Abstract
Background
We examined whether primary prevention with statins and high adherence to statins reduce the associated risk of cardiovascular events or death in a low‐risk population with type 2 diabetes mellitus (T2D).
Methods and Results
Using Danish nationwide registers, we included patients with new‐onset T2D, aged 40 to 89 years, between 2005 and 2011, who were alive 18 months following the T2D diagnosis (index date). In patients who purchased statins within 6 months following T2D diagnosis, we calculated the proportion of days covered (PDC) within 1 year after the initial 6‐month period. We studied the combined end point of myocardial infarction, stroke, or all‐cause mortality, whichever came first, with Cox regression. Reported were standardized 5‐year risk differences for fixed comorbidity distribution according to statin treatment history, stratified by sex and age. Among 77 170 patients, 42 975 (56%) were treated with statins, of whom 31 061 (72%) had a PDC ≥80%. In men aged 70 to 79 years who were treated with statins, the standardized 5‐year risk was 22.9% (95% CI, 21.5%–24.3%), whereas the risk was 29.1% (95% CI, 27.4%–30.7%) in men not treated, resulting in a significant risk reduction of 6.2% (95% CI, 4.0%–8.4%), P<0.0001. The risk reduction associated with statins increased with advancing age group (women: age 40–49 years, 0.0% [95% CI, −1.0% to 1.0%]; age 80–89 years, 10.8% [95% CI, 7.2%–14.4%]). Standardizing to all patients treated with statins, PDC <80% was associated with increased risk difference (reference PDC ≥80%; PDC <20%, 4.2% [95% CI, 2.9%–5.6%]).
Conclusions
This study supports the use of statins as primary prevention against cardiovascular diseases or death in 18‐month surviving low‐risk patients with T2D, with the highest effect in the elderly and adherent patients.
Keywords: cardiovascular disease, diabetes mellitus, statins
Subject Categories: Cardiovascular Disease, Primary Prevention, Epidemiology
Nonstandard Abbreviations and Acronyms
- PDC
proportion of days covered
- T2D
type 2 diabetes mellitus
Clinical Perspective
What Is New?
In a low‐risk, nationwide, contemporary population with type 2 diabetes mellitus, use of statins was associated with a lower 5‐year risk of a major adverse cardiovascular events or death in all age groups for men and from age >50 years in women, and the risk reduction increased with advancing age.
A low adherence of statins was associated with a higher 5‐year risk of major adverse cardiovascular events or death.
What Are the Clinical Implications?
This nationwide study supports the use of statins as primary prevention against major adverse cardiovascular events or death in low‐risk patients with type 2 diabetes mellitus, with the highest effect in elderly patients.
A high adherence of statins was important to maintain this effect.
Statins are commonly used in both primary and secondary prevention of cardiovascular disease (CVD) in patients with diabetes mellitus. Prior studies have elucidated the beneficial effect of statin therapy in primary prevention of CVD in patients with diabetes mellitus.1, 2, 3, 4 Although a low proportion had diabetes mellitus, a meta‐analysis further observed a CVD rate reduction of almost 40% per 1‐mmol/L reduction of low‐density lipoprotein (LDL) cholesterol in patients at low CVD risk.5 Studies exploring the cardiovascular risks associated with treatment with statins in a low‐risk population with diabetes mellitus are, however, limited. In addition, there are discrepancies between European6 and American7, 8 guidelines on the use of LDL levels to guide primary prevention treatment of dyslipidemia in patients with diabetes mellitus. Generally, suboptimal adherence to primary prevention therapies of CVD may contribute significantly to increased risk of CVD and death,9 but whether high levels of adherence to primary prevention therapy with statins are associated with reduced risk of CVD or death in patients with diabetes mellitus is unknown. Last, emerging evidence for the potential benefits of statins in elderly people remains limited and to date only examined in relative rather than absolute terms, which limits the understanding of the size of the statin treatment effect.
The aims of this study were, therefore, first to investigate whether statins are associated with reduced 5‐year risks of myocardial infarction, ischemic stroke, or all‐cause death by sex and age groups in individuals with short type 2 diabetes mellitus (T2D) duration and without prior CVD, chronic kidney failure, or cancer, and second, to investigate the associations between adherence levels to statins and 5‐year risk of the composite outcome.
Methods
Data obtained through the nationwide registers in Denmark can be made available only through research on Danish servers hosted in highly protected research environments where researchers can be granted access and permission with encrypted person identification. Access to raw data can be gained only through collaboration with the authors or other Danish institutions that already have been granted access. Please contact the first author with any questions on data access.
