Abstract
Background
Stroke is a major comorbidity in patients with heart failure (HF), especially in those with decreased left atrial (LA) function, and thus, identifying patients highly at risk of stroke can prevent its occurrence. We evaluated the predictive value of global longitudinal strain of LA (LAGLS) in patients with acute HF and sinus rhythm.
Methods and Results
In this retrospective study, 2461 patients (53.3% men, 69.7±14.4 years old) with sinus rhythm and LAGLS among 4312 consecutive patients with acute HF from 3 tertiary hospitals were included. HF phenotypes were defined as HF with reduced ejection fraction (EF) (left ventricular EF ≤40%), HF with midrange EF (40% <left ventricular EF <50%), and HF with preserved ejection fraction (left ventricular EF ≥50%). Primary outcome was new‐onset stroke. The mean left ventricular EF was 39.4%±15.6%. Moreover, 1388 (57.5%), 342 (14.2%), and 682 (28.3%) were classified with HF with reduced EF, HF with midrange EF, and HF with preserved EF, retrospectively. LAGLS was 17.2%±10.4%. During the follow‐up duration (mean: 30.3±25.4 months), 100 patients experienced stroke. Patients with stroke had higher LA diameter (P=0.031) and lower LAGLS (P=0.010) than those without stroke. In the univariate analysis, age, diabetes mellitus, LA diameter, LA volume index, and LAGLS were significant risk factors for stroke. In the multivariate analysis, each 1% decrease in LAGLS was associated with a 3.8% increased risk for stroke (hazard ratio [HR], 1.038; 95% CI, 1.013–1.065; P=0.003). When applying a LAGLS cutoff point of 14.5%, patients with LAGLS <14.5% had approximately twice the risk for stroke after adjusting other significant variables (HR, 1.940; 95% CI, 1.269–2.965; P=0.002).
Conclusions
In patients with acute HF and sinus rhythm, decreased LAGLS (<14.5%) was associated with an increased risk for stroke, with an annual incidence of 2.38%.
Keywords: heart failure, strain echocardiography, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Echocardiography, Heart Failure
Nonstandard Abbreviations and Acronyms
- AHF
acute heart failure
- HFrEF
heart failure with reduced ejection fraction
- LAGLS
left atrial global longitudinal strain
Clinical Perspective
What Is New?
Decreased left atrial global longitudinal strain (<14.5%) was associated with an increased risk for stroke, with an annual incidence of 2.38%, in patients with acute heart failure and sinus rhythm.
The risk of stroke corresponds to a CHA2DS2‐VASc score of 2 to 3 in patients with nonvalvular atrial fibrillation.
What Are the Clinical Implications?
Further clinical evaluation is needed to evaluate the cost‐effectiveness of anticoagulant therapy to prevent future stroke in patients with acute heart failure and lower left atrial global longitudinal strain.
Stroke is a major cardiovascular disease with high mortality and morbidity. The estimated prevalence of stroke was 2.5% in 2016, and the prevalence of stroke increases with age.1 Approximately 87% of patients are diagnosed with ischemic stroke, and approximately 91% are associated with modifiable risk factors including hypertension, obesity, hyperglycemia or diabetes mellitus, dyslipidemia, and chronic kidney disease.2
Among the risk factors for stroke, atrial fibrillation (AF) is a powerful risk factor that can increase the risk of stroke approximately 5‐fold.3, 4 Heart failure (HF) is the second most common risk factor for stroke after AF and accounts for approximately 9% of stroke.5, 6 Specifically, patients with HF with reduced ejection fraction (HFrEF) have increased risk of thromboembolism because of blood stasis in the dilated left ventricle, impaired release of endothelium‐derived vasodilators, hemoconcentration, increased inflammation and thrombin‐related pathways, and increased risk of AF.7, 8, 9 The cerebral blood flow may be reduced in patients with HF, which may contribute to further cerebral ischemia in patients with stroke.10
Although the anticoagulation value is not well studied, identifying patients highly at risk of stroke can prevent its occurrence. As left atrial (LA) enlargement and dysfunction are associated with an increased risk of AF,11, 12 decreased LA function assessed by LA global longitudinal strain (LAGLS) was associated with increased risk of new‐onset AF and mortality.13, 14, 15 Although decreased LAGLS can be associated with LA thrombus formation and subsequent embolic infarction, few studies have shown the association between LAGLS and stroke.
