Abstract
Background
Despite its clinical significance, the risk of severe infection requiring hospitalization among outpatients with severe acute respiratory syndrome coronavirus 2 infection who receive angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) remains uncertain.
Methods and Results
In a propensity score–matched outpatient cohort (January–May 2020) of 2263 Medicare Advantage and commercially insured individuals with hypertension and a positive outpatient SARS‐CoV‐2, we determined the association of ACE inhibitors and ARBs with COVID‐19 hospitalization. In a concurrent inpatient cohort of 7933 hospitalized with COVID‐19, we tested their association with in‐hospital mortality. The robustness of the observations was assessed in a contemporary cohort (May–August). In the outpatient study, neither ACE inhibitors (hazard ratio [HR], 0.77; 0.53–1.13, P=0.18) nor ARBs (HR, 0.88; 0.61–1.26, P=0.48) were associated with hospitalization risk. ACE inhibitors were associated with lower hospitalization risk in the older Medicare group (HR, 0.61; 0.41–0.93, P=0.02), but not the younger commercially insured group (HR, 2.14; 0.82–5.60, P=0.12; P‐interaction 0.09). Neither ACE inhibitors nor ARBs were associated with lower hospitalization risk in either population in the validation cohort. In the primary inpatient study cohort, neither ACE inhibitors (HR, 0.97; 0.81–1.16; P=0.74) nor ARBs (HR, 1.15; 0.95–1.38, P=0.15) were associated with in‐hospital mortality. These observations were consistent in the validation cohort.
Conclusions
ACE inhibitors and ARBs were not associated with COVID‐19 hospitalization or mortality. Despite early evidence for a potential association between ACE inhibitors and severe COVID‐19 prevention in older individuals, the inconsistency of this observation in recent data argues against a role for prophylaxis.
Keywords: angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, COVID‐19, hypertension
Subject Categories: Hypertension, Mortality/Survival, Epidemiology, Quality and Outcomes
Clinical Perspective
What Is New?
In this national cohort study of Medicare Advantage and commercial insurance enrollees with hypertension and SARS‐CoV‐2 infection, the use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, compared with the use of other antihypertensive agents, was not associated with an increased risk of hospitalization among outpatients and in‐hospital mortality among inpatients.
While early estimates in the pandemic found 40% lower risk of hospitalizations in an older Medicare population testing positive with SARS‐CoV‐2 as an outpatient, this effect could not be replicated in more recent data.
What Are the Clinical Implications?
Our study findings do not support a change to the current use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers among patients with hypertension being managed in the outpatient setting and at risk of infection with SARS‐CoV‐2.
Given the inconsistent association of angiotensin‐converting enzyme inhibitors with lower risk of severe disease in older patients, our study does not support their use as prophylaxis against SARS‐CoV‐2.
Hypertension is a risk factor for severe infection with COVID‐19.1 During the early months of the spread of COVID‐19, there was controversy regarding the use of 2 first‐line antihypertensive agents—angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)—and whether their use exacerbated or mitigated the infection.2, 3, 4, 5 There have since been a series of studies that evaluated the association of these drugs with outcomes of patients hospitalized with COVID‐19 and those studies did not find an increase in the risk of in‐hospital mortality.5, 6, 7, 8, 9 Other reports have also not found an association with the risk of infection with SARS‐CoV‐2.7, 10, 11 In this present study, we describe the results of a national study that evaluated the association of ACE inhibitors and ARBs among patients with hypertension, using an active comparator design using high‐quality national data, which were validated with updated data during the course of the pandemic.
The studies that have evaluated the association of ACE inhibitors and ARBs have been examined by systematic reviews that report vast variations in quality, with more than half not accounting for confounding through gaps in risk‐adjustment, thus limiting their ability to make an inference on an association.7, 11 There are also frequent limitations in the design of studies because they lack an active comparator,12, 13 which is essential to account for the confounding effect of receiving a treatment of any kind on outcomes. Some of the studies evaluating the association of COVID‐19 have also been limited by data sourced from a limited number of health facilities, from questionnaires rather than prescription data,14 or drawn from the proportional use of these drugs among hospitalized individuals relative to the general population.12 Specifically, most studies lack the ability to track a large number of individuals regardless of where they seek care,10 which is particularly important in assessing hospitalization risk following SARS‐CoV‐2 infection. Furthermore, the studies, thus far, have also been single‐time investigations, and have not assessed the consistency of the association as more data emerged. Of note, a recently published randomized trial did not find a deleterious effect of continuing ACE inhibitors/ARB during a COVID‐19 hospitalization,15 but included only 152 patients and does not provide information about risk of severe disease requiring hospitalization.
Our national study assessed the association of ACE inhibitors and ARBs with outcomes in individuals who had hypertension and who tested positive for SARS‐CoV‐2 in the outpatient setting. We specifically evaluated the association among those with hypertension who were receiving another antihypertensive agent, ensuring that we had an active comparator. Also, to provide information about the association in inpatients, we conducted a study of the association of ACE inhibitors and ARBs on mortality among individuals who had hypertension and who were hospitalized with COVID‐19. We also validated the findings in a period following the initial evaluation.
METHODS
Data Sources
We used de‐identified administrative claims for Medicare Advantage and commercially insured enrollees in a research database from a single large health insurance provider in the United States. The database contains medical (emergency, inpatient, and outpatient) and pharmacy claims for services submitted for third‐party reimbursement, available as International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) and National Drug Codes claims, respectively.
We used 2 additional data sources. First, a limited outpatient data set included results for enrollees undergoing outpatient testing for SARS‐CoV‐2 at 49 hospital‐based, freestanding outpatient, and third‐party laboratories across the United States. Second, the inpatient COVID‐19 data set included a daily updated record of COVID‐19 inpatient admissions for all insurance enrollees with claims information, representing those admitted to a hospital with a primary or secondary diagnosis of COVID‐19 (Table S1), along with their current disposition (admitted, discharged, transferred, or died).
The data are proprietary and are not available for public use but can be made available to Editors and their approved auditors under a data use agreement to confirm the findings of the current study. The statistical code is available from the first and corresponding authors.
Primary Study Population
We constructed cohorts of enrollees for each of the 2 studies. First, for the outpatient study, we included individuals ≥18 years old with 6 or more months of enrollment in Medicare Advantage or commercial insurance from January through December 2019 and available claims data, a diagnosis of hypertension in claims and receiving 1 or more antihypertensive agents, and a positive test for SARS‐CoV‐2 in an outpatient setting between March 6, 2020 and May 3, 2020 (Figure S1). The Medicare Advantage and commercially insured individuals in the study represented all individuals with available claims in the UnitedHealth Group Clinical Discovery Database who satisfied the inclusion criteria.
Second, for the inpatient study, we identified an inpatient cohort of adults hospitalized with COVID‐19. This included all patients (age ≥18 years) with at least 6 months of health insurance enrollment in 2019 with available claims data, a diagnosis of hypertension in 1 or more claims, who were receiving 1 or more antihypertensive agents, and were hospitalized with a primary or secondary diagnosis of COVID‐19 between January 5, 2020 and May 10, 2020 (Figure S2).
For both studies, a diagnosis of hypertension was based on ICD‐10 codes (Table S1), and drug treatment for hypertension was defined by the receipt of 1 or more agents included in the 2017 American Heart Association hypertension guidelines.16 These include first‐line agents of ACE inhibitors, ARBs, thiazide and thiazide‐like diuretics, and dihydropyridine and nondihydropyridine calcium channel blockers, as well as second‐line agents of β‐adrenergic antagonists, α blockers, centrally acting α agonists, loop diuretics, potassium‐sparing diuretics, mineralocorticoid receptor antagonists, and direct vasodilators (individual drugs listed in Table S2). Fixed‐dose drug combinations were considered equivalent to taking the component drugs as individual drugs (Table S2). This information was defined by a pharmacy claim corresponding to a cumulative supply >30 days between July 1, 2019 and December 31, 2019.
Validation Study Population
We created 2 secondary outpatient and inpatient cohorts to assess the robustness of observations in a more contemporary population when lockdowns for COVID‐19 were progressively relaxed nationally, and people potentially had different patterns of healthcare‐seeking behavior compared with the early pandemic. These were defined in a manner identical to the primary cohorts but were drawn between May 4 and August 2, 2020.
Study Exposures
We identified 2 mutually exclusive exposure groups, including individuals receiving (1) ACE inhibitors, and (2) ARBs, with or without other agents. We used an active comparator for these analyses that included all remaining individuals with a diagnosis of hypertension who received 1 or more antihypertensive agents from drug classes other than ACE inhibitor or ARB. These agents include all first‐ and second‐line antihypertensive agents based on the 2017 American Heart Association guidelines for hypertension.16 In sensitivity analyses, we restricted the control group to individuals receiving at least 1 first‐line antihypertensive agent.
Study Covariates
We combined information from inpatient and outpatient claims in 2019 to identify potential confounders of the association of the ACE inhibitors and ARB use and clinical outcomes. We included age, sex, race, conditions that would represent potential indications for selective use of ACE inhibitors or ARBs (diabetes mellitus, myocardial infarction, heart failure, and chronic kidney disease), and each of the additional comorbidities included in the Charlson Comorbidity Index (Table S1). Race was available only for Medicare Advantage enrollees. We included information on the total number of antihypertensive agents prescribed to enrollees by using pharmacy claims.
