Abstract
Background
Functional assessment of myocardial bridging (MB) remains clinically challenging because of the dynamic nature of the extravascular coronary compression with a certain degree of intraluminal coronary reduction. The aim of our study was to assess performance and diagnostic value of diastolic‐fractional flow reserve (d‐FFR) during dobutamine provocation versus conventional‐FFR during adenosine provocation with exercise‐induced myocardial ischemia as reference.
Methods and Results
This prospective study includes 60 symptomatic patients (45 men, mean age 57±9 years) with MB on the left anterior descending artery and systolic compression ≥50% diameter stenosis. Patients were evaluated by exercise stress‐echocardiography test, and both conventional‐FFR and d‐FFR in the distal segment of left anterior descending artery during intravenous infusion of adenosine (140 μg/kg per minute) and dobutamine (10–50 μg/kg per minute), separately. Exercise–stress‐echocardiography test was positive for myocardial ischemia in 19/60 patients (32%). Conventional‐FFR during adenosine and peak dobutamine had similar values (0.84±0.04 versus 0.84±0.06, P=0.852), but d‐FFR during peak dobutamine was significantly lower than d‐FFR during adenosine (0.76±0.08 versus 0.79±0.08, P=0.018). Diastolic‐FFR during peak dobutamine was significantly lower in the exercise‐stress‐echocardiography test –positive group compared with the exercise‐ stress‐echocardiography test –negative group (0.70±0.07 versus 0.79±0.06, P<0.001), but not during adenosine (0.79±0.07 versus 0.78±0.09, P=0.613). Among physiological indices, d‐FFR during peak dobutamine was the only independent predictor of functionally significant MB (odds ratio, 0.870; 95% CI, 0.767–0.986, P=0.03). Receiver‐operating characteristics curve analysis identifies the optimal d‐FFR during peak dobutamine cut‐off ≤0.76 (area under curve, 0.927; 95% CI, 0.833–1.000; P<0.001) with a sensitivity, specificity, and positive and negative predictive value of 95%, 95%, 90%, and 98%, respectively, for identifying MB associated with stress‐induced ischemia.
Conclusions
Diastolic‐FFR, but not conventional‐FFR, during inotropic stimulation with high‐dose dobutamine, in comparison to vasodilatation with adenosine, provides more reliable functional significance of MB in relation to stress‐induced myocardial ischemia.
Keywords: adenosine, dobutamine, fractional flow reserve, myocardial bridging, myocardial ischemia, stress‐echocardiography
Subject Categories: Diagnostic Testing, Coronary Circulation, Hemodynamics, Ischemia, Angina
Nonstandard Abbreviations and Acronyms
- d‐Pa
mean aortic blood pressure obtained duringdiastole
- d‐Pd
mean distal intracoronary pressure obtained during diastole
- d‐FFR
diastolic fractional flow reserve
- DS
diameter stenosis
- FFR
fractional flow reserve
- HR
heart rate;
- MB
myocardial bridging
- MLD
minimal luminal diameter
- Pa
mean aortic blood pressure obtained during the whole cardiac cycle
- Pd
mean distal intracoronary pressure obtained during the whole cardiac cycle;
- SE
stress‐echocardiography test
Clinical Perspective
What Is New?
Diastolic fractional flow reserve during inotropic stimulation with high‐dose dobutamine, in comparison to vasodilatation with adenosine, provides more reliable functional significance of myocardial bridging in relation to exercise stress‐induced myocardial ischemia.
The cut‐off value of ≤0.76 for diastolic fractional flow reserve during dobutamine provocation has the best sensitivity and specificity for identifying myocardial bridging associated with stress‐induced myocardial ischemia.
What Are the Clinical Implications?
Diastolic fractional flow reserve using dobutamine provocation appears to be a useful index in detecting functionally significant myocardial bridging and may play an important role as a guide to therapeutic approach.
Further studies are needed to determine the prognostic value of diastolic fractional flow reserve during dobutamine provocation in patients with myocardial bridging managed without revascularization, with respect to the rate of major adverse cardiac events in long‐term follow‐up.
Further studies are needed for the validation of other more accessible and routinely performed diastole‐specific indices against diastolic fractional flow reserve during dobutamine provocation for the functional assessment of myocardial bridging.
Myocardial bridging (MB) is dynamic stenosis characterized by systolic compression of the intramyocardial arterial segment (“milking effect”) with delayed early diastolic artery relaxation leading to more or less reduction of vessel luminal diameter in diastole.1, 2, 3, 4, 5, 6 This coronary anomaly is commonly considered a benign anatomic variation, but when located in the left anterior descending (LAD) artery it may cause myocardial ischemia, acute coronary syndrome, life‐threatening ventricular arrhythmias, systolic dysfunction of the left ventricle, or even sudden cardiac death.7, 8, 9, 10 Thus, the occurrence of severe cardiac events has raised the issue on the most appropriate diagnostic test/s that could identify functionally significant MB. Still, there is no established standard for the functional evaluation of MB, and a number of diagnostic modalities have been proposed.1, 11, 12, 13, 14, 15
Although conventional fractional flow reserve (FFR) obtained during adenosine‐induced maximal hyperemia is the “gold” standard for the functional assessment of fixed coronary stenosis, the role of FFR in evaluation of MB remains challenging.11, 12, 14, 15 In fact, several authors have suggested that adequate invasive hemodynamic assessment of MB should include inotropic stimulation with dobutamine because of its dynamic nature that depends on a degree of extravascular coronary compression.1, 11, 12 Furthermore, a study by Escaned et al showed that the functional assessment of MB should include diastolic‐FFR (d‐FFR) measurement during dobutamine provocation because decrease and negativization of systolic pressure gradient across the MB during inotropic stimulation leads to a paradoxical increase in conventional‐FFR values.12 Based on a previous study, a d‐FFR ≤0.76 obtained during dobutamine infusion was used for the hemodynamic significance of MB, but this cut‐off value has not been validated in the context of objective signs of myocardial ischemia as induced by physiologic exercise. Additionally, there are no studies correlating both conventional‐FFR and d‐FFR using adenosine and dobutamine infusion with objective signs of myocardial ischemia during physiological exercise stress in patients with isolated‐MB. Therefore, the aim of our study was to assess performance and diagnostic value of d‐FFR during dobutamine provocation versus conventional‐FFR during adenosine provocation with exercise‐induced myocardial ischemia as reference.
Methods
All data and supporting materials have been provided with the published article.
Study Population
This prospective, single‐center study included 60 symptomatic patients (45 male [75%], mean age 57±9 years, range, 36–77 years) scheduled for coronary angiography. To be eligible for the study, each patient was required to have chest pain (typical or atypical), and angiographically significant isolated‐MB on the LAD defined as systolic compression of intramyocardial arterial segment ≥50% diameter stenosis (DS) after intracoronary administration of 200 µg of nitroglycerin, as measured by quantitative coronary angiography. Key exclusion criteria included (1) patients aged ≤18 years old; (2) asymptomatic patients with MB; (3) angiographically nonsignificant systolic compression at MB‐site; (4) presence of fixed stenosis/es ≥50% DS in the LAD or other coronary arteries; (5) any previous myocardial infarction; (6) any previous percutaneous coronary intervention; and (7) previous aortocoronary bypass grafting surgery. Full inclusion and exclusion criteria are presented in Table S1.
Study Protocol
Standard 2‐dimensional echocardiography, treadmill‐exercise stress‐echocardiography (SE) test, and coronary angiography with invasive functional evaluation of MB were performed in all patients. The latter was performed after all noninvasive tests were done. Antianginal drugs including beta‐blockers, calcium‐channel blockers, long‐acting nitrates, trimetazidine and ranolazine, as well as xanthine‐containing foods or beverages (coffee, tea, sugar drinks, sweets, chocolate, and fruits) were discontinued 24 to 48 hours before the examinations. The study protocol was presented and approved by the Ethical Committees of Clinical Center of Serbia and Faculty of Medicine University of Belgrade (Belgrade, Serbia). Informed consent was obtained from all patients.
