation and amplification processes.1, 2 The pivotal role of this platelet signaling pathway is supported by a plethora of studies conducted over the past 2 decades showing that the use of P2Y12 receptor inhibitors in adjunct to aspirin, in high‐risk patients with coronary artery disease (CAD), such as those undergoing percutaneous coronary interventions (PCIs) or presenting with an acute coronary syndrome (ACS), significantly reduces short‐ and long‐term ischemic events.3, 4 Most investigations have been conducted with oral formulations of P2Y12 inhibitors. Although clopidogrel is the most commonly used oral P2Y12 inhibitor, it is characterized by impaired platelet inhibitory effects in a considerable number of patients.5, 6 Prasugrel and ticagrelor are more potent oral P2Y12 inhibitors compared with clopidogrel and associated with greater efficacy, albeit at the expense of increased bleeding risk.7, 8, 9 However, pharmacodynamic studies have shown a gap in their onset of action, especially in patients with ST‐segment–elevation myocardial infarction (STEMI) or hemodynamic impairment, underlining the need for intravenous therapies with a prompt and potent onset of action.10, 11, 12
Cangrelor is an intravenous platelet P2Y12 antagonist characterized by a rapid onset of action and achieving potent P2Y12 inhibitory effects.13 Moreover, because of its short half‐life and reversibly binding properties, cangrelor has a fast offset of effects.2, 14 Cangrelor was approved on the basis of its superior efficacy in reducing thrombotic complications compared with clopidogrel in patients undergoing PCI.15 Accordingly, its use has increased in real‐life world practice.16 Although its clinical efficacy compared with potent oral P2Y12 inhibitors (ie, prasugrel and ticagrelor) has not been explored, pharmacodynamic studies have shown that cangrelor overcomes limitations of oral therapies by achieving fast and potent platelet inhibition.10, 11, 17 Pharmacodynamic studies have also allowed to better define the optimal approach to transition from cangrelor to oral P2Y12 inhibiting therapy.18, 19, 20, 21 The current article provides a review of pharmacology, clinical studies, contemporary use of cangrelor in real‐world practice, a description of ongoing studies, and futuristic insights on potential strategies on how to improve outcomes of patients undergoing PCI.
Cangrelor: From Pharmacology to Clinical Outcomes Data
Pharmacology
Cangrelor is the only intravenous P2Y12 receptor antagonist approved for use in patients with CAD undergoing PCI.22 Cangrelor is a nonthienopyridine ATP analog acting as a direct, reversible P2Y12 receptor antagonist.23 Maximum concentrations of cangrelor, which are associated with extensive platelet blockade, are rapidly achieved with the use of an intravenous bolus, followed by a continuous infusion, reaching maximum serum concentration (Cmax) within 2 minutes.23 Cangrelor has a half‐life of 3 to 6 minutes because of its relatively rapid hydrolysis to its inactive metabolite.23 Cangrelor markedly inhibits ADP‐induced platelet aggregation throughout the duration of infusion.23 It has a rapid offset of effect after discontinuation of its infusion, with platelet function returning to normal within 60 minutes (Figure 1).23 These pharmacologic properties make cangrelor not only an attractive agent for protection of ischemic events in patients undergoing PCI, but also a safe one in case of procedural complications, such as bleeding or need for emergent surgery, given its fast offset of effects, obviating the need for an antidote for reversal.24, 25, 26
Figure 1. Mechanism of action of oral and intravenous P2Y12 inhibitors on platelet receptors (A) and transition to oral platelet P2Y12 receptor inhibitors in cangrelor‐treated patients (B).

CAD indicates coronary artery disease; CYP, cytochrome P450; PCI, percutaneous coronary intervention; and PLT, platelet.
Cangrelor is associated with high P2Y12 receptor occupancy, thus not allowing for other agents to bind with the receptor.22 The active metabolites of the thienopyridines, clopidogrel and prasugrel, are unstable and have a limited half‐life. For this reason, if thienopyridines are given during cangrelor infusion or when cangrelor is still present at a high concentration in the blood, the active metabolites will not be able to bind to the P2Y12 receptor, preventing them from achieving any antiplatelet effects and ischemic protection.27 Accordingly, thienopyridines, in particular clopidogrel, should be administered immediately after discontinuation of cangrelor infusion.18, 28 Prasugrel can be administered immediately after or up to 30 minutes before cangrelor infusion is discontinued.18, 21 The reason for the latter is prasugrel generates more active metabolite than clopidogrel, which remains in circulation for a slightly longer time.18 Although some investigations did support the feasibility of administering prasugrel at the start of cangrelor infusion, these studies were not designed to rule out a drug interaction29, 30 and thus this is a strategy that is not recommended. On the other hand, ticagrelor is a derivative of ATP, with a half‐life ranging from 8 to 12 hours, and, like cangrelor, it binds reversibly to the platelet P2Y12 receptor. For these reasons, ticagrelor can be administered before or during the infusion of cangrelor without resulting in a drug interaction.18, 21
The ongoing SWAP (Switching Anti Platelet)‐5 (ClinicalTrials.gov Identifier: NCT04634162) and SWAP‐6 (ClinicalTrials.gov Identifier: NCT04668144) studies will further clarify the pharmacodynamic effect of the transition from cangrelor to ticagrelor and prasugrel, respectively.
Registration Trials Leading to Approval of Cangrelor
The efficacy of cangrelor was assessed in the large phase 3 CHAMPION (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) program that included 3 randomized controlled trials and >25 000 patients: PCI,31 PLATFORM,32 and PHOENIX15 (Table). The first 2 studies, CHAMPION PCI31 and CHAMPION PLATFORM,32 randomized patients to cangrelor (bolus of 30 µg/kg plus infusion of 4 µg/kg per minute) or clopidogrel (loading dose of 600 mg) either before or soon after PCI in patients with ACS, but both were stopped prematurely for futility. No difference in the primary composite of death, myocardial infarction (MI), or ischemia‐driven revascularization (IDR) at 48 hours was observed in either study. These neutral outcomes were mostly attributed to the definition of MI, a key driver of outcomes in PCI trials. Indeed, MI was defined as the presence of new Q waves in 2 contiguous ECG leads, cardiac biomarkers at least 3 times the upper limit of normal, or ≥50% increase above baseline when biomarkers were initially elevated.
Table 1.