Data Sources
All residents in Denmark receive a unique and permanent civil registration number at birth or immigration that enables individual‐level linkage between nationwide registries. We obtained data from the following: (1) the Danish Civil Registration System registry (sex, date of birth, immigration, emigration, and vital status), (2) the Danish National Patient Registry (discharge diagnoses coded according to the International Classification of Diseases, Eighth Revision [ICD‐8] since 1977, and International Classification of Diseases, Tenth Revision [ICD‐10] system since 1994), (3) the Danish National Prescription Registry (all prescriptions claimed since 1995 according to the Anatomical Therapeutic Classification, including date of dispensing, strength, and quantity), and (4) the Danish National Causes of Death Registry (date of death as well as assumed primary and contributing causes of death from death certificates). All registries have been validated previously.10, 11, 12, 13
Population
The index date was defined as 18 months following the diagnosis of T2D (Figure S1). The study population comprised all individuals in Denmark with incident T2D at age 40 to 89 years between January 1, 2005, and December 31, 2011, who were alive at the index date. Incident T2D was defined as either initiation of treatment with an antidiabetic medication (Anatomical Therapeutic Classification=A10; positive predictive value, 95%; sensitivity, 72%14) or discharge diagnosis of T2D (ICD‐10 code E11; positive predictive value, 97%; sensitivity, 64%14), whichever came first. We excluded individuals with assumed type 1 diabetes mellitus (monotherapy of insulin [Anatomical Therapeutic Classification code A10A] before the age of 30 years) as well as patients with a diagnosis of coronary artery disease, heart failure, ischemic stroke, peripheral arterial disease, and chronic kidney failure before index, and further excluded patients who were diagnosed with any cancer 6.5 years before index or emigrated before the index date (diagnosis codes in Table S1). Antidiabetic drug dispensations that were prescribed for possible polycystic ovary syndrome or gestational diabetes mellitus were excluded, as done previously.15
Exposure
The method used to determine the dose and treatment duration is shown in Data S1 and has been described previously.16 Individuals were considered treated with statins, if they were covered at diagnosis of T2D or initiated treatment within the first 6 months (365.25 days/2) following T2D diagnosis. Among individuals who were treated, we used consecutive claimed prescriptions to calculate the drug adherence level as proportion of days covered (PDC), which was calculated as days exposed to statins within 1 year before index (PDC=days covered/365.25).
Comorbidities
Comorbidities (atrial fibrillation and chronic obstructive pulmonary disease) and medications (antidiabetic agents, antihypertensive agents, lipid‐lowering drugs, and anticoagulant drugs) at index were identified based on ICD‐8 and ICD‐10 codes and Anatomical Therapeutic Classification codes (Table S1). Medications were defined as dispensed prescriptions within 180 days before the index date.
Outcomes
The primary outcome was the composite of first myocardial infarction, first ischemic stroke, or all‐cause death (ICD‐10 codes in Table S1), whichever came first. The diagnoses for myocardial infarction and ischemic stroke have been validated in Danish registers with high positive predictive values (ie, 97% for myocardial infarction and 97% for ischemic stroke).17, 18 The definition of ischemic stroke included diagnoses of ischemic stroke and unspecified stroke, as most unspecified strokes have been observed to be of ischemic origin.18
Statistical Analysis
All individuals were followed up from the index date until the primary event, emigration, or 5 years following the index date, whichever came first.
We present population characteristics at the index date as medians with 25th and 75th percentiles for continuous variables, and as counts with percentages for categorical variables. We calculated the probability of initiating treatment within the first 6‐month period by sex and age at time of T2D diagnosis (age, 40–49, 50–59, 60–69, 70–79, and 80–89 years). The main analyses were based on a multiple Cox regression model for the hazard rate of the composite outcome in subgroups defined by sex and age. The model was adjusted for comorbidities and use of comedications at the index date (Table S2). On the basis of the Cox regression analyses, we computed the standardized 5‐year risks of the composite outcome according to possible drug adherence levels (ie, untreated, PDC levels <20%, 20%–40%, 40%–60%, 60%–80%, or ≥80%), keeping the observed values of the other patient characteristics. We reported average treatment effects as differences of the crude and standardized 5‐year risk. We set the significance level at 5%.
In sensitivity analyses, we used multivariable Cox model with the same set of exposure variables for the rate of "any hospital discharge due to a skin lesion" (ICD‐10 codes S00–99). We further moved the index date further away from the date of first T2D diagnosis and repeated all analyses at landmark times set 30, 42, 54, 66, and 78 months following T2D diagnosis. Last, we used a nested case‐control design with 10 age‐ and sex‐matched controls from the risk set of each case to fit a Cox regression model with time‐dependent exposure and time‐dependent covariates and baseline hazard function stratified for age and sex (Table S2).19, 20 The current statin exposure was defined in an 18‐month long exposure window before case date, and the comorbidities and comedication were evaluated before the exposure window (Figure S1). Population characteristics at the index date, including comorbidities, medications, as well as coverage and PDC level during the 18 months before the case date, were registered (Tables S1 and S3).
All statistical analyses were conducted using R, version 3.6.1.21
Ethical Approval
Retrospective register studies do not need ethical approval in Denmark. The Danish Data Protection Agency has approved the project (approval number P‐2019‐393).
