Abstract
Background
SGLT‐2 (sodium glucose transporter‐2) inhibitors and GLP‐1RAs (glucagon‐like peptide‐1 receptor agonists) effectively lowered cardiovascular risk in large clinical trials for patients with type 2 diabetes mellitus at high risk for these complications, and have been recommended by guidelines. To evaluate the contemporary landscape in which these recommendations would be implemented, we examined the use of these medications according to clinical guideline practice.
Methods and Results
In the National Health and Nutrition Examination Survey for 2017 to 2018, we defined compelling indications for SGLT‐2 inhibitors by the presence of atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease, and for GLP‐1RAs by the presence of established or high‐risk atherosclerotic cardiovascular disease, based on large clinical trials that have been incorporated in guideline recommendations of the American College of Cardiology and American Diabetes Association. We then evaluated use of these medications among patients with physician‐diagnosed type 2 diabetes mellitus. All analyses incorporated complex survey design to produce nationally representative estimates. A total 1104 of 9254 sampled individuals had type 2 diabetes mellitus, representing 10.6% (95% CI, 9.7%–11.6%) of the US population or 33.2 million adults nationally. Of these, 52.6% (95% CI, 47.7%–57.5%) had an indication for SGLT‐2 inhibitors, 32.8% (95% CI, 28.8%–37.2%) for GLP‐1RAs, and 26.6% (95% CI, 22.2%–31.7%) for both medications. During 2017 to 2018, 4.5% (95% CI, 2.4%–8.2%) were treated with SGLT‐2 inhibitors and 1.5% (95% CI, 0.7%–3.2%) with GLP‐1RAs. Atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease were not independently associated with SGLT‐2 inhibitor or GLP‐1RA use in patients with diabetes mellitus.
Conclusions
Despite a large number of patients being eligible for guideline‐recommended cardiorenal protective therapies, there are substantial gaps in the use of SGLT‐2 inhibitors and GLP‐1RAs, limiting their public health benefits.
Keywords: cardioprotective medications, diabetes mellitus, GLP1‐RAs, novel agents, sodium glucose transporter‐2 inhibitors
Subject Categories: Quality and Outcomes, Statements and Guidelines
Nonstandard Abbreviations and Acronyms
- GLP‐1RA
glucagon like peptide‐1 receptor agonist
- NHANES
National Health and Nutrition Examination Survey
- SGLT‐2
sodium glucose cotransporter‐2
Clinical Perspective
What Is New?
Nearly half of US patients with diabetes mellitus have guideline recommendations for the use of SGLT‐2 (sodium glucose transporter‐2) inhibitors, and over a third are eligible for GLP‐1RAs (glucagon‐like peptide‐1 receptor agonists) based on current clinical practice guidelines.
Among patients with diabetes mellitus, <5% received SGLT‐2 inhibitors, and only 1% received GLP‐1RAs in the US from 2017 to 2018.
What Are the Clinical Implications?
Substantial gaps in the use of these medications limit their potential public health benefits.
The emergence of SGLT‐2 (sodium glucose cotransporter‐2) inhibitors and GLP‐1RAs (glucagon like peptide‐1 receptor agonists) has transformed the therapeutic landscape for type 2 diabetes mellitus. These medications represent the only drug classes with evidence for reduced risk of adverse cardiovascular events in patients with type 2 diabetes mellitus in large randomized clinical trials, with their important role increasingly recognized by clinical practice guidelines.1 The expanding indications for treatment with these medications build upon significant reduction in major adverse cardiovascular events noted with the use of these drugs in patients with diabetes mellitus and atherosclerotic cardiovascular disease (ASCVD), heart failure, or chronic kidney disease (CKD).2
However, for these large therapeutic effects to have an impact on public health, it is critical to ensure their use in patients with compelling indications. Prior assessment of patients who qualify for therapy and use of these medications relied on older data from 2015 to 2016 and does not reflect the expanding indications with emerging evidence or their potential uptake in contemporary practice. Other assessments based on clinical registries were also limited to select group of patients and may not be generalizable to the US population on a national scale.3
In this contemporary, nationally representative US study based on clinical and laboratory data, we evaluated the proportion of patients with diabetes mellitus who have compelling indications for SGLT‐2 inhibitors, GLP‐1RAs, or both, and the patterns of current use among those with and without indications.
Methods
The data used in the study are publicly available from National Center for Health Statistics.
Data Source
We used the most recent National Health and Nutrition Examination Survey (NHANES) for the years 2017 to 2018. NHANES is a nationally representative database of cross‐sectional surveys that gather demographic, socioeconomic, dietary, medical history, prescription drug use, and laboratory information of a systematically selected random sample of individuals through interview, physical examination, and laboratory testing.4 NHANES uses a probability‐based sampling approach with clustering and stratification methods that allow for national estimates representative of the US population.