Summary of Published and Ongoing Randomized and Observational Studies Assessing the Clinical Benefits of Cangrelor
| Study Name | No. of Patients | Treatment | Type of Patients | Outcomes |
|---|---|---|---|---|
| Randomized studies | ||||
| CHAMPION PCI31 | 8877 | Cangrelor (arm A) vs clopidogrel, 600 mg (arm B), before PCI | Patients undergoing PCI (SA, 5.2%; UA, 35.4%; NSTEMI, 59.4%) |
Death/MI/IDR at 48 h Arm A vs arm B: 7.5% vs 7.1%; P=0.59 Major bleeding at 48 h (arm A vs arm B): ACUITY criteria: 3.6% vs 2.9%; P=0.06 GUSTO criteria: 0.2% vs 0.3%; P=0.82 TIMI criteria: 0.4% vs 0.3%; P=0.39 |
| CHAMPION PLATFORM32 | 5362 | Cangrelor (arm A) vs placebo (arm B) during PCI, followed by clopidogrel, 600 mg | Patients undergoing PCI (SA, 15.1%; UA, 24.7%; NSTEMI, 49.2%; STEMI, 11.0%) |
Death/MI/IDR at 48 h Arm A vs arm B: 7.0% vs 8.0%; P=0.17* ST at 48 h Arm A vs arm B: 0.2% vs 0.6%; P=0.02 Death from any cause at 48 h Arm A vs arm B: 0.2% vs 0.7%; P=0.02 Severe bleeding (GUSTO) at 48 h Arm A vs arm B: 0.3% vs 0.2%; P=0.45 |
| CHAMPION PHOENIX15 | 10 942 | Cangrelor (arm A) vs clopidogrel, 300/600 mg (arm B), before PCI | Patients undergoing PCI (SA, 57.0%; NSTEMI, 25.4%; STEMI, 17.6%) |
Death/MI/IDR/ST at 48 h Arm A vs arm B: 4.7% vs 5.9%; P=0.005 ST at 48 h Arm A vs arm B: 0.8% vs 1.4%; P=0.01 Severe bleeding (GUSTO) at 48 h Arm A vs arm B: 0.2% vs 0.1%; P=0.44 |
| BRIDGE33 | 210 | Cangrelor (arm A) vs placebo (arm B) | Patients with ACS or with a coronary stent on a thienopyridine awaiting CABG (NSTEMI, 44.5%; STEMI, 11.9%; SA, 44.6%) |
Proportion of patients with PRU <240 Arm A vs arm B: 98.8% vs 19.0%; P<0.001 Excessive CABG surgery‐related bleeding Arm A vs arm B: 11.8% vs 10.4%; P=0.76 |
| Observational studies | ||||
| Vaduganathan et al34 |
100 |
Cangrelor by US SPC | Patients with ACS (STEMI, 52%; NSTEMI, 40%; SA, 7%; other, 6%) |
At 48 h 1 ST; no deaths or major bleeding (GUSTO criteria) |
| Vaduganathan et al35 |
38 |
Cangrelor by US SPC | Patients with CS (PCI for ACS, 82%; bridging to surgery, 13%; other reasons, 5%) |
At 48 h: No ST, deaths, or major bleeding (GUSTO criteria) |
| Grimfjärd et al36 |
915 |
Cangrelor by EU SPC | Patients undergoing PCI (STEMI, 98.2%; NSTEMI, 1.8%) |
At 30 d All‐cause mortality: 15.1%; ST: 0.7% |
| Ongoing randomized studies | ||||
| Cangrelor OHCA (NCT04005729) | 30 | Cangrelor+ticagrelor vs ticagrelor | Comatose survivors of OHCA undergoing PCI | PRU; bleeding (BARC criteria); final TIMI flow; ST at 30 d; mortality at 90 d |
| MONET BRIDGE (NCT03862651) | 140 | Cangrelor vs placebo | Patients requiring discontinuation of P2Y12 inhibitor because of a significant bleeding risk | Residual PRU; bleeding (BARC criteria) |
| DAPT‐SHOCK‐AMI (NCT03551964) | 304 | Cangrelor vs ticagrelor | Patients with MI and CS requiring PCI | Composite death/MI/stroke; PRU; composite death/MI/urgent revascularization; bleeding (BARC criteria); ST; death; MI; stroke; urgent revascularization; duration of hospitalization; surgery delay because of bleeding |
| Ongoing observational studies | ||||
| CAMEO (NCT04076813) | 3000 | Cangrelor | NSTEMI, STEMI undergoing PCI | Number of antiplatelet medications used and bleedings occurring during hospitalization |
| MARS (NCT03981835) | 1492 | DAPT | Patients post‐PCI on DAPT undergoing NCS and CS | NACE; intravenous antiplatelet bridging; death; ST; length of hospital stay; health economic analysis |
| ARCANGELO (NCT04471870) | 1000 | Cangrelor | ACS undergoing PCI | Bleeding (BARC criteria); MACE |
Enrollment was stopped when an interim analysis concluded that the trial would be unlikely to show superiority for the primary end point.
Italics indicate 'title' for the outcome.
ACS indicates acute coronary syndrome; ACUITY, Acute Catheterization and Urgent Intervention Triage Strategy; ARCANGELO, Italian Prospective Study on Cangrelor; BARC, Bleeding Academic Research Consortium; BRIDGE, The Bridging Antiplatelet Therapy With Cangrelor in Patients Undergoing Cardiac Surgery; CABG, coronary artery bypass grafting; CAMEO, Cangrelor in Acute Myocardial Infarction: Effectiveness and Outcomes; CHAMPION, Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition; CS, cardiogenic shock; DAPT, dual‐antiplatelet therapy; DAPT‐SHOCK‐AMI, DAPT for Shock Patients With Acute Myocardial Infarction; EU, European Union; GUSTO, Global Use of Strategies to Open Occluded Coronary Arteries; IDR, ischemia‐driven revascularization; MACE, major adverse cardiac event; MARS, Management of Antiplatelet Regimen During Surgical Procedures; MI, myocardial infarction; MONET BRIDGE, Maintenance of Antiplatelet Therapy in Patients With Coronary Stenting Undergoing Surgery; NACE, net adverse clinical event; NCS, noncardiac surgery; NSTEMI, non–ST‐segment–elevation myocardial infarction; OHCA, out‐of‐hospital cardiac arrest; PCI, percutaneous coronary intervention; PRU, platelet reactivity (measured in P2Y12 reaction units); SA, stable angina; ST, stent thrombosis; STEMI, ST‐segment–elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; and UA, unstable angina.
In a post hoc analysis, data from 13 000 patients enrolled in both studies were pooled, and the prevalence of periprocedural MI was calculated according to the universal definition37 (ie, elevations of cardiac biomarkers ≥3 times the 99th percentile upper limit of normal in patients with normal baseline troponin values). Instead, in case of abnormal troponin levels at baseline, only Q‐wave MIs were included.38 Notably, compared with clopidogrel, treatment with cangrelor resulted in significant reduction in early ischemic events under the universal definition of MI (odds ratio [OR], 0.82; 95% CI, 0.68–0.99; P=0.037). This finding has important clinical implications, because periprocedural MI, according to contemporary definitions, is associated with an increase in all‐cause mortality rate at 10 years following PCI.39, 40 In this regard, in CHAMPION PLATFORM,32 which was a true placebo‐controlled trial in that clopidogrel loading was performed after the PCI, there were significant reductions in the secondary end points of stent thrombosis (ST) and mortality.