Results
We included 88 175 individuals with incident T2D between January 1, 2005, and December 31, 2011, and following exclusion of another 11 005 individuals during the 18‐month period following T2D diagnosis (23% attributable to death); the final population comprised 77 170 patients (Figure S2). During our study period, 10 209 patients (13.2%) had a first‐time myocardial infarction, had a first ischemic stroke, or died. Compared with patients not treated, patients treated with statins as well as treated patients with a high adherence level (PDC ≥80%) compared with lower PDC levels were slightly older, were more frequently ethnically Danish, had lower level of education, and generally claimed prescriptions for antidiabetic agents (except insulin), antihypertensive agents, and anticoagulants more frequently (Table).
Table 1.
Population Characteristics According to Treatment With Statins
| Characteristics | Coverage | Proportion of Days Covered | |||||
|---|---|---|---|---|---|---|---|
| Not Treated | Treated | <20% | 20%–40% | 40%–60% | 60%–80% | >80% | |
| Count | 34 195 | 42 975 | 2651 | 1486 | 2099 | 5678 | 31 061 |
| Women | 15 473 (45.2) | 20 287 (47.2) | 1149 (43.3) | 661 (44.5) | 925 (44.1) | 2501 (44.0) | 15 051 (48.5) |
| Age, Q1–Q3, y | 59 (50–68) | 62 (54–68) | 59 (50–67) | 58 (50–65) | 59 (51–67) | 60 (52–67) | 62 (55–69) |
| Ethnic Danish | 30 383 (88.9) | 39 319 (91.5) | 2225 (83.9) | 1222 (82.2) | 1778 (84.7) | 5064 (89.2) | 29 030 (93.5) |
| Comorbidities | |||||||
| Atrial fibrillation | 1311 (3.8) | 1662 (3.9) | 92 (3.5) | 41 (2.8) | 70 (3.3) | 198 (3.5) | 1261 (4.1) |
| COPD | 1640 (4.8) | 1759 (4.1) | 116 (4.4) | 51 (3.4) | 88 (4.2) | 242 (4.3) | 1262 (4.1) |
| Highest attained education* | |||||||
| Basic school | 14 255 (41.7) | 18 064 (42.0) | 1025 (38.7) | 601 (40.4) | 830 (39.5) | 2307 (40.6) | 13 301 (42.8) |
| Upper secondary | 1141 (3.3) | 1109 (2.6) | 91 (3.4) | 57 (3.8) | 69 (3.3) | 156 (2.7) | 736 (2.4) |
| Vocational | 12 794 (37.4) | 16 765 (39.0) | 1055 (39.8) | 568 (38.2) | 803 (38.3) | 2222 (39.1) | 12 117 (39.0) |
| Short‐ or medium‐length higher education | 4583 (13.4) | 5559 (12.9) | 357 (13.5) | 192 (12.9) | 311 (14.8) | 764 (13.5) | 3935 (12.7) |
| Master's degree or higher | 1422 (4.2) | 1478 (3.4) | 123 (4.6) | 68 (4.6) | 86 (4.1) | 229 (4.0) | 972 (3.1) |
| Medication | |||||||
| Metformin | 22 340 (65.3) | 34 583 (80.5) | 1414 (53.3) | 1017 (68.4) | 1532 (73.0) | 4653 (81.9) | 25 967 (83.6) |
| Insulin | 2492 (7.3) | 1665 (3.9) | 150 (5.7) | 72 (4.8) | 105 (5.0) | 234 (4.1) | 1104 (3.6) |
| Sulfonylureas | 6723 (19.7) | 7266 (16.9) | 340 (12.8) | 224 (15.1) | 337 (16.1) | 964 (17.0) | 5401 (17.4) |
| DPP‐4 inhibitor | 1289 (3.8) | 2107 (4.9) | 104 (3.9) | 62 (4.2) | 104 (5.0) | 324 (5.7) | 1513 (4.9) |
| GLP‐1 analogue | 442 (1.3) | 706 (1.6) | 43 (1.6) | 33 (2.2) | 37 (1.8) | 115 (2.0) | 478 (1.5) |
| Aspirin | 5233 (15.3) | 13 030 (30.3) | 433 (16.3) | 275 (18.5) | 559 (26.6) | 1647 (29.0) | 10 116 (32.6) |
| ADP inhibitor | 61 (0.2) | 209 (0.5) | 6 (0.2) | 10 (0.7) | 7 (0.3) | 25 (0.4) | 161 (0.5) |
| Anticoagulants | 1089 (3.2) | 1546 (3.6) | 74 (2.8) | 37 (2.5) | 54 (2.6) | 176 (3.1) | 1205 (3.9) |
| RASi | 14 153 (41.4) | 26 245 (61.1) | 1039 (39.2) | 671 (45.2) | 1105 (52.6) | 3410 (60.1) | 20 020 (64.5) |
| Cholesterol‐lowering drugs (nonstatins) | 251 (0.7) | 206 (0.5) | 103 (3.9) | 44 (3.0) | 24 (1.1) | 22 (0.4) | 13 (0.0) |
| β Blocker | 4623 (13.5) | 7916 (18.4) | 286 (10.8) | 190 (12.8) | 297 (14.1) | 922 (16.2) | 6221 (20.0) |
| Calcium channel blockers | 6149 (18.0) | 11 477 (26.7) | 436 (16.4) | 272 (18.3) | 447 (21.3) | 1407 (24.8) | 8915 (28.7) |
| Thiazides | 5591 (16.4) | 8778 (20.4) | 338 (12.7) | 239 (16.1) | 322 (15.3) | 986 (17.4) | 6893 (22.2) |
| Furosemide | 2826 (8.3) | 3193 (7.4) | 130 (4.9) | 67 (4.5) | 132 (6.3) | 365 (6.4) | 2499 (8.0) |
| Aldosterone | 917 (2.7) | 887 (2.1) | 40 (1.5) | 15 (1.0) | 39 (1.9) | 105 (1.8) | 688 (2.2) |
Data are given as number (percentage), unless otherwise indicated. COPD indicates chronic obstructive pulmonary disease; DPP4, dipeptidyl peptidase 4; GLP1, glucagon‐like peptide 1; Q1–Q3, 25th percentile–75th percentile; and RASi, renin‐angiotensin system inhibitors.