Study Population and Exposure Groups
We used a combination of strategies to identify patients with type 2 diabetes mellitus. These included patients reporting a history of diabetes mellitus, receiving glucose‐lowering medications, or having a hemoglobin A1C above 6.5%. Patients younger than 40 years old who were only receiving insulin were excluded. We used American Diabetes Association and American College of Cardiology clinical practice guidelines for standards of care in patients with diabetes mellitus to define the indications for SGLT‐2 inhibitors and GLP‐1RAs.5, 6 We posited that the guideline recommendations represent a synthesis of landmark trials that identified cardiorenal indications for these drug classes.
For SGLT‐2 inhibitors, this included individuals with diabetes mellitus who also had established ASCVD, heart failure, or CKD with estimated glomerular filtration rate 30 to 60 mL/min per 1.73 mm2 (stage III) or urine albumin to creatinine ratio >30 mg/g (categories A2–A3). The Modification of Diet in Renal Disease formula was used to calculate estimated glomerular filtration rate.7 Patients with established ASCVD were identified by a reported history of coronary heart disease, heart attack, stroke, or angina on a standardized validated questionnaire delivered by a trained interviewer. Patients with heart failure were similarly identified on the basis of in‐person interviews.
Patients with established or at high risk for ASCVD were considered to have a compelling indication for GLP‐1RAs. American Diabetes Association and American College of Cardiology clinical practice guidelines define patients at high risk for ASCVD as those aged 55 years or older with coronary, carotid, or lower extremity stenosis >50%, left ventricular hypertrophy, retinopathy, or with multiple cardiovascular risk factors. NHANES does not collect angiography or echocardiography data of participants. We limited our definition to patients with diabetes mellitus, aged 60 years or older, with at least 2 of the following conditions: central obesity, smoking, hypertension, or dyslipidemia, following the protocol of clinical trials on GLP‐1RAs8 and the American College of Cardiology guideline definition of high‐risk ASCVD.6
Central obesity was defined as waist‐to‐hip ratio above 88 cm in women and 102 cm in men. Smoking was defined as self‐reported current cigarette smoking. Hypertension was defined as self‐reported history of high blood pressure, or 3 consecutive measurements with systolic blood pressure above 130 mm Hg and/or diastolic blood pressure above 80 mm Hg. Dyslipidemia was defined on the basis of either a self‐reported history of high cholesterol or abnormalities on the lipid panel, including triglycerides above 200 mg/dL, low‐density lipoprotein cholesterol above 130 mg/dL, or high‐density lipoprotein cholesterol below 40 mg/dL in men or 50 mg/dL in women.
Patients with contraindications for each medication based on US Food and Drug Administration product information were considered noneligible. This included those with end‐stage renal disease, on dialysis, or with estimated glomerular filtration rate below 30 mL/min per 1.73 mm2 for SGLT‐2 inhibitors, and pregnant and breastfeeding women for GLP‐1RAs.
Study Covariates
Demographic characteristics of the study population, including age, sex, and race were identified on the basis of standard definitions in NHANES. Notably, we identified participants' race and ethnicity as non‐Hispanic White, non‐Hispanic Black, and others, which included Mexican American individuals, other Hispanic individuals, multiracial individuals, and participants from other races. Health insurance status was assessed by self‐reported coverage during interviews. Other study covariates are outlined in the Study Population and Exposure Groups section above.
Study Outcome
The outcome of the study was the use of SGLT‐2 inhibitors and GLP‐1RAs across eligibility based on clinical practice guidelines. Patients taking any of the following medications were identified as SGLT‐2 inhibitor users: empagliflozin, canagliflozin, dapagliflozin, or ertugliflozin. Those taking any of the following medications were identified as GLP‐1RA users: liraglutide, semaglutide, dulaglutide, albiglutide, exenatide, or lixisenatide. The primary analyses focused on drug class. In exploratory analyses, we evaluated the use of individual drugs. SGLT‐2 inhibitor and GLP‐1RA use were assessed through in‐person interviews as described above.
Statistical Analysis
We used survey‐specific methods that account for the complex design of the NHANES. First, we characterized patients with diabetes mellitus in the United States. Given the clustered and stratified sampling of the NHANES study, we used subject weights that take into account differential probabilities of selection for each individual, rates of survey nonresponse, and representativeness of the sampled individuals for the target population. The weighted analyses provide national estimates representative of the US population and are reported with a 95% CI consistent with the recommendations for the National Center for Health Statistics.9
We assessed demographic characteristics and comorbidities that identified patients with diabetes mellitus eligible for SGLT‐2 inhibitors and/or GLP‐1RAs. We also assessed eligibility across subgroups of age, sex, and race. We then identified the proportion of patients who reported taking SGLT‐2 inhibitors and GLP‐1RAs among both individuals with indications and those without a strong recommendation for therapy based on clinical practice guidelines.