On that basis, another trial was designed, the CHAMPION PHOENIX (Figure 2), where a scrupulous assessment of MI, according to the universal definition, was prospectively implemented.41 The trial was conducted across the spectrum of CAD manifestations (ie, stable CAD and ACS) in patients who were P2Y12 naïve and undergoing PCI. Cangrelor significantly reduced the primary end point of death, MI, IDR, or ST at 48 hours (OR, 0.78; 95% CI, 0.66– 0.93; P=0.005) and the key secondary end point of ST (OR, 0.62; 95% CI, 0.43–0.90; P=0.01) compared with clopidogrel. In particular, cangrelor decreased the occurrence of intraprocedural ST (defined as the development of new or increasing thrombus in or adjacent to an implanted stent during the PCI procedure) that is associated with a significant increase in mortality, MI, IDR, and definite or probable ST at 48 hours and at 30 days.42 A large‐scale, blinded angiographic core laboratory‐based analysis studied the association between clinical outcomes of the CHAMPION PHOENIX trial and high‐risk PCI target lesion features. It showed that cangrelor consistently reduced the rate of major adverse cardiac events at 48 hours compared with clopidogrel, and it showed a greater absolute effect with the increase of complex coronary lesions treated.43 These findings suggest that the clinical benefits of cangrelor could be greatest during PCI in patients with complex coronary anatomy. The rate of the primary safety end point of site‐reported Global Use of Strategies to Open Occluded Coronary Arteries–defined severe bleeding or in the rate of transfusions was not increased in patients randomized to cangrelor,44 even in patients who received unfractionated heparin or glycoprotein IIb/IIIa inhibitors (GPIs) during PCI.45, 46 Notably, the incidence of major bleeding events, according to the Global Use of Strategies to Open Occluded Coronary Arteries or the more sensitive Acute Catheterization and Urgent Intervention Triage Strategy definition, was comparable between cangrelor and clopidogrel, even when PCI was performed via the radial artery (26% of the overall population).47
Figure 2. Clinical results of the CHAMPION (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) PHOENIX trial.

GUSTO indicates Global Use of Strategies to Open Occluded Coronary Arteries; IDR, ischemia‐driven revascularization; MI, myocardial infarction; mITT, modified intention‐to‐treat; NSTE‐ACS, non–ST‐segment–elevation acute coronary syndrome; OR, odds ratio; PCI, percutaneous coronary intervention; ST, stent thrombosis; and STEMI, ST‐segment–elevation myocardial infarction.
A patient‐level meta‐analysis of the 3 CHAMPION studies confirmed the efficacy of cangrelor in terms of death, MI, IDR, or ST without significant increase in Global Use of Strategies to Open Occluded Coronary Arteries severe bleeding.44 A following post hoc adjudication of site‐reported bleeding showed a not significant increase of minor bleeding events in patients randomized to cangrelor, according to the TIMI (Thrombolysis in Myocardial Infarction) classification compared with clopidogrel (hazard ratio, 3.01; 95% CI, 1.52–5.96; P<0.001).44 In several post hoc and sensitivity analyses, the effectiveness of cangrelor was consistent, according to alternative end point definitions and patients' subgroups,48 including those with a diagnosis of ACS.49
Additional Studies of Cangrelor
Recent studies assessed the pharmacodynamic efficacy of cangrelor in patients with STEMI. The CANTIC (Platelet Inhibition With Cangrelor and Crushed Ticagrelor in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention) study was a prospective, randomized, double‐blind, placebo‐controlled investigation of the pharmacodynamic effects of cangrelor versus placebo in patients undergoing primary PCI treated with crushed 180‐mg loading dose of ticagrelor. Cangrelor reduced platelet inhibition after just 5 minutes, with an effect that persisted throughout the infusion and without any drug interactions with ticagrelor given concomitantly with cangrelor at the start of the PCI, proving to be an effective strategy in bridging the latency of platelet inhibition of oral drugs during primary PCI.20 These findings are consistent with other investigations supporting prompt, potent, and sustained platelet inhibition of cangrelor during primary PCI,50, 51 with important practical implications, especially for patients needing opioids that decrease gastrointestinal motility, contributing to delays in absorption and action of oral P2Y12 inhibitors.
Most recently, however, a randomized prospective investigation (FABOLUS‐FASTER [Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open‐Label Trial in Patients With ST‐Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention]) failed to show potent platelet inhibitory effects associated with cangrelor, resulting in lower platelet inhibition compared with tirofiban, yet greater than that achieved with prasugrel.52 The counterintuitive finding with respect to tirofiban versus cangrelor may have to do with issues pertaining to the suboptimal methods used to assess platelet inhibition.
The rapid onset and offset of action of cangrelor make it an attractive agent for bridging among patients with recent stent implantation who need to undergo nondeferrable surgery and in whom discontinuation of oral P2Y12 inhibition is required. To this extent, the prospective, randomized, double‐blind, placebo‐controlled, multicenter BRIDGE (The Bridging Antiplatelet Therapy With Cangrelor in Patients Undergoing Cardiac Surgery) trial was conducted, involving 210 patients treated with a thienopyridine awaiting coronary artery bypass grafting. This trial was preceded by a dose‐findings study that identified the optimal bridging regimen of cangrelor to be 0.75 μg/kg per minute. In a trial comparing cangrelor with placebo in bridging antiplatelet therapy, infusion was maintained for at least 48 hours and up to 7 days during washout from oral thienopyridine therapy; the infusion was discontinued 1 to 6 hours before coronary artery bypass grafting. A greater proportion of patients treated with cangrelor had low levels of platelet reactivity throughout the entire treatment period compared with placebo. Despite numerically higher incidence of minor bleeding with cangrelor, results demonstrated no significant differences in major bleeding before or during coronary artery bypass grafting surgery.33 Although the use of cangrelor as a bridging agent is not an approved indication by the Food and Drug Administration or European Medical Agency, it is commonly used with this intent in patients with recent stent implantation requiring both cardiac and noncardiac surgery.22, 53, 54, 55, 56 Moreover, its use as a bridging agent is currently recommended in several expert consensus recommendations.57, 58 Nevertheless, recent data suggesting the safety of early discontinuation of dual antiplatelet therapy after PCI59 might change future consensus recommendations for bridging.