Therefore, we evaluated the predictive value of LAGLS for stroke in patients with acute heart failure (AHF) and sinus rhythm.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Goo‐Yeong Cho, MD, PhD (cardioch@snu.ac.kr).
Study Population
The STRATS‐AHF (Strain for Risk Assessment and Therapeutic Strategies in Patients With Acute Heart Failure) registry (ClinicalTrials.gov Identifier 03513653, https://clinicaltrials.gov/ct2/show/NCT03513653) is a large strain registry including 4312 acute HF patients from 3 tertiary university teaching hospitals in Korea between January 2009 and December 2016. Detailed information and primary outcomes are reported elsewhere.16, 17 In this registry, we retrospectively enrolled all admitted AHF patients with symptoms or signs of AHF with either pulmonary edema or objective findings of structural heart disease. We excluded patients with acute coronary syndrome or severe valvular heart disease requiring surgical correction at the time of admission.
This study included only patients with sinus rhythm and LAGLS value. Those with AF at the time of admission were excluded. Although the cardiac rhythm was normal at the time of hospitalization, patients of AF confirmed by the medical history were also excluded from this study. For the detection of new‐onset AF during the follow‐up period, electrocardiographs of all patients were reviewed. New‐onset AF was confirmed when documented in electrocardiographs or when the patient had I48 codes from the International Classification of Diseases, Tenth Revision (ICD‐10) in their diagnosis. Approximately 16 electrocardiographs per patient (mean: 16.5±15.2 electrocardiographs) were reviewed for AF detection. A total of 397 patients with new‐onset AF (16.1%) during the follow‐up were also included in the analysis. The institutional review board of each hospital approved this study protocol and waived the consent from the participants.
The study complied with the principles of the Declaration of Helsinki.
Study Variables and Definitions
Based on echocardiographic findings at the index admission for AHF, patients were categorized as having HFrEF (left ventricular EF [LVEF] ≤40%), HF with midrange EF (40% <LVEF <50%), and HF with preserved EF (LVEF ≥50%).
The primary outcome was ischemic stroke after discharge from the index admission.
Stroke diagnosis was identified from data in the medical records with patients with regular follow‐up. Ischemic stroke was identified as an episode of neurological dysfunction caused by embolism.
Echocardiographic Examination
We performed echocardiographic examinations using commercial echocardiographic machines and a 2.5‐MHz probe using standard echocardiographic techniques, including M‐mode, 2‐dimensional, and Doppler modalities, as suggested by the American Society of Echocardiography.18 End‐diastolic and end‐systolic LV volumes were measured by 2‐dimensional Simpson’s method from the apical 4‐ and 2‐chamber views, and the LVEF was calculated from these values. We recorded mitral inflow velocities using the pulsed‐wave Doppler method at the mitral valve coaptation point and mitral annular velocities using the tissue Doppler method of the septum of the mitral annulus. We assessed LV diastolic function with the mitral E and A velocities, E/A ratio, deceleration time, and tissue Doppler analysis of septal mitral annular E′ velocity, and E/e′ ratio. Pulmonary artery systolic pressure was calculated from the continuous‐wave Doppler–derived peak tricuspid regurgitation jet velocity. The anteroposterior LA diameter was measured from the parasternal long‐axis view, and the LA maximal volume was measured at the end‐systole frame using the area‐length method from the apical 4‐ and 2‐chamber views and presented as LA volume index after adjusting for body surface area.
Strain Analysis
We measured the strains including LAGLS from the stored echocardiographic images using TomTec‐Arena version 4.6 (TomTec, Munich, Germany) from digitally stored echocardiographic images.19, 20 The TomTec program is a vendor‐independent software used to measure strain. After the endocardial border was manually traced on the end‐systolic frame in the selected image, the software tracked speckles along the endocardial border and myocardium throughout the cardiac cycle automatically.
