Abstract
Background
Different adiposity traits may be causally related to hypertension in different ways. By using genetic variants as randomly allocated proxies for studying the effect of modifying adiposity traits, the Mendelian randomization approach can be used to investigate this.
Methods and Results
In this study, we used 4 different genetic risk scores (GRS; GRS‐BMI565, GRS‐WHR324, GRS‐VAT208, GRS‐BF81) including hundreds of single nucleotide polymorphisms associated with body mass index, waist‐to‐hip ratio, visceral adipose tissue, and body fat, respectively. These were applied as instrumental variables in Mendelian randomization analyses. Two Swedish urban‐based cohort studies, the Malmö Diet and Cancer, and the Malmö Preventive 795Projects were used to obtain genetic association estimates with blood pressure (BP). In both the Malmö Preventive Projects and Malmö Diet and Cancer studies, except for that for body fat, all of the genetic risk scores were significantly associated with systolic BP and diastolic BP, but with different magnitudes. In particular, in both cohorts, each standard deviation increase in the genetic risk score made up by the 324 single nucleotide polymorphisms associated with waist‐to‐hip ratio was associated with doubling of the likelihood of hypertension prevalence at baseline. However, only the genetic risk score made up by the 565 SNPs associated with body mass index was significantly associated with hypertension incidence during 23.6±4.3 years of follow‐up in the Malmö Preventive Project.
Conclusions
We support a causal link between genetically mediated adiposity, especially waist‐to‐hip ratio and body mass index, and BP traits including hypertension prevalence and, for the first time to our knowledge, hypertension incidence. The differences in magnitude between these associations might suggest different mechanisms by which different adiposity affects BP/hypertension and consequently may indicate that tailored interventions are needed to reduce cardiovascular risk.
Keywords: adiposity, blood pressure, genetics, Mendelian randomization, polymorphisms
Subject Categories: Genetics, Hypertension, Risk Factors, Blood Pressure
Nonstandard Abbreviations and Acronyms
- 2SLS
2‐step least squares
- BF
body fat
- DBP
diastolic blood pressure
- GRS
genetic risk score
- GRS‐BF81
genetic risk score made up by 84 SNPs associated with body fat
- GRS‐BMI565
genetic risk score made up by 565 SNPs associated with body mass index
- GRS‐VAT208
genetic risk score made up by 208 SNPs associated with visceral adiposity tissue
- GRS‐WHR324
genetic risk score made up by 324 SNPs associated with waist‐to‐hip ratio
- IV
instrumental variable
- MDC
Malmö Diet and Cancer
- MPP
Malmö Preventive Project
- MR
Mendelian randomization
- SBP
systolic blood pressure
- VAT
visceral adiposity tissue
- WHR
waist‐to‐hip ratio
Clinical Perspective
What Is New?
Different genetically determined adiposity traits (eg, body mass index, waist‐to‐hip ratio) are potentially causally linked to blood pressure and hypertension but with different magnitudes.
What Are the Clinical Implications?
Interventions targeted to body mass index and waist‐to‐hip ratio may also decrease blood pressure.
Obesity and hypertension are 2 of the main causes of noncommunicable diseases and mortality worldwide.1, 2 It is well known that adiposity itself is a risk factor for hypertension,3 but how different measures of obesity influence blood pressure (BP) is less clear.
On one hand, genome‐wide association studies have now pinpointed hundreds of genetic variants that are associated not only with body mass index (BMI), but also with waist‐to‐hip ratio (WHR), visceral fat mass, and body mass distribution.4, 5, 6, 7 On the other hand, Mendelian randomization (MR) studies have supported that BMI and other adiposity measures are causally related with hypertension.8, 9
Adiposity is a complex trait, whose genetic component is influenced by the contribution of many different loci. From the latest genome‐wide association studies, the number of genetic variants (single nucleotide polymorphisms [SNPs]) found to be associated with different measures of body fat distribution have exponentially increased.5, 7 A genetic risk score (GRS), obtained by summing up weights and risk alleles of the SNPs associated with the trait, is a powerful tool to capture the genetic component of complex phenotypes.10
However, even if the GRSs obtained in large genome‐wide association studies and meta‐analyses were validated in well‐powered studies, they need replication in other independent samples to assess the reproducibility of the genome–phenotype association.11 The objective of an MR analysis is to test a causal hypothesis, and the genetic information that we used in the form of a GRS represents the instrumental variable (IV) that is linked to the outcomes only through the endogenous variables (the adiposity measures).12, 13 An MR design can reduce reverse causality and confounding.
The MDC (Malmö Diet and Cancer) and the MPP (Malmö Preventive Projects) studies are 2 large urban‐based cohort studies based in Malmö in southern Sweden in which >27 000 participants were genotyped. In the 2 samples, different measures of adiposity (BMI, waist circumference, hip, and body fat) as well as BP were recorded. In the MPP study, there is also the possibility to evaluate hypertension incidence (not only prevalence) during follow‐up, because this cohort was reexamined 23.6±4.3 years after the baseline exam.