We also identified patient characteristics that were associated with the use of SGLT‐2 inhibitors and GLP‐1RAs in survey‐specific logistic regression models. Eligibility for each drug class was also separately defined as a composite variable per guideline indications as described above. In univariate analyses, age, sex, race, smoking, hypertension, dyslipidemia, preexisting ASCVD, heart failure, CKD, and composite eligibility were tested for associations with SGLT‐2 inhibitor and GLP‐1RA use. Multivariate models were then used to assess whether eligibility for a SGLT‐2 inhibitor or GLP‐1RA was associated with their use, adjusted for age, sex, and race. All variables were categorical except for age, which was continuous, for which odds ratio (OR) per 1 standard deviation change is reported.
Because NHANES is publicly available deidentified data, it was exempt from the preview of the Yale Institutional Review Board. All statistical tests were 2‐sided, and α=0.05 was set as the significancy level. Statistical analysis was performed using survey tools in Stata 16 (StataCorp, College Station, TX).
Results
Characteristics of Study Population and National Estimates
From 2017 to 2018, 1104 of 9254 sampled individuals in NHANES had type 2 diabetes mellitus, representing 33.2 million adults or 10.6% (95% CI, 9.7%–11.6%) of the US population. Among patients with diabetes mellitus, 21 sampled individuals with type 1 diabetes mellitus were excluded from analysis. The mean age for patients with diabetes mellitus was 60.6 years (95% CI, 58.7–62.5 years) and 42.4% (95% CI, 36.2%–48.9%) were aged 65 years or older. A total of 48.6% (95% CI, 42.8%–54.4%) of patients with diabetes mellitus were female patients, 58.8% (95% CI, 52.9%–64.4%) were non‐Hispanic White, 13.1% (95% CI, 9.2%–18.4%) were non‐Hispanic Black, and 90.6% (95% CI, 86.3%–93.7%) had health insurance coverage. Furthermore, 12.1% (95% CI, 8.5%–16.9%) of patients with diabetes mellitus reported current smoking, 79.0% (95% CI, 73.9%–83.3%) had central obesity, 74.7% (95% CI, 70.5%–78.5%) had hypertension, and 80.9% (95% CI, 76.3%–84.7%) had dyslipidemia. Among patients with diabetes mellitus, 26.3% (95% CI, 21.0%–32.4%) had ASCVD, 8.5% (95% CI, 5.8%–12.3%) reported history of heart failure, and 39.2% (95% CI, 35.0%–43.6%) had CKD stage III/A2‐3 (Table S1).
Indication for SGLT‐2 Inhibitors and GLP‐1RAs
Overall, 548 of 1104 sampled individuals with diabetes mellitus, representing 52.6% (95% CI, 47.7%–57.5%) of the US patients with diabetes mellitus, had a guideline indication for SGLT‐2 inhibitors based on concomitant ASCVD, heart failure, or CKD (Figure 1). This population included 64.4% (95% CI, 56.9%–71.2%) of patients aged 65 years or older, 47.8% (95% CI, 39.8%–55.9%) were female patients, 57.4% (95% CI, 50.4%–64.2%) were non‐Hispanic White, and 42.3% (95% CI, 35.4%–49.5%) were non‐Hispanic Black. (Figure 2). In sensitivity analyses that narrowly defined eligibility based on ASCVD and CKD alone, 52.1% (95% CI, 47.2%–56.9%) of patients with diabetes mellitus and without heart failure had a guideline indication for SGLT‐2 inhibitors use.
Figure 1. Flow diagram of exclusion and eligibility criteria for SGLT‐2 inhibitors and GLP‐1RAs.