Indications and Dosage
Cangrelor is currently available in the United States and most European countries. According to the Food and Drug Administration, cangrelor is approved as an adjunct to PCI for reducing the risk of periprocedural MI, repeated coronary revascularization, and ST in patients not treated with an oral P2Y12 inhibitor and not planned to receive a GPI.60 Cangrelor should be administered as a bolus of 30 µg/kg, before initiation of the PCI procedure, followed by an infusion of 4 µg/kg per minute for at least 2 hours or through the duration of the intervention, whichever is longer.60 To maintain platelet inhibition after discontinuation of cangrelor infusion, an oral P2Y12 platelet inhibitor should be administered as follows60: clopidogrel, 600 mg, immediately after discontinuation of cangrelor; prasugrel, 60 mg, immediately after discontinuation of cangrelor; or ticagrelor, 180 mg, at any time during cangrelor infusion or immediately after discontinuation. The American College of Cardiology/American Heart Association guidelines do not provide any recommendations on the use of cangrelor because the drug was approved only after the most recent guideline updates3 (Figure 3).
Figure 3. Use of cangrelor in patients with acute coronary syndrome (ACS).
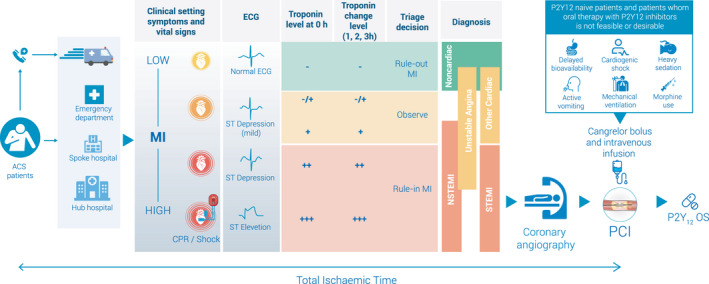
MI indicates myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; OS, oral somministration; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Cangrelor was approved by European Medical Agency for the reduction of thrombotic cardiovascular events in patients with CAD undergoing PCI who have not received an oral P2Y12 inhibitor before PCI and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable.61 The European Medical Agency additionally specifies that the infusion must not exceed 4 hours.61 The 2020 European Society of Cardiology Guidelines for the management of ACS without persistent ST‐segment elevation suggest the use of cangrelor during PCI (class of recommendation IIb; level of evidence A)and confirm that the timing of administration of oral P2Y12 inhibitors in patients receiving cangrelor infusion at the time of PCI should be drug specific.62, 63
Real‐World Use of Cangrelor
Real‐world evidence on cangrelor includes initial clinical experiences and large health system analyses. In a US single‐center analysis of 147 consecutive cangrelor‐treated patients undergoing coronary angiography with the intent of PCI, loading doses of oral P2Y12 inhibitors were given before cangrelor in a few patients, whereas the vast majority received oral P2Y12 inhibitor loading doses during or at the end of cangrelor infusion. About 90% of patients were treated with a 30‐µg/kg bolus, followed by 4 µg/kg per minute, whereas the lower dose of 0.75 µg/kg per minute was used in 6% of them, for a median duration of 70.5 hours. A total of 18 mild to moderate bleeding events were observed, whereas severe, life‐threatening, or intracranial bleeding was not observed, confirming cangrelor is effective and well tolerated when used in high‐risk patients undergoing PCI.64 Another report from the same center, including 38 patients with cardiogenic shock (81% with STEMI), suggested that cangrelor is associated with low rates of clinically significant ischemic or bleeding events, even in this setting.34
In a study analyzing the data from the Swedish Coronary Angiography and Angioplasty Registry, cangrelor was used by 16% of the 5513 patients with STEMI treated with primary PCI; about one third of these patients had a cardiac arrest. Among hospitals, the use of cangrelor in primary PCI varied dramatically, ranging from 4% to 36%. Notably, unlike registration trials, cangrelor was mostly used in STEMI, or during left main PCI or thrombus aspiration. In two thirds of patients, cangrelor was used in combination with ticagrelor; in more than half of them, this combination happened before the hospitalization. Prehospital ticagrelor loading dose was used in 5% of the patients with cardiac arrest treated with cangrelor, compared with 39% of the non–cangrelor‐treated cardiac arrest cases. Mean times from diagnostic ECG to PCI were shorter in the cangrelor‐treated patients (1.35 hours) than non–cangrelor‐treated patients (2.27 hours). Even if cangrelor was more commonly used in high‐risk patients, ST rates were low and similar in cangrelor‐ and non–cangrelor‐treated patients at 30 days.35
Therefore, the data available evaluating its real‐world use show that physicians are using cangrelor in high‐risk patients undergoing PCI for STEMI, such as those needing endotracheal intubation or complicated by cardiac arrest or cardiogenic shock, independently from their geographic location.35, 36 Accordingly, a recent survey of the American College of Clinical Pharmacy's Cardiology Practice and Research Network, aimed to evaluate the opinion of cardiovascular clinical pharmacists on the current role of GPIs in ACS, highlighted that cangrelor would be the ideal agent for the management of patients with STEMI undergoing PCI.65 Indeed, for those with STEMI and nausea or other gastrointestinal symptoms, a route of administration other than oral could be preferable.
Ongoing Studies on Cangrelor
There are several ongoing research studies (Table), including national and international registries,66, 67, 68, 69 that will provide insights on the use of cangrelor in patients undergoing contemporary PCI. In particular, more data are desirable on the transition to potent oral P2Y12 receptor inhibitors, or for patients who need a quick‐acting intravenous agent like cangrelor in emergent situations, such as cardiac arrest or cardiogenic shock, or for those who have been preloaded with oral antiplatelet agents or GPI and present angiographic findings requiring an additional antiplatelet agent. Nevertheless, because registries will have no comparator or randomization, they will provide limited insight into the clinical value of cangrelor in combination with the newer P2Y12 agents.
The CAMEO (Cangrelor in Acute Myocardial Infarction: Effectiveness and Outcomes Registry; ClinicalTrials.gov Identifier: NCT04076813) is an ongoing multicenter US registry aimed to retrospectively address optimal platelet inhibition during the early management of patients with MI before coronary angiography or coronary artery bypass grafting.
The MARS (Management of Antiplatelet Regimen During Surgical Procedures; ClinicalTrials.gov Identifier: NCT03981835) and the MONET BRIDGE (Maintenance of Antiplatelet Therapy in Patients With Coronary Stenting Undergoing Surgery; ClinicalTrials.gov Identifier: NCT03862651) studies will study the area of perioperative antiplatelet therapy management. In particular, the MARS registry is a US multicenter observational registry designed to collect preoperative, intraoperative, and postoperative clinical strategies, therapeutic interventions, and 30‐day outcomes data of ≈1500 patients post‐PCI scheduled to undergo cardiac or noncardiac surgery. The MONET BRIDGE study is a randomized, placebo‐controlled study aimed to assess if a prolonged cangrelor infusion is safe and able to maintain an effective platelet inhibition in patients who discontinue an oral P2Y12 inhibitor for cardiac or noncardiac procedures within 1 year from PCI.