Thus, the aim of the present study was to evaluate the association of different measures of genetically mediated adiposity with different BP‐related traits, including hypertension prevalence and incidence, using an MR design.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Cohorts
Malmö Diet and Cancer Study
Cross‐sectional BP and hypertension prevalence at baseline were studied in the MDC study, a large‐scale urban population‐based cohort consisting of 30 447 individuals (aged 58±7.6 years) from Malmö, Sweden. Of these, 29 386 were genotyped for genome‐wide association studies. Men aged 46 to 73 years and women aged 45 to 73 years, included between 1991 and 1996, were included.14, 15 Ethical permission for the MDC study was obtained from the ethical committee at Lund University (LU 51–90 and DNR 652/2005), and all participants provided written informed consent.
Malmö Preventive Project
Hypertension prevalence and incidence were studied in the MPP, an urban‐based prospective study with BP measurements available, both at a baseline exam, including 33 346 citizens from Malmö, Sweden between 1974 to 1992, and 18 240 at a reexamination between 2002 and 2006.16 Of those, 9367, who were included also in the MDC study, underwent genome‐wide association studies genotyping. The ethics committee of Lund University approved the study protocols (DNR 2009/633), and all participants provided written informed consent.
Characteristics of the 2 cohorts are presented in Table S1.
BP Measurement
In the MDC cohort at baseline, BP was measured manually once in the supine position after 5 minutes of rest. To deal with the cofounding of the antihypertensive treatment on BP, we used a stepped addition method, in which 8/4, 14/10, 20/16, and 26/22 mm Hg were added to systolic BP (SBP)/diastolic BP (DBP) in the presence of 1, 2, 3, or 4 medications, respectively.17
In the MPP cohort, at the baseline investigation, BP was measured after 1 minute of resting in the supine position, followed by another measurement after 1 minute in an upright standing position. This procedure was repeated also after 10 minutes of resting. For those subjects with a least 3 valid measurements, BP values were averaged and used for the analysis. At reinvestigation (after a mean follow‐up time of 23.0±4.7 years), 2 measures in the supine position were recorded. For those subjects with at least 2 valid measures, we used the averaged BP values. Both SBP and DBP were increased respectively, using a fixed addition adjustment method by 15 and 10 mm Hg in the presence of antihypertensive treatment,17 because the number of medications was not available.
In both cohorts, Korotkoff sound Phase I was defined as SBP and Phase V as DBP.18
In both cohorts, hypertension was defined as having either SBP or DBP >140 or >90 mm Hg, respectively, or taking antihypertensive drugs, in line with current European guidelines.2 Hypertension prevalence could be evaluated in both the MDC and MPP cohorts at baseline, whereas in the MPP cohort, hypertension incidence at the reinvestigation survey was also investigated. For this analysis, individuals who were already hypertensive at baseline (n=3135) were excluded.
Adiposity Measurements
In both cohorts, height and weight were measured by trained nurses, as previously described.19, 20 BMI (in kilograms divided by height in meters squared) was computed as weight divided by squared height. Waist circumference (in centimeters) was measured between the lowest rib margin and the iliac crest, and the hip circumference (in centimeters) as the largest circumference between the waist and thighs. WHR was computed. In the MDC cohort, the percentage of body fat (BF) was estimated with bioelectrical impedance analyzers according to the manufacture's algorithm (BIA 103 single‐frequency analyzer; RJL Systems, Detroit, MI) (Table S2).
Statistical Analysis
Instrumental Variables
A GRS for BMI (GRS‐BMI565), a GRS for WHR (GRS‐WHR324), a GRS for predicted visceral adiposity tissue (VAT) (GRS‐VAT208), and a GRS for BF distribution (GRS‐BF81) were constructed including, respectively, 565, 324, 208, and 81 independent SNPs retrieved from the most recent meta‐analyses.5, 6, 7 The scores were weighted for the β coefficients reported in the primary studies.5, 6, 7 Information about the studies can be found in Table S3.
The 4 GRSs (GRS‐BMI565, GRS‐WHR324, GRS‐VAT208, and GRS‐BF81) were used as IVs in the MR analysis.
Exposure
BMI was used as the exposure trait for the GRS‐BMI565 and GRS‐VAT208 in both the MPP and MDC cohorts, because VAT measure was not available in the 2 cohorts; and the WHR was used as the exposure trait for GRS‐WHR324. In the MPP cohort, BMI was used as the exposure trait for GRS‐BF81 instead of BF, because that measure was not available.
In the MDC cohort, the percentage of body fat was used as the exposure trait for GRS‐BF81.
Individual‐Level Data
For continuous outcome (SBP and DBP at baseline and at reinvestigation), 2‐step least squares (2SLS) regression was used to assess the causal association of the 4 GRSs. The 2SLS consists of 2 regression steps. In the first step, the exposure is regressed on the instrumental variable, and in the second step, the fitted values obtained from the first regression step are used as independent variables in the regression on the outcome.21
For binary outcomes (hypertension prevalence and incidence), we used the analog of 2SLS consisting of 2 sequential regressions, with the difference that the second step consists of logistic regression of the fitted values from the first step on the outcome.21
The exposure traits (BMI, WHR, BF) and the outcomes (SBP, DBP) have been used as standardized residuals resulting after adjustment for age, sex, age2, and age×sex.