Study Outcomes
In the outpatient study, the primary outcome was inpatient hospitalization for COVID‐19, defined as a hospitalization with a principal or secondary diagnosis of COVID‐19 in a linked inpatient data set (Table S1). We assessed mortality during this inpatient hospitalization as a secondary outcome. In the inpatient study, the primary outcome was in‐hospital mortality. In addition, we evaluated a secondary composite outcome of death or discharge to hospice and hospital length of stay.
Propensity Score Matching
In both outpatient and inpatient studies, we created propensity score–matched cohorts of individuals with hypertension, treated with ACE inhibitors, ARBs, or other antihypertensive medications. For example, we modeled the receipt of ACE inhibitor or another antihypertensive (excluding ARB) to determine each person's likelihood of receiving these agents based on their measured clinical characteristics. We applied this strategy to different pairs of treatment comparisons (ACE inhibitor versus others, ARB versus others, and ACE inhibitor versus ARB). We pursued 100 iterations to find the lowest mean absolute standardized difference among matched variables. We matched our cohorts on age, sex, race, insurance type, conditions that may lead to selective use of ACE inhibitors and ARBs (ie, diabetes mellitus, myocardial infarction, heart failure, and chronic kidney disease), each of the comorbidities in the Charlson Comorbidity Index, and the number of antihypertensive agents present in claims. To account for regional clustering of care practices and response to the COVID‐19 pandemic, we explicitly accounted for census region of laboratory testing site or inpatient facility in our models.
We evaluated the performance of propensity score matching using several strategies. First, we assessed the propensity score distributions in the unmatched and matched cohorts and calculated an equipoise metric to summarize the degree of overlap in characteristics of individuals receiving these drugs.17, 18 A value >0.5 implies 2 drugs are in empirical equipoise, with a higher value indicating a lower likelihood of confounding by indication.18 Next, we evaluated whether our matching algorithm achieved a standardized difference of <10% between matched cohorts, suggestive of adequately matched groups.17, 19 Third, we evaluated the success of our matching algorithm using negative control outcomes that are unlikely to be affected by the treatment assignment.17 These strategies were designed to evaluate the potential for residual confounding after creating propensity score–matched cohorts. Finally, we evaluated our observations for robustness by assessing treatment effects in 100 iterations of the propensity score matching–algorithm. Further details are included in Data S1.
Statistical Analysis
We describe differences between individuals treated with ACE inhibitors and ARBs compared with other antihypertensive agents, and between those treated with ACE inhibitors using χ2 test for categorical variables and t test for continuous variables. Because the duration of follow‐up was expected to vary across individuals in both outpatient and inpatient COVID‐19 cohorts, we evaluated their effects in time‐to‐event analyses with Cox‐Proportional Hazards models in both unadjusted and propensity score–matched cohorts. To reduce bias from residual differences in matched covariates in our evaluation of outcomes, we included the covariates included in our propensity score–matching algorithm as independent variables in these models.20 We repeated these analyses without this additional covariate adjustment.
For the outpatient study, the index date was represented by the day of positive SARS‐CoV‐2 test as an outpatient, the period of the study was measured in days from the positive SARS‐CoV‐2 test, and the outcome of interest was hospitalization. For the outpatient analysis, we used Cox proportional hazards to assess pairwise hazards of hospitalization in propensity‐matched groups of patients receiving ACE inhibitor, ARB, or controls.
For the inpatient study, the index date was represented by the first day of hospitalization with COVID‐19, the period of the study was measured in days from admission, and the outcome of interest was death. Since hospitalization could end with either a person's death or being discharged alive, we created a cause‐specific Cox proportional hazards model, which is a competing risk analysis.21, 22, 23 In this model, patients still hospitalized at the end of the observation period were right censored. Among those who were no longer hospitalized, we assessed the hazards of the 2 competing outcomes: in‐hospital death and alive‐discharge, because these represent mutually exclusive events, wherein one precludes the other. Therefore, in the cause‐specific Cox proportional hazards, the occurrence of death is treated as a right‐censoring event for the outcome of being discharged alive, and an alive‐discharge is treated as a right‐censoring event for the death outcome. The design of our study focusing on comparative effectiveness favored the cause‐specific hazard model as the appropriate analysis for competing‐risk assessment.
Both outpatient analyses for hospitalization risk and inpatient analyses for mortality risk were right censored at the end of the observation period on May 10, 2020 for the primary analysis.
We evaluated quantitative and qualitative interactions between insurance type and treatment groups for the assessment of our outcomes. We also created propensity score–matched cohorts within each of the 2 insurance subgroups.
All analyses were repeated in the validation cohort, which included individuals who tested positive with SARS‐CoV‐2 or were hospitalized with COVID‐19 between May and August 2020.
Analyses were performed using R 3.4.0 (CRAN) and Python 3.8.2. All hypothesis tests were 2‐sided, with a level of significance set at 0.05, except for interaction tests where the level of significance was set at 0.10. Given the exploratory nature of study, statistical tests were not adjusted for multiple testing. The Yale Institutional Review Board and the UnitedHealth Group Office of Human Research Affairs exempted this study from other review, because all activities were limited to retrospective analysis of de‐identified data and accessed in accordance with Health Insurance Portability and Accountability Act regulations.
RESULTS
Characteristics of the Outpatient Cohort
Among 6885 individuals who tested positive for SARS‐CoV‐2 between January and May 2020 and had at least 6 months of enrollment in Medicare Advantage or commercial insurance, and in pharmacy benefits with their insurance, 2263 had a diagnosis of hypertension with the use of at least 1 antihypertensive drug (Figure S1). The primary outpatient study cohort included individuals from 44 states (Figure S3). A total of 1467 (64.8%) were Medicare Advantage enrollees and 796 (35.2%) of the cohort were commercially insured.
The characteristics of the 3 groups of patients receiving ACE inhibitors, ARBs, and other antihypertensive agents are compared in Table 1. Medicare Advantage and commercial insurance enrollees are compared in Table S3 and Figure S4. Medicare Advantage enrollees are older (median age 75 years; interquartile range [IQR], 70.0–82.0) (versus 46 years [IQR, 49.0–61.0] in commercially insured, P<0.001), with a higher prevalence of all comorbid conditions and median Charlson comorbidity score of 2 (IQR, 0–3), compared with 0 (IQR, 0–1) for the commercially insured (P<0.001) (Table S3). Hospitalization rates were also substantially higher in Medicare enrollees, compared with the commercially insured (14.5% versus 9.3%, P<0.001).
Table 1.
Characteristics of the Primary Outpatient Cohort
| Variable | Overall | Antihypertensive Drug Cohorts | |||||
|---|---|---|---|---|---|---|---|
| Cohort | P Value | ||||||