Basic school (primary, lower secondary; 9 years); upper secondary (general secondary, technical secondary; "high‐school"); vocational (eg, electrician or chef); short‐ or medium‐length higher education (academy professional degree, professional bachelor's degree, university bachelor's degree; 2–4 years following upper secondary); master's degree or higher.
Initiation of Medication, Coverage, and Adherence Level
During the 6 months following T2D diagnosis, the proportion of individuals initiating treatment of statins increased rapidly during the first few days after T2D diagnosis and subsequently stabilized, leaving 56% treated with statins following 6 months (Figure 1). Overall, a higher proportion of women (except those aged 40–49 years; Figure 1 and Figure S3) and a higher proportion of patients aged 50 to 79 years initiated treatment with statins. In patients who initiated treatment with statins, most had a high adherence level (PDC ≥80%: 72%; Table and Figure 2), and the adherence level of statins between men and women was largely similar (Figure S3).
Figure 1. Initiation of statins according to time since diagnosis of type 2 diabetes mellitus, stratified by age at time of diagnosis.
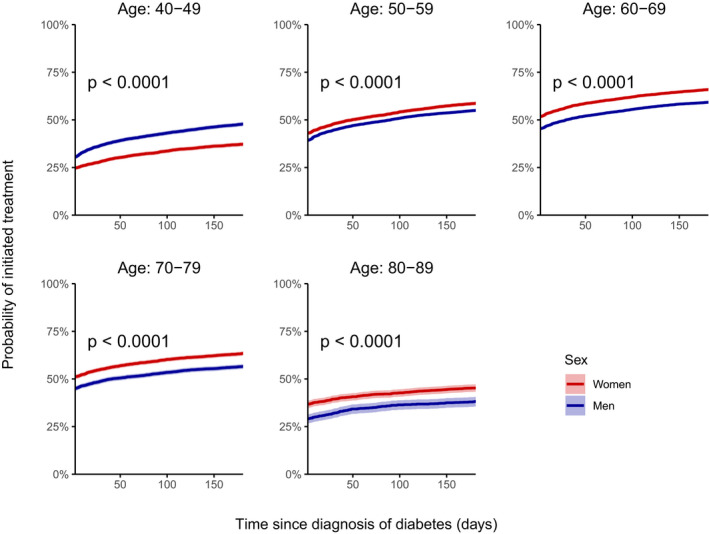
Figure 2. Coverage of statins at index date.
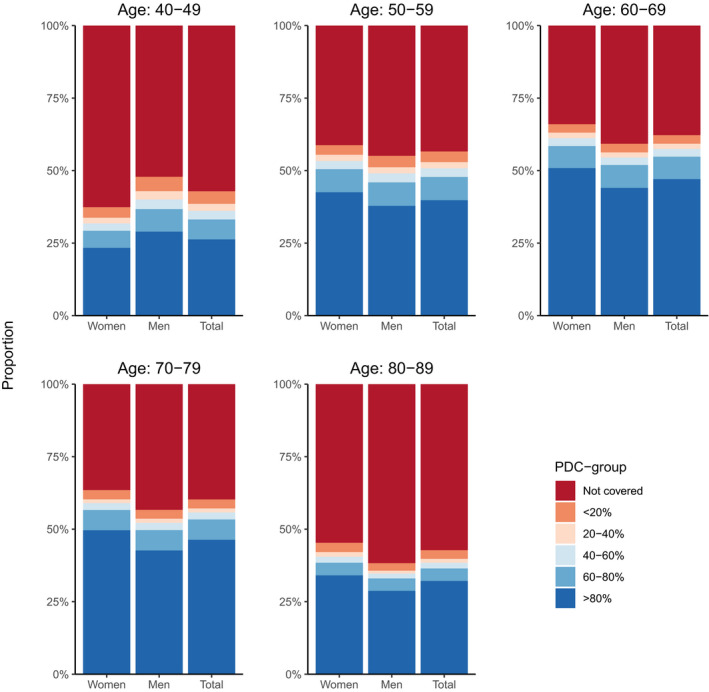
PDC indicates proportion of days covered.