When dealing with multiple instrumental variables, an alternative approach to the aggregation in a GRS is to use the estimates from each of the instrumental variables using summarized data analysis, as in a meta‐analysis.22 With the increasing number of variants used in MR investigations, it is increasingly unlikely that all variants are valid IVs.23
Sensitivity Analysis
The inverse‐variance weighted method, MR‐Egger, and weighted median were used as sensitivity analyses in the MDC cohort to assess the robustness of any casual finding of the multiple genetic variants involved in the construction of the 4 adiposity‐trait GRSs to avoid IV assumption.24 The inverse‐variance weighted method is a weighted mean of the ratio estimates and is equal to estimates of 2SLS,22 and it is liable to bias if only one IV does not satisfy the IV assumption in the presence of pleiotropy.23 The weighted‐median estimate is similar to the inverse‐variance weighted method, except for the use of a weighted median instead of a weighted mean, and consistent up to 50% invalid instruments.23 The MR‐Egger allows for estimating the causal effect, taking into account the instrument strength independent of direct effect assumption, which states that the pleiotropic associations of genetic variants must be uncorrelated with the genetic variant–exposure association.23
Software
All analysis was performed using R software (packages ivpack, MendelianRandomization; R Foundation for Statistical Computing, Vienna, Austria)25 and SPSS software (version 22.0; IBM, Armonk, NY).
Results
Association of IVs With the Exposures
In Table 1 and Figure S1, the association of each of the 4 GRSs used as IVs, with their related exposure traits, are presented. The contribution to model in terms of R 2 and the F statistic is also reported.
Table 1.
Association of the 4 GRSs (Expressed as per 1‐SD Increment) With Their Related Exposure Traits (Expressed as SD) at Baseline in the MPP and MDC Cohorts
| MPP | MDC | |||||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P Value | R 2 | F | β (95% CI) | P Value | R 2 | F | |
| BMI | BMI | |||||||
| GRS‐BMI565 | 0.201 (0.179–0.218) | 8.0E−86 | 0.005 | 393 | 0.200 (0.189–0.211) | 3.4E−263 | 0.04 | 1226 |
| WHR | WHR | |||||||
| GRS‐WHR324 | 0.086 (0.065–0.107) | 4.8E−16 | 0.007 | 66 | 0.046 (0.034–0.057) | 4.7E−15 | 0.002 | 61 |
| BMI | % BF | |||||||
| GRS‐BF81 | 0.063 (0.043–0.083) | 9.5E−10 | 0.004 | 37 | 0.045 (0.034–0.057) | 1.5E−14 | 0.002 | 59 |
| BMI | BMI | |||||||
| GRS‐VAT208 | 0.128 (0.107–0.147) | 1.19E−35 | 0.016 | 156 | 0.136 (0.125–0.147) | 1.12E−121 | 0.02 | 555 |
BF indicates body fat; BMI, body mass index; GRS, genetic risk score; GRS‐BF81, GRS for BF associated with 81 SNPs; GRS‐BMI565,GRS for BMI associated with 565 SNPs; GRS‐VAT208, GRS for visceral adipose tissue associated with 208 SNPs; GRS‐WHR324, GRS for WHR associated with 324 SNPs; MDC, Malmö Diet and Cancer; MPP, Malmö Preventive Project; SNPs, single nucleotide polymorphisms; and WHR, waist‐to‐hip ratio.
Association With BP Traits
In Table S4, the results from the MR analysis of the 4 adiposity GRSs with the continuous traits SBP/DBP at baseline are shown for MPP (Table S4 a.) and MDC (Table S4 b.). In the MPP cohort (Table S4 a. and Figure 1 A), all of the GRSs with the exception of the GRS‐BF81 identify significant associations with baseline SBP and DBP. In the MDC cohort (Table S4 b. and Figure 2), the associations with baseline measurements were in line with the results in the MPP cohort. In both studies, the GRS‐WHR324 shows the strongest association, in particular with DBP. At the MPP follow‐up, only the GRS‐BMI565 remains significantly associated with the BP‐related traits (Table S4 a. and Figure 1 B).
Figure 1. Causal association from the 2‐step least squares (2SLS) Mendelian randomization between the genetic risk scores (GRSs) and blood pressure traits in the MPP (Malmö Preventive Project) cohort.
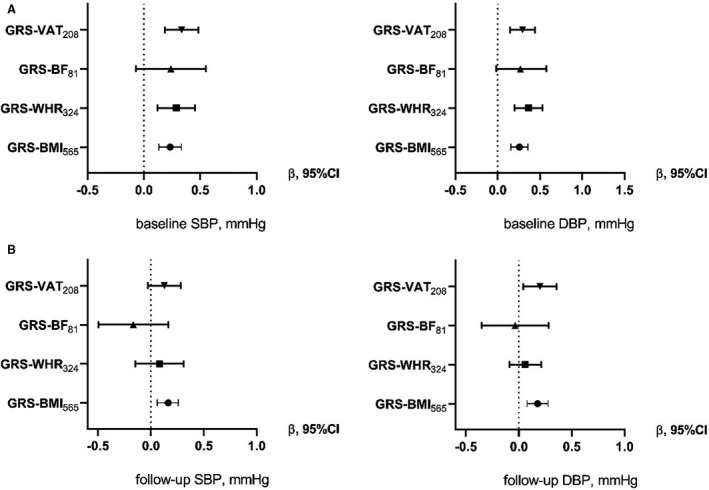
Each forest plot shows the causal estimates (β) from the 2SLS regression of the 4 GRSs with baseline (A) and follow‐up (B) blood pressure outcomes (SBP or DBP) in the MPP cohort for Model 2 (adjustment for age, sex, age2, age×sex). An increase of 1 SD in GRS‐VAT208 is associated with an SD increase of 0.326 of SBP. DBP indicates diastolic blood pressure; GRS‐BF81, GRS for body fat associated with 81 SNPs; GRS‐BMI565, GRS for body mass index associated with 565 SNPs; GRS‐VAT208, GRS for visceral adipose tissue associated with 208 SNPs; GRS‐WHR324, GRS for waist‐to‐hip ratio associated with 324 SNPs; SBP, systolic blood pressure; and SNPs, single nucleotide polymorphisms.