| ACE Inhibitor | ARB | Other | ACE Inhibitor vs Other | ARB vs Other | ACE Inhibitor vs ARB | ||
| Number of patients | 2263 (100.0%) | 722 (100.0%) | 731 (100.0%) | 810 (100.0%) | … | … | … |
| Age, median (IQR) | 69.0 (59.0–78.0) | 68.0 (57.0–76.0) | 69.0 (59.0–76.0) | 71.0 (60.2–80.0) | <0.0001 | 0.00012 | 0.086 |
| Age range | |||||||
| 18–30 y | * | * | * | * | 0.88 | 0.78 | 0.99 |
| 31– 40 y | 63 (2.8%) | 25 (3.5%) | 14 (1.9%) | 24 (3.0%) | 0.68 | 0.25 | 0.096 |
| 41–50 y | 171 (7.6%) | 69 (9.6%) | 56 (7.7%) | 46 (5.7%) | 0.0055 | 0.14 | 0.23 |
| 51–60 y | 408 (18.0%) | 138 (19.1%) | 140 (19.2%) | 130 (16.0%) | 0.13 | 0.13 | 0.96 |
| 61–70 y | 570 (25.2%) | 189 (26.2%) | 203 (27.8%) | 178 (22.0%) | 0.062 | 0.010 | 0.53 |
| 71–80 y | 606 (26.8%) | 185 (25.6%) | 194 (26.5%) | 227 (28.0%) | 0.32 | 0.55 | 0.74 |
| >80 y | 435 (19.2%) | 112 (15.5%) | 121 (16.6%) | 202 (24.9%) | <0.0001 | <0.0001 | 0.64 |
| Female sex | 1189 (52.5%) | 318 (44.0%) | 392 (53.6%) | 479 (59.1%) | <0.0001 | 0.033 | 0.00032 |
| Medicare Advantage | 1467 (64.8%) | 434 (60.1%) | 452 (61.8%) | 581 (71.7%) | <0.0001 | <0.0001 | 0.54 |
| Location | |||||||
| Urban | 1059 (46.8%) | 333 (46.1%) | 336 (46.0%) | 390 (48.1%) | 0.46 | 0.42 | 0.99 |
| Rural | 434 (19.2%) | 130 (18.0%) | 142 (19.4%) | 162 (20.0%) | 0.35 | 0.83 | 0.53 |
| Suburban | 747 (33.0%) | 244 (33.8%) | 248 (33.9%) | 255 (31.5%) | 0.36 | 0.33 | 1.00 |
| Unknown | 23 (1.0%) | * | * | * | 0.0043 | 0.62 | 0.040 |
| Race† | |||||||
| White | 863 (38.1%) | 270 (37.4%) | 250 (34.2%) | 343 (42.3%) | 0.055 | 0.0012 | 0.22 |
| Black | 434 (19.2%) | 113 (15.7%) | 131 (17.9%) | 190 (23.5%) | 0.00017 | 0.0091 | 0.28 |
| Hispanic | 69 (3.0%) | 21 (2.9%) | 25 (3.4%) | 23 (2.8%) | 0.94 | 0.61 | 0.68 |
| Asian | 46 (2.0%) | 15 (2.1%) | 19 (2.6%) | 12 (1.5%) | 0.49 | 0.17 | 0.63 |
| Native American | * | * | * | * | 0.53 | 0.52 | |
| Other | 36 (1.6%) | * | * | * | 0.63 | 0.041 | 0.19 |
| Unknown | 813 (35.9%) | 293 (40.6%) | 288 (39.4%) | 232 (28.6%) | <0.0001 | <0.0001 | 0.68 |
| Geography | |||||||
| Region of test site | |||||||
| Northeast | 847 (37.4%) | 241 (33.4%) | 242 (33.1%) | 364 (44.9%) | <0.0001 | <0.0001 | 0.96 |
| South | 711 (31.4%) | 229 (31.7%) | 275 (37.6%) | 207 (25.6%) | 0.0090 | <0.0001 | 0.021 |
| Midwest | 175 (7.7%) | 64 (8.9%) | 53 (7.3%) | 58 (7.2%) | 0.26 | 0.98 | 0.30 |
| West | 202 (8.9%) | 81 (11.2%) | 64 (8.8%) | 57 (7.0%) | 0.0057 | 0.25 | 0.14 |
| Unknown | 328 (14.5%) | 107 (14.8%) | 97 (13.3%) | 124 (15.3%) | 0.85 | 0.29 | 0.44 |
| State of test site | |||||||
| New York | 230 (10.2%) | 54 (7.5%) | 79 (10.8%) | 97 (12.0%) | 0.0042 | 0.52 | 0.035 |
| New Jersey | 303 (13.4%) | 86 (11.9%) | 93 (12.7%) | 124 (15.3%) | 0.064 | 0.17 | 0.70 |
| Connecticut | 136 (6.0%) | 43 (6.0%) | 36 (4.9%) | 57 (7.0%) | 0.45 | 0.10 | 0.45 |
| Georgia | 183 (8.1%) | 58 (8.0%) | 67 (9.2%) | 58 (7.2%) | 0.58 | 0.18 | 0.50 |
| Florida | 124 (5.5%) | 38 (5.3%) | 58 (7.9%) | 28 (3.5%) | 0.11 | 0.00021 | 0.052 |
| Other | 959 (42.4%) | 336 (46.5%) | 301 (41.2%) | 322 (39.8%) | 0.0086 | 0.61 | 0.045 |
| Unknown | 328 (14.5%) | 107 (14.8%) | 97 (13.3%) | 124 (15.3%) | 0.85 | 0.29 | 0.44 |
| Comorbid conditions | |||||||
| Diabetes mellitus without complications | 911 (40.3%) | 320 (44.3%) | 321 (43.9%) | 270 (33.3%) | <0.0001 | <0.0001 | 0.92 |
| Myocardial infarction | 81 (3.6%) | 16 (2.2%) | 20 (2.7%) | 45 (5.6%) | 0.0013 | 0.0087 | 0.64 |
| Chronic heart failure | 326 (14.4%) | 72 (10.0%) | 99 (13.5%) | 155 (19.1%) | <0.0001 | 0.0039 | 0.042 |
| Chronic pulmonary disease | 410 (18.1%) | 100 (13.9%) | 139 (19.0%) | 171 (21.1%) | 0.00026 | 0.34 | 0.0098 |
| Peptic ulcer disease | 19 (0.8%) | * | * | * | 0.85 | 0.46 | 0.81 |
| AIDS | 22 (1.0%) | * | * | * | 0.85 | 0.23 | 0.22 |
| Rheumatologic disease | 120 (5.3%) | 28 (3.9%) | 40 (5.5%) | 52 (6.4%) | 0.034 | 0.50 | 0.19 |
| Diabetes mellitus, chronic complications | 625 (27.6%) | 225 (31.2%) | 210 (28.7%) | 190 (23.5%) | 0.00087 | 0.022 | 0.34 |
| Metastatic cancer | 20 (0.9%) | * | * | * | 0.41 | 0.40 | 0.77 |
| Hemiplegia or paraplegia | 92 (4.1%) | 29 (4.0%) | 15 (2.1%) | 48 (5.9%) | 0.11 | 0.00021 | 0.04 |
| Liver disease, mild | 106 (4.7%) | 28 (3.9%) | 34 (4.7%) | 44 (5.4%) | 0.19 | 0.56 | 0.55 |
| Solid tumor without metastases | 181 (8.0%) | 41 (5.7%) | 61 (8.3%) | 79 (9.8%) | 0.0041 | 0.38 | 0.059 |
| Liver disease, moderate to severe | * | * | * | * | 0.70 | 0.93 | 0.99 |
| Dementia | 250 (11.0%) | 60 (8.3%) | 43 (5.9%) | 147 (18.1%) | <0.0001 | <0.0001 | 0.089 |
| Peripheral vascular disease | 467 (20.6%) | 122 (16.9%) | 121 (16.6%) | 224 (27.7%) | <0.0001 | <0.0001 | 0.92 |
| Renal failure, moderate to severe | 359 (15.9%) | 100 (13.9%) | 93 (12.7%) | 166 (20.5%) | 0.00078 | <0.0001 | 0.58 |
| Cerebrovascular disease | 289 (12.8%) | 73 (10.1%) | 83 (11.4%) | 133 (16.4%) | 0.00040 | 0.0053 | 0.50 |
| Charlson Score, median (IQR) | 2.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 2.0 (0.0–4.0) | <0.0001 | 0.00014 | 0.29 |
| Drug therapy | |||||||
| Antihypertensives | |||||||
| β‐blockers | 911 (40.3%) | 243 (33.7%) | 265 (36.3%) | 403 (49.8%) | <0.0001 | <0.0001 | 0.33 |
| Non‐dihydropyridine calcium channel blockers | 99 (4.4%) | 19 (2.6%) | 23 (3.1%) | 57 (7.0%) | <0.0001 | 0.00089 | 0.67 |
| Dihydropyridine calcium channel blockers | 813 (35.9%) | 215 (29.8%) | 253 (34.6%) | 345 (42.6%) | <0.0001 | 0.0016 | 0.056 |
| Thiazide or thiazide‐like diuretics | 709 (31.3%) | 236 (32.7%) | 300 (41.0%) | 173 (21.4%) | <0.0001 | <0.0001 | 0.0012 |
| Loop diuretics | 328 (14.5%) | 73 (10.1%) | 84 (11.5%) | 171 (21.1%) | <0.0001 | <0.0001 | 0.45 |
| Centrally acting α agonists | 54 (2.4%) | * | * | * | 0.0062 | 0.49 | 0.056 |
| Potassium‐sparing diuretics | 56 (2.5%) | * | * | * | 0.064 | 0.00046 | 0.40 |
| Mineralocorticoid aldosterone antagonists | 85 (3.8%) | 15 (2.1%) | 28 (3.8%) | 42 (5.2%) | 0.0021 | 0.25 | 0.069 |
| Renin inhibitors | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| α‐adrenergic blocking agents | 40 (1.8%) | * | * | * | 0.18 | 0.91 | 0.22 |
| Direct vasodilators | 99 (4.4%) | 27 (3.7%) | 27 (3.7%) | 45 (5.6%) | 0.12 | 0.11 | 0.93 |
| Place in therapy | |||||||
| First‐line | 1964 (86.8%) | 722 (100.0%) | 731 (100.0%) | 511 (63.1%) | <0.0001 | <0.0001 | |
| Second‐line | 1135 (50.2%) | 290 (40.2%) | 308 (42.1%) | 537 (66.3%) | <0.0001 | <0.0001 | 0.48 |
| Number of antihypertensive classes | |||||||
| 1 | 822 (36.3%) | 206 (28.5%) | 148 (20.2%) | 468 (57.8%) | <0.0001 | <0.0001 | 0.00030 |
| 2 | 780 (34.5%) | 271 (37.5%) | 288 (39.4%) | 221 (27.3%) | <0.0001 | <0.0001 | 0.50 |
| 3+ | 661 (29.2%) | 245 (33.9%) | 295 (40.4%) | 121 (14.9%) | <0.0001 | <0.0001 | 0.013 |
| Number, median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 1.0 (1.0–2.0) | <0.0001 | <0.0001 | 0.00019 |
| Other drug therapies | |||||||
| Statins | 1210 (53.5%) | 418 (57.9%) | 401 (54.9%) | 391 (48.3%) | 0.00020 | 0.011 | 0.26 |
| Other lipid‐lowering agents | 113 (5.0%) | 37 (5.1%) | 42 (5.7%) | 34 (4.2%) | 0.46 | 0.20 | 0.68 |
| Oral anticoagulants | 201 (8.9%) | 48 (6.6%) | 58 (7.9%) | 95 (11.7%) | 0.00089 | 0.016 | 0.40 |
| Insulins | 215 (9.5%) | 77 (10.7%) | 70 (9.6%) | 68 (8.4%) | 0.15 | 0.47 | 0.55 |
| Oral antihyperglycemic agents | 581 (25.7%) | 234 (32.4%) | 217 (29.7%) | 130 (16.0%) | <0.0001 | <0.0001 | 0.29 |
| Follow‐up | |||||||
| Follow‐up days, median (IQR) | 30.0 (19.0–40.0) | 30.0 (19.0–40.0) | 31.0 (21.0–40.0) | 29.0 (19.0–38.0) | 0.35 | 0.011 | 0.13 |
| Test to hospitalization, median (IQR) | 4.0 (2.0–7.0) | 4.0 (2.0–6.0) | 4.0 (2.0–7.0) | 5.0 (3.0–7.0) | 0.40 | 0.51 | 0.83 |
| Total hospitalized | 287 (12.7%) | 77 (10.7%) | 93 (12.7%) | 117 (14.4%) | 0.032 | 0.36 | 0.25 |
The cohort includes individuals who had a positive test for SARS‐CoV‐2 in the outpatient setting. ACE indicates angiotensin‐converting enzyme; ARB indicates angiotensin receptor blocker; and IQR, interquartile range.