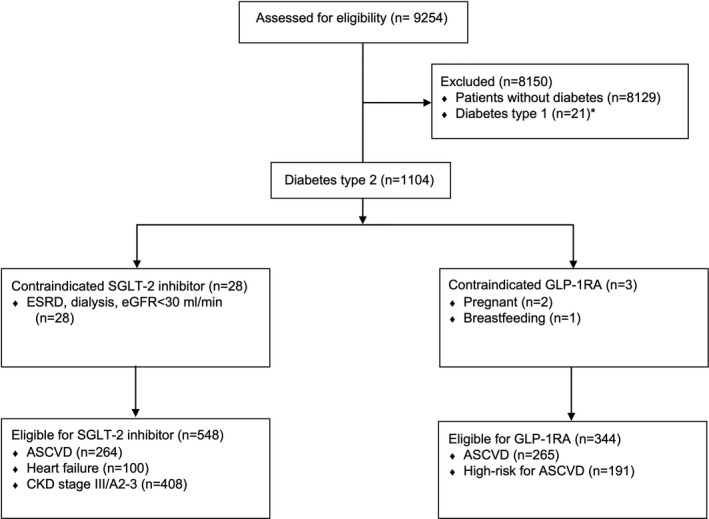
ASCVD indicates atherosclerotic cardiovascular disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; and SGLT‐2, sodium glucose transporter‐2. *Probable type 1 diabetes mellitus was defined as patients younger than 40 years who were only receiving insulin.
Figure 2. Use of SGLT‐2 (sodium glucose transporter‐2) inhibitors among patients with type 2 diabetes mellitus with guideline indications (A) and without guideline indications (B).
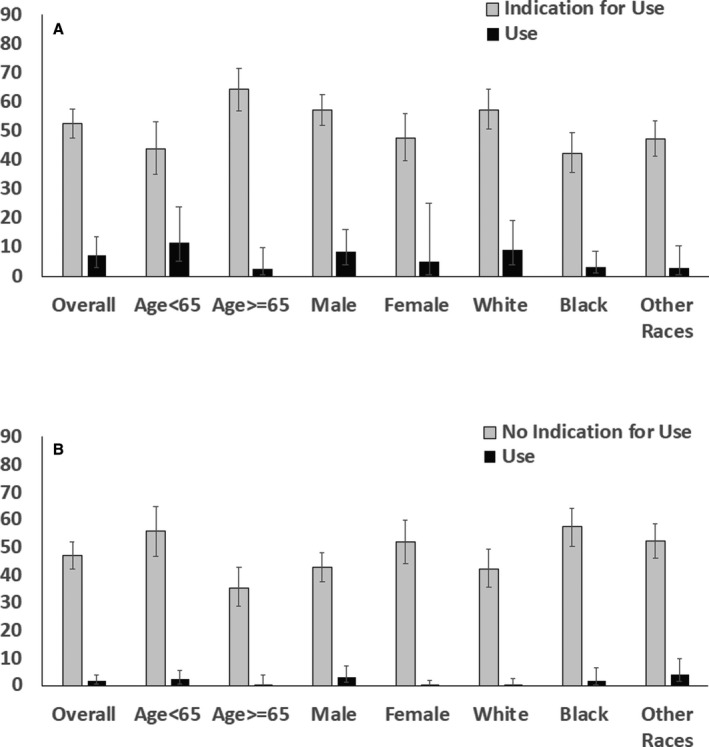
Error bars represent upper and lower limits of 95% CI for weighted percentage.
For GLP‐1RAs, 344 of 1104 sampled participants with diabetes mellitus, or one third of individuals with diabetes mellitus nationally (32.8%; 95% CI, 28.8%–37.2%), were eligible for therapy (Figure 1). This eligible population included 40.6% (95% CI, 34.2%–47.2%) of those aged 65 years or older, 27.0% (95% CI, 21.6%–33.3%) were women, 38.2% (95% CI, 31.2%–45.8%) of non‐Hispanic White, and 24.7% (95% CI, 18.7%–31.9%) of non‐Hispanic Black patients with diabetes mellitus (Figure 3).
Figure 3. Use of GLP‐1RAs (glucagon like peptide‐1 receptor agonists) among patients with type 2 diabetes mellitus with guideline indications (A) and without guideline indications (B).
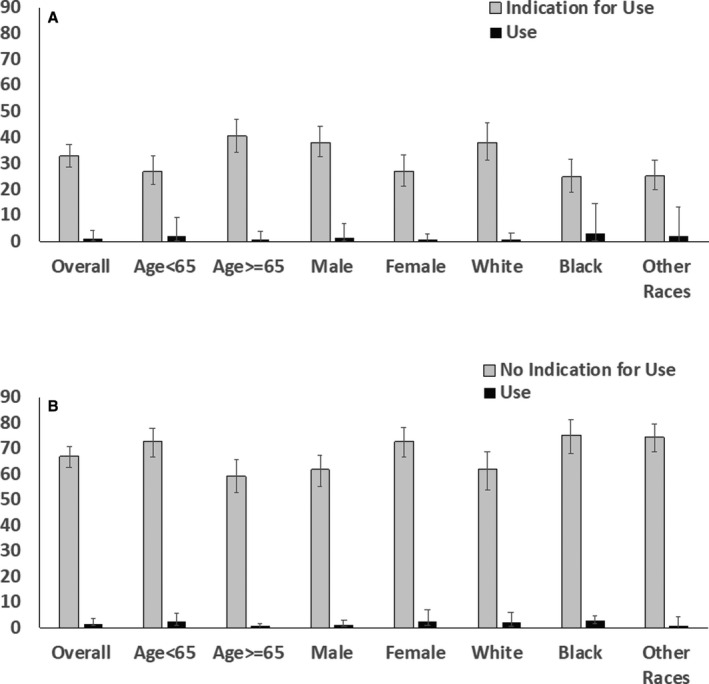
Error bars represent upper and lower limits of 95% CI for weighted percentage.