Figure 2. Causal association from the 2‐step least squares (2SLS) Mendelian randomization between the genetic risk scores (GRSs) and blood pressure traits in the MDC (Malmö Diet and Cancer) cohort.
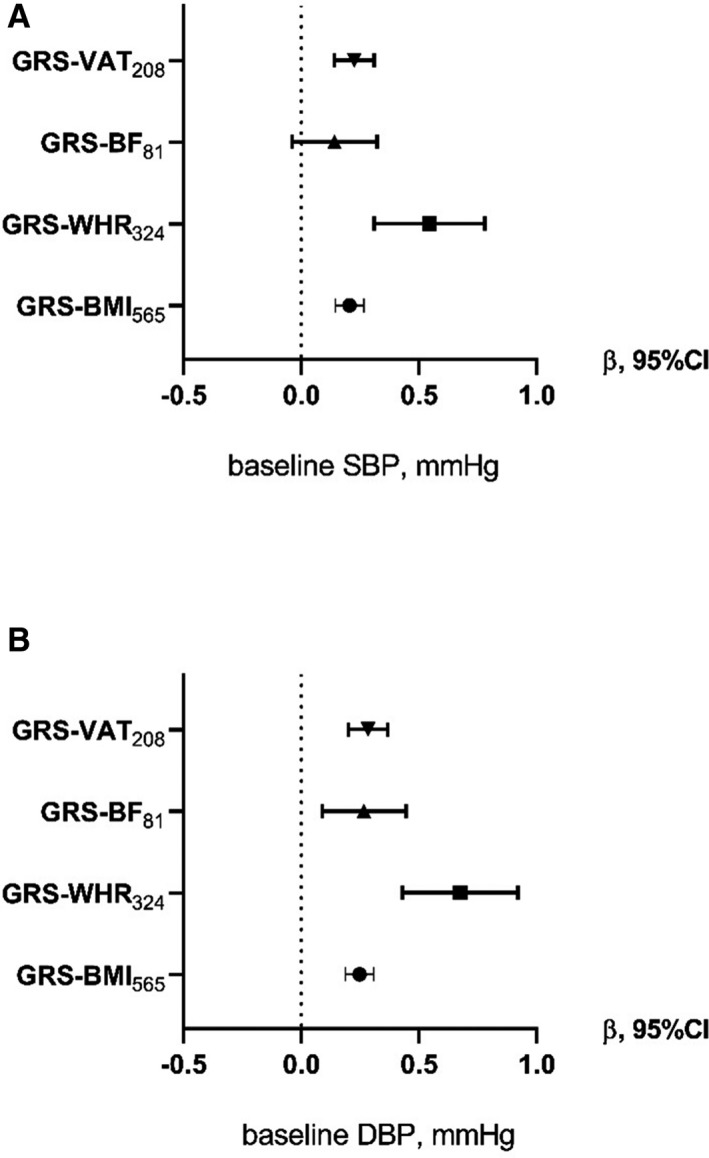
Each forest plot shows the causal estimates (β) from the 2SLS regression of the 4 GRSs with blood pressure outcomes SBP (A) or DBP (B) in the MDC cohort for Model 2 (adjustment for age, sex, age2, age×sex). An increase of 1 SD in GRS‐VAT208 is associated with an SD increase of 0.326 of SBP. DBP indicates diastolic blood pressure; GRS‐BF81, GRS for body fat associated with 81 SNPs; GRS‐BMI565, GRS for body mass index associated with 565 SNPs; GRS‐VAT208, GRS for visceral adipose tissue associated with 208 SNPs; GRS‐WHR324, GRS for waist‐to‐hip ratio associated with 324 SNPs; SBP, systolic blood pressure; and SNPs, single nucleotide polymorphisms.
In Table 2, the associations of the 4 GRSs with the prevalence of hypertension are reported. A higher odds ratio was found with GRS‐WHR324 as compared with other adiposity GRSs for both the MPP (Table 2 and Figure 3A) and MDC cohorts (Table 2 and Figure 3C).
Table 2.