Finally, the Cangrelor OHCA (Out‐of‐Hospital Cardiac Arrest; ClinicalTrials.gov Identifier: NCT04005729) and the DAPT‐SHOCK‐AMI (Dual Antiplatelet Therapy for Shock Patients With Acute Myocardial Infarction; ClinicalTrials.gov Identifier: NCT03551964) randomized controlled studies will assess the efficacy of cangrelor compared with ticagrelor in high‐risk subgroups, such as comatose survivors of OHCA and patients with cardiogenic shock undergoing PCI.
The ARCANGELO
Most real‐world evidence on the use of cangrelor is derived from retrospective analyses.36, 68 Such assessment may lack systematic collection of safety data. Furthermore, registration trials were performed only with the use of clopidogrel as an oral P2Y12 inhibitor. However, in real‐world practice, cangrelor is more commonly used in association with ticagrelor,65 underscoring the need for real‐world prospective registries.
The ARCANGELO (Italian Prospective Study on Cangrelor) (ClinicalTrials.gov Identifier: NCT04471870) is a multicenter, observational, prospective cohort study, including patients with ACS undergoing PCI who receive cangrelor and transitioning to any oral P2Y12 inhibitor aimed to collect information about the safety of cangrelor in real clinical practice (Figure 4). The primary end point is the incidence of any hemorrhage, according to Bleeding Academic Research Consortium criteria,70 in the 30 days following the PCI, calculated as the ratio between the number of patients experiencing at least one event during the 30‐day observation period/the total number of evaluable patients. This evaluation will be of added value because bleeding data will be collected and scored in a prespecified standardized manner.
Figure 4. Design of the ARCANGELO (Italian Prospective Study on Cangrelor).
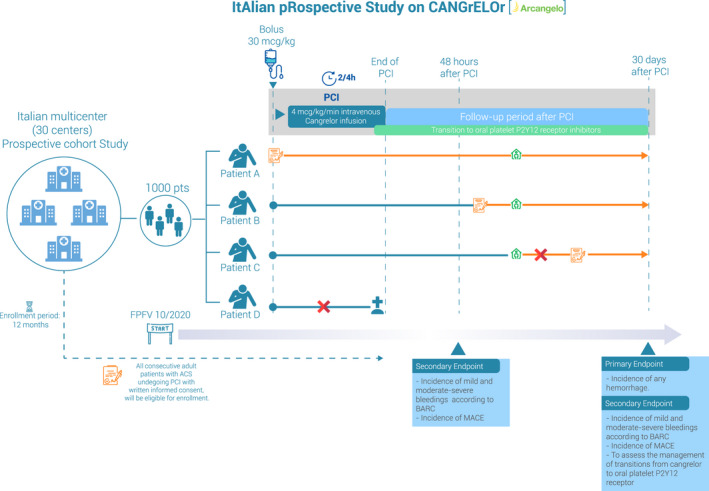
Each letter (A, B, C, and D) represents a patient prototype. Orange boxes identified the Informed and Privacy Consent Form. Green diamonds identify time of discharge. Each horizontal solid line represents the period of observation of each patient, which can be either mainly entirely prospective (orange lines) or could also include, for a small proportion of patients, a retrospective period (blue lines). Cangrelor intravenous infusion could end after percutaneous coronary intervention (PCI) conclusion. Even if adherence to European Medical Agency indications41 is not required, the transition to oral platelet P2Y12 receptor inhibitors may occur before the end of cangrelor infusion, according to the product's approved summary of product characteristics (SPC).41 In these examples, patients A and B were both eligible, because the Informed and Privacy Consent Form was signed before patient discharge; in particular, patient B was not able to give consent before start of cangrelor and PCI. On the contrary, patient C provided consent after being discharged; therefore, the patient was not eligible. Also, patient D was not eligible because death occurred before being able to obtain Informed and Privacy Consent Form. ACS indicates acute coronary syndrome; BARC, Bleeding Academic Research Consortium; FPFV, first patient first visit; MACE, major adverse cardiac event; and pts, patients.
The secondary outcomes will include the evaluation of the incidence of major adverse cardiac events, including death, MI, IDR, and ST, and different types of bleedings, the type and timing of administration of oral platelet P2Y12 inhibitors, and the use of GPI from 48 hours to 30 days after PCI (Figure 4). The study plans to enroll ≈1000 patients from the 30 participating centers in Italy, until September 2021.
Conclusions
There are several antithrombotic drugs currently being developed for the treatment of ACS, targeting multiple pathways, with the potential of reducing recurrent ischemic events without significantly increasing bleeding complications, compared with standard therapies71 (Figure 5). Cangrelor is the only intravenous platelet P2Y12 inhibitor currently available for clinical use. Cangrelor provides prompt, potent, and reliable antiplatelet effects. Such pharmacologic properties allow to overcome limitations of oral P2Y12 inhibitors characterized by inevitable delay in their onset of action, which is enhanced in high‐risk short‐term settings in which their gastrointestinal absorption is further compromised. Cangrelor therefore represents an ideal agent to reduce the risk of thrombotic complications in patients undergoing PCI who have not been pretreated with an oral P2Y12 inhibitor as well as in settings in which absorption of an oral agent is impeded or impaired (eg, hemodynamically unstable or intubated patients who are unable to swallow or who might not fully absorb an oral antiplatelet agent because of STEMI or cardiogenic shock). The introduction of cangrelor in clinical practice has seen its use expand and differ from how this was investigated in registration trials. These observations underscore the need for prospective evaluations that will provide insights on the safety and efficacy of cangrelor in real‐world clinical practice.
Figure 5. Antithrombotic drugs currently developed for the treatment of acute coronary syndrome.
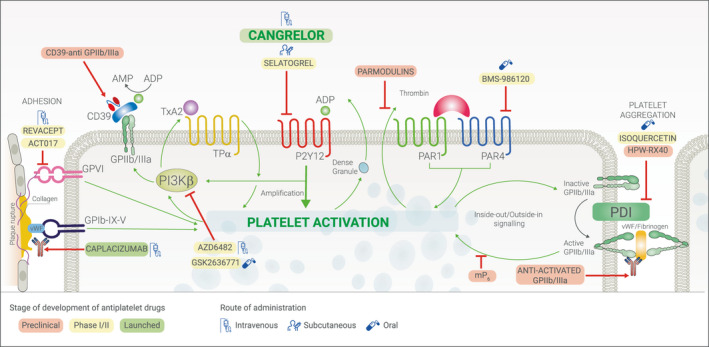
GP indicates glycoprotein; PAR, protease‐activated receptors; and PDI, protein disulfide isomerase.