Exercise Stress‐Echocardiography
After standard 2‐dimensional echocardiographic examination, all patients underwent treadmill exercise‐SE according to maximal Bruce protocol, as previously described.16, 17 Echocardiographic studies were performed with a digital ultrasound system (Acuson Sequoia C256; Siemens Medical Solutions USA, Inc, Mountain View, CA), with a 3V2C multifrequency transducer using second‐harmonic technology. Briefly, all standard echocardiographic views were obtained with patients in the left lateral decubitus position before and immediately after exercise. Echocardiographic images were interpreted and analyzed by a senior echocardiographer blinded for patients' clinical, angiographic, and functional status. For the purpose of this analysis, left ventricle walls were divided into the 17‐segment model and segmental wall motion was graded as follows: 1=normal, 2=hypokinetic, 3=akinetic, and 4=dyskinetic.18 Exercise‐SE test was considered positive for myocardial ischemia when new wall‐motion abnormalities were observed in at least 2 adjacent left ventricle segments in the LAD territory. Only SE‐findings of new wall‐motion abnormalities were considered as objective evidence of myocardial ischemia. Neither the presence of angina, nor the presence of ECG changes during exercise‐SE were considered as positive markers of myocardial ischemia, because of its limited sensitivity and specificity.
Invasive Coronary Angiography and Evaluation of Conventional‐FFR
Invasive coronary angiography was performed in all patients with the femoral or radial artery approach using standard Judkins technique with 6‐Fr catheters. After intracoronary administration of 200 µg of nitroglycerin, the baseline coronary angiogram was acquired in multiple projections, and 2 views of LAD showing the best visualization of MB were chosen for further angiographic analysis. After intravenous administration of 70 IU/kg of unfractioned heparin, a 6‐Fr Judkins left (JL) or extra backup (EBU) guiding catheter without side holes was engaged in the left coronary ostium. A pressure–temperature sensor‐tipped 0.014‐in guidewire (PressureWire Certus, St. Jude Medical, St. Paul, MN), previously flushed and calibrated to zero pressure, was advanced to the tip of the guiding catheter where an equalization was performed to ensure identical pressures by the guiding catheter and pressure guidewire. The pressure guidewire was then passed across the MB and positioned into the distal segment of LAD with the sensor located ≈3 cm below the bridged segment. Mean aortic blood pressure (Pa) and mean intracoronary blood pressure distal to MB (Pd, distal intracoronary pressure) were simultaneously measured with the guiding catheter and the pressure guidewire, respectively, at basal conditions and during sustained hyperemia achieved by intravenous infusion of adenosine at a dose of 140 µg/kg per minute for at least 1 minute (Figure S1). Conventional‐FFR was calculated as the ratio of mean distal intracoronary pressure to mean aortic blood pressure (Pd/Pa) obtained during the whole cardiac cycle, and pressure gradient (ΔP) across the MB was calculated as the maximal difference between Pa and Pd (Pa−Pd).19 At the end of the procedure, the guidewire was pulled back to the tip of the guiding catheter, and the presence of pressure drift was assessed. When a drift of ±0.02 was observed, the measurements were repeated.
Fifteen minutes after the evaluation of conventional‐FFR during adenosine, all measurements were repeated during dobutamine infusion, starting at a dosage of 10 µg/kg per minute, and increasing by 10 µg/kg per minute every 3 minutes, to the maximal dosage of 50 µg/kg per minute (Figure S2). The primary goal of dobutamine provocation was to achieve an increase in heart rate (HR) of at least 85% of the maximum age‐predicted HR. If not, dobutamine infusion was also considered hemodynamically adequate if increase in HR was at least 50 beats per minute (bpm) from baseline‐HR (delta‐HR).20 In case of inadequate increase in HR, and in the absence of ischemia or other side effects, a bolus of atropine 0.5 mg was injected during the last minute of the test and repeated up to a maximal dose of 2.0 mg if necessary. At the end of the procedure, and after intravenous administration of metoprolol (2.5–5.0 mg over 1–2 minutes), the presence of pressure drift was assessed in the same manner as described previously.
HR, blood pressure, and 12‐lead ECG were monitored continuously and recorded under basal conditions, during adenosine and each dose of dobutamine infusion, separately. Rate–pressure product was calculated as HR multiplied by systolic blood pressure (bpm×mm Hg).
Postprocessing of Acquired Physiologic Data and Evaluation of Diastolic‐FFR
The raw data of the pressure tracings from which conventional‐FFR was calculated were automatically stored on RadiAnalyzer Xpress (St. Jude Medical, St. Paul, MN), and transferred to the RadiView software package (RadiView 2.2), previously installed on a personal computer. Raw data were then exported from the RadiView 2.2 to a Microsoft Office Excel database for further off‐line analysis by a senior investigator, who was blinded to the patient's clinical status. Mean diastolic‐Pa (d‐Pa) and mean diastolic‐Pd (d‐Pd) were calculated using diastolic components of pressure values (Pa and Pd), starting from the dicrotic notch until the end of diastole on aortic pressure waveform (Figure 1, Figure S3). All measurements represented an average of 3 consecutive cardiac cycles. Diastolic‐FFR was calculated as the ratio of mean distal intracoronary pressure to mean aortic blood pressure obtained during diastole (mean diastolic‐Pd/mean diastolic‐Pa), after intravenous infusions of adenosine and each dose of dobutamine. Diastolic‐ΔP across the MB was calculated as the maximal difference between diastolic‐Pa and diastolic‐Pd, while systolic‐ΔP was calculated as the maximal difference between systolic‐Pa and systolic‐Pd.12, 21, 22
Figure 1. Schematic chart of conventional‐FFR and diastolic‐FFR measurements.
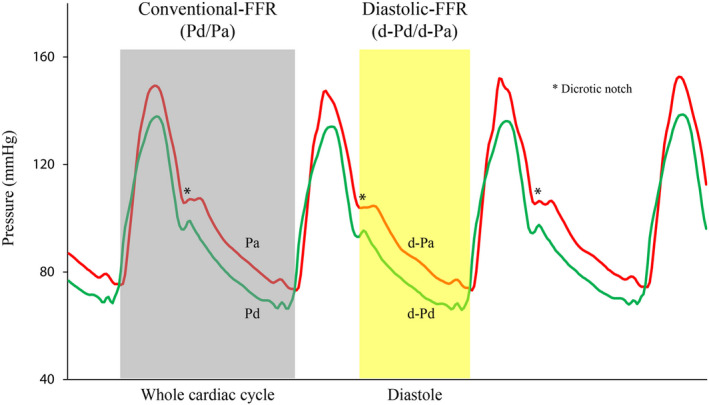
The onset of diastole was defined from the nadir of dicrotic notch on the aortic pressure signal (Pa), while the end of diastole was defined as the point of the lowest pressure just before the Pa upstroke. d‐Pa indicates aortic blood pressure waveform during diastole; d‐Pd, distal intracoronary pressure waveform during diastole; FFR, fractional flow reserve; Pa (red line), aortic blood pressure waveform during the whole cardiac cycle; and Pd (green line), distal intracoronary pressure waveform during the whole cardiac cycle.
Quantitative Coronary Angiography
We performed a detailed frame‐by‐frame quantitative coronary angiography analysis of the interpolated reference diameter, minimal luminal diameter (MLD), and percent DS, at the most severe site of MB, at end‐systole (peak of T wave), and end‐diastole (beginning of QRS complex).5 All angiographic images were obtained at least 1 minute after intracoronary administration of 200 µg of nitroglycerin, and analyzed in exactly the same views and with the same positioning of the cursor under both phases of the cardiac cycle. The images were analyzed off‐line by a dedicated system for quantitative coronary angiography (Siemens Quantcor QCA), and by a senior investigator blinded to the patients' clinical, functional, and echocardiographic status.