Furthermore, an estimated 26.6% (95% CI, 22.2%–31.7%) of the US patients with diabetes mellitus were eligible for both SGLT‐2 inhibitors and GLP‐1RAs. Distribution of eligibility for SGLT‐2 inhibitors, GLP‐1RAs, and both medications across demographic subgroups and comorbidities are outlined in Table 1.
Table 1.
Distribution of Eligibility for SGLT‐2 Inhibitors, GLP‐1RAs, or Both in Patients With Diabetes Mellitus Across Demographics and Comorbidities
| Total | SGLT‐2 Inhibitor | GLP‐1RA | SGLT‐2 Inhibitor and GLP‐1RA | ||||
|---|---|---|---|---|---|---|---|
| No. | No. | Weighted % [95% CI] | No. | Weighted % [95% CI] | No. | Weighted % [95% CI] | |
| Overall | 1104 | 548 | 52.6 [47.7–57.5] | 344 | 32.8 [28.8–37.2] | 267 | 26.6 [22.2–31.7] |
| Age, y | |||||||
| <65 | 577 | 234 | 44.0 [35.3–53.1] | 149 | 27.2 [21.8–33.2] | 103 | 20.9 [15.7–27.3] |
| ≥65 | 527 | 314 | 64.4 [56.9–71.2] | 195 | 40.6 [34.2–47.2] | 164 | 34.4 [27.6–41.9] |
| Sex | |||||||
| Women | 537 | 228 | 47.8 [39.8–55.9] | 135 | 27.0 [21.6–33.3] | 97 | 20.0 [14.0–27.7] |
| Men | 567 | 320 | 57.2 [51.8–62.5] | 209 | 38.3 [32.4–44.7] | 170 | 32.9 [26.9–39.5] |
| Race/ethnicity | |||||||
| Non‐Hispanic White | 345 | 208 | 57.4 [50.4–64.2] | 148 | 38.2 [31.2–45.8] | 124 | 32.6 [25.0–41.3] |
| Non‐Hispanic Black | 271 | 122 | 42.3 [35.4–49.5] | 71 | 24.7 [18.7–31.9] | 58 | 18.9 [14.7–24.0] |
| Others | 488 | 218 | 47.4 [41.4–53.5] | 125 | 25.4 [20.3–31.2] | 85 | 17.7 [13.4–23.1] |
| Health insurance | 985 | 493 | 52.3 [46.7–57.9] | 319 | 33.4 [28.7–38.5] | 248 | 26.7 [21.5–32.7] |
| Comorbidities | |||||||
| Smoking | 144 | 81 | 53.0 [39.4–66.1] | 49 | 34.4 [24.2–46.3] | 45 | 31.6 [22.0–43.2] |
| Central obesity | 787 | 382 | 50.7 [44.8–56.7] | 220 | 30.0 [25.8–34.6] | 175 | 24.1 [18.7–30.5] |
| Hypertension | 817 | 452 | 61.5 [56.0–66.8] | 241 | 32.3 [26.8–38.4] | 223 | 31.2 [25.7–37.2] |
| Dyslipidemia | 863 | 446 | 55.1 [49.3–60.7] | 230 | 28.5 [22.7–35.2] | 212 | 27.2 [21.7–33.6] |
GLP‐1RA indicates glucagon like peptide‐1 receptor agonist; and SGLT‐2, sodium glucose transporter‐2.