For the LAGLS analysis, we traced the LA endocardial border manually on the LV end‐systolic frame. Subsequently, the software tracked speckles along the endocardial border and myocardium automatically throughout the cardiac cycle. We used R‐R gating as the zero‐reference point. We defined the LAGLS as the first peak positive deflection demonstrating LA reservoir function. The LAGLS was calculated as the mean value of the 4 segments of each apical view as the average of the GLS values from the apical 4‐ and 2‐ chamber views.13, 18 The roof of the LA was not analyzed because of poor delineation and contamination of pulmonary veins. All LAGLS values were analyzed on a single cardiac cycle, and an echocardiographic specialist (PJH) blinded to the clinical data independently measured all LAGLS values.
Statistical Analysis
We presented continuous variables as means±SDs and categorical variables as frequencies. We performed the Student’s t‐test for continuous variables and the χ 2 test for categorical variables for comparisons between groups. We used the Kaplan–Meier method with comparison using the log‐rank test in the analysis of survival and multivariate time‐dependent Cox‐proportional hazard analysis to determine the independent predictors of stroke in the time to first adverse clinical events. We included all significant variables (with P value less than 0.05) in the univariate analysis as covariates in the multivariate analysis. However, we excluded variables with multicollinearity with others from the multivariate analysis. Two independent investigators calculated intra‐ and interobserver variabilities of the LAGLS in 20 random patients by calculating the intraclass correlation coefficient. The data were analyzed using the SPSS version 22 (IBM, Chicago, IL, USA). A 2‐sided P value of <0.05 was considered statistically significant.
Results
Patient Characteristics
Initially, we screened all 4312 patients with AHF. We excluded 494 patients without LA strain values and 1357 patients with prior AF or missing values of cardiac rhythm (Figure 1). Thus, we analyzed a total of 2461 admitted patients (1311 men, 69.7±14.4 years old) with sinus rhythm and adequate echocardiographic images suitable for the measurement of LAGLS. Their baseline characteristics are summarized in Table 1. The mean LVEF was 39.1%±15.6%, and 1388 (57.5%), 342 (14.2%), and 682 (28.3%) patients were classified as having HFrEF, HF HF with midrange EF, and HF with preserved EF, retrospectively. Hypertension was the most common associated cardiovascular risk factor (56.8%), and diabetes mellitus was found in 912 patients (37.1%). LAGLS was 17.2%±10.4%.
Figure 1. Of the 4312 patients who were included in the STRATS‐AHF (Strain for Risk Assessment and Therapeutic Strategies in Patients With Acute Heart Failure) registry, 2461 patients with sinus rhythm and left atrial (LA) strain value were analyzed.
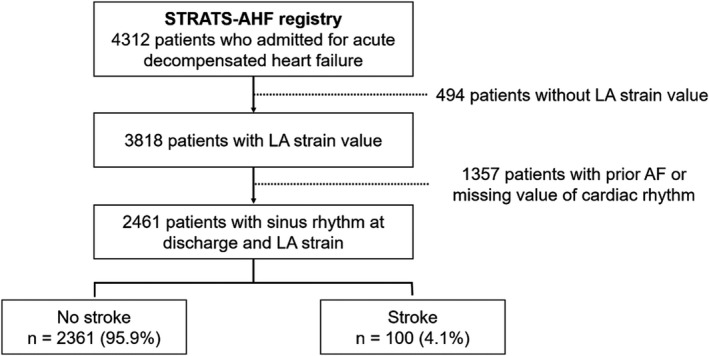
During the 5‐year follow‐up, 100 (4.1%) patients had stroke. AF indicates atrial fibrillation.
Table 1.