Statistical Analysis
All data were entered into a database and then processed in the statistical program IBM SPSS Statistics for Windows, version 26.0 (IBM Corporation, Armonk, NY). Categorical variables are reported as count with percentages, and compared using a χ2 or Fischer exact test, depending on group sizes. Normal distribution of continuous variables was confirmed by both Kolmogorov–Smirnov and Levene tests, and expressed as mean±SD. Continuous variables were compared with Student t test (paired or unpaired) and 1‐way ANOVA followed by the post hoc Tukey HSD test, as appropriate. Relation between continuous variables was estimated using Pearson correlation coefficient and additionally modeled with linear regression analysis. Differences in hemodynamic parameters between baseline, during adenosine and peak dobutamine infusion were analyzed using ANOVA for repeated measures with Sidak correction for multiple pairwise comparison. Additionally, differences in hemodynamic parameters between baseline and each dose of dobutamine infusion were analyzed using linear mixed model, which was set by unstructured covariance matrix and subjects as random effect, and using Sidak for multiple comparison P value adjustment. Variables were tested for their ability to predict stress‐induced myocardial ischemia in univariate binary logistic regression analyses. Univariate predictors with a value of P<0.05 were considered for inclusion in multivariate regression models using backward method to determine correlates independently associated with stress‐induced ischemia in patients with MB. Receiver operating characteristics curves with the 95% CI were used to estimate diagnostic accuracy, while the highest Youden's index (sensitivity+specificity−1) was used to assess the best cut‐off values of conventional‐FFR and d‐FFR during adenosine and peak dobutamine infusion, for discrimination of patients with MB with and without stress‐induced myocardial ischemia. Cohen's kappa coefficient was used for assessing the agreement between dichotomous variables, while measurement error of continuous variables and 95% limits of agreement were assessed by Bland–Altman analysis. A 2‐sided value of P<0.05 was considered statistically significant.
Results
In all patients, isolated‐MB was located in the medial segment of LAD with mean systolic arterial compression of 64±10% DS (range, 51%–95% DS). Exercise‐SE was positive for myocardial ischemia in 19/60 patients (32%). In all patients with stress‐induced ischemia, wall‐motion abnormalities occurred in medial and distal segments of anterior septum. Ischemic ST depression ≥1.0 mm during exercise was found in 7 of 19 patients with stress‐induced ischemia, and 1 of 41 patients without stress‐induced ischemia (36.8% versus 2.4%, P=0.001). Chest discomfort during exercise was present in 7 of 19 patients with stress‐induced ischemia and 9 of 41 patients without stress‐induced ischemia (36.8% versus 22%, P=0.225).
Demographic, clinical, and angiographic characteristics of patients with MB in relation to exercise‐SE results and presence of myocardial ischemia are presented in Table 1. There were no significant differences in observed demographic and clinical variables between the SE‐positive and SE‐negative group.
Table 1.
Demographic, Clinical, and Angiographic Characteristics of the Whole Study Group and in Patients With and Without Stress‐Induced Wall Motion Abnormalities in the LAD Territory
| Variable | All (n=60) | SE − (n=41) | SE + (n=19) | P Value |
|---|---|---|---|---|
| Age±SD, y | 57±9 | 56±8 | 59±10 | 0.167 |
| Sex, men (%) | 45 (75) | 30 (73) | 15 (79) | 0.755 |
| BMI±SD, kg/m2 | 27.7±4.4 | 27.4±3.4 | 28.5±5.8 | 0.382 |
| Hypertension, n (%) | 51 (85) | 37 (90) | 14 (74) | 0.126 |
| Diabetes mellitus, n (%) | 8 (13) | 6 (15) | 2 (10.5) | 0.716 |
| Smoking, n (%) | 29 (48) | 18 (44) | 11 (58) | 0.313 |
| Hyperlipidemia, n (%) | 48 (80) | 34 (83) | 14 (74) | 0.493 |
| Family history, n (%) | 33 (55) | 24 (58.5) | 9 (47) | 0.419 |
| LVEF±SD, % | 65±8 | 65±8 | 63±7 | 0.287 |
| RD (end‐systole) ±SD, mm | 2.58±0.40 | 2.64±0.40 | 2.44±0.35 | 0.068 |
| RD (end‐diastole) ±SD, mm | 2.62±0.40 | 2.67±0.40 | 2.49±0.38 | 0.106 |
| MLD (end‐systole) ±SD, mm | 0.93±0.32 | 0.98±0.32 | 0.82±0.32 | 0.07 |
| MLD (end‐diastole) ±SD, mm | 1.81±0.40* | 1.95±0.37* | 1.54±0.32* | <0.001 |
| DS (end‐systole) ±SD, % | 64±10 | 62±9 | 66±13 | 0.118 |
| DS (end‐diastole) ±SD, % | 31±9* | 27±7* | 38±7* | <0.001 |
Data are expressed as mean±SD or as number (%). BMI indicates body‐mass index; DS, diameter stenosis; LAD, left anterior descending artery; LVEF, left ventricle ejection fraction; MB, myocardial bridging; MLD, minimal luminal diameter; RD, reference diameter; SE−, group of patients with MB without stress‐induced ischemia; and SE+, group of patients with MB with stress‐induced ischemia.
P<0.05 vs end‐systole.
At the end‐systole, percent DS was significantly higher, and MLD significantly lower in comparison to correspondent values at end‐diastole (64±10% versus 31±9%, P<0.001; 0.93±0.32 versus 1.81±0.40 mm, P<0.001, respectively). At end‐diastole, percent DS was significantly higher, and MLD significantly lower in the SE‐positive in comparison to the SE‐negative group (38±7% versus 27±7%, P<0.001; 1.54±0.32 versus 1.95±0.37 mm, P<0.001, respectively), but not at end‐systole (66±13% versus 62±9%, P=0.118; 0.82±0.32 versus 0.98±0.32 mm, P=0.07, respectively).
Feasibility and Safety of Invasive Functional Testing During Adenosine and Dobutamine Infusion
Invasive functional testing with adenosine provocation was not performed in 4 patients (performance 56/60=93%) because of chronic obstructive pulmonary disease, whereas all patients (60/60=100%) underwent dobutamine testing, and HR ≥85% of the maximum age‐predicted HR was achieved in 92% (55/60) of them. In 5 patients (8%), HR ≥85% of the maximum age‐predicted HR was not achieved because of the occurrence of ischemic ECG changes and chest pain, requiring early discontinuation of the test. However, HR during peak dobutamine dose was increased ≥50 bpm from baseline‐HR in all patients, including those with ischemic ECG changes during dobutamine provocation (mean delta‐HR: 66±12 bpm, range, 50–92 bpm).
No major complications occurred. One patient presented with a transient complete atrioventricular block during adenosine infusion, and 2 patients presented with ventricular bigeminy during dobutamine infusion. Full patient recovery was achieved after discontinuation of dobutamine and concomitant intravenous administration of metoprolol (2.5–5.0 mg).
Comparative Analysis of Coronary Physiological Parameters During Adenosine and Dobutamine Infusion
All systemic and coronary parameters are presented in Table 2. Regarding dobutamine infusion, smaller changes of coronary hemodynamic changes were observed with dobutamine dose >30 mg/kg per minute, despite further significant increase in HR and rate–pressure product. However, d‐FFR during peak dobutamine dose was significantly lower in comparison to d‐FFR during adenosine (0.76±0.07 versus 0.79±0.08, P=0.018), but not for conventional‐FFR (0.84±0.06 versus 0.84±0.04, P=0.852) (Figure S4).
Table 2.