Association of the 4 Adiposity GRSs (Expressed as SD Increase) With Hypertension Prevalence in the MPP
| Hypertension Prevalence, n=9137 | Hypertension Incidence, n=5971 | Hypertension Prevalence, n=29 262 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| GRS‐BMI565 | 1.51 (1.2–1.88) | 0.0003 | 1.48 (1.19–1.83) | 0.0003 | 1.34 (1.03–1.74) | 0.029 | 1.33 (1.03–1.72) | 0.03 | 1.70 (1.50–1.93) | 1.1E−16 | 1.56 (1.39–1.75) | 8.5E−14 |
| GRS‐WHR324 | 2.46 (1.46–4.14) | 0.001 | 1.85 (1.33–2.58) | 0.0003 | 1.07 (0.58–1.98) | 0.820 | 1.05 (0.70–1.57) | 0.820 | 3.81 (2.28–6.37) | 3.5E−7 | 2.95 (1.95–4.47) | 3.5E−7 |
| GRS‐BF81 | 1.46 (0.71–2.99) | 0.274 | 1.45 (0.74–2.83) | 0.274 | 0.48 (0.21–1.11) | 0.079 | 0.49 (0.22–1.09) | 0.079 | 1.12 (1.03–1.21) | 0.006 | 1.57 (1.08–2.28) | 0.02 |
| GRS‐VAT208 | 2.01 (1.43–2.83) | 6.2E−5 | 1.95 (1.41–2.70) | 6.2E−5 | 1.28 (0.85–1.94) | 0.235 | 1.27 (0.86–1.89) | 0.235 | 1.61 (1.35–1.91) | 7.6E−8 | 1.59 (1.34–1.88) | 7.6E−8 |
Model 1: raw association (without adjustment). Model 2: the exposure trait was used as the residual from linear regression with age, sex, age2, and age×sex. GRS indicates genetic risk score; GRS‐BF81, GRS for body fat associated with 81 SNPs; GRS‐BMI565, GRS for body mass index associated with 565 SNPs; GRS‐VAT208, GRS for visceral adipose tissue associated with 208 SNPs; GRS‐WHR324, GRS for waist‐to‐hip ratio associated with 324 SNPs; OR, odds ratio; and SNPs, single nucleotide polymorphisms.
Figure 3. Forest plot of the association of the 4 genetic risk scores (GRSs) with the prevalence of hypertension at baseline in the 2 cohorts and with the incidence of hypertension at follow‐up in the MPP (Malmö Preventive Project) cohort.
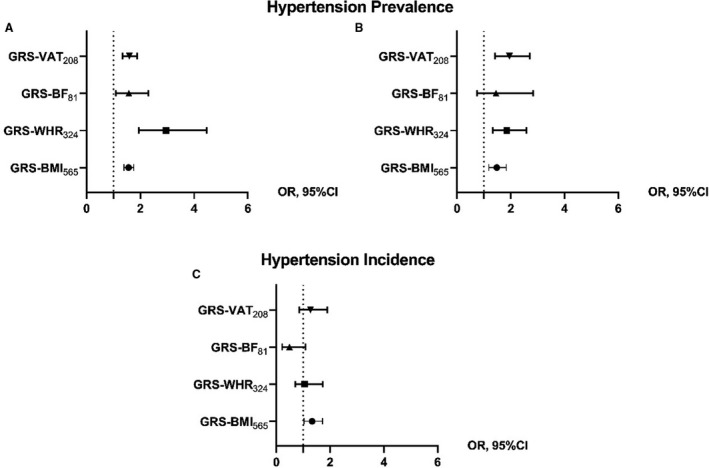
Each forest plot shows the causal estimates in OR from the 2‐stage logistic regression of the 4 GRSs with the prevalence of hypertension at baseline in the Malmö Diet and Cancer cohort (A) and the MPP cohort (B), and with the incidence of hypertension at follow‐up in the MPP cohort (C) for Model 2 (adjusted for age, sex, age2, age×sex). The increase of 1 SD in GRS‐VAT208 is associated with an OR of 2.01 for hypertension prevalence. GRS‐BF81, GRS for body fat associated with 81 SNPs; GRS‐BMI565, GRS for body mass index associated with 565 SNPs; GRS‐VAT208, GRS for visceral adipose tissue associated with 208 SNPs; GRS‐WHR324, GRS for waist‐to‐hip ratio associated with 324 SNPs; OR, odds ratio; and SNPs, single nucleotide polymorphisms.
For hypertension incidence in the MPP cohort, after the exclusion of participants already hypertensive at baseline, only the GRS‐BMI565 remained significantly associated with incident hypertension (Table 2 and Figure 3B).
Results from sensitivity analysis are presented in Tables S5 through S7. As expected, the inverse‐variance weighted method reflects the results obtained by the 2SLS regression of the GRSs on the traits (Table S4). The latter shows significant associations for all scores with both traits except for the GRS‐BF81. Among the 4 scores, the only score that showed a significant MR‐Egger method was the GRS‐WHR324, both for SBP and DBP (Table S8 and Figures S2 through S5).
DISCUSSION
Summary of Main Findings
The main finding of the present study is that genetically proxied measures of adiposity, as condensed in specific GRSs, are associated with BP‐related traits but with different magnitude. In particular, the GRS‐BMI565 and the GRS‐WHR324 were potentially causally associated with all BP‐related traits measured at baseline, but the WHR had a higher estimate size. On the contrary, at follow‐up, the genetically predicted association of WHR with BP tends to be attenuated and is no longer significant, whereas that of BMI is still significant, although all of the associations tended to be weaker than in the prevalence analysis. For more complex measures of adiposity, such as BF and visceral fat, their genetic component seems to be more limited in determining BP but still significant when hypertension prevalence is evaluated, at least in the MDC cohort.