The 5‐Year Risks According to Coverage and Adherence Level
Use of statins was associated with a significantly lower standardized 5‐year risk of the composite outcome of myocardial infarction, ischemic stroke, and all‐cause mortality in all age groups for men and from age >50 years in women (ie, men aged 70–79 years: treated, 22.9% [95% CI, 21.5%–24.3%]; not treated, 29.1% [95% CI, 27.4%–30.7%]; risk difference, 6.2% [95% CI, 4.0%–8.4%]; and number needed to treat, 16; Figures 3 and 4 and Figures S4 and S5). Crude 5‐year risks and risk differences of the composite according to use of statins are presented in Figures S4 and S5. Although the standardized 5‐year risk reduction associated with statins increased with advancing age group in men (age 40–49 years, 1.1% [95% CI, 0.0%–2.3%]; 50–59 years, 2.4% [95% CI, 1.3%–3.4%]; 60–69 years, 3.6% [95% CI, 2.4%–4.8%]; 70–79 years, 6.2% [95% CI, 4.0%–8.4%]; 80–89 years, 12.9% [95% CI, 7.6%–18.2%]) and in women (age 40–49 years, −0.0% [95% CI, −1.0% to 1.0%]; 50–59 years, 1.6% [95% CI, 0.6%–2.7%]; 60–69 years, 2.3% [95% CI, 1.2%–3.5%]; 70–79 years, 7.1% [95% CI, 5.1%–9.2%]; 80–89 years, 10.8% [95% CI, 7.2%–14.4%]), the standardized risk ratio remained largely constant with advancing age for both sexes (except women aged 40–49 years; Figure 4 and Figure S6).
Figure 3. Standardized 5‐year risk difference of the composite of myocardial infarction, ischemic stroke, or all‐cause death, according to treatment with statins (reference [ref.]=risktreated) and proportion of days covered (PDC; reference=riskPDC 80%–100%) and stratified by sex.
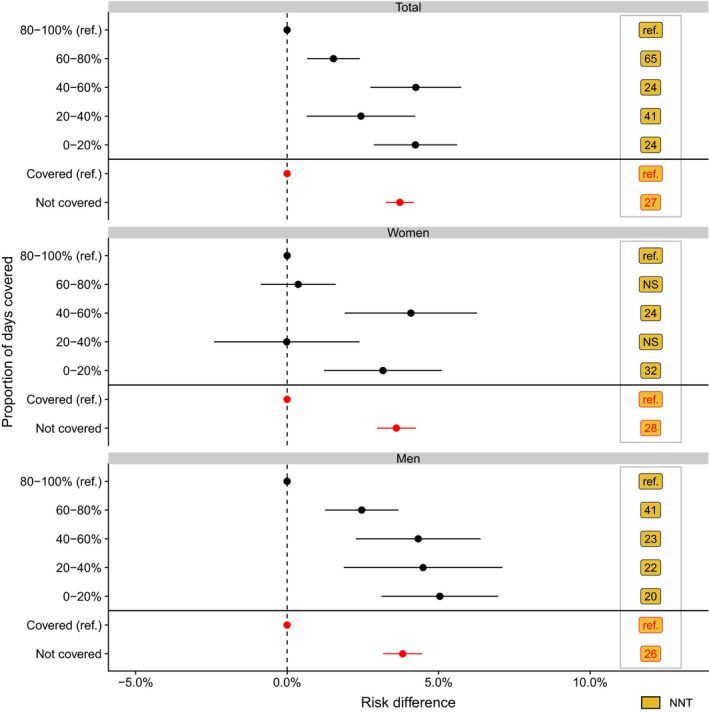
Number needed to treat (NNT) is the NNT with PDC level 80% to 100% or NNT with statins (yes/no) to prevent a cardiovascular disease event. NS indicates not significant.
Figure 4. Standardized 5‐year risk ratios and risk difference of the composite of myocardial infarction, ischemic stroke, or all‐cause death, according to treatment with statins (reference=risktreated) and stratified by sex and age group.