Comparative Effects of Adenosine and Dobutamine Infusion on Systemic and Coronary Hemodynamic Parameters in the Study Population (n=60)
| BL1 | ADO | BL2 | DOB 10 | DOB 20 | DOB 30 | DOB 40/50/ATR | DOBmax | |
|---|---|---|---|---|---|---|---|---|
| HR, bpm | 73±12 | 90±13* | 73±12 | 83±13* | 103±16* , † | 124±15* , † | 140±8* , † | 139±8* , ‡ |
| Systolic‐BP, mm Hg | 126±11 | 115±14* | 126±11 | 127±12 | 130±14* , † | 133±15* , † | 140±15* , † | 136±16* , ‡ |
| Diastolic‐BP, mm Hg | 69±7 | 69±8 | 69±7 | 69±7 | 70±8 | 70±8 | 70±8 | 70±8 |
| RPP×103, bpm∙mm Hg | 9.2±1.7 | 10.3±1.9* | 9.2±1.7 | 10.5±1.8* | 13.4±2.6* , † | 16.4±3.4* , † | 19.6±2.4* , † | 18.9±2.7* , ‡ |
| Pa, mm Hg | 88±7 | 84±8* | 88±7 | 89±7 | 90±7* , † | 91±8* , † | 92±8* | 92±8* , ‡ |
| Pd, mm Hg | 81±7 | 70±8* | 81±7 | 79±7* | 77±8* , † | 77±8* | 77±8* | 77±8* , ‡ |
| Overall‐ΔP, mm Hg | 7±3 | 14±4* | 7±3 | 10±3* | 13±4* , † | 14±5* , † | 15±4* , † | 15±5* |
| Conventional‐FFR | 0.92±0.03 | 0.84±0.04* | 0.92±0.03 | 0.89±0.03* | 0.87±0.05* , † | 0.85±0.06* , † | 0.84±0.04* , † | 0.84±0.06* |
| s‐Pa, mm Hg | 103±10 | 93±11* | 103±10 | 103±10 | 104±10 | 105±10* | 107±9* | 106±11* , ‡ |
| s‐Pd, mm Hg | 96±10 | 83±11* | 96±10 | 96±10 | 97±11 | 98±12* | 99±11* | 99±11* , ‡ |
| Systolic‐ΔP, mm Hg | 7±3 | 10±5* | 7±3 | 7±4 | 7±7 | 7±7 | 8±5 | 7±7‡ |
| d‐Pa, mm Hg | 84±11 | 72±12* | 84±11 | 83±12 | 79±14* , † | 76±13* , † | 75±13* , † | 75±13* |
| d‐Pd, mm Hg | 75±11 | 57±12* | 75±11 | 71±12* | 64±15* , † | 59±14* , † | 58±13* , † | 57±13* |
| Diastolic‐ΔP, mm Hg | 9±3 | 15±6* | 9±3 | 12±4* | 15±6* , † | 17±6* , † | 17±4* | 18±6* , ‡ |
| Diastolic‐FFR | 0.89±0.04 | 0.79±0.08* | 0.89±0.04 | 0.86±0.05* | 0.81±0.08* , † | 0.78±0.08* , † | 0.77±0.05* , † | 0.76±0.08* , ‡ |
Data are expressed as mean±SD. ADO indicates adenosine (140 µg/kg/min); BL1, basal conditions before adenosine infusion; BL2, basal conditions before dobutamine infusion; BP, blood pressure; bpm, beats per minute; diastolic‐ΔP, mean pressure gradient across the MB during diastole; DOB 10, 10 µg/kg/min of DOB; d‐Pa, mean aortic blood pressure obtained during diastole; d‐Pd, mean distal intracoronary pressure obtained during diastole; DOB, dobutamine; DOB 20, 20 µg/kg/min of DOB; DOB 30, 30 µg/kg/min of DOB; DOB 40/50/ATR, 40 or 50 µg/kg/min of DOB, or atropine; DOBmax, peak DOB dose; FFR, fractional flow reserve; HR, heart rate; MB, myocardial bridging; overall‐ΔP, mean overall pressure gradient across the MB; Pa, mean aortic blood pressure obtained during the whole cardiac cycle; Pd, mean distal intracoronary pressure obtained during the whole cardiac cycle; RPP, rate–pressure product; s‐Pa, mean aortic blood pressure obtained during systole; s‐Pd, mean distal intracoronary pressure obtained during systole; and systolic‐ΔP, mean pressure gradient across the MB during systole.
P<0.05 vs BL.
P<0.05 vs preceding value.
P<0.05 vs ADO.
There was a significant correlation between conventional‐FFRs, as well as between diastolic‐FFRs obtained during adenosine and peak dobutamine infusion (r=0.370, P=0.005;r=0.545, P<0.001, respectively) (Figure 2A and 2B). However, Bland–Altman scatterplots demonstrate better agreement between conventional‐FFR measured by either adenosine or dobutamine, in comparison to d‐FFR values (Figure 2C and 2D). Using paired samples t test, a significant difference was observed between d‐FFR values using both tests (0.0240±0.0727, P=0.018), but not between conventional‐FFR values (−0.0021±0.0575, P=0.781), meaning on a clinical level that if conventional‐FFR is used, it is irrelevant which provocation test is used. On the contrary, in case d‐FFR has been evaluated, it becomes important which diagnostic test is utilized for the functional assessment of MB.
Figure 2. Comparative analysis of invasive physiological indices during adenosine and dobutamine infusions.
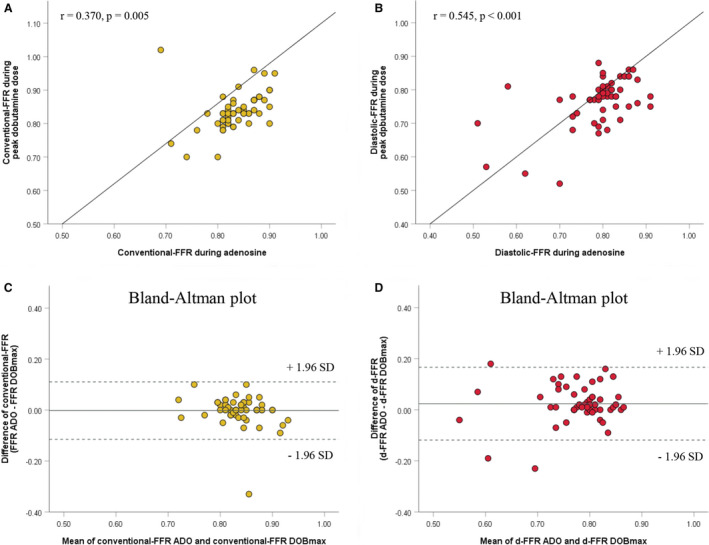
Correlations between conventionalfractional flow reserves (FFRs) (A) and diastolic‐FFRs (B) obtained by adenosine (ADO) and peak dobutamine infusion (DOBmax). The Bland‐Altman scatterplots demonstrate good agreement between conventional‐FFR obtained by ADO and DOBmax against their mean (C), but not between diastolic‐FFR using both methods (D). Dotted lines represent boundaries of mean±1.96 SD. d‐FFR indicates diastolic fractional flow reserve.
In addition, only d‐FFR at peak dobutamine dose had a significant correlation with percent DS and MLD at end‐diastole (r=−0.370, P=0.004; r=0.362, P=0.005, respectively), but not at end‐systole (r=−0.087, P=0.513; r=0.163, P=0.218, respectively).
Comparison of Conventional‐FFR and Diastolic‐FFR During Adenosine and Dobutamine Infusion in Relation to Stress‐Induced Myocardial Ischemia
Conventional‐FFR during adenosine and peak dobutamine infusion did not differ significantly in relation to SE results (FFR‐adenosine: 0.84±0.04 versus 0.83±0.06, P=0.396; FFR‐maximal [peak] dobutamine dose: 0.84±0.04 versus 0.83±0.08, P=0.584, respectively) (Figure 3A). Diastolic‐FFR during peak dobutamine was significantly lower in SE‐positive compared with the SE‐negative group (0.70±0.05 versus 0.79±0.06, P<0.001), but not during adenosine (0.78±0.09 versus 0.79±0.07, P=0.613) (Figure 3B). In both groups of patients, both conventional‐FFR and d‐FFR significantly and gradually decreased during dobutamine infusion, but in patients with stress‐induced myocardial ischemia, obvious and significant decrease in d‐FFR is observed with higher dobutamine dose exceeding 20 µg/kg per minute (Figure 3C and 3D). Coronary physiological parameters during both adenosine and dobutamine infusions in patients with and without myocardial ischemia are presented in Table S2.