Disclosures
Dr De Luca declares that he has received consulting fees or honoraria from Amgen, Aspen, AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Daiichi Sankyo, Eli Lilly, Menarini, Pfizer/Bristol‐Myers Squibb, Sanofi, Servier, and The Medicines Company, outside the present work; Dr Steg declares that he has received consulting fees or honoraria from Amgen, Myokardia, Novo‐Nordisk, and Regeneron, and has received research grants from Amarin, Bayer, Sanofi, and Servier, outside the present work; he has also received payments for participation in clinical trials (Steering Committee, Clinical Events Committee [CEC], Data Safety Monitoring Board [DSMB]) of Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Idorsia, Novartis, Pfizer, Sanofi, and Servier. Dr Bhatt discloses the following relationships: Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED [CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis Requiring Aortic Valve Replacement] trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE‐II [Protection Against Emboli During Carotid Artery Stenting Using the Neuroguard IEP System]), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE [Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation] trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (ACC) (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI [Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting] clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE [A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease] trial, funded by Ferring Pharmaceuticals), HMP Global (Editor‐in‐Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co‐Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS [Cardiovascular Outcomes for People Using Anticoagulation Strategies] operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR (National Cardiovascular Data Registry)‐ACTION (Acute Coronary Treatment and Intervention Outcomes) Registry Steering Committee (Chair), Veterans Affairs Clinical Assessment Reporting and Tracking (VA CART) Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, Chiesi (including for his role as co‐Chair of CHAMPION PHOENIX), CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company (including for his role as co‐Chair of the CHAMPION trials); Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, and Takeda. Dr Capodanno declares that he has received consulting fees or honoraria from AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Daiichi‐Sankyo, and Sanofi, outside the present work; Dr Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company, and has received payments for participation in review activities from CeloNova and St. Jude Medical, outside the present work. Dr Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation.
Acknowledgments
The authors would like to acknowledge Andrea Rossi, who provided editorial assistance with the preparation of the article, with funding from Chiesi. However, no funding was provided to the authors, who were responsible for the content of the article and made the decision to submit the article.
(J Am Heart Assoc. 2021;10:e022125. DOI: 10.1161/JAHA.121.022125.)
For Disclosures, see page 10.
References
- 1.Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607. DOI: 10.1253/circj.CJ-09-0982. [DOI] [PubMed] [Google Scholar]
- 2.Damman P, Woudstra P, Kuijt WJ, De Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143–153. DOI: 10.1007/s11239-011-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72:2915–2931. DOI: 10.1016/j.jacc.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 4.De Luca L, Leonardi S, Cavallini C, Lucci D, Musumeci G, Caporale R, Abrignani MG, Lupi A, Rakar S, Gulizia MM, et al. Contemporary antithrombotic strategies in patients with acute coronary syndrome admitted to cardiac care units in Italy: the EYESHOT Study. Eur Heart J Acute Cardiovasc Care. 2015;4:441–452. DOI: 10.1177/2048872614560505. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Fernandez‐Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–1516. DOI: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. DOI: 10.1016/j.jcin.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. DOI: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 8.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. DOI: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F‐J, Ardissino D, De Servi S, Murphy SA, et al; TRITON‐TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. DOI: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 10.Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol. 2017;14:361–379. DOI: 10.1038/nrcardio.2017.18. [DOI] [PubMed] [Google Scholar]
- 11.Tavenier AH, Hermanides RS, Fabris E, Angiolillo DJ, van 't Hof AWJ. Bridging the gap: current and future insights for improving suboptimal platelet inhibition in STEMI. Int J Cardiol. 2021;328:40–45. DOI: 10.1016/j.ijcard.2020.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Koul S, Smith JG, Götberg M, Omerovic E, Alfredsson J, Venetsanos D, Persson J, Jensen J, Lagerqvist BO, Redfors B, et al. No benefit of ticagrelor pretreatment compared with treatment during percutaneous coronary intervention in patients with ST‐segment‐elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2018;11:e005528. DOI: 10.1161/CIRCINTERVENTIONS.117.005528. [DOI] [PubMed] [Google Scholar]
- 13.Ferreiro JL, Ueno M, Angiolillo DJ. Cangrelor: a review on its mechanism of action and clinical development. Expert Rev Cardiovasc Ther. 2009;7:1195–1201. DOI: 10.1586/erc.09.101. [DOI] [PubMed] [Google Scholar]
- 14.Angiolillo DJ, Schneider DJ, Bhatt DL, French WJ, Price MJ, Saucedo JF, Shaburishvili T, Huber K, Prats J, Liu T, et al. Pharmacodynamic effects of cangrelor and clopidogrel: the platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J Thromb Thrombolysis. 2012;34:44–55. DOI: 10.1007/s11239-012-0737-3. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–1313. DOI: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 16.Capodanno D, Milluzzo RP, Angiolillo DJ. Intravenous antiplatelet therapies (glycoprotein IIb/IIIa receptor inhibitors and cangrelor) in percutaneous coronary intervention: from pharmacology to indications for clinical use. Ther Adv Cardiovasc Dis. 2019;13:1753944719893274. DOI: 10.1177/1753944719893274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexopoulos D, Varlamos C, Mpahara A, Lianos I. P2Y12 inhibitors for the treatment of acute coronary syndrome patients undergoing percutaneous coronary intervention: current understanding and outcomes. Expert Rev Cardiovasc Ther. 2019;17:717–727. DOI: 10.1080/14779072.2019.1675513. [DOI] [PubMed] [Google Scholar]
- 18.Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, et al. International expert consensus on switching platelet P2Y12 receptor‐inhibiting therapies. Circulation. 2017;136:1955–1975. DOI: 10.1161/CIRCULATIONAHA.117.031164 [DOI] [PubMed] [Google Scholar]