Even if some reasons for these discrepancies can stand on the relative weakness of the instrumental variables used with some GRSs being stronger than others, it is likely that our study can give some clues to understanding more deeply the biology of the well‐known influence of BF on BP. Our data may indicate that the effect of BMI is important, but with low magnitude throughout midlife to old age being recognizable in people aged in their 40s and 50s (the average age in the MPP cohort was 45±7.4 years and in the MDC cohort was 58±7.6 years), but also in older people; the average age in the MPP cohort at reinvestigation was 68±5.8 years). Instead, the contribution to high BP of genetically determined abdominal fat, represented by WHR, could seem much higher in midlife, overcoming the effect of BMI, than later on. However, an analysis done by stratifying participants by age in the MDC cohort showed an inverse trend for the GRS‐WHR324, with older individuals more strongly associated with prevalent hypertension than younger individuals, suggesting a possible survival bias for the analysis in MPP, where participants with especially deleterious GRS‐WHR324 could have died before the examination (Table S8 and Figure S6).
Explanation in the Context of Existing Research
Our results are in line with those of 2 recent studies but with some differences.8, 9 In an MR analysis, including ≈400 000 individuals from the UK Biobank, GRS‐BMI was found to be associated with BP (β [95% CI], 0.19 [0.18–0.21] for SBP and 0.27 [0.26–0.29] for DBP) and to hypertension prevalence (odds ratio [OR] [95% CI], 1.10 [1.07–1.12]) of the same order of magnitude of the results we found in the MDC.8 Also, the association of the genetic component of body fat and VAT with hypertension that we found in the MDC cohort is consistent with a previous MR performed in the UK Biobank study,6, 9 despite our using BMI instead of VAT as the exposure trait. Though the association of the instrumental variable GRS‐WHR with BP/hypertension is reported in a previous study,8 the magnitude of the association is much higher in our samples.
A reason for such a difference in magnitude is not clear. Differences between the 2 samples in age (slightly lower in the UK Biobank study with respect to the MDC cohort), ethnicity (more homogeneous in the MDC cohort), distribution of sex (higher prevalence of women in the MDC cohort), and adiposity and BP traits (obesity is more prevalent in the UK Biobank study but hypertension is in the MDC cohort) can be postulated as a partial explanation.
The debate over which adiposity measure could better predict BP or hypertension is still ongoing, even in nongenetic studies. There are several pieces of research comparing BMI, waist, WHR, waist‐to‐height ratio (another proxy for central obesity), and VAT showing somewhat contradictory results. Some studies, including a couple of meta‐analyses, conclude that waist‐to‐height and waist circumference are stronger predictors than BMI of future cardiovascular events and hypertension.26, 27 However, other studies28, 29, 30 show that BMI is preferentially associated with the incidence of hypertension with respect to WHR. A study in young adults (aged 18–36 years) showed that BMI has an equal or better ability to predict adult hypertension as compared with WHR and other adiposity measures.31 Moreover, other well‐powered analyses found that either VAT or BF are strongly associated with blood pressure traits32 and the incidence of hypertension.33, 34 One problem in this type of study is that the adiposity measures are highly correlated with each other and associate with differing levels of measurement error that could affect the strength of the observed associations.32 MR could be considered an advantage in inferring causality, because the use of GRS is less susceptible to cofounding from environmental factors or reverse causation with respect to the raw trait measures.
Possible Mechanisms and Clinical Relevance
Even if it is widely accepted that obesity and hypertension are interrelated and, in particular, the former is one of the causes of the latter, from a pathophysiological perspective, it has not been clarified how adiposity can influence BP at different ages. It is widely accepted that arterial stiffness is one of the main factors contributing to higher SBP in older ages. Other pathophysiological mechanisms potentially linking obesity to high BP refer to metabolic factors, endothelial and vascular dysfunction, the hyperactivation of the sympathetic nervous and the renin–angiotensin–aldosterone systems, sodium retention with a shift of the well‐known pressure–natriuresis curve toward higher BP levels, and/or other renal dysfunctions.35, 36 A pivotal player also seems to be that VAT becomes resistant to insulin and leptin and is the site of altered secretion of molecules and hormones such as adiponectin, leptin, resistin, tumor necrosis factor‐α, and interleukin‐6.37, 38
From the data of our study, it is possible to speculate that adipokines especially produced in visceral fat and insulin resistance are more important for BP and the development of hypertension in midlife, whereas total fat is important throughout life and still determinant when arterial stiffness becomes prominent.
In previous MR studies, BMI was also suggested to be causally associated with metabolic traits such as insulin and inflammation markers such as interleukin‐6, which could be associated with hypertension development over time.39
Recently, it was shown that SNPs related to VAT fat (ie, that is found around abdominal organs) is strongly associated also with hypertension. In the same study, the heritability of VAT was found to be as high as >35%,6 and the genetically determined VAT was associated with hypertension spanning between 1.81 and 1.89 in men and women, respectively, for the rank‐transformed VAT (ie, the VAT after rank transformation to standard normal distributions for women and men separately) to between 2.17 and 3.41 for the bias‐corrected VAT (ie, VAT corrected for measurement errors in a covariate‐adjusted model). These results are fairly in line with the results in our study and similar to that of genetically determined WHR. The similar magnitude of the result for WHR and VAT underlines the pivotal role of visceral fat for high BP.