Cells with values <11 were suppressed in accordance with the Centers for Medicare and Medicare Services cell size suppression policy for values from 1 to 10.
Race is unknown in all commercially insured enrollees.
The characteristics of patients in the validation cohort were similar to the primary cohort (Table S4), though substantial geographic variation in case distribution occurred, with cases in the secondary study cohort shifting away from the Northeast (10.5% versus 37.4%) and into the South (66.9% versus 31.4%).
In the primary cohort, we matched 441 patients receiving ACE inhibitors to 441 patients receiving other antihypertensive agents (Figure S5), achieving <10% standardized differences for all covariates (Figure S6). Similarly, we matched 412 patients receiving ARB to 412 patients receiving other antihypertensive agents (Figure S5). The equipoise for comparisons of ACE inhibitors to other drugs, and for ACE inhibitors to ARB were >0.5 but were lower for the ARB comparisons. There were 1144 patients receiving ACE inhibitors and 995 patients who were receiving ARB and who were successfully matched to the same number of controls, respectively, in the secondary outpatient cohort.
Characteristics of the Inpatient Cohort
Among 12 566 patients who were hospitalized for COVID‐19 with linked claims data, 7933 had had a diagnosis of hypertension and had an outpatient prescription for at least 1 antihypertensive drug (Figure S2). The primary inpatient cohort included patients from 47 states (Figure S3). Of the included patients, 92.0% were Medicare Advantage enrollees. The median age of hospitalized individuals was 77.0 years, and 54.6% were women; 29.9% of Medicare Advantage enrollees were Black. Groups are compared in Table 2. In the inpatient cohort, 1731 patients receiving ACE inhibitors and 1560 patients receiving ARBs were propensity score–matched to patients receiving other antihypertensive agents (Figure S7), with covariate standardized differences of <10% after matching (Figure S6). The equipoise for comparisons of ACE inhibitors to other drugs, and for ACE inhibitors to ARB were >0.5 but were lower for the ARB comparisons (Table 3). The inpatient validation cohort was similar to the primary cohort across all exposure groups (Table S5) except for geography (15.1% versus 42.0% in the Northeast; 61.9% versus 34.7% in the South).
Table 2.
Characteristics of the Primary Inpatient Cohort
| Variable | Overall | Cohort | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ACE Inhibitor | ARB | Other | ACE Inhibitor vs Other | ARB vs Other | ACE Inhibitor vs ARB | ||||
| Number of patients | 7933 | 2361 | 2226 | 3346 | <0.0001 | <0.0001 | <0.0001 | ||
| Age, median (IQR) | 77.0 (69.0–85.0) | 76.0 (68.0–83.0) | 76.0 (69.0–84.0) | 78.0 (70.0–86.0) | <0.0001 | <0.0001 | 0.12 | ||
| Age range | |||||||||
| 18–30 y | 11 (0.1%) | * | * | * | 0.39 | 0.93 | 0.79 | ||
| 31–40 y | 30 (0.4%) | * | * | * | 0.86 | 0.40 | 0.69 | ||
| 41–50 y | 173 (2.2%) | 60 (2.5%) | 47 (2.1%) | 66 (2.0%) | 0.18 | 0.79 | 0.39 | ||
| 51–60 y | 544 (6.9%) | 172 (7.3%) | 177 (8.0%) | 195 (5.8%) | 0.031 | 0.0022 | 0.43 | ||
| 61–70 y | 1523 (19.2%) | 499 (21.1%) | 415 (18.6%) | 609 (18.2%) | 0.0064 | 0.70 | 0.038 | ||
| 71–80 y | 2627 (33.1%) | 822 (34.8%) | 786 (35.3%) | 1019 (30.5%) | 0.00058 | 0.00017 | 0.75 | ||
| >80 y | 3025 (38.1%) | 794 (33.6%) | 792 (35.6%) | 1439 (43.0%) | <0.0001 | <0.0001 | 0.17 | ||
| Female sex | 4332 (54.6%) | 1171 (49.6%) | 1246 (56.0%) | 1915 (57.2%) | <0.0001 | 0.37 | <0.0001 | ||
| Medicare Advantage | 7296 (92.0%) | 2152 (91.1%) | 1991 (89.4%) | 3153 (94.2%) | <0.0001 | <0.0001 | 0.057 | ||
| Location | |||||||||
| Urban | 3574 (45.1%) | 1047 (44.3%) | 1048 (47.1%) | 1479 (44.2%) | 0.94 | 0.037 | 0.067 | ||
| Rural | 1623 (20.5%) | 488 (20.7%) | 463 (20.8%) | 672 (20.1%) | 0.61 | 0.54 | 0.94 | ||
| Suburban | 2714 (34.2%) | 822 (34.8%) | 708 (31.8%) | 1184 (35.4%) | 0.68 | 0.0062 | 0.033 | ||
| Unknown | 22 (0.3%) | * | * | * | 0.37 | 0.88 | 0.48 | ||
| Race† | |||||||||
| White | 4486 (56.5%) | 1352 (57.3%) | 1117 (50.2%) | 2017 (60.3%) | 0.0024 | <0.0001 | <0.0001 | ||
| Black | 2181 (27.5%) | 633 (26.8%) | 636 (28.6%) | 912 (27.3%) | 0.73 | 0.30 | 0.19 | ||
| Hispanic | 209 (2.6%) | 70 (3.0%) | 67 (3.0%) | 72 (2.2%) | 0.063 | 0.054 | 1.00 | ||
| Asian | 137 (1.7%) | 21 (0.9%) | 73 (3.3%) | 43 (1.3%) | 0.20 | <0.0001 | <0.0001 | ||
| Native American | * | * | * | * | 0.012 | 0.31 | 0.33 | ||
| Other | 156 (2.0%) | 38 (1.6%) | 64 (2.9%) | 54 (1.6%) | 0.93 | <0.001 | 0.0050 | ||
| Unknown | 756 (9.5%) | 241 (10.2%) | 267 (12.0%) | 248 (7.4%) | 0.00024 | <0.0001 | 0.060 | ||
| Geographic region | |||||||||
| Region of inpatient facility | |||||||||
| Northeast | 3335 (42.0%) | 950 (40.2%) | 947 (42.5%) | 1438 (43.0%) | 0.041 | 0.77 | 0.12 | ||
| South | 2750 (34.7%) | 807 (34.2%) | 829 (37.2%) | 1114 (33.3%) | 0.50 | 0.0027 | 0.033 | ||
| Midwest | 1528 (19.3%) | 482 (20.4%) | 378 (17.0%) | 668 (20.0%) | 0.70 | 0.0058 | 0.0033 | ||
| West | 320 (4.0%) | 122 (5.2%) | 72 (3.2%) | 126 (3.8%) | 0.013 | 0.33 | 0.0015 | ||
| State of inpatient facility | |||||||||
| New York | 1226 (15.5%) | 320 (13.6%) | 386 (17.3%) | 520 (15.5%) | 0.040 | 0.081 | 0.00045 | ||
| New Jersey | 796 (10.0%) | 212 (9.0%) | 260 (11.7%) | 324 (9.7%) | 0.39 | 0.019 | 0.0031 | ||
| Connecticut | 779 (9.8%) | 238 (10.1%) | 206 (9.3%) | 335 (10.0%) | 0.97 | 0.37 | 0.37 | ||
| Georgia | 666 (8.4%) | 188 (8.0%) | 208 (9.3%) | 270 (8.1%) | 0.92 | 0.11 | 0.11 | ||
| Florida | 542 (6.8%) | 141 (6.0%) | 184 (8.3%) | 217 (6.5%) | 0.46 | 0.014 | 0.0030 | ||
| Other | 3924 (49.5%) | 1262 (53.5%) | 982 (44.1%) | 1680 (50.2%) | 0.017 | <0.0001 | <0.0001 | ||
| Comorbid conditions | |||||||||
| Diabetes mellitus without complications | 4022 (50.7%) | 1339 (56.7%) | 1237 (55.6%) | 1446 (43.2%) | <0.0001 | <0.0001 | 0.45 | ||
| Myocardial infarction | 425 (5.4%) | 109 (4.6%) | 123 (5.5%) | 193 (5.8%) | 0.064 | 0.75 | 0.18 | ||
| Chronic heart failure | 2469 (31.1%) | 626 (26.5%) | 656 (29.5%) | 1187 (35.5%) | <0.0001 | <0.0001 | 0.028 | ||
| Chronic pulmonary disease | 2266 (28.6%) | 576 (24.4%) | 588 (26.4%) | 1102 (32.9%) | <0.0001 | <0.0001 | 0.12 | ||
| Peptic ulcer disease | 133 (1.7%) | 36 (1.5%) | 39 (1.8%) | 58 (1.7%) | 0.61 | 0.96 | 0.62 | ||
| AIDS | 33 (0.4%) | * | * | * | 0.60 | 0.50 | 0.21 | ||
| Rheumatologic disease | 435 (5.5%) | 91 (3.9%) | 146 (6.6%) | 198 (5.9%) | 0.00058 | 0.36 | <0.0001 | ||
| Diabetes mellitus, chronic complications | 3081 (38.8%) | 984 (41.7%) | 963 (43.3%) | 1134 (33.9%) | <0.0001 | <0.0001 | 0.29 | ||