- 19.Badreldin HA, Carter D, Cook BM, Qamar A, Vaduganathan M, Bhatt DL. Safety and tolerability of transitioning from cangrelor to ticagrelor in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017;120:359–361. DOI: 10.1016/j.amjcard.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Franchi F, Rollini F, Rivas A, Wali M, Briceno M, Agarwal M, Shaikh Z, Nawaz A, Silva G, Been L, et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST‐segment‐elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of the CANTIC Study. Circulation. 2019;139:1661–1670. DOI: 10.1161/CIRCULATIONAHA.118.038317. [DOI] [PubMed] [Google Scholar]
- 21.Schneider DJ, Agarwal Z, Seecheran N, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and ticagrelor. JACC Cardiovasc Interv. 2014;7:435–442. DOI: 10.1016/j.jcin.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Franchi F, Rollini F, Muñiz‐Lozano A, Cho RJ, Angiolillo DJ. Cangrelor: a review on pharmacology and clinical trial development. Expert Rev Cardiovasc Ther. 2013;11:1279–1291. DOI: 10.1586/14779072.2013.837701. [DOI] [PubMed] [Google Scholar]
- 23.Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50:27–35. DOI: 10.1177/0091270009344986. [DOI] [PubMed] [Google Scholar]
- 24.Leonardi S, Bhatt DL. Practical considerations for cangrelor use in patients with acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. 2019;8:39–44. DOI: 10.1177/2048872617707960. [DOI] [PubMed] [Google Scholar]
- 25.Angiolillo DJ, Bhatt DL, Steg PG, Stone GW, White HD, Gibson CM, Hamm CW, Price MJ, Prats J, Liu T, et al. Impact of cangrelor overdosing on bleeding complications in patients undergoing percutaneous coronary intervention: insights from the CHAMPION trials. J Thromb Thrombolysis. 2015;40:317–322. DOI: 10.1007/s11239-015-1233-3. [DOI] [PubMed] [Google Scholar]
- 26.Franchi F, Rollini F, Park Y, Angiolillo DJ. A safety evaluation of cangrelor in patients undergoing PCI. Expert Opin Drug Saf. 2016;15:275–285. DOI: 10.1517/14740338.2016.1133585. [DOI] [PubMed] [Google Scholar]
- 27.Dovlatova NL, Jakubowski JA, Sugidachi A, Heptinstall S. The reversible P2Y antagonist cangrelor influences the ability of the active metabolites of clopidogrel and prasugrel to produce irreversible inhibition of platelet function. J Thromb Haemost. 2008;6:1153–1159. DOI: 10.1111/j.1538-7836.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 28.Steinhubl SR, Oh JJ, Oestreich JH, Ferraris S, Charnigo R, Akers WS. Transitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effect. Thromb Res. 2008;121:527–534. DOI: 10.1016/j.thromres.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Schneider DJ, Seecheran N, Raza SS, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and prasugrel. Coron Artery Dis. 2015;26:42–48. DOI: 10.1097/MCA.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 30.Hochholzer W, Kleiner P, Younas I, Valina CM, Löffelhardt N, Amann M, Bömicke T, Ferenc M, Hauschke D, Trenk D, et al. Randomized comparison of oral P2Y 12‐receptor inhibitor loading strategies for transitioning from cangrelor: the ExcelsiorLOAD2 Trial. JACC Cardiovasc Interv. 2017;10:121–129. DOI: 10.1016/j.jcin.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Harrington RA, Stone GW, McNulty S, White HD, Lincoff AM, Gibson CM, Pollack CV, Montalescot G, Mahaffey KW, Kleiman NS, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329. DOI: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt DL, Lincoff AM, Gibson CM, Stone GW, McNulty S, Montalescot G, Kleiman NS, Goodman SG, White HD, Mahaffey KW, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330–2341. DOI: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 33.Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, Voeltz MD, Chandna H, Ramaiah C, Brtko M, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012;307:265–274. DOI: 10.1001/jama.2011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaduganathan M, Qamar A, Singh A, Venkateswaran RV, Szumita PM, Croce KJ, Mauri L, Leopold JA, Shah PB, Sobieszczyk P, et al. Cangrelor use since FDA approval: a single‐center, real‐world experience at a tertiary care hospital. J Am Coll Cardiol. 2017;69:463–464. DOI: 10.1016/j.jacc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Vaduganathan M, Qamar A, Badreldin HA, Faxon DP, Bhatt DL. Cangrelor use in cardiogenic shock: a single‐center real‐world experience. JACC Cardiovasc Interv. 2017;10:1712–1714. DOI: 10.1016/j.jcin.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Grimfjärd P, Lagerqvist B, Erlinge D, Varenhorst C, James S. Clinical use of cangrelor: nationwide experience from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J Cardiovasc Pharmacother. 2019;5:151–157. DOI: 10.1093/ehjcvp/pvz002. [DOI] [PubMed] [Google Scholar]
- 37.Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. DOI: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 38.White HD, Chew DP, Dauerman HL, Mahaffey KW, Gibson CM, Stone GW, Gruberg L, Harrington RA, Bhatt DL. Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163:182–190. DOI: 10.1016/j.ahj.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Hara H, Serruys PW, Takahashi K, Kawashima H, Ono M, Gao C, Wang R, Mohr FW, Holmes DR, Davierwala PM, et al; SYNTAX Extended Survival Investigators . Impact of peri‐procedural myocardial infarction on outcomes after revascularization. J Am Coll Cardiol. 2020;76:1622–1639. DOI: 10.1016/j.jacc.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Gregson J, Stone GW, Ben‐Yehuda O, Redfors B, Kandzari DE, Morice M‐C, Leon MB, Kosmidou I, Lembo NJ, Brown WM III, et al. Implications of alternative definitions of peri‐procedural myocardial infarction after coronary revascularization. J Am Coll Cardiol. 2020;76:1609–1621. DOI: 10.1016/j.jacc.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Leonardi S, Mahaffey KW, White HD, Gibson CM, Stone GW, Steg GW, Hamm CW, Price MJ, Todd M, Dietrich M, et al. Rationale and design of the cangrelor versus standard therapy to achieve optimal management of platelet inhibition PHOENIX trial. Am Heart J. 2012;163:768–776.e2. DOI: 10.1016/j.ahj.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Généreux P, Stone GW, Harrington RA, Gibson CM, Steg PG, Brener SJ, Angiolillo DJ, Price MJ, Prats J, LaSalle L, et al. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol. 2014;63:619–629. DOI: 10.1016/j.jacc.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Stone GW, Généreux P, Harrington RA, White HD, Gibson CM, Steg PG, Hamm CW, Mahaffey KW, Price MJ, Prats J, et al. Impact of lesion complexity on peri‐procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: core laboratory analysis from 10 854 patients from the CHAMPION PHOENIX trial. Eur Heart J. 2018;39:4112–4121. DOI: 10.1093/eurheartj/ehy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient‐level data. Lancet. 2013;382:1981–1992. DOI: 10.1016/S0140-6736(13)61615-3. [DOI] [PubMed] [Google Scholar]