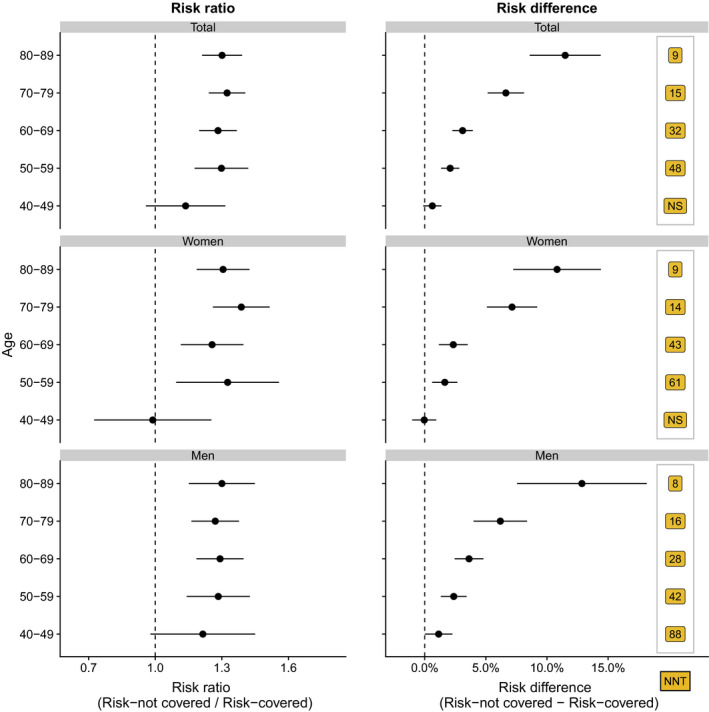
Number needed to treat (NNT) is the NNT with statins (yes/no) to prevent a cardiovascular disease event. NS indicates not significant.
Although the standardized risks were higher in men than in women (P for interaction: statins, <0.001; Figure S4), the standardized risk differences were largely similar in men and women (Figure 4). When standardizing to all patients treated with statins, we observed higher standardized risks with a PDC level of <80% compared with a PDC level of 80% to 100% (reference PDC ≥80%; PDC=60%–80%, 1.5% [95% CI, 0.7%–2.4%]; PDC=40%–60%, 4.2% [95% CI, 2.7%–5.7%]; PDC=20%–40%, 2.4% [95% CI, 0.6%–4.2%]; PDC <20%, 4.2% [95% CI, 2.9%–5.6%]; Figure 3 and Figure S4).
Exchanging the main outcome showed lower crude and standardized 5‐year risks of hospital discharge attributable to any skin lesion associated with statins as well as lower crude and standardized 5‐year risks associated with an increasing adherence level of statins (Figure S7). However, when stratifying by age group, the standardized 5‐year risks were largely similar between individuals treated and not treated with statins (Figure S7).
Sensitivity Analyses: Nested Case‐Control Population
In event‐free survivors, we observed that the proportion of patients treated with statins as well as individuals with a PDC level of 80% to 100% increased over study time regardless of age and sex (Figure S8). The associations between both treatment initiation (yes/no) and PDC levels and associated risk of the primary outcome were comparable in main analyses and the nested case‐control population (Figure S9; hazard ratios [HRs] for adjustment variables for main analyses in Tables S4 and S5 and for nested case‐control population in Tables S6 and S7).
Discussion
We observed that in a low‐risk, nationwide, contemporary population with T2D, use of statins was associated with a lower 5‐year risk of a composite outcome of first myocardial infarction, first ischemic stroke, or all‐cause mortality in all age groups for men and from age >50 years in women, and that the risk reduction increased with advancing age group. Second, a high adherence of statins was important to maintain this effect. Finally, we observed that women were more frequently treated with statins, and that a high proportion of patients (44%) were not treated with statins 6 months following T2D diagnosis.
Although no prior study has used exactly the same outcome as our study, prior clinical trials1, 2, 3 and meta‐analyses4, 5 have demonstrated beneficial effects of statins as primary prevention of CVD in patients with T2D. However, data are sparse for the effects of statins in a contemporary low‐risk population with T2D. A meta‐analysis observed that reducing LDL levels resulted in a reduction in cardiovascular rate in individuals with predicted <5% and 5% to 10% 5‐year cardiovascular risk (HR, 0.61 and 0.66 per 1‐mmol/L reduction in LDL, respectively),5 but only a low proportion of these patients had type 1+2 diabetes mellitus (7% and 18%, respectively), and competing risks of noncardiovascular death were not taken into account when stratifying by the calculated cardiovascular risk groups. Another meta‐analysis with patients with type 1+2 diabetes mellitus only observed a beneficial effect of reducing the LDL level on CVD rate in individuals without prior myocardial infarction, stroke, and peripheral arterial disease, but diabetes mellitus duration was unknown.4 As in our study, both meta‐analyses observed slightly higher associations in men than in women. In both meta‐analyses, the results were reported in relative terms (HRs), and not on an absolute scale. Although we did not have access to the patients' lipid profile, the latter meta‐analysis showed a similar effect of statins regardless of the baseline lipid profile.4
To date, meta‐analyses4, 5, 22 and a recent retrospective cohort study23 have only examined the statin treatment effect on major vascular events by age in relative rather than absolute terms, which makes it difficult to interpret the size of the treatment effect.24 Although one meta‐analysis stated that there is less direct evidence of benefit among patients aged >75 years without evidence of occlusive vascular disease,22 all 4 studies have largely observed a benefit of statins in primary prevention of major vascular events in relative terms in various subpopulations, excluding patients with heart failure and chronic renal failure in all age groups. We observed similar results in relative terms. In our study, we focused on absolute risk differences and observed a clear and increasingly beneficial effect of statins with advancing age in absolute terms, which resulted in lower numbers needed to treat in elderly patients. Thus, the conclusions of the referred studies may have been different if a risk difference had been used. Of note, we did not have access to information on LDL levels, the outcomes were slightly different, and our study used risks, whereas the meta‐analyses used hazards limiting the comparison.