Strengths and Limitations
Among the strengths of this study are the use of the more recently validated GRSs associated with adiposity and the MR design that can preserve issues of confounding and reverse causality. Additionally, the results were supported in sensitivity analyses that are more robust to the presence of variants with pleiotropic associations.40, 41 Finally, to our knowledge, this is the first study performing MR between adiposity traits and the incidence of hypertension, not only prevalence.
Our study has limitations. There is overlap between the MDC and MPP samples, so that even if the 2 studies had their own examinations at different time points, one cannot be considered a real replication of the other. Some GRSs used as instrumental variables were weaker than others, especially when proxy traits were used as exposure. Thus, some of the differences in the associations we found between adiposity‐related GRSs and BP‐related traits could be because GRS‐BMI565 and GRS‐WHR324 were better IVs than GRS‐BF81 and GRS‐VAT208. In addition, GRS‐BF81 and GRS‐VAT208 were regressed on BMI in the MPP study, necessarily dampening their predictive value. Moreover, we have different statistical power for the different BP‐related traits (ie, hypertension incidence and prevalence). In addition, the hypertension incidence outcome, apart from the diminished sample size, could be blurred by the fact that the analysis is restricted to people less prone to develop hypertension (ie, the ones who have not developed hypertension at baseline).
Finally, there is the possible presence of pleiotropy among the studied variants. A clear dissection between all of the observed adiposity‐related traits is not possible, and several SNPs, especially those encompassed in loci that have previously been associated with obesity and BMI, such as the well‐known FTO and MC4R loci, are common among the different GRSs. All of the above‐mentioned points, which in some ways could be considered intrinsic limitations of the use of genetic variants as IVs,42 contribute to reduce the strength of the causal inference of our analysis. Any causal inference drawn from MR analyses alone should be considered tentative.
Conclusions
We have demonstrated that genetically predicted adiposity, and especially genetically predicted WHR and BMI, are potentially causally linked to BP and hypertension. Tailored interventions to block this deleterious relationship should be pursued and, in particular, intervention to decrease waist circumference and BMI throughout life are likely to be most effective.
Sources of Funding
This work was supported by the Lund University Infrastructure grant “Malmö Population‐Based Cohorts” (STYR 2019/2046). Dr Gill is supported by the British Heart Foundation Centre of Research Excellence (RE/18/4/34215) at Imperial College London and a National Institute for Health Research Clinical Lectureship at St. George's, University of London (CL‐2020‐16‐001).
Disclosures
Dr Gill is employed part time by Novo Nordisk, outside of the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S8
Figures S1–S6
Acknowledgments
Author contributions: All authors contributed to the management and coordination of all research activities, planning, and execution. All authors contributed to the review process for the final version of the article.
(J Am Heart Assoc. 2021;10:e020405. DOI: 10.1161/JAHA.120.020405.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020405
For Sources of Funding and Disclosures, see page 9.
References
- 1.Obesity and overweight. Available at: https://www.who.int/en/news‐room/fact‐sheets/detail/obesity‐and‐overweight. Accessed February 17, 2020.
- 2.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. DOI: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 3.Hall JE, Do Carmo JM, Da Silva AA, Wang Z, Hall ME. Obesity‐induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. DOI: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. DOI: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, Yengo L, Ferreira T, Marouli E, Ji Y, et al. Meta‐analysis of genome‐wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166–174. 10.1093/hmg/ddy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson T, Rask‐Andersen M, Pan G, Höglund J, Wadelius C, Ek WE, Johansson Å. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. 2019;25:1390–1395. 10.1038/s41591-019-0563-7 [DOI] [PubMed] [Google Scholar]
- 7.Rask‐Andersen M, Karlsson T, Ek WE, Johansson Å. Genome‐wide association study of body fat distribution identifies adiposity loci and sex‐specific genetic effects. Nat Commun. 2019;10:1–10. DOI: 10.1038/s41467-018-08000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Censin JC, Peters SAE, Bovijn J, Ferreira T, Pulit SL, Mägi R, Mahajan A, Holmes MV, Lindgren CM. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 2019;15:e1008405. DOI: 10.1371/journal.pgen.1008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41:221–226. DOI: 10.1093/eurheartj/ehz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan L, Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, Peterson R, Domingue B. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019;10:3328. DOI: 10.1038/s41467-019-11112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffman JE. Examining the current standards for genetic discovery and replication in the era of mega‐biobanks. Nat Commun. 2018;9:1–4. DOI: 10.1038/s41467-018-07348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess S, Smith DG, Davies NM, Dudbridge F, Gill D, Glymour MM, Hartwig FP, Holmes MV, Minelli C, Relton CL, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020;4:186. DOI: 10.12688/wellcomeopenres.15555.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehby GL, Ohsfeldt RL, Murray JC. “Mendelian randomization” equals instrumental variable analysis with genetic instruments. Stat Med. 2008;27:2745–2749. DOI: 10.1002/sim.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berglund G, Elmstähl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;233:45–51. DOI: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O. Atrial fibrillation in the Malmö Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. DOI: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 16.Fava C, Sjögren M, Montagnana M, Danese E, Almgren P, Engström G, Nilsson P, Hedblad B, Guidi GC, Minuz P, et al. Prediction of blood pressure changes over time and incidence of hypertension by a genetic risk score in Swedes. Hypertension. 2013;61:319–326. DOI: 10.1161/HYPERTENSIONAHA.112.202655. [DOI] [PubMed] [Google Scholar]