Figure 3. Conventional‐FFR and diastolic‐FFR changes during both adenosine and dobutamine provocation in relations to stress‐echocardiography (SE) results.
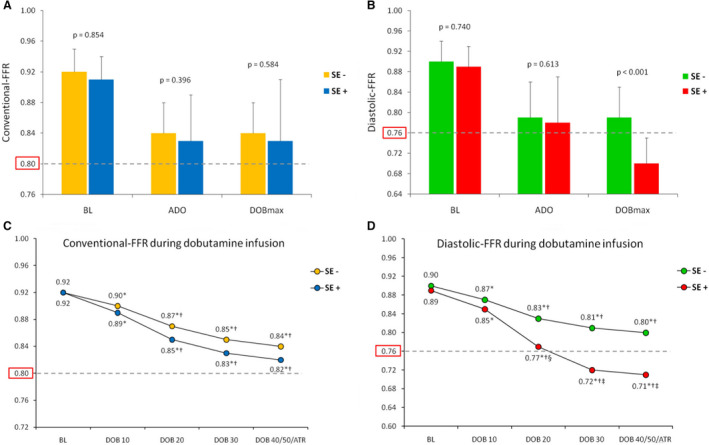
A, Conventional‐FFR, and (B) diastolic‐FFR during both adenosine (ADO) and peak dobutamine infusion (DOBmax) in relation to SE results. C, Conventional‐FFR and (D) diastolic‐FFR during dobutamine infusion (10–50 µg/kg/min) in relation to SE results. Dotted lines in all figures represent the ischemic tresholds for conventional‐FFR (0.80) and diastolic‐FFR (0.76). BL indicates basal conditions, before dobutamine infusion; DOB, dobutamine; DOB 10, 10 µg/kg/min of DOB; DOB 20, 20 µg/kg/min of DOB; DOB 30, 30 µg/kg/min of DOB; DOB 40/50/ATR, 40 or 50 µg/kg/min of DOB, or atropine; FFR, fractional flow reserve; SE−, group of patients without stress‐induced ischemia; and SE+, group of patients with stress‐induced ischemia. *P<0.05 vs BL; † P<0.05 vs preceding value; ‡ P<0.05 vs SE− group.
Specific Hemodynamic Patterns in Patients With MB
Two local hemodynamic features specific to MB, so‐called “overshooting” and “sucking” effects, occurred in a number of patients during dobutamine provocation (Figure 4). The “overshooting” effect existed when systolic‐ΔP reached negative value, meaning that the average intracoronary pressure distal to the MB overshooted the average aortic blood pressure during systole.4, 12 This effect appeared in 9 patients during dobutamine (mean: −9±6 mm Hg, range, from −1 to −16 mm Hg), but not during adenosine infusion. Seventeen more patients had a lower systolic‐ΔP at peak dobutamine dose in comparison to its baseline value (5±2 versus 8±2 mm Hg, P=0.001), but without “overshooting” effect.
Figure 4. Example of MB invasive functional assessment with schematic graphics.
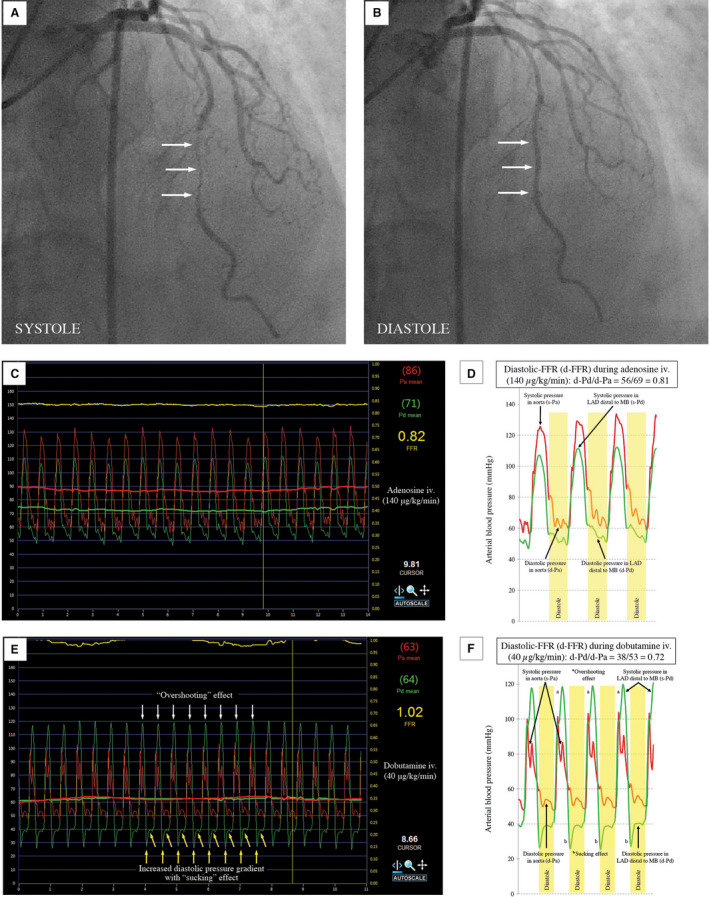
Coronary angiography revealed MB in the medial segment of left anterior descending (LAD) artery with systolic compression (A) and diastolic decompression (B) of intramyocardial segment (“milking effect”). Conventional‐FFR and diastolic‐FFR (d‐FFR) measurements during adenosine (C and D), and peak dobutamine infusion (E and F). d‐Pa indicates aortic blood pressure waveform during diastole; d‐Pd, distal intracoronary pressure waveform during diastole; FFR, fractional flow reserve; MB, myocardial bridging; Pa (red line), aortic blood pressure waveform during the whole cardiac cycle; and Pd (green line), distal intracoronary pressure waveform during the whole cardiac cycle.
Conventional‐FFR at peak dobutamine dose was significantly higher in the group of patients with “overshooting” effect in comparison to the group of patients without this effect (0.91±0.07 versus 0.82±0.05, P=0.006), but not for d‐FFR (0.76±0.08 versus 0.77±0.06, P=0.722) (Table S3). Figure 5 shows that decrease and negativization of the systolic‐ΔP across the MB during dobutamine infusion leads to a significant increase in conventional‐FFR values, which may be even >1.0 (FFR‐paradox).
Figure 5. Influence of systolic pressure gradient (A, C, and E), and diastolic pressure gradient (B, D, and F) across the myocardial bridging (MB) on the discrepancy between conventional‐fractional flow reserve (FFR) and diastolic‐FFR (d‐FFR) measurements after adenosine and peak dobutamine infusion, expressed as the conventional‐FFR/d‐FFR ratio.
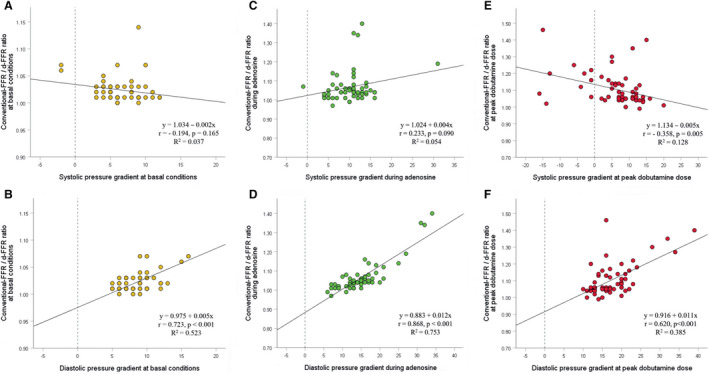
Diastolic pressure gradient had a significant direct influence on the discrepancy in all conditions, meaning that its increase leads to a significant decrease in d‐FFR values. Systolic pressure gradient had a significant inverse influence on the discrepancy only at peak dobutamine dose, meaning that its decrease or negativization during dobutamine provocation leads to a significant increase in conventional‐FFR values, reaching maximal in patients with negative systolic pressure gradient across the MB.
The “sucking” effect, defined as an abrupt pressure drop of Pd at the beginning of diastole,23 appeared in 48 patients (80%) during peak dobutamine, and in only 9 patients (16%) during adenosine infusion (Figure 4). There were no differences between conventional‐FFR and d‐FFR during both tests in relation to the presence of “sucking” effect (Table S3).