Use of SGLT‐2 Inhibitors and GLP‐1RAs From 2017 to 2018
Among individuals with diabetes mellitus in the United States, an estimated 4.5% (95% CI, 2.4%–8.2%) of patients overall and 7.0% (95% CI, 3.4%–13.7%) of patients eligible for SGLT‐2 inhibitors were receiving a drug in this class. Similarly, 1.5% (95% CI, 0.7%–3.2%) of patients with diabetes mellitus overall and 1.3% (95% CI, 0.3%–4.5%) of patients eligible for GLP‐1RAs were receiving one of the medications in this drug class. Three patients were receiving both medications, representing 0.2% (95% CI, 0.1%–0.3%) of patients with diabetes mellitus. The most commonly used medications in each drug class were canagliflozin (weighted use rate 2.6% [95% CI, 1.3%–5.1%] among eligible patients) and empagliflozin (1.9% [95% CI, 0.5%–6.9%]) for SGLT‐2 inhibitors, and liraglutide (0.6% [95% CI, 0.1%–2.8%]) and dulaglutide (0.4% [95% CI, 0.07%–2.1%]) for GLP‐1RAs. Distribution of SGLT‐2 inhibitors and GLP‐1RAs use among patients with and without compelling indications across age, sex, and race/ethnicity domains are represented in Figures 2 and 3. The degree of underuse for SGLT‐2 inhibitors was highest among patients with CKD/albuminuria, followed by ASCVD and heart failure. The use of SGLT‐2 inhibitors and GLP1‐RAs by their individual indication are summarized in Table S2.
History of established ASCVD, heart failure, and CKD were not associated with use of either drug class in univariate models (Table S3). In multivariate models, the composite of any compelling indication for treatment was significantly associated with SGLT‐2 inhibitors use (adjusted OR, 4.2 [95% CI, 1.23–14.8], P=0.02) among patients with diabetes mellitus but not the use of GLP‐1RAs (adjusted OR, 0.97 [95% CI, 0.28–3.3], P=0.97; Table 2). Univariate and multivariate analyses of demographic factors and comorbidities in predicting use of each drug class in patients with diabetes mellitus are presented in Table S3 and Figure S1.
Table 2.
Multivariate Logistic Regression Analysis of Predictors of SGLT‐2 Inhibitor and GLP‐1RA Use in Patients With Diabetes Mellitus
| SGLT‐2 Inhibitor | GLP‐1RA | |||
|---|---|---|---|---|
| Odds Ratio [95% CI] | P Value | Odds Ratio [95% CI] | P Value | |
| Age | 0.77 [0.34–1.17] | 0.25 | 0.61 [0.41–0.82] | 0.002 |
| Women | 0.50 [0.08–3.05] | 0.42 | 1.4 [0.26–7.7] | 0.65 |
| Non‐Hispanic White | 1.5 [0.56–4.1] | 0.37 | 1.4 [0.17–12.0] | 0.70 |
| Non‐Hispanic Black | 0.79 [0.33–1.8] | 0.58 | 2.7 [0.43–17.3] | 0.26 |
| Any compelling indication | 4.2 [1.23–14.8] | 0.02 | 0.97 [0.28–3.3] | 0.97 |
Compelling indications included established atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease stage III/A2–3 for SGLT‐2 inhibitors, and established or high‐risk atherosclerotic cardiovascular disease for GLP‐1RAs. GLP‐1RA indicates glucagon like peptide‐1 receptor agonist; and SGLT‐2, sodium glucose transporter‐2.
Discussion
In the United States, based on current national practice guidelines, 1 in 2 patients with diabetes mellitus has a strong recommendation for treatment with SGLT‐2 inhibitors, 1 in 3 for GLP‐1RAs, and 1 in 4 for both medications. However, despite multiple large randomized clinical trials suggesting their role in reducing the risk of cardiovascular events and progression of renal disease, only 7% of eligible patients received SGLT‐2 inhibitors within 3 years of obtaining specific approval for these indications. For GLP‐1RAs, only 1% of patients with indications received these medications, and fewer than 1% of individuals received both medications. Furthermore, there is no consistent use among individuals most likely to benefit from these drug classes, including low rates of use among those with cardiovascular or renal disease.