- 45.Vaduganathan M, Harrington RA, Stone GW, Steg PG, Gibson CM, Hamm CW, Price MJ, Deliargyris EN, Prats J, Mahaffey KW, et al. Cangrelor versus clopidogrel on a background of unfractionated heparin (from CHAMPION PHOENIX). Am J Cardiol. 2017;120:1043–1048. DOI: 10.1016/j.amjcard.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 46.Vaduganathan M, Harrington RA, Stone GW, Deliargyris EN, Steg PG, Gibson CM, Hamm CW, Price MJ, Menozzi A, Prats J, et al. Cangrelor with and without glycoprotein IIb/IIIa inhibitors in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2017;69:176–185. DOI: 10.1016/j.jacc.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez JA, Harrington RA, Blankenship JC, Stone GW, Steg PG, Gibson CM, Hamm CW, Price MJ, Généreux P, Prats J, et al; CHAMPION PHOENIX Investigators . The effect of cangrelor and access site on ischaemic and bleeding events: insights from CHAMPION PHOENIX. Eur Heart J. 2016;37:1122–1130. DOI: 10.1093/eurheartj/ehv498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaduganathan M, Harrington R, Stone G, Steg G, Gibson C, Hamm C, Price M, Lopes R, Leonardi S, Deliargyris E, et al. Short‐ and long‐term mortality following bleeding events in patients undergoing percutaneous coronary intervention: insights from four validated bleeding scales in the CHAMPION trials. EuroIntervention. 2018;13:e1841–e1849. DOI: 10.4244/EIJ-D-17-00723. [DOI] [PubMed] [Google Scholar]
- 49.Abtan J, Steg PG, Stone GW, Mahaffey KW, Gibson CM, Hamm CW, Price MJ, Abnousi F, Prats J, Deliargyris EN, et al. Efficacy and safety of cangrelor in preventing periprocedural complications in patients with stable angina and acute coronary syndromes undergoing percutaneous coronary intervention: the CHAMPION PHOENIX Trial. JACC Cardiovasc Interv. 2016;9:1905–1913. DOI: 10.1016/j.jcin.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad MA, Andell P, Koul S, James S, Scherstén F, Götberg M, Erlinge D. Cangrelor in combination with ticagrelor provides consistent and potent P2Y12‐inhibition during and after primary percutaneous coronary intervention in real‐world patients with ST‐segment‐elevation myocardial infarction. Platelets. 2017;28:414–416. DOI: 10.1080/09537104.2016.1246714. [DOI] [PubMed] [Google Scholar]
- 51.Ubaid S, Ford TJ, Berry C, Murray HM, Wrigley B, Khan N, Thomas MR, Armesilla AL, Townend JN, Khogali SS, et al. Cangrelor versus ticagrelor in patients treated with primary percutaneous coronary intervention: impact on platelet activity, myocardial microvascular function and infarct size: a randomized controlled trial. Thromb Haemost. 2019;119:1171–1181. DOI: 10.1055/s-0039-1688789. [DOI] [PubMed] [Google Scholar]
- 52.Gargiulo G, Esposito G, Avvedimento M, Nagler M, Minuz P, Campo G, Gragnano F, Manavifar N, Piccolo R, Tebaldi M, et al. Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST‐segment‐elevation myocardial infarction: primary results of the FABOLUS‐FASTER Trial. Circulation. 2020;142:441–454. DOI: 10.1161/CIRCULATIONAHA.120.046928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossini R, Masiero G, Fruttero C, Passamonti E, Calvaruso E, Cecconi M, Carlucci C, Mojoli M, Guido P, Talanas G, et al. Antiplatelet therapy with cangrelor in patients undergoing surgery after coronary stent implantation: a real‐world bridging protocol experience. TH Open. 2020;4:e437–e445. DOI: 10.1055/s-0040-1721504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sible AM, Nawarskas JJ. Cangrelor: a new route for P2Y12 inhibition. Cardiol Rev. 2017;25:133–139. DOI: 10.1097/CRD.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 55.Keating GM. Cangrelor: a review in percutaneous coronary intervention. Drugs. 2015;75:1425–1434. DOI: 10.1007/s40265-015-0445-3. [DOI] [PubMed] [Google Scholar]
- 56.Stern G, Rimsans J, Qamar A, Vaduganathan M, Bhatt DL. Contemporary parenteral antiplatelet bridging strategies: a single‐centre real‐world experience at a tertiary care centre. EuroIntervention. 2018;14:e333–e335. DOI: 10.4244/EIJ-D-18-00036. [DOI] [PubMed] [Google Scholar]
- 57.Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P, Capodanno D, Leonardi S, Lettino M, Limbruno U, et al. A multidisciplinary approach on the perioperative antithrombotic management of patients with coronary stents undergoing surgery: surgery after stenting 2. JACC Cardiovasc Interv. 2018;11:417–434. DOI: 10.1016/j.jcin.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 58.Cao D, Chandiramani R, Capodanno D, Berger JS, Levin MA, Hawn MT, Angiolillo DJ, Mehran R. Non‐cardiac surgery in patients with coronary artery disease: risk evaluation and periprocedural management. Nat Rev Cardiol. 2021;18:37–57. DOI: 10.1038/s41569-020-0410-z. [DOI] [PubMed] [Google Scholar]
- 59.De Luca L, Valgimigli M. Unravelling the puzzle of antithrombotic therapies for complex percutaneous coronary intervention. Eur Heart J Cardiovasc Pharmacother. 2020. Sep 16 [epub ahead of print]. DOI: 10.1093/ehjcvp/pvaa107. [DOI] [PubMed] [Google Scholar]
- 60.Cangrelor United States full prescribing information [online]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204958lbl.pdf. Accessed January 21, 2021.
- 61.Kangrexal EPAR summary for the public [online]. https://www.ema.europa.eu/en/medicines/human/EPAR/kengrexal. Accessed January 21, 2021.
- 62.Collet J‐P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2021;42:1289–1367. DOI: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 63.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–254. DOI: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 64.Beavers CJ, Jennings DL. Use of glycoprotein IIb/IIIa inhibitors in the modern era of acute coronary syndrome management: a survey of cardiovascular clinical pharmacists. J Pharm Pract. 2021;34:372–377. DOI: 10.1177/0897190019872386. [DOI] [PubMed] [Google Scholar]
- 65.Droppa M, Vaduganathan M, Venkateswaran RV, Singh A, Szumita PM, Roberts RJ, Qamar A, Hack L, Rath D, Gawaz M, et al. Cangrelor in cardiogenic shock and after cardiopulmonary resuscitation: a global, multicenter, matched pair analysis with oral P2Y12 inhibition from the IABP‐SHOCK II trial. Resuscitation. 2019;137:205–212. DOI: 10.1016/j.resuscitation.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Ashrafi R. Clinical disease registries in acute myocardial infarction. World J Cardiol. 2014;6:415. DOI: 10.4330/wjc.v6.i6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeymer U, Ludman P, Danchin N, Kala P, Ferrari R, Maggioni AP, Weidinger F. The European Society of Cardiology ACCA‐EAPCI Registry on ST elevation myocardial infarction. Eur Heart J. 2017;38:138–139. DOI: 10.1093/eurheartj/ehw619. [DOI] [PubMed] [Google Scholar]
- 68.Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013;99:297–303. DOI: 10.1136/heartjnl-2012-303379. [DOI] [PubMed] [Google Scholar]
- 69.Bagai A, Lu D, Lucas J, Goyal A, Herzog CA, Wang TY, Goodman SG, Roe MT. Temporal trends in utilization of cardiac therapies and outcomes for myocardial infarction by degree of chronic kidney disease: a report from the NCDR Chest Pain‐MI Registry. J Am Heart Assoc. 2018;7:e010394. DOI: 10.1161/JAHA.118.010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. DOI: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 71.Zwart B, Parker WAE, Storey RF. New antithrombotic drugs in acute coronary syndrome. J Clin Med. 2020;9:2059. DOI: 10.3390/jcm9072059. [DOI] [PMC free article] [PubMed] [Google Scholar]