Moreover, both “overshooting” and “sucking” effects after peak dobutamine infusion had no influence on the occurrence of stress‐induced myocardial ischemia (Table S4).
Diagnostic Value of All Invasive Physiological Indices With Stress‐Induced Myocardial Ischemia as the Reference Standard
Two separate multivariate logistic regression analyses (backward method) were performed for all significant univariate predictors: one with invasive physiological indices obtained during adenosine, and the other with invasive indices obtained during peak dobutamine infusion (Model 1, Table 3). In this manner, percent DS MB at end‐diastole and d‐FFR during dobutamine provocation were the only independent predictors of stress‐induced myocardial ischemia in patients with MB. The same result was obtained when all significant predictors were placed together in multivariate regression analysis (Model 2, Table 3). The value of MLD MB at end‐diastole was not taken into account in multivariate analyses because of high correlation and multicollinearity with the value of percent DS MB at end diastole (r=−0.728, P<0.001). The same principle was applied in Model 2 to both d‐FFR with adenosine and conventional‐FFR with dobutamine because of high correlation and multicollinearity with the value of both conventional‐FFR with adenosine and d‐FFR with dobutamine (conventional‐FFR adenosine versus d‐FFR adenosine: r=0.855, P<0.001; conventional‐FFR maximal [peak] dobutamine dose versus d‐FFR maximal [peak] dobutamine dose: r=0.665, P<0.001, respectively).
Table 3.
Multivariate Logistic Regression Analyses for All Significant Univariate Variables (P≤0.05) Predicting Stress‐Induced Myocardial Ischemia in Patients With MB
| OR (95% CI for OR) | P Value | R 2 | HL Test P Value | |
|---|---|---|---|---|
| Univariate analysis | ||||
| MLD MB (end‐systole) | 0.207 (0.036–1.192) | 0.078 | 0.075 | 0.531 |
| MLD MB (end‐diastole) | 0.029 ( 0.003–0.244) | 0.001 | 0.330 | 0.763 |
| %DS MB (end‐systole) | 1.035 (0.964–1.090) | 0.191 | 0.040 | 0.581 |
| %DS MB (end‐diastole) | 1.222 (1.096–1.363) | <0.001 | 0.446 | 0.270 |
| Conventional‐FFR ADO | 0.004 (0.000001–1328.866) | 0.392 | 0.018 | 0.867 |
| Conventional‐FFR DOBmax | 0.033 (0.000002–594.865) | 0.495 | 0.011 | 0.169 |
| Diastolic‐FFR ADO | 0.127 (0.0001–138.290) | 0.563 | 0.008 | 0.708 |
| Diastolic‐FFR DOBmax* | 0.803 (0.701–0.919) | 0.002 | 0.393 | 0.200 |
| Model 1. Backward method with %DS MB (end‐diastole) and: | ||||
| Conventional‐FFR ADO† | … | … | … | … |
| %DS MB (end‐diastole) | 1.227 (1.095–1.376) | <0.001 | 0.468 | 0.795 |
| Conventional‐FFR DOBmax† | … | … | … | … |
| %DS MB (end‐diastole) | 1.222 (1.096–1.363) | <0.001 | 0.446 | 0.270 |
| Diastolic‐FFR ADO† | … | … | … | … |
| %DS MB (end‐diastole) | 1.226 (1.093–1.374) | <0.001 | 0.461 | 0.656 |
| Diastolic‐FFR DOBmax* , † | 0.851 (0.750–0.967) | 0.013 | 0.586 | 0.436 |
| %DS MB (end‐diastole) | 1.201 (1.062–1.358) | 0.004 | 0.586 | 0.436 |
| Model 2. Backward method with %DS MB (end‐diastole), conventional‐FFR ADO, and diastolic‐FFR DOBmax | ||||
| Diastolic‐FFR DOBmax* | 0.870 (0.767–0.986) | 0.030 | 0.567 | 0.891 |
| %DS MB (end‐diastole) | 1.208 (1.065–1.370) | 0.003 | 0.567 | 0.891 |
Dependent variable: stress‐induced wall‐motion abnormalities in the left anterior descending coronary artery territory. Multivariate logistic regression analyses were adjusted for all variables with P≤0.05 in univariate analysis. ADO indicates adenosine; DOBmax, peak dobutamine dose; DS, diameter stenosis; FFR, fractional flow reserve; HL, Hosmer and Lemeshow test; MB, myocardial bridging; MLD, minimal luminal diameter; OR, odds ratio; and R 2, Nagelkerke R square.
Because of small values of diastolic‐FFR DOBmax, in model, this variable is multiplied by 100 to obtain OR with 95% CI that can be evaluated.
Only variable in the model.
Receiver operating characteristics curves analyses identified d‐FFR obtained during high‐dose dobutamine infusion as the best diagnostic test for discrimination of patients with MB with and without stress‐induced myocardial ischemia (Figure 6). The optimal d‐FFR cut‐off value at peak dobutamine dose for detection of patients with MB with stress‐induced ischemia was ≤0.76, with a sensitivity and specificity of 95%, positive predictive value of 90%, and negative predictive value of 98% (area under curve 0.927; 95% CI, 0.833–1.000; P<0.001). The overall diagnostic value of the test was 95%. The classification agreement between dichotomized values of 2 categorical variables (d‐FFR maximal [peak] dobutamine dose: 0=>0.76; 1=≤0.76; SE results: 0=without stress‐induced ischemia, 1=with stress‐induced ischemia) was high (κ value=0.886, P<0.001).
Figure 6. ROC analysis for assessing the accuracy of diagnostic tests for detection of stress‐induced myocardial ischemia in patients with MB.

Dash line represents a random chance line, while solid colored line represents a test. (A) Conventional‐FFR during adenosine provocation (FFR ADO); (B) Conventional‐FFR at peak dobutamine provocation (FFR DOBmax); (C) Diastolic‐FFR during adenosine provocation (d‐FFR ADO); (D) Diastolic‐FFR at peak dobutamine provocation (d‐FFR DOBmax). ADO indicates adenosine; AUC, area under curve; d‐FFR, diastolic fractional flow reserve; DOBmax, peak dobutamine infusion; FFR, fractional flow reserve; MB, myocardial bridging; ROC, receiver‐operating characteristics curve; SE, standard error; Sn, sensitivity; and Sp, specificity.
Discussion
The main finding of this study is that d‐FFR during inotropic stimulation with high doses of dobutamine, in comparison to vasodilation with adenosine, provides a more reliable assessment of functional significance of MB correspondent to stress‐induced myocardial ischemia. The optimal cut‐off of ≤0.76 for d‐FFR at peak dobutamine dose has the best sensitivity, specificity, and positive and negative predictive value for discriminating the MB in relation to exercise stress‐induced myocardial ischemia. In addition, our study confirmed previous findings by Escaned et al that MB predominantly affects diastolic hemodynamics and the value of d‐FFR over conventional‐FFR, because of development of significant diastolic‐ΔP across the MB, even after low‐dose dobutamine provocation.12 They also emphasized the role of a specific coronary hemodynamic pattern in patients with MB, including both “overshooting” and “sucking” effects, on the interpretation of conventional‐FFR and d‐FFR tracings and values (ie, the study indicated that the decrease or negativization of the systolic‐ΔP across the MB during dobutamine provocation was a major determinant of the discrepancies between conventional‐FFR and d‐FFR).12 These discrepancies could be explained by the fact that, during inotropic stimulation, MB produces an increase of diastolic‐ΔP but artificially negative systolic‐ΔP secondary to distal intracoronary pressure overshooting.12, 15 The latter phenomenon may lead to an artificial increase in conventional‐FFR values (as shown in Figure 4), which is the ratio between average Pd and Pa. This is the main reason for the inability of conventional‐FFR to identify patients with functionally significant MB according to ischemic threshold ≤0.80. Furthermore, by utilizing higher doses of both dobutamine and adenosine, our study demonstrated in a large number of patients that both “overshooting” and “sucking” effects had no effects on the occurrence of stress‐induced myocardial ischemia, but only pressure gradient during the diastolic phase of cardiac cycle as translated into the value of d‐FFR. Therefore, d‐FFR has an advantage over conventional‐FFR because of the exclusion of the systolic phase of the cardiac cycle and the elimination of the systolic gradient influence on the overall pressure measurements.