We did not observe a significant effect of statins in women aged 40 to 49 years, which particularly in the youngest age group could be because cardiovascular events are rare in these age groups in women, which may explain why we were unable to detect an effect of statins. Furthermore, expected to be a minor limitation, this age category includes pregnant women with T2D, and possibly some women with polycystic ovary syndrome, for whom statins are not recommended. This is supported by more men than women aged 40 to 49 years initiating treatment with statins in our study.
Similar to our study, some studies have previously shown a beneficial effect of a high adherence of statin therapy as primary prevention of CVD and death,9 but none of the studies was based on a population of patients with diabetes mellitus only, and the methods varied greatly between the studies. Many studies only stratified by good versus poor adherence (typically defined as PDC <80% versus ≥80%); however, this might not really exist because the dose‐response phenomenon is more likely a continuum. Although dose‐response effects are difficult to mimic in a real‐life setting, they are crucial for decision‐making on operational adherence thresholds for different therapies.25 In this context, our study showed that the risk of the composite outcome increased with decreasing PDC group for statins. Because more than every fourth individual claiming prescriptions for statins had a PDC level of <80%, there is an urgent need to improve the adherence to statins, so that patients can benefit fully from the protective effects of statin therapy.
Our study used coverage status and PDC level of statins at the index date to calculate a 5‐year risk of myocardial infarction, ischemic stroke, or all‐cause mortality, but coverage status and PDC level may change over time, which could have influenced the outcome. We therefore conducted a sensitivity analysis exploring use in the 18 months before outcome event, which confirmed our overall findings for statins, as well as the associations with PDC level of statins.
Strengths and Limitations
The major strengths of this contemporary nationwide study include minimal risk of selection bias, minimal loss to follow‐up ensured by the comprehensive Danish registries, and the large sample size. However, important limitations need to be addressed.
The main limitation of the study is its observational nature; thus, only hypothesis‐generating associations and not causal relations can be explored. As with any statistical analysis, our ability to adjust for potential confounding is limited to data availability. In this study, we did not have access to information on metabolic control (glucose levels, lipids, blood pressure, and urine albumin levels), imaging findings (echocardiography and computed tomographic angiography, including coronary artery calcium scores), or lifestyle factors (smoking, alcohol consumption, body mass index, physical activity level, or diet). Although the Danish registries do not have information on smoking, we included chronic obstructive pulmonary disease as we consider it a strong marker of smoking. Similarly, we did not have information on hypertension, which is largely diagnosed from general practitioners. Discharge diagnoses of hypertension would therefore strongly underestimate the number of patients with hypertension. Instead, we chose to adjust for claimed prescriptions of antihypertensive medication groups separately. In addition, the group of cholesterol‐lowering drugs (nonstatins) was considered as a common group. These drugs were claimed only by a small number of patients at index, and we would therefore not expect subgrouping of this medication group to have a large impact on our results. PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors are not registered in the Danish National Prescription Registry as they have been delivered from hospital lipid clinics directly since October 2015, using rather restrictive rules because of the high price. Because the population of the current study was a low‐risk population with T2D, PCSK9 inhibitors may only have been given to a minor group of patients toward the end of the study period, which we consider unlikely to have affected our results.
Furthermore, a "healthy adherer" effect cannot be excluded,26 because exchanging the outcome to hospital discharge attributable to any skin lesion showed greater reduction of the composite outcome, particularly with increasing PDC level. However, when stratifying by age, a healthy adherer effect was less obvious. It has previously been suggested that, on the basis of the differential class effects of drug adherence on long‐term survival, adherence‐related benefits associated with evidence‐based pharmacotherapies are mediated by drug effects more than by healthy adherer behaviors.27, 28 Last, others have observed that individuals who are more ill appear to adhere to statins better.29 These findings challenge the concept of the "healthy adherer effect."
Third, we did not know the indication for the statins, and the calculations of dose and treatment periods represent approximations. During our study period, Danish guidelines recommended initiation of statins as primary prevention at time of T2D diagnosis at LDL levels >2.5 mmol/L, which was in line with European Association for the Study of Diabetes/European Society of Cardiology guidelines.30 To minimize inclusion of secondary prevention statin therapy, we excluded individuals with coronary heart disease, ischemic stroke, peripheral arterial disease, heart failure, cancer, and chronic kidney disease before and 18 months following a T2D diagnosis. Although these diagnoses have high positive predictive values17, 18, 31, 32 in the Danish registers, and high sensitivity for myocardial infarction,33 the sensitivity for heart failure is low32 and unknown for the remaining diseases. Thus, we cannot rule out that the indications for the claimed prescriptions of statins have been prescribed because of indications other than primary prevention of CVD.
Last, most of the Danish population is White individuals, and we did not include individuals immigrating to Denmark during our study period because of unknown medical history; thus, our results may not be generalizable to non‐White individuals.