The favorable cardiorenal benefits of SGLT‐2 inhibitors and GLP‐1RAs have been reported in large randomized trials from 2015 to 2016,10, 11, 12 with expedited inclusion of these indications by the US Food and Drug Administration. They have also been recommended for patients with diabetes mellitus and concomitant cardiovascular disease and/or CKD in clinical practice guidelines from 2017 to 2018.13 The large randomized clinical trials that evaluated effects of these drugs had broad eligibility, and more than a third of the US population with diabetes mellitus would have been potentially eligible for SGLT‐2 inhibitor trials,14 and over half would have been eligible to enroll in those for GLP‐1RAs.15
Despite multiple investigations highlighting the unique indications of these novel agents in a large proportion of patients with diabetes mellitus, we found that even in 2018 their use was restricted to only 1 in 14 of eligible individuals for SGLT‐2 inhibitors and 1 in 100 of those eligible for GLP‐1RAs. Our national study confirms the generalizability of prior observations in clinical registries, including the GOULD (Getting to an Improved Understanding of Low‐Density Lipoprotein Cholesterol and Dyslipidemia Management) study, in a selected number of outpatient practices that reported only 5% to 6% and 8% to 9% of patients eligible for enrollment in trials were receiving SGLT‐2 inhibitors and GLP1‐RAs, respectively.16
Affordability of therapy likely remains a major barrier to expand use of both drug classes. The monthly out‐of‐pocket cost of treatment is estimated to be $338 to $593 for SGLT‐2 inhibitors and $744 to $1106 for GLP‐1RAs, varying across health insurance coverage.17 Of note, 90% of patients with diabetes mellitus had access to health insurance, but barriers with insurance prior authorization for novel agents likely represent an impediment to their wider use among the insured.18, 19 Of note, we observed a trend toward lower use of SGLT‐2 inhibitors in non‐Hispanic Black patients, which may represent underlying racial disparities in prescription or healthcare access that lead to worsened outcomes in this population.20, 21 The slower adoption of these drugs may also suggest clinical inertia toward using novel agents or lack of information on these updated guidelines among practicing clinicians, but this aspect requires dedicated investigations.22
Our study has limitations that merit consideration. First, our assessment of comorbid conditions and treatments is partly based on interviews. However, the questionnaire used in NHANES is a validated instrument delivered by trained interviewers and was used in multiple prior studies.23, 24 Furthermore, we supplemented our diagnostic criteria to include objective measures, such as blood pressure readings and laboratory values of hemoglobin A1C, serum creatinine, and urine albumin to strengthen the robustness of our interpretations. Our estimated 10.6% national prevalence of diabetes mellitus is consistent with estimates from other national agencies.25 Second, we cannot definitively distinguish type 1 and type 2 diabetes mellitus, because only the latter group is eligible for these therapies. We attempted to distinguish these patients by excluding those who were younger than age 40 years and were taking only insulin. However, only 5% of US patients with diabetes mellitus have type 1 diabetes mellitus, and therefore, it is unlikely to meaningfully change the estimates. Third, our data do not inform adherence with treatment among those receiving these therapies. We are also unable to assess patient's preferences, affordability, or discontinuation of treatment because of adverse events. Fourth, the most recent data from NHANES represent practice in 2018, and their use may have increased in recent years. However, our data highlight the slow uptake of therapy in clinical practice even years after robust data supported a strong protective role for SGLT‐2 inhibitors and GLP‐1RAs. Nevertheless, the slow uptake several years after the publication of landmark trials demonstrating their efficacy suggests that they are likely to continue to be underused. Fifth, certain conditions that were included in guidelines as a component of the indications, including left ventricular hypertrophy or carotid or peripheral artery stenosis, could not be identified in NHANES. There is likely to be overlap among these and included conditions, and nevertheless would suggest that our analysis likely underestimates the eligibility and overestimates the use of these medications. Finally, the small number of patients taking SGLT‐2 inhibitors and GLP‐1RAs precluded an assessment of heterogeneity in their use. This also limited a drug‐level analysis, adjustment for further confounding factors in multivariable models, and those evaluating the role of factors such as income and comorbidities, which may be associated with underuse of these drug classes. However, the low uptake in contemporary practice suggests a widespread underuse of these drugs across clinical and demographic groups.
In conclusion, nearly half of US patients with diabetes mellitus have guideline recommendations for the use of SGLT‐2 inhibitors, and over a third are eligible for GLP‐1RAs based on current clinical practice guidelines. Despite these recommendations, there are substantial gaps in the use of these medications, limiting their potential public health benefits.
Sources of Funding
Dr Khera received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under the award K23HL153775‐01A1. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
Dr Desai has reported working under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay‐for‐performance program; and has received research grants from and consulting for Amgen, Astra Zeneca, Boehringer Ingelheim, Cytokinetics, Medicines Company, Relypsa, Novartis, and SCPharmaceuticals. Dr Lipska received funding from National Institute of Aging and the American Federation for Aging Research through the Paul Beeson Career Development Award (K23AG048359), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and from the Centers for Medicare and Medicaid Services to develop and maintain publicly reported quality measures. Dr Krumholz reports receiving personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, F‐Prime, Siegfried & Jensen Law Firm, Arnold & Porter Law Firm, Martin/Baughman Law Firm, and the National Center for Cardiovascular Diseases, Beijing; serving as cofounder of HugoHealth and Refactor Health, and works under contract with Centers for Medicare and Medicaid Services; and grants from Medtronic and the Food and Drug Administration, Medtronic, Johnson & Johnson, and Shenzhen Center for Health Information outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figure S1
(J Am Heart Assoc. 2021;10:e021084. DOI: 10.1161/JAHA.121.021084.)
This study was presented at the American College of Cardiology Scientific Sessions, May 15 to 17, 2021.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021084
For Sources of Funding and Disclosures, see page 8.