Interestingly, the same cut‐off point of 0.76 for d‐FFR has been suggested earlier by Abe et al for the functional evaluation of fixed coronary stenosis.24 In that study, d‐FFR during adenosine with ischemic threshold of ≤0.76 had a higher sensitivity and the same specificity for detecting fixed stenoses with inducible ischemia in comparison to conventional‐FFR (96% versus 83%; 100% versus 100%, respectively). This implies that myocardial ischemia is a unique phenomenon, but with different pathophysiological mechanisms in different pathoanatomical coronary entities (fixed stenosis versus MB). Higher diagnostic significance of d‐FFR during both tests is because coronary blood flow occurs mainly during the diastolic phase of the cardiac cycle (80%–85%), and is mainly dependent on diastolic driving pressure.
However, d‐FFR calculation is complex, time‐consuming, and not routinely performed in most cardiac laboratories, and these disadvantages might be overcome by another diastole‐specific index—instantaneous wave‐free ratio (iFR), which has also been shown to be superior to conventional‐FFR in MB hemodynamic assessment, according to a study by Tarantini et al.15 This study also showed that iFR obtained during dobutamine provocation had even higher consistency with angina and/or positive noninvasive tests in comparison to iFR at rest.15 These findings might be explained by the fact that the iFR, similar to d‐FFR, is not hampered by decrease or negativization of the systolic‐ΔP across the MB, which appears during dobutamine infusion.15 Still, ischemic cut‐off values of iFR at rest and during dobutamine provocation in patients with MB are yet to be defined. Our study clearly demonstrated that a cut‐off value ≤0.76 for d‐FFR during dobutamine provocation has the highest diagnostic accuracy for identifying patients with functionally significant MB, which may play an important role not only as a guide to therapeutic approach, but also as a useful index in further studies that are needed for the validation of other diastole‐specific indices against d‐FFR for MB assessment.
Our study also demonstrated that both stress‐induced ischemia and d‐FFR during dobutamine provocation correlate well with both percent DS and MLD at end‐diastole, but not at end‐systole, suggesting that MB decompression is probably slow and incomplete.1, 2, 3, 4, 5, 6 These findings support the hypothesis that the underlying mechanism of stress‐induced ischemia in patients with MB is probably a delay in early diastolic artery relaxation with persistent reduction of vessel luminal diameter in diastole, which may worsen during inotropic provocation with dobutamine or exercise stress because of tachycardia and shortening of diastolic perfusion time.1, 2, 3, 4, 5, 6, 7, 25 At the beginning of diastole, when the MB compression still persists, ventricular relaxation with decompression of coronary microcirculation occurs, resulting in a significant decrease and negativization of instantaneous diastolic‐Pd with the creation of a “sucking” effect, which was more prominent during invasive evaluation of MB with dobutamine provocation in our study.7, 12, 23 Accordingly, the occurrence of highest instantaneous pressure gradient across the MB leads to rapid coronary flow acceleration (early diastolic hyperemic flow) and, therefore, early diastolic hyperemic myocardial perfusion (“finger‐tip” phenomenon).1, 2, 3, 4, 5, 7, 25, 26 This hypothesis is supported by the study of Escaned et al, which demonstrated that the instantaneous pressure gradient across the MB resulting from the difference between Pa and Pd during dobutamine provocation mimics this characteristic diastolic coronary flow velocity pattern.1, 2, 3, 4, 5, 7, 12, 25 A prolongation of severe MB compression beyond systole into diastole because of severe impairment of MB decompression, which is more prominent during inotropic provocation with dobutamine or exercise stress, may impair early diastolic hyperemic flow, and thereby myocardial perfusion, especially at the subendocardial level with consequent ischemia.7, 26
Therefore, the use of beta‐blockers is generally considered first‐line therapy for patients with MB with stress‐induced ischemia, because of their negative chronotropic and inotropic effects, with consequently: (1) decrease in contractility and systolic and early diastolic compression of both intramyocardial arterial segment and coronary microcirculation, and (2) prolongation of diastolic perfusion time, which leads to normalization of early diastolic hyperemic flow and myocardial perfusion, especially at the subendocardial level.2, 7, 8, 9, 10, 26 Nondihydropyridine calcium‐channel blockers can also be used as an alternative to beta‐blockers in those patients with MB who cannot tolerate beta‐blockers because of active bronchospasm or other contraindications.2, 7, 8, 9, 10, 26 In patients with MB with stress‐induced ischemia not responding to medical therapy, surgical treatment options, such as surgical unroofing or supra‐arterial myotomy, are favorable over MB stenting, because the latter is associated with worse outcomes during short‐ and long‐term follow‐up, including coronary perforation, in‐stent restenosis, stent fracture, and stent thrombosis.27, 28, 29 However, large and prospective randomized clinical trials assessing the short‐ and long‐term effect of antianginal drugs versus surgical treatment options or percutaneous coronary intervention in patients with MB with stress‐induced ischemia are lacking.
Our study also confirms previous findings by Bartunek et al that high doses of dobutamine infusion (>20 µg/kg per minute) have the same effect as adenosine on the microcirculation, suggesting that dobutamine‐induced hyperemia is similar to adenosine‐induced hyperemia, regardless of the presence of ischemia.22 However, 2 studies using transthoracic Doppler echocardiography in patients with fixed stenosis and MB, respectively, showed that maximal coronary flow reserve was similar during adenosine‐ and dobutamine‐induced hyperemia only in those with wall motion abnormalities detected on exercise stress echocardiography or dobutamine‐SE.1, 30 Conversely, these studies noticed that although coronary flow reserve can adequately compensate further marked increase in myocardial oxygen demand at higher dose of dobutamine only in coronary arteries without significant lesion, it was significantly lower after peak dobutamine dose compared with adenosine. These findings are consistent with our results where d‐FFR is associated with myocardial ischemia only when higher doses of dobutamine are used (>20 µg/kg per minute).
Study Limitations
All patients were divided into binary positive or negative groups according to exercise SE‐results. Exercise‐SE as a physiological test has a well‐defined and proven diagnostic role in detection of myocardial ischemia. However, because of the semiquantitative nature of assessment and small areas of wall‐motion abnormalities, certain patients might be misdiagnosed in regard to development of myocardial ischemia.17
It is possible that some of our patients were susceptible to the exercise‐induced vasospasm during exercise‐SE, because the study protocol includes the cessation of all antianginal drugs for 24 to 48 hours before the test. However, the intracoronary administration of nitroglycerin just before invasive physiological measurements could eliminate the vasospasm of the LAD.
The development of d‐FFR is complex and therefore more vulnerable to measurement errors, although all measurements were performed at least 3 times in the study. Additionally, hyperemia induced by adenosine and dobutamine is not necessarily equivalent to exercise‐induced maximal hyperemia, suggesting that some patients with stress‐induced ischemia could have nonischemic d‐FFR values, especially during dobutamine provocation.
Since all antianginal drugs have been discontinued, it remains unclear whether treatment with these medications, especially with beta‐blockers, alters the effects of dobutamine on MB physiology.
Conclusions
Diastolic‐FFR, but not conventional‐FFR, during inotropic stimulation with high‐dose dobutamine, in comparison to vasodilatation with adenosine, provides more reliable functional significance of myocardial bridging in relation to exercise stress–induced myocardial ischemia. Diastolic‐FFR during dobutamine provocation appears to be a useful index of the functional severity of isolated MB, but data on prognostic implications are yet to be determined.
Sources of Funding
This work was supported by Ministry of Education, Sciences and Technological Development of Republic of Serbia (Grant: III41022).