| Metastatic cancer | 146 (1.8%) | 37 (1.6%) | 36 (1.6%) | 73 (2.2%) | 0.12 | 0.16 | 0.99 | ||
| Hemiplegia or paraplegia | 596 (7.5%) | 189 (8.0%) | 110 (4.9%) | 297 (8.9%) | 0.27 | <0.0001 | <0.0001 | ||
| Liver disease, mild | 477 (6.0%) | 120 (5.1%) | 129 (5.8%) | 228 (6.8%) | 0.0084 | 0.14 | 0.32 | ||
| Solid tumor without metastases | 923 (11.6%) | 265 (11.2%) | 252 (11.3%) | 406 (12.1%) | 0.31 | 0.38 | 0.95 | ||
| Liver disease, moderate to severe | 66 (0.8%) | 17 (0.7%) | 12 (0.5%) | 37 (1.1%) | 0.18 | 0.038 | 0.56 | ||
| Dementia | 1645 (20.7%) | 481 (20.4%) | 344 (15.5%) | 820 (24.5%) | 0.00028 | <0.0001 | <0.0001 | ||
| Peripheral vascular disease | 2687 (33.9%) | 755 (32.0%) | 624 (28.0%) | 1308 (39.1%) | <0.0001 | <0.0001 | 0.0040 | ||
| Renal failure, moderate to severe | 2351 (29.6%) | 592 (25.1%) | 641 (28.8%) | 1118 (33.4%) | <0.0001 | <0.0001 | 0.0050 | ||
| Cerebrovascular disease | 1744 (22.0%) | 507 (21.5%) | 445 (20.0%) | 792 (23.7%) | 0.055 | 0.0014 | 0.23 | ||
| Charlson Score, median (IQR) | 3.0 (2.0–5.0) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | 3.0 (2.0–6.0) | <0.0001 | <0.0001 | 0.77 | ||
| Drug therapy | |||||||||
| Antihypertensives | |||||||||
| β‐blockers | 4277 (53.9%) | 1112 (47.1%) | 1095 (49.2%) | 2070 (61.9%) | <0.0001 | <0.0001 | 0.17 | ||
| Nondihydropyridine calcium channel blockers | 3438 (43.3%) | 929 (39.3%) | 959 (43.1%) | 1550 (46.3%) | <0.0001 | 0.019 | 0.011 | ||
| Dihydropyridine calcium channel blockers | 2972 (37.5%) | 826 (35.0%) | 848 (38.1%) | 1298 (38.8%) | 0.0037 | 0.62 | 0.031 | ||
| Thiazide or thiazide‐like diuretics | 1650 (20.8%) | 512 (21.7%) | 702 (31.5%) | 436 (13.0%) | <0.0001 | <0.0001 | <0.0001 | ||
| Loop diuretics | 2400 (30.3%) | 570 (24.1%) | 612 (27.5%) | 1218 (36.4%) | <0.0001 | <0.0001 | 0.010 | ||
| Centrally acting α agonists | 303 (3.8%) | 85 (3.6%) | 98 (4.4%) | 120 (3.6%) | 0.96 | 0.14 | 0.19 | ||
| Potassium‐sparing diuretics | 112 (1.4%) | 21 (0.9%) | 22 (1.0%) | 69 (2.1%) | 0.00069 | 0.0028 | 0.85 | ||
| Mineralocorticoid aldosterone antagonists | 435 (5.5%) | 93 (3.9%) | 135 (6.1%) | 207 (6.2%) | 0.00023 | 0.90 | 0.0012 | ||
| Renin inhibitors | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | <0.0001 | <0.0001 | <0.0001 | ||
| α‐adrenergic blocking agents | 247 (3.1%) | 70 (3.0%) | 76 (3.4%) | 101 (3.0%) | 0.97 | 0.46 | 0.43 | ||
| Direct vasodilators | 515 (6.5%) | 110 (4.7%) | 159 (7.1%) | 246 (7.4%) | <0.0001 | 0.81 | 0.00044 | ||
| Place in therapy | |||||||||
| First‐line | 6399 (80.7%) | 2361 (100.0%) | 2226 (100.0%) | 1812 (54.2%) | <0.0001 | <0.0001 | <0.0001 | ||
| Second‐line | 5478 (69.1%) | 1405 (59.5%) | 1388 (62.4%) | 2685 (80.2%) | <0.0001 | <0.0001 | 0.052 | ||
| Number of antihypertensive classes | |||||||||
| 1 | 2322 (29.3%) | 442 (18.7%) | 312 (14.0%) | 1568 (46.9%) | <0.0001 | <0.0001 | <0.0001 | ||
| 2 | 2625 (33.1%) | 850 (36.0%) | 692 (31.1%) | 1083 (32.4%) | 0.0047 | 0.33 | 0.00048 | ||
| 3+ | 2986 (37.6%) | 1069 (45.3%) | 1222 (54.9%) | 695 (20.8%) | <0.0001 | <0.0001 | <0.0001 | ||
| Number, median (IQR) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (1.0–2.0) | <0.0001 | <0.0001 | <0.0001 | ||
| Other drug therapies | |||||||||
| Statins | 4772 (60.2%) | 1528 (64.7%) | 1408 (63.3%) | 1836 (54.9%) | <0.0001 | <0.0001 | 0.32 | ||
| Other lipid‐lowering agents | 423 (5.3%) | 119 (5.0%) | 145 (6.5%) | 159 (4.8%) | 0.66 | 0.0055 | 0.038 | ||
| Oral anticoagulants | 1375 (17.3%) | 384 (16.3%) | 333 (15.0%) | 658 (19.7%) | 0.0012 | <0.0001 | 0.24 | ||
| Insulin | 1373 (17.3%) | 461 (19.5%) | 421 (18.9%) | 491 (14.7%) | <0.0001 | <0.0001 | 0.62 | ||
| Oral antihyperglycemic agents | 2188 (27.6%) | 820 (34.7%) | 738 (33.2%) | 630 (18.8%) | <0.0001 | <0.0001 | 0.27 | ||
| Follow‐up | |||||||||
| Follow‐up days, median (IQR) | 6.0 (3.0–11.0) | 6.0 (3.0–11.0) | 6.0 (3.0–11.0) | 7.0 (3.0–11.0) | 0.80 | 0.96 | 0.78 | ||
| Days to death, median (IQR) | 6.0 (3.0–11.0) | 7.0 (3.0–11.0) | 7.0 (3.0–13.0) | 5.0 (2.0–10.0) | 0.0092 | <0.0001 | 0.15 | ||
| Total mortality | 1130 (14.2%) | 319 (13.5%) | 345 (15.5%) | 466 (13.9%) | 0.70 | 0.088 | 0.048 | ||
The cohort includes individuals who were hospitalized with COVID‐19. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; and IQR, interquartile range.
Cells with values <11 were suppressed in accordance with the Centers for Medicare and Medicare Services cell size suppression policy for values from 1 to 10.
Race is unknown in all commercially insured enrollees.
Table 3.
Hazard Ratio for Hospitalization Among Individuals Testing Positive for SARS‐CoV‐2 in the Outpatient Setting and for In‐Hospital Death and Survival to Discharge Among Individuals Hospitalized for COVID‐19 Between January and May, 2020
| Comparison Group | Treatment | Control | Matched Treatment | Matched Control | Hazard Ratio (95% CI, P Value) | Equipoise Metric |
|---|---|---|---|---|---|---|
| Primary outpatient cohort—outcome: hospitalization | ||||||
| Overall population | ||||||
| ACE inhibitor vs other | 722 | 810 | 441 | 441 | 0.77 (0.53, 1.13); P=0.18 | 0.68 |
| ARB vs other | 731 | 810 | 412 | 412 | 0.88 (0.61, 1.26); P=0.48 | 0.68 |
| ACE inhibitor vs ARB | 722 | 731 | 591 | 591 | 0.91 (0.65, 1.29); P=0.60 | 0.96 |
| Medicare Advantage enrollees | ||||||
| ACE vs other | 581 | 434 | 296 | 296 | 0.61 (0.40, 0.93); P=0.02 | 0.67 |
| ARB vs other | 581 | 452 | 283 | 283 | 0.89 (0.59, 1.36); P=0.59 | 0.68 |
| ACE vs ARB | 452 | 434 | 352 | 352 | 0.88 (0.57, 1.36); P=0.56 | 0.96 |
| Primary inpatient cohort—outcomes: in‐hospital death/alive discharge | ||||||
| Overall population | ||||||
| ACE inhibitor vs other | 2360 | 3338 | 1731 | 1731 |
In‐hospital death: 0.97 (0.81, 1.16); P=0.74 Alive discharge: 1.03 (0.94, 1.12); P=0.57 |
0.56 |
| ARB vs other | 2224 | 3338 | 1560 | 1560 |
In‐hospital death: 1.15 (0.95, 1.38); P=0.15 Alive discharge: 1.01 (0.93, 1.11); P=0.76 |
0.46 |
| ACE inhibitor vs ARB | 2360 | 2224 | 1882 | 1882 |
In‐hospital death: 0.89 (0.75, 1.05); P=0.16 Alive discharge: 1.03 (0.95, 1.12); P=0.47 |
0.95 |
| Medicare Advantage enrollees | ||||||
| ACE vs other | 2151 | 3145 | 1580 | 1580 |
In‐hospital death: 0.89 (0.74, 1.07); P=0.20 Alive discharge: 1.03 (0.94, 1.13); P=0.48 |
0.56 |
| ARB vs other | 1989 | 3145 | 1425 | 1425 |
In‐hospital death: 1.19 (0.99, 1.44); P=0.066 Alive discharge: 1.03 (0.93, 1.13); P=0.58 |
0.46 |
| ACE vs ARB | 2151 | 1989 | 1704 | 1704 |
In‐hospital death: 0.88 (0.74, 1.04); P=0.14 Alive discharge: 1.01 (0.92, 1.10); P=0.89 |
0.95 |
Pairwise comparisons from propensity score–matched cohorts. ACE indicates angiotensin‐converting enzyme; and ARB, angiotensin receptor blocker.