- 17.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. DOI: 10.1161/01.HYP.0000044938.94050.E3. [DOI] [PubMed] [Google Scholar]
- 18.Fava C, Ohlsson T, Sjögren M, Tagetti A, Almgren P, Engström G, Nilsson P, Hedblad B, Minuz P, Melander O. Cardiovascular consequences of a polygenetic component of blood pressure in an urban‐based longitudinal study: the Malmo Diet and Cancer. J Hypertens. 2014;32:1424–1428. DOI: 10.1097/HJH.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 19.Borné Y, Nilsson PM, Melander O, Hedblad B, Engström G. Multiple anthropometric measures in relation to incidence of diabetes: a Swedish population‐based cohort study. Eur J Public Health. 2015;25:1100–1105. DOI: 10.1093/eurpub/ckv044. [DOI] [PubMed] [Google Scholar]
- 20.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all‐cause mortality and coronary events in middle‐aged individuals (the Malmo Preventive Project). Eur Heart J. 2010;31:85–91. DOI: 10.1093/eurheartj/ehp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26:2333–2355. DOI: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35:1880–1906. DOI: 10.1002/sim.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44:313–329. DOI: 10.1002/gepi.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yavorska OO, Burgess S.MendelianRandomization: An R package for performing Mendelian randomizationanalyses using summarized data. Int J Epidemiol. 2017;46(6):1734‐1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team . R: a language and environment for statistical computing. R Foundation for Staistical Computing; 2019. Available at: https://www.r‐project.org/. Accessed May 4, 2020 [Google Scholar]
- 26.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist‐to‐height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 05 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–269. 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 27.Lee CMY, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta‐analysis. J Clin Epidemiol. 2008;61:646–653. 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Chei CL, Iso H, Yamagishi K, Tanigawa T, Cui R, Imano H, Kiyama M, Kitamura A, Sato S, Shimamoto T. Body fat distribution and the risk of hypertension and diabetes among Japanese men and women. Hypertens Res. 2008;31:851–857. DOI: 10.1291/hypres.31.851. [DOI] [PubMed] [Google Scholar]
- 29.Nyamdorj R, Qiao Q, Söderberg S, Pitkäniemi J, Zimmet P, Shaw J, Alberti G, Nan H, Uusitalo U, Pauvaday V, et al. Comparison of body mass index with waist circumference, waist‐to‐hip ratio, and waist‐to‐stature ratio as a predictor of hypertension incidence in Mauritius. J Hypertens. 2008;26:866–870. DOI: 10.1097/HJH.0b013e3282f624b7. [DOI] [PubMed] [Google Scholar]
- 30.Panagiotakos DB, Chrysohoou C, Pitsavos C, Skoumas J, Lentzas Y, Katinioti A, Stefanadis C. Hierarchical analysis of anthropometric indices in the prediction of 5‐year incidence of hypertension in apparently healthy adults: the ATTICA study. Atherosclerosis. 2009;206:314–320. DOI: 10.1016/j.atherosclerosis.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Fan H, Zhu Q, Medrano‐Gracia P, Zhang X. Comparison of child adiposity indices in prediction of hypertension in early adulthood. J Clin Hypertens. 2019;21:1858–1862. DOI: 10.1111/jch.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malden D, Lacey B, Emberson J, Karpe F, Allen N, Bennett D, Lewington S. Body fat distribution and systolic blood pressure in 10,000 adults with whole‐body imaging: UK Biobank and Oxford BioBank. Obesity (Silver Spring). 2019;27:1200–1206. DOI: 10.1002/oby.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, De Lemos JA, Turer AT. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64:997–1002. DOI: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 34.Park SK, Ryoo J, Oh C, Choi J, Chung P, Jung JY. Body fat percentage, obesity, and their relation to the incidental risk of hypertension. J Clin Hypertens. 2019;21:1496–1504. DOI: 10.1111/jch.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity‐associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–137. DOI: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Vaněčková I, Maletínská L, Behuliak M, Nagelová V, Zicha J, Kuneš J. Obesity‐related hypertension: possible pathophysiological mechanisms. J Endocrinol. 2014;223:R63–R78. DOI: 10.1530/JOE-14-0368. [DOI] [PubMed] [Google Scholar]
- 37.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. 10.1038/nrendo.2014.44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity‐induced hypertension. Hypertens Res. 2010;33:386–393. [DOI] [PubMed] [Google Scholar]
- 39.Lyall DM, Celis‐Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, Smith DJ, Ntuk UE, Mackay DF, Holmes MV, et al. Association of body mass index with cardiometabolic disease in the UK Biobank: a Mendelian randomization study. JAMA Cardiol. 2017;2:882–889. DOI: 10.1001/jamacardio.2016.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. DOI: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. DOI: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Hinke S, Davey Smith G, Lawlor DA, Propper C, Windmeijer F. Genetic markers as instrumental variables. J Health Econ. 2016;45:131–148. DOI: 10.1016/j.jhealeco.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S6