References
- 1.Hussein H, Zaccardi F, Khunti K, Seidu S, Davies MJ, Gray LJ. Cardiovascular efficacy and safety of sodium‐glucose co‐transporter‐2 inhibitors and glucagon‐like peptide‐1 receptor agonists: a systematic review and network meta‐analysis. Diabet Med. 2019;36:444–452. DOI: 10.1111/dme.13898. [DOI] [PubMed] [Google Scholar]
- 2.Filion KB, Lix LM, Yu OHY, Dell’Aniello S, Douros A, Shah BR, St‐Jean A, Fisher A, Tremblay E, Bugden SC, et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi‐database retrospective cohort study. BMJ. 2020;370:m3342. DOI: 10.1136/bmj.m3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold SV, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Mues KE, Alam S, Elliott‐Davey M, Bhatt DL, Cannon CP, et al. Use of guideline‐recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140:618–620. DOI: 10.1161/CIRCULATIONAHA.119.041730. [DOI] [PubMed] [Google Scholar]
- 4.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. DOI: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Suppl 1):S98–S110. DOI: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 6.Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr, Kalyani RR, Kosiborod M, Magwire M, Morris PB, Neumiller JJ, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117–1145. DOI: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. DOI: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121–130. DOI: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) . National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 10.Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. DOI: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. DOI: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. DOI: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Introduction: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41(Suppl 1):S1–S2. DOI: 10.2337/dc18-Sint01. [DOI] [PubMed] [Google Scholar]
- 14.Castellana M, Procino F, Sardone R, Trimboli P, Giannelli G. Generalizability of sodium‐glucose co‐transporter‐2 inhibitors cardiovascular outcome trials to the type 2 diabetes population: a systematic review and meta‐analysis. Cardiovasc Diabetol. 2020;19:87. DOI: 10.1186/s12933-020-01067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittbrodt ET, Eudicone JM, Bell KF, Enhoffer DM, Latham K, Green JB. Generalizability of glucagon‐like peptide‐1 receptor agonist cardiovascular outcome trials enrollment criteria to the US type 2 diabetes population. Am J Manag Care. 2018;24(suppl):S146–S155. [PubMed] [Google Scholar]
- 16.Arnold SV, Inzucchi SE, Tang F, McGuire DK, Mehta SN, Maddox TM, Goyal A, Sperling LS, Einhorn D, Wong ND, et al. Real‐world use and modeled impact of glucose‐lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR(R) Research to Practice project. Eur J Prev Cardiol. 2017;24:1637–1645. DOI: 10.1177/2047487317729252. [DOI] [PubMed] [Google Scholar]
- 17.Vardeny O, Vaduganathan M. Practical guide to prescribing sodium‐glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019;7:169–172. DOI: 10.1016/j.jchf.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Resneck JS Jr. Refocusing medication prior authorization on its intended purpose. JAMA. 2020;323:703–704. DOI: 10.1001/jama.2019.21428. [DOI] [PubMed] [Google Scholar]
- 19.Bergeson JG, Worley K, Louder A, Ward M, Graham J. Retrospective database analysis of the impact of prior authorization for type 2 diabetes medications on health care costs in a Medicare Advantage Prescription Drug Plan population. J Manag Care Pharm. 2013;19:374–384. DOI: 10.18553/jmcp.2013.19.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canedo JR, Miller ST, Schlundt D, Fadden MK, Sanderson M. Racial/ethnic disparities in diabetes quality of care: the role of healthcare access and socioeconomic status. J Racial Ethn Health Disparities. 2018;5:7–14. DOI: 10.1007/s40615-016-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whyte MB, Hinton W, McGovern A, van Vlymen J, Ferreira F, Calderara S, Mount J, Munro N, de Lusignan S. Disparities in glycaemic control, monitoring, and treatment of type 2 diabetes in England: a retrospective cohort analysis. PLoS Med. 2019;16:e1002942. DOI: 10.1371/journal.pmed.1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schernthaner G, Shehadeh N, Ametov AS, Bazarova AV, Ebrahimi F, Fasching P, Janež A, Kempler P, Konrāde I, Lalić NM, et al. Worldwide inertia to the use of cardiorenal protective glucose‐lowering drugs (SGLT2i and GLP‐1 RA) in high‐risk patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:185. DOI: 10.1186/s12933-020-01154-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS One. 2013;8:e76632. DOI: 10.1371/journal.pone.0076632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel CJ, Pho N, McDuffie M, Easton‐Marks J, Kothari C, Kohane IS, Avillach P. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci Data. 2016;3:160096. DOI: 10.1038/sdata.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention . National Diabetes Statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