Conclusions
This nationwide study supports the use of statins as primary prevention against CVDs or death in 18‐month surviving low‐risk patients with T2D, with the highest effect in the elderly patients. Second, a high adherence of statins was important to maintain this effect. Finally, women were more frequently treated with statins, and a high proportion of patients were not treated with statins 6 months following T2D diagnosis.
Sources of Funding
This work was funded by the Danish Heart Foundation (grant number: 18‐R121‐A8218).
Disclosures
None.
Supporting information
Data S1
Tables S1–S7
Figures S1–S9
(J Am Heart Assoc. 2021;10:e020395. DOI: 10.1161/JAHA.120.020395.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020395
For Sources of Funding and Disclosures, see page 10.
References
- 1.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, Thomason MJ, Mackness MI, Charlton‐Menys V, Fuller JH; CARDS Investigators . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364:685–696. DOI: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003;361:2005–2016. DOI: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 3.Knopp RH, d’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non‐insulin‐dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485. DOI: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists’ (CTT) Collaborators , Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. DOI: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists’ (CTT) Collaborators , Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. DOI: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. DOI: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association . 10: Cardiovascular disease and risk management: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S103–S123. DOI: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 8.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. DOI: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78:684–698. DOI: 10.1111/bcp.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25. DOI: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 11.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. DOI: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 12.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. DOI: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 13.Helweg‐Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–29. DOI: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 14.Carstensen B, Kristensen JK, Marcussen MM, Borch‐Johnsen K. The national diabetes register. Scand J Public Health. 2011;39:58–61. DOI: 10.1177/1403494811404278. [DOI] [PubMed] [Google Scholar]
- 15.Malmborg M, Schmiegelow MDS, Nørgaard CH, Munch A, Gerds T, Schou M, Kistorp C, Torp‐Pedersen C, Hlatky MA, Gislason G. Does type 2 diabetes confer higher relative rates of cardiovascular events in women compared with men? Eur Heart J. 2020;41:1346–1353. DOI: 10.1093/eurheartj/ehz913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.tagteam/heaven. GitHub. https://github.com/tagteam/heaven. Accessed March 27, 2020.
- 17.Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. DOI: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krarup L‐H, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28:150–154. DOI: 10.1159/000102143. [DOI] [PubMed] [Google Scholar]
- 19.Borgan O, Goldstein L, Langholz B. Methods for the analysis of sampled cohort data in the cox proportional hazards model. Ann Stat. 1995;23:1749–1778. DOI: 10.1214/aos/1176324322. [DOI] [Google Scholar]
- 20.Borgan O, Samuelsen SO. Nested case‐control and case‐cohort. In: Klein JP, van Houwelingen HC, Ibrahim JG, Scheike TH, eds. Handbook of Survival Analysis. Routledge Handbooks Online; 2013:343–367. https://www.routledgehandbooks.com/doi/10.1201/b16248‐22. Accessed March 10, 2021. [Google Scholar]
- 21.R: the R project for statistical computing. https://www.r‐project.org/. Accessed March 23, 2020.
- 22.Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of statin therapy in older people: a meta‐analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. DOI: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orkaby AR, Driver JA, Ho Y‐L, Lu B, Costa L, Honerlaw J, Galloway A, Vassy JL, Forman DE, Gaziano JM, et al. Association of statin use with all‐cause and cardiovascular mortality in US veterans 75 years and older. JAMA. 2020;324:68–78. DOI: 10.1001/jama.2020.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akobeng AK. Understanding measures of treatment effect in clinical trials. Arch Dis Child. 2005;90:54–56. DOI: 10.1136/adc.2004.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organisation mondiale de la santé, Organization WH, WHO . Adherence to Long‐Term Therapies: Evidence for Action. World Health Organization; 2003:1–230. https://www.who.int/chp/knowledge/publications/adherence_report/en/. Accessed March 10, 2021. [Google Scholar]
- 26.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta‐analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. DOI: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. DOI: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 28.Daskalopoulou SS, Delaney JAC, Filion KB, Brophy JM, Mayo NE, Suissa S. Discontinuation of statin therapy following an acute myocardial infarction: a population‐based study. Eur Heart J. 2008;29:2083–2091. DOI: 10.1093/eurheartj/ehn346. [DOI] [PubMed] [Google Scholar]
- 29.Hope HF, Binkley GM, Fenton S, Kitas GD, Verstappen SMM, Symmons DPM. Systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease. PLoS One. 2019;14:e0201196. DOI: 10.1371/journal.pone.0201196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer M‐J, Cosentino F, Jönsson B, Laakso M, Malmberg K, et al; Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC), European Association for the Study of Diabetes (EASD) . Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28:88–136. DOI: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 31.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. DOI: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kümler T, Gislason GH, Kirk V, Bay M, Nielsen OW, Køber L, Torp‐Pedersen C. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10:658–660. DOI: 10.1016/j.ejheart.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003;56:124–130. DOI: 10.1016/S0895-4356(02)00591-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S7
Figures S1–S9