Hospitalizations in the Outpatient Cohort
In the primary outpatient cohort, over a median 30 (IQR, 19–40) days from SARS‐CoV‐2 testing, individuals receiving ACE inhibitors were less frequently hospitalized than those receiving other antihypertensive agents (10.7% versus 14.4%, P=0.03). There was no significant association between ARB therapy and hospitalization rates (12.7% versus 14.4% in individuals receiving other antihypertensive agents, P=0.36). In propensity score–matched cohorts, use of neither ACE inhibitors nor ARB was significantly associated with risk of hospitalization (hazard ratio [HR], 0.77; 95% CI, 0.53–1.13, P=0.18 for ACE inhibitors, and 0.88; 0.61–1.26, P=0.48 for ARB, versus other antihypertensive agents) (Figure 1, Table 3, and Figure S8). There were no differences in falsification end points between propensity score–matched populations (Table S6).
Figure 1. Cumulative event curves for hospitalization among hypertensive individuals with a positive SARS‐CoV‐2 test in the outpatient setting during January to May, 2020 (primary outpatient cohort) comparing (A) ACE inhibitor vs other; (B) ARB vs other; and (C) ACE inhibitor vs ARB.
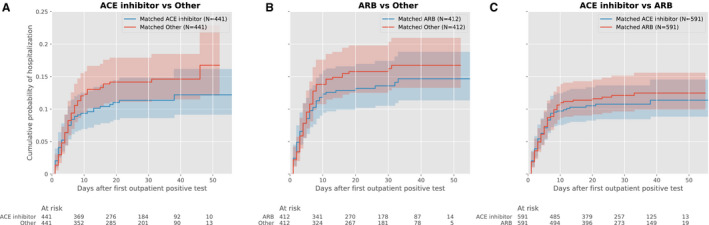
Plots represent propensity score–matched groups. ACE indicates angiotensin‐converting enzyme; and ARB, angiotensin receptor blocker.
There were differences between the association of ACE inhibitors and hospitalization risk across insurance groups (P=0.09 for interaction), with a lower risk of hospitalization in Medicare Advantage enrollees (HR, 0.61; 0.41–0.93, P=0.02) that was not observed in commercially insured individuals (HR, 2.14; 0.82–5.60, P=0.12) (Table 3).
In propensity score–matched analyses, ARB use was not significantly associated with lower hospitalization risk than in individuals receiving other antihypertensive agents (HR, 0.88; 0.61–1.26, P=0.48) (Figure 1). There were no significant differences in hospitalization rates between propensity score–matched cohorts of individuals receiving an ACE inhibitor, compared with ARB (HR, 0.91; 0.65–1.29, P=0.60). There were no significant interactions by insurance type and the association of ARB with outcomes (P=0.55 for interaction).
In the outpatient validation cohort, neither ACE inhibitor nor ARB was associated with hospitalization risk, overall (HR in propensity‐matched cohorts, ACE inhibitor, 0.98; 0.76–1.26; P=0.87, and ARB, 0.77; 0.59–1.02; P=0.07) or in the Medicare Advantage or commercially insured population (Figure 2, Table 4).
Figure 2. Cumulative event curves for hospitalization among hypertensive individuals with a positive SARS‐CoV‐2 test in the outpatient setting during May to August, 2020 (secondary outpatient cohort) comparing (A) ACE inhibitor vs other; (B) ARB vs other; (C) ACE inhibitor vs ARB.
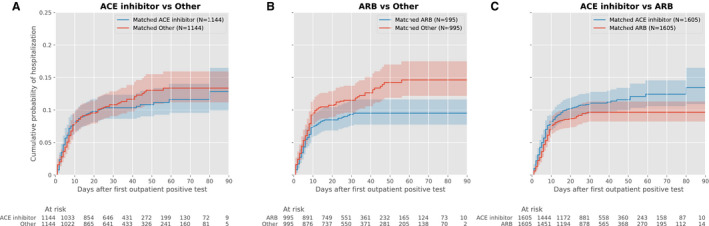
Plots represent propensity score–matched groups. ACE indicates angiotensin‐converting enzyme; and ARB, angiotensin receptor blocker.
Table 4.
Hazard Ratio for Hospitalization Among Individuals Testing Positive for SARS‐CoV‐2 in the Outpatient Setting and for In‐Hospital Death and Survival to Discharge Among Individuals Hospitalized for COVID‐19 Between May and August, 2020
| Comparison Group | Treatment | Control | Matched Treatment | Matched Control | Hazard Ratio (95% CI, P Value) | Equipoise Metric |
|---|---|---|---|---|---|---|
| Secondary outpatient cohort—outcome: hospitalization | ||||||
| Overall population | ||||||
| ACE inhibitor vs other | 2152 | 1592 | 1144 | 1144 | 0.98 (0.76, 1.26); P=0.87 | 0.73 |
| ARB vs other | 1808 | 1592 | 995 | 995 | 0.77 (0.59, 1.02); P=0.067 | 0.69 |
| ACE inhibitor vs ARB | 2152 | 1808 | 1605 | 1605 | 1.26 (1.01, 1.57); P=0.040 | 0.97 |
| Medicare Advantage enrollees | ||||||
| ACE vs other | 1181 | 972 | 673 | 673 | 0.91 (0.70, 1.19); P=0.51 | 0.73 |
| ARB vs other | 1077 | 972 | 569 | 569 | 0.77 (0.57, 1.04); P=0.084 | 0.64 |
| ACE vs ARB | 1181 | 1077 | 905 | 905 | 1.22 (0.94, 1.57); P=0.13 | 0.96 |
| Secondary inpatient cohort—outcome: in‐hospital death/alive discharge | ||||||
| Overall population | ||||||
| ACE inhibitor vs other | 2660 | 3119 | 1819 | 1819 |
In‐hospital death: 1.06 (0.85, 1.33); P=0.59 Alive discharge: 1.03 (0.96, 1.11); P=0.41 |
0.59 |
| ARB vs other | 2323 | 3119 | 1518 | 1518 |
In‐hospital death: 1.04 (0.83, 1.31); P=0.72 Alive discharge: 0.97 (0.89, 1.06); P=0.52 |
0.44 |
| ACE inhibitor vs ARB | 2660 | 2323 | 1976 | 1976 |
In‐hospital death: 1.01 (0.83, 1.24); P=0.89 Alive discharge: 1.02 (0.95, 1.1); P=0.59 |
0.93 |
| Medicare Advantage enrollees | ||||||
| ACE vs other | 2352 | 2905 | 1659 | 1659 |
In‐hospital death: 1.02 (0.82, 1.26); P=0.89 Alive discharge: 1.00 (0.92, 1.09); P=0.95 |
0.59 |
| ARB vs other | 2028 | 2905 | 1357 | 1357 |
In‐hospital death: 1.03 (0.81, 1.31); P=0.78 Alive discharge: 1.03 (0.94, 1.13); P=0.53 |
0.44 |
| ACE vs ARB | 2352 | 2028 | 1721 | 1721 |
In‐hospital death: 0.93 (0.75, 1.15); P=0.51 Alive discharge: 1.01 (0.93, 1.09); P=0.82 |
0.93 |
Pairwise comparisons from propensity score–matched cohorts. ACE indicates angiotensin‐converting enzyme; and ARB, angiotensin receptor blocker.
Among the individuals in the outpatient cohort who were hospitalized, there was no association with ACE inhibitor or ARB use with subsequent in‐hospital mortality (Table S7).
Mortality in the Inpatient Cohort
Of the 7933 individuals hospitalized with COVID‐19, 1128 (14.2%) died during hospitalization, 4722 (59.5%) were discharged alive, and 2083 (26.3%) were still hospitalized at the end of the observation period. A majority of deaths (90.1%) were among the Medicare Advantage population. The median length of stay (including individuals who died as well as those discharged alive) for COVID‐19 hospitalizations was 6 (IQR, 3–11) days, which was similar across individuals who died (6 (IQR, 3–10) days) or were discharged alive (6 [IQR, 3–11] days) during the observation period.
Overall, the proportion of COVID‐19 inpatients who died did not differ significantly in those on ACE inhibitor therapy before hospitalization compared with those on other antihypertensive agents (13.5% versus 13.9%, P=0.68). In the propensity matched–cohort of individuals receiving ACE inhibitors before hospitalization (Figures S6 and S7), in‐hospital mortality was not significantly different from that of individuals on other antihypertensive drugs (HR, 0.97; 0.81–1.16, P=0.74; Figure 3, Table 3). Similarly, treatment with ARB did not have a significantly different risk of mortality compared with other antihypertensive agents (1.15; 0.95–1.38, P=0.15). There were no significant differences in mortality between individuals receiving ACE inhibitors and ARBs in the overall population, without a significant interaction between insurance group and treatment assignment and patient outcome (Figure 2, Table 3). These findings were consistent in our secondary outcome of in‐hospital death or discharge to hospice (Table S8). There was also no association between treatment with ACE inhibitor or ARB on hospital length of stay (Table S9). There were no significant differences between our falsification outcomes in matched groups (Table S10). There were no significant differences in mortality across propensity‐matched groups of hospitalized patients in the inpatient validation cohort (Figure 4, Table 4).