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S4
(J Am Heart Assoc. 2021;10:e020597. DOI: 10.1161/JAHA.120.020597.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020597
For Sources of Funding and Disclosures, see page 14.
References
- 1.Aleksandric S, Djordjevic‐Dikic A, Beleslin B, Parapid B, Teofilovski‐Parapid G, Stepanovic J, Simic D, Nedeljkovic I, Petrovic M, Dobric M, et al. Noninvasive assessment of myocardial bridging by coronary flow velocity reserve with transthoracic Doppler echocardiography: vasodilator vs. inotropic stimulation. Int J Cardiol. 2016;225:37–45. DOI: 10.1016/j.ijcard.2016.09.101. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz ER, Gupta R, Haager PK, vom Dahl J, Klues HG, Minartz J, Uretsky BF. Myocardial bridging in absence of coronary artery disease: proposal of a new classification based on clinical‐angiographic data and long‐term follow‐up. Cardiology. 2009;112:13–21. DOI: 10.1159/000137693. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional characteristics of myocardial bridging: a combined angiographic and intracoronary Doppler flow study. Eur Heart J. 1997;18:434–442. DOI: 10.1093/oxfordjournals.eurheartj.a015263. [DOI] [PubMed] [Google Scholar]
- 4.Klues HG, Schwarz ER, vom Dahl J, Reffelmann T, Hl R, Potthast K, Schmitz C, Minartz J, Krebs W, Hanrath P. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997;96:2905–2913. DOI: 10.1161/01.CIR.96.9.2905. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short‐term intravenous beta‐blocker medication. J Am Coll Cardiol. 1996;27:1637–1645. DOI: 10.1016/0735-1097(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 6.Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Görge G, Haude M, Meyer J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–1732. DOI: 10.1161/01.CIR.89.4.1725. [DOI] [PubMed] [Google Scholar]
- 7.Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left anterior descending artery myocardial bridging: a clinical approach. J Am Coll Cardiol. 2016;68:2887–2899. DOI: 10.1016/j.jacc.2016.09.973. [DOI] [PubMed] [Google Scholar]
- 8.Corban MT, Hung OY, Eshtehardi P, Rasoul‐Arzrumly E, McDaniel M, Mekonnen G, Timmins LH, Lutz J, Guyton RA, Samady H. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63:2346–2355. DOI: 10.1016/j.jacc.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alegria JR, Herrmann J, Holmes DR Jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. 2005;26:1159–1168. DOI: 10.1093/eurheartj/ehi203. [DOI] [PubMed] [Google Scholar]
- 10.Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–359. DOI: 10.1016/S0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 11.Hakeem A, Cilingiroglu M, Leesar MA. Hemodynamic and intravascular ultrasound assessment of myocardial bridging: fractional flow reserve paradox with dobutamine versus adenosine. Catheter Cardiovasc Interv. 2010;75:229–236. DOI: 10.1002/ccd.22237. [DOI] [PubMed] [Google Scholar]
- 12.Escaned J, Cortés J, Flores A, Goicolea J, Alfonso F, Hernández R, Fernández‐Ortiz A, Sabaté M, Bañuelos C, Macaya C. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42:226–233. DOI: 10.1016/S0735-1097(03)00588-6. [DOI] [PubMed] [Google Scholar]
- 13.Lin S, Tremmel JA, Yamada R, Rogers IS, Yong CM, Turcott R, McConnell MV, Dash R, Schnittger I. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097. DOI: 10.1161/JAHA.113.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uusitalo V, Saraste A, Pietilä M, Kajander S, Bax JJ, Knuuti J. The functional effects of intramural course of coronary arteries and its relation to coronary atherosclerosis. JACC Cardiovasc Imaging. 2015;8:697–704. DOI: 10.1016/j.jcmg.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Tarantini G, Barioli A, Fovino LN, Fraccaro C, Masiero G, Iliceto S, Napodano M. Unmasking myocardial bridge‐related ischemia by intracoronary functional evaluation. Circ Cardiovasc Interv. 2018;11:e006247. DOI: 10.1161/CIRCINTERVENTIONS.117.006247. [DOI] [PubMed] [Google Scholar]
- 16.Beleslin BD, Ostojic M, Stepanovic J, Djordjevic‐Dikic A, Stojkovic S, Nedeljkovic M, Stankovic G, Petrasinovic Z, Gojkovic L, Vasiljevic‐Pokrajcic Z. Stress‐echocardiography in the detection of myocardial ischemia. Head‐to‐head comparison of exercise, dobutamine, and dipyridamole tests. Circulation. 1994;90:1168–1176. DOI: 10.1161/01.CIR.90.3.1168. [DOI] [PubMed] [Google Scholar]
- 17.Pellikka PA, Arruda‐Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:1–41.e8. DOI: 10.1016/j.echo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Pijls NHJ, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JJ, Koolen J, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. DOI: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 20.Forte EH, Rousse MG, Lowenstein JA. Target heart rate to determine the normal value of coronary flow reserve during dobutamine stress echocardiography. Cardiovasc Ultrasound. 2011;9:10. DOI: 10.1186/1476-7120-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van't Veer M, Pijls NHJ, Hennigan B, Watkins S, Ali ZA, De Bruyne B, Zimmermann FM, van Nunen LX, Barbato E, Berry C, et al. Comparison of different diastolic resting indexes to iFR: are they all equal? J Am Coll Cardiol. 2017;70:3088–3096. 10.1016/j.jacc.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 22.Bartunek J, Wijns W, Heyndrickx GR, de Bruyne B. Effects of dobutamine on coronary stenosis physiology and morphology: comparison with intracoronary adenosine. Circulation. 1999;100:243–249. DOI: 10.1161/01.CIR.100.3.243. [DOI] [PubMed] [Google Scholar]
- 23.Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J. 1995;73:462–465. DOI: 10.1136/hrt.73.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe M, Tomiyama H, Yoshida H, Doba N. Diastolic fractional flow reserve to assess the functional severity of moderate coronary stenoses. Comparison with fractional flow reserve and coronary flow velocity reserve. Circulation. 2000;102:2365–2370. 10.1161/01.cir.102.19.2365. [DOI] [PubMed] [Google Scholar]
- 25.Ge J, Jeremias A, Rupp A, Abels M, Baumgart D, Liu F, Haude M, Goorge G, von Birgelen C, Sack S, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–1716. DOI: 10.1053/euhj.1999.1661. [DOI] [PubMed] [Google Scholar]
- 26.Gould LK, Johnson NP. Myocardial bridges: lessons in clinical coronary pathophysiology. JACC Cardiovasc Imaging. 2015;8:705–709. 10.1016/j.jcmg.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Murtaza G, Mukherjee D, Gharacholou SM, Nanjundappa A, Lavie CJ, Khan AA, Shanmugasundaramf M, Paul TP. An updated review on myocardial bridging. Cardiovasc Revasc Med. 2020;21:1169–1179. DOI: 10.1016/j.carrev.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Cerrato E, Barbero U, D’Ascenzo F, Taha S, Biondi‐Zoccai G, Omedè P, Bianco M, Echavarria‐Pinto M, Escaned J, Gaita F, et al. What is the optimal treatment for symptomatic patients with isolated coronary myocardial bridge? A systematic review and pooled analysis. J Cardiovasc Med (Hagerstown). 2017;18:758–770. DOI: 10.2459/JCM.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 29.Boyd JH, Pargaonkar VS, Scoville DH, Rogers IS, Kimura T, Tanaka S, Yamada R, Fischbein MP, Tremmel JA, Mitchell RS, et al. Surgical unroofing of hemodynamically significant left anterior descending myocardial bridges. Ann Thorac Surg. 2017;103:1443–1450. DOI: 10.1016/j.athoracsur.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Meimoun P, Sayah S, Tcheuffa JC, Benali T, Luycx‐Bore A, Levy F, Tribouilloy C. Transthoracic coronary flow velocity reserve assessment: comparison between adenosine and dobutamine. J Am Soc Echocardiogr. 2006;19:1220–1228. DOI: 10.1016/j.echo.2006.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S4