Figure 3. Cumulative event curves for in‐hospital mortality among hypertensive individuals with hospitalization for COVID‐19 during January to May, 2020 (primary inpatient cohort) comparing (A) ACE inhibitor vs other; (B) ARB vs other; and (C) ACE inhibitor vs ARB.
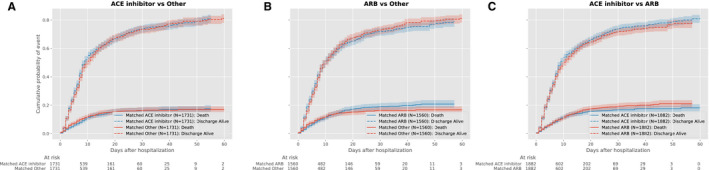
Plots represent propensity score–matched groups. ACE indicates angiotensin‐converting enzyme; and ARB, angiotensin receptor blocker.
Figure 4. Cumulative event curves for in‐hospital mortality among hypertensive individuals with hospitalization for COVID‐19 during May to August, 2020 (secondary inpatient cohort) comparing (A) ACE inhibitor vs other; (B) ARB vs other; and (C) ACE inhibitor vs ARB.
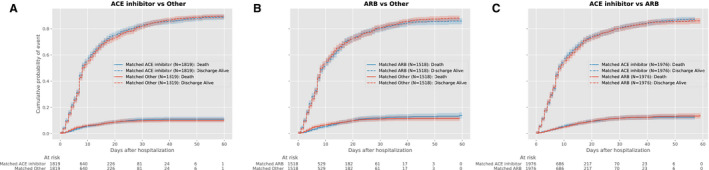
Plots represent propensity score–matched groups. ACE indicates angiotensin‐converting enzyme; and ARB, angiotensin receptor blocker.
Sensitivity analyses that focused on individuals receiving at least 1 first‐line antihypertensive agent in the control group, and that varied the covariate adjustment strategies, were consistent with the primary analysis (Tables S11 through S14).
DISCUSSION
In this national study of ACE inhibitors and ARBs among patients with hypertension in the outpatient setting testing positive for SARS‐CoV‐2, we found that overall these drugs did not confer additional risk or benefit. While early data indicated that ACE inhibitors may be associated with a lower risk of hospitalization for COVID‐19, more recent data did not demonstrate this association. Moreover, such an effect was not observed with ARBs. Among inpatients with COVID‐19, we did not find a benefit or a harm of these medications. Collectively, the findings do not support a change to the current use of these medications or evaluating the use of ACE inhibitors to reduce the risk of severe SARS‐CoV‐2 infection.
Our study was restricted to individuals with hypertension who were receiving at least 1 antihypertensive agent, thereby limiting our assessment to individuals receiving treatment for the same chronic illness, and therefore, equally likely to seek care for healthcare needs for COVID‐19. In all analyses, we explicitly compared individuals with equipoise for receiving either drug treatment. Moreover, we did not find any evidence of confounding by disease severity in choice of therapy in our assessment of falsification end points. Furthermore, our study included individuals from across the United States, thereby limiting the effect of hospital or regional care practices that may bias an evaluation of treatment effects.
Our observations extend the prior evidence of supporting safety of ACE inhibitor treatment in COVID‐19.8, 13, 24 Many studies thus far have had limitations with their data sources and study designs to adequately address the hypotheses focusing on the safety of ACE inhibitors and ARBs in COVID‐19, and their potential efficacy in reducing the severity of the disease.7, 11 Our study adds to the literature by focusing on a large national population spanning the entire adult age range and including individuals across the United States, thereby overcoming the challenge of generalizability of studies that are based on single centers or hospitals in the same region. We show that these agents are not associated with harm in outpatient SARS‐CoV‐2‐infected individuals and were able to track the same individuals across different outpatient and inpatient settings. We also use robust methods to account for confounding. This complements the studies of those who were hospitalized and focused on severity of disease and mortality in these patient groups.13, 25
Our original study that focused on data from January through May had an intriguing finding. In the subgroup of individuals enrolled in Medicare Advantage, we found that ACE inhibitors were associated with a significantly lower risk of hospitalization following an infection with SARS‐CoV‐2 in the outpatient setting. Medicare is a federal health insurance program in the United States for adults aged 65 years and older, and certain younger individuals with disability and end‐stage renal disease. Medicare Advantage is a subtype that includes coverage of inpatient, outpatient, and often prescriptions and is administered in conjunction with commercial insurance providers. Since Medicare predominantly includes individuals over 65 years of age, Medicare beneficiaries are older, more frequently have comorbidities, and are more vulnerable to severe COVID‐19 disease.13 These observations had prompted our team to plan a clinical trial for the prophylactic use of ACE inhibitors to prevent severe disease. However, our analyses in more contemporary data demonstrate that the original results do not represent a consistent association.
Our results inform the discussion of preclinical evidence that had suggested a possible protective role for ACE inhibitors in COVID‐19. ACE inhibitors, but not ARBs, are associated with the upregulation of ACE‐2 receptors.3, 4 Of note, these receptors modulate the renin‐angiotensin‐aldosterone system, in the lung tissue.26 The presence of ACE‐2 receptors is, therefore, suggested to exert a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage.27, 28 Moreover, ACE‐2 receptors are also present in the vascular endothelium, as well as in the renal tubular and intestinal epithelia, with uncertain role in the pathogenicity of SARS‐CoV‐2.29, 30 Our study did not, however, include an assessment of ACE‐2 levels in study participants. Recent studies have also suggested a lower level of cytokines and peripheral blood T cells among patients with COVID‐19 with hypertension who were receiving renin‐angiotensin system inhibitors.31 Prior evidence from randomized clinical trials and observational studies of identified a reduced risk of pneumonia with ACE inhibitors that is not observed with ARBs.32,33
Our observations do not support a clinical effect corresponding to either enhanced or decreased virulence of the virus with use of either ACE inhibitors or ARBs after accounting for confounding.
Our study of in‐hospital outcomes adds to the literature on studies that have reached contrasting conclusions regarding the role of ACE inhibitor therapy and in‐hospital mortality among hospitalized patients with COVID‐19. We did not find a significant association with mortality, consistent with others who have not found such an association.6, 13, 25, 34, 35 Our findings contrast with certain studies that have found lower mortality in hospitalized patients with COVID‐19 treated with ACE inhibitors.25, 36 Notably, most studies that have evaluated mortality risk with COVID‐19 before have not consistently been designed to detect potential causal association of drug therapy with outcomes, relied on case–control designs,12, 24 pursued potentially biased assessment by using comparators not receiving any therapy,12, 13 or are based on data from single health centers.13, 25 Among studies that are pending peer review, there is similarly no evidence of increased hospitalization or mortality risk (Table S15).10, 37
Our study has important implications for 4 ongoing randomized trials because none of them align with the observations of our study.7 Of the 4 trials, 3 are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized patients with COVID‐19, and 1 is using a 10‐day course of ARBs after a positive SARS‐CoV‐2 test to prevent hospitalization.7 However, our study suggests that ACE inhibitors are unlikely to play a role in reducing COVID‐19‐related hospitalizations or mortality.
The qualitative differences in the effect estimates observed in our primary and secondary analyses also highlight the challenges with observational studies designed to identify drugs that may have a role in the management of COVID‐19, or for any other disease using real‐world data, particularly during the rapidly evolving pandemic. While our primary analyses favored the role of ACE inhibitors in reducing hospitalization risk in SARS‐CoV‐2, this was not observed in larger more recent data. This emphasizes the need for independent validation of effectiveness findings in observational studies, which in spite of robust designs can lead to erroneous conclusions because of chance alone. The Observational Health Data Sciences and Informatics framework that iteratively tests each hypothesis in multiple discrete data sets to determine treatment effects is less prone to erroneous conclusions because of chance.10, 38
The findings of our study should be interpreted in light of the following limitations. First, the study is observational, and despite robust methods, we cannot exclude the effects of residual confounding, which is a limitation for causal inference. Nevertheless, our assessment of the observations in 2 discrete data periods with potentially different care‐seeking patterns allowed us to assess for consistency of effects over time. Second, we do not know the proportion of individuals who are receiving ACE inhibitors and ARBs and who continued to be treated with these drugs during the illness and the association of their continued use or cessation with patient outcomes. This is a limitation of most observational assessments.39 Third, while the present study is one of the largest US studies on the association of ACE inhibitor and ARB use with both hospitalization and mortality risk with COVID‐19, the number of individuals in the propensity‐matched groups is smaller with a more limited number of events. Fourth, we focused on patients with hypertension receiving at least 1 antihypertensive agent to limit unmeasured confounding because these would represent individuals with comparable underlying health status. We also explicitly accounted for measured differences between groups through our propensity score matching. However, our focus may limit the generalizability of our comparisons to those not receiving any antihypertensive agents.
Fifth, all included data elements are contingent upon individuals seeking care for that ailment or filling a medication using their insurance provider and would not be captured if they chose to self‐pay. Sixth, we cannot account for differences in timing of presentation relative to symptom onset. However, we limited the effect of differential presentation by individuals across exposure groups by focusing on those receiving treatment for the same medical comorbidity, ie, hypertension, and only varying the class of drugs. Moreover, we included individuals across the United States and accounted for clustering of cases, thereby limiting the effect of local practice patterns that may affect hospitalization thresholds. Therefore, it is unlikely that an individual's care‐seeking behavior would be affected by knowledge of their underlying disease. Additionally, while our analyses of hospitalization used all available evidence for disease severity, we do not have granular details on real‐time inpatient treatment of patients with COVID‐19 and whether certain presentation or care characteristics are associated with in‐hospital outcomes. Instead, our study evaluates the association of only prehospital factors with outcomes during hospitalization. Finally, we do not account for a possible dose–response relationship of ACE inhibitors or ARBs on SARS‐CoV‐2 hospitalization risk or COVID‐19 mortality risk because we were unable to evaluate the effects of the drugs as a function of the total daily dose.
In conclusion, the use of ACE inhibitors and ARBs was not associated with the risk of hospitalization or mortality among those infected with SARS‐CoV‐2. Despite early evidence for a potential protective effect of ACE inhibitors in preventing severe disease in older individuals, the inconsistency of this observation in recent data argues against a role as prophylaxis against severe disease.
Sources of Funding
The study was funded by Research & Development (R&D) at UnitedHealth Group. The authors from UnitedHealth Group R&D participated in each aspect of the study, including its design and conduct, data management and analysis, interpretation of the results, as well as provided input on the manuscript for submission. Dr Khera reports support from the National Heart, Lung, and Blood Institute (K23HL153775‐01A1) and the National Center for Advancing Translational Sciences (UL1TR001105) of the United States National Institutes of Health. Dr Lu is supported by the National Heart, Lung, and Blood Institute (K12HL138037) of the United States National Institutes of Health and the Yale Center for Implementation Science. The National Institutes of Health and the Yale Center for Implementation Science had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
Dr Krumholz was a recipient of a research grant, through Yale, from Medtronic and the US Food and Drug Administration to develop methods for postmarket surveillance of medical devices; was a recipient of a research grant with Medtronic and is the recipient of a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; was a recipient of a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; receives payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation, from the Martin/Baughman Law Firm for work related to the Cook Celect IVC filter litigation, and from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; was a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is a co‐founder of HugoHealth, a personal health information platform, and co‐founder of Refactor Health, an enterprise healthcare artificial intelligence–augmented data management company. He is also an advisor to FPrime. Drs Lin, Spatz, and Murugiah work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures that are publicly reported. Dr Spatz receives support from the US Food and Drug Administration to support projects within the Yale‐Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI); the National Institute on Minority Health and Health Disparities (U54MD010711‐01) to study precision‐based approaches to diagnosing and preventing hypertension; and the National Institute of Biomedical Imaging and Bioengineering (R01EB028106‐01) to study a cuff‐less blood pressure device. Drs Clark, Ren, Vojta, and Mr Guo and Mr Truax are full‐time employees in Research & Development at UnitedHealth Group and own stock in the company. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S15
Figures S1–S8
Reference 40
Acknowledgments
Author contributions: Khera, Clark, Vojta, and Krumholz were responsible for the study concept and design. Guo, Ren, and Truax were responsible for the acquisition and analysis of data. All authors contributed to the interpretation of the data. Khera and Clark drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version. Vojta and Krumholz are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
(J Am Heart Assoc. 2021;10:e018086. DOI: 10.1161/JAHA.120.018086.)
Preprint posted on MedRxiv May 19, 2020. doi: https://doi.org/10.1101/2020.05.17.20104943.
For Sources of Funding and Disclosures, see page 19.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. DOI: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8:e21. DOI: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. DOI: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382:1653–1659. DOI: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. DOI: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. DOI: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, Sonnen P, Kansagara D. Risks and impact of angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers on SARS‐CoV‐2 infection in adults: a living systematic review. Ann Intern Med. 2020;173:195–203. DOI: 10.7326/M20-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431–2440. DOI: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palazzuoli A, Mancone M, De Ferrari GM, Forleo G, Secco GG, Ruocco GM, D'Ascenzo F, Monticone S, Paggi A, Vicenzi M, et al. Antecedent administration of angiotensin‐converting enzyme inhibitors or angiotensin II receptor antagonists and survival after hospitalization for COVID‐19 syndrome. J Am Heart Assoc. 2020;9:e017364. DOI: 10.1161/JAHA.120.017364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales DR, Conover MM, You SC, Pratt N, Kostka K, Duarte‐Salles T, Fernandez‐Bertolin S, Aragon M, DuVall SL, Lynch K, et al. Renin‐angiotensin system blockers and susceptibility to COVID‐19: an international, open science, cohort analysis. Lancet Digit Health. 2021;3:e98–e114. DOI: 10.1016/S2589-7500(20)30289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpe M, Battistoni A. Systematic review of the role of renin‐angiotensin system inhibitors in late studies on Covid‐19: a new challenge overcome? Int J Cardiol. 2020;321:150–154. DOI: 10.1016/j.ijcard.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Abajo FJ, Rodriguez‐Martin S, Lerma V, Mejia‐Abril G, Aguilar M, Garcia‐Luque A, Laredo L, Laosa O, Centeno‐Soto GA, Ángeles Gálvez M, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395:1705–1714. DOI: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441–2448. DOI: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M; Investigators S‐R . Age and multimorbidity predict death among COVID‐19 patients: results of the SARS‐RAS study of the Italian Society of Hypertension. Hypertension. 2020;76:366–372. DOI: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JB, Hanff TC, William P, Sweitzer N, Rosado‐Santander NR, Medina C, Rodriguez‐Mori JE, Renna N, Chang TI, Corrales‐Medina V, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID‐19: a prospective, randomised, open‐label trial. Lancet Respir Med. 2021;9:275–284. DOI: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. DOI: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 17.Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen R, Pratt N, Reich CG, Duke J, Madigan D, Hripcsak G, et al. Comprehensive comparative effectiveness and safety of first‐line antihypertensive drug classes: a systematic, multinational, large‐scale analysis. Lancet. 2019;394:1816–1826. DOI: 10.1016/S0140-6736(19)32317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida K, Solomon DH, Haneuse S, Kim SC, Patorno E, Tedeschi SK, Lyu H, Hernandez‐Diaz S, Glynn RJ. A tool for empirical equipoise assessment in multigroup comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2019;28:934–941. DOI: 10.1002/pds.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941–950. DOI: 10.1001/jamainternmed.2014.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen TL, Collins GS, Spence J, Daures JP, Devereaux PJ, Landais P, Le Manach Y. Double‐adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17:78. DOI: 10.1186/s12874-017-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. DOI: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–2308. DOI: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SL, Sen S, Greenhalgh DG, Lawless M, Curri T, Palmieri TL. A competing risk analysis for hospital length of stay in patients with burns. JAMA Surg. 2015;150:450–456. DOI: 10.1001/jamasurg.2014.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Zhu L, Cai J, Lei F, Qin J‐J, Xie J, Liu Y‐M, Zhao Y‐C, Huang X, Lin L, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671–1681. DOI: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona‐Rubio AE, Jacob M, Procop GW, Harrington S, et al. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1020–1026. DOI: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin‐converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. DOI: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan YI, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875–879. DOI: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181:281–292.e6. DOI: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardenberg JB, Luft FC. Covid‐19, ACE2 and the kidney. Acta Physiol (Oxf). 2020;230:e13539. DOI: 10.1111/apha.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W, Derclaye S, Vincent SP, Soumillion P, Alsteens D. Molecular interaction and inhibition of SARS‐CoV‐2 binding to the ACE2 receptor. Nat Commun. 2020;11:4541. DOI: 10.1038/s41467-020-18319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. DOI: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldeira D, Alarcao J, Vaz‐Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta‐analysis. BMJ. 2012;345:e4260. DOI: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, Good C, Restrepo MI, Downs JR, Frei CR, et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;55:1466–1473. DOI: 10.1093/cid/cis733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarcho JA, Ingelfinger JR, Hamel MB, D'Agostino RB Sr, Harrington DP. Inhibitors of the renin‐angiotensin‐aldosterone system and Covid‐19. N Engl J Med. 2020;382:2462–2464. DOI: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usman MS, Siddiqi TJ, Khan MS, Ahmed A, Ali SS, Michos ED, Hall ME, Krasuski RA, Greene SJ, Butler J, et al. A meta‐analysis of the relationship between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19. Am J Cardiol. 2020;130:159–161. DOI: 10.1016/j.amjcard.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fosbøl EL, Butt JH, Østergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168–177. DOI: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bean DM, Kraljevic Z, Searle T, Bendayan R, Pickles A, Folarin A, Roguski L, Noor K, Shek A, O’Gallagher K, et al. ACE‐inhibitors and angiotensin‐2 receptor blockers are not associated with severe SARS‐ COVID19 infection in a multi‐site UK acute Hospital Trust. Eur J Heart Fail. 2020;22:967–974. DOI: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hripcsak G, Ryan PB, Duke JD, Shah NH, Park RW, Huser V, Suchard MA, Schuemie MJ, DeFalco FJ, Perotte A, et al. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci USA. 2016;113:7329–7336. DOI: 10.1073/pnas.1510502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UnitedHealth Group . The PACE Study. Available at: https://www.unitedinresearch.com/studies/2. Accessed July 13, 2020.
- 40.Yoshida K, Solomon DH, Haneuse S, Kim SC, Patorno E, Tedeshi SK, Lyu H, Hernandez‐Diaz S, Glynn RJ. Original article: a tool for empirical equipoise assessment in multi‐group comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2019;28:934–941. DOI: 10.1002/pds.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S15
Figures S1–S8
Reference 40