Comparison of Baseline Clinical Characteristics and Echocardiographic Data According to the Presence of Stroke
| Total (n=2461) | No stroke (n=2361) | Stroke (n=100) | P Value | |
|---|---|---|---|---|
| Male sex, % | 1311 (53.3%) | 1262 (53.5%) | 49 (49.0%) | 0.414 |
| Age, y | 69.7±14.4 | 69.6±14.5 | 71.3±12.4 | 0.188 |
| Body mass index, kg/m2 | 23.3±4.1 | 23.3±4.1 | 23.7±4.2 | 0.323 |
| New York Heart Association functional class IV, % | 840 (34.1%) | 800 (33.9%) | 40 (40.0%) | 0.138 |
| Physical examination | ||||
| Systolic blood pressure, mm Hg | 129.4±27.2 | 129.2±27.3 | 132.8±24 | 0.198 |
| Diastolic blood pressure, mm Hg | 74.0±16.2 | 74±16.3 | 74.9±13.7 | 0.583 |
| Heart rate, bpm | 84.8±21.7 | 84.9±21.7 | 84.2±19.9 | 0.765 |
| Past medical history | ||||
| Hypertension, % | 1399 (56.8%) | 1337 (56.6%) | 62 (62.0%) | 0.304 |
| Diabetes mellitus, % | 912 (37.1%) | 867 (36.7%) | 45 (45.0%) | 0.112 |
| Ischemic heart disease, % | 912 (37.1%) | 871 (36.9%) | 41 (41.0%) | 0.400 |
| Laboratory findings | ||||
| Total cholesterol, mg/dL | 158.9±44.8 | 159±44.9 | 156.5±43.1 | 0.593 |
| Triglyceride, mg/dL | 114.9±75.9 | 114.9±76.4 | 115.3±64.6 | 0.966 |
| High‐density lipoprotein‐cholesterol, mg/dL | 43.4±13.3 | 43.4±13.4 | 42.7±11.6 | 0.668 |
| Hemoglobin, g/dL | 12.0±2.3 | 12.0±2.3 | 12.1±2.2 | 0.603 |
| Serum urea nitrogen, mg/dL | 25.8±16.8 | 25.8±16.9 | 25.4±14.9 | 0.819 |
| Creatinine, mg/dL | 1.7±2.0 | 1.7±2.0 | 1.9±2.5 | 0.361 |
| Glucose, mg/dL | 155.1±77.4 | 154.8±77.3 | 162.4±79.1 | 0.347 |
| Echocardiographic findings | ||||
| LV end‐diastolic dimension, mm | 54.1±9.6 | 54.0±9.6 | 56.0±10.1 | 0.044 |
| LV end‐systolic dimension, mm | 41.8±11.9 | 41.8±11.8 | 43.2±13.5 | 0.273 |
| LV end‐diastolic volume, mL | 130.4±66.2 | 129.9±65.7 | 144.1±76.9 | 0.107 |
| LV end‐systolic volume, mL | 86.4±57.8 | 86.0±57.2 | 98.3±69.3 | 0.119 |
| LVEF, % | 39.1±15.6 | 39.1±15.6 | 38.2±16.5 | 0.564 |
| LA diameter, mm | 42.5±8.1 | 42.4±8.1 | 44.2±8.6 | 0.031 |
| LA volume index, mL/m2 | 50.1±25 | 49.8±24.8 | 56.6±29.8 | 0.059 |
| Mitral E/Eʹ ratio | 18.8±11.1 | 18.9±11.2 | 18.4±9.3 | 0.739 |
| Tricuspid regurgitation velocity, m/s | 3.0±0.6 | 3.0±0.6 | 3.0±0.7 | 0.735 |
| LA global peak systolic longitudinal strain, % | 17.2±10.4 | 17.3±10.5 | 14.5±8.8 | 0.010 |
| Phenotype of HF, % | 0.647 | |||
| HF with reduced EF, % | 1437 (58.4%) | 1377 (58.3%) | 60 (60.0%) | |
| HF with midrange EF, % | 342 (14.2%) | 331 (14.3%) | 11 (11.0%) | |
| HF with preserved EF, % | 682 (28.3%) | 653 (28.2%) | 29 (29.0%) | |
| New‐onset atrial fibrillation, % | 397 (16.1%) | 365 (15.5%) | 32 (32.0%) | <0.001 |
EF indicates ejection fraction; HF, heart failure; LA, left atrial; and LV, left ventricular.
Stroke and Its Determinants
In our registry, 108 patients were lost within 1 year, and additional 157 patients were lost within 2 years. We found 100 stroke events during the follow‐up duration (mean duration: 30.3±25.4 months) from medical records with regular clinical follow‐ups and compared variables between the 2 groups according to the presence of stroke (Table 1).
LV end‐diastolic dimension and LA diameter in patients with stroke were significantly higher than that in patients without stroke. Although the difference in stroke incidence was not statistically significant according to HF phenotypes, it was highest in the HFrEF group (60 patients [60%] in HFrEF, 11 [11%] in HF with midrange EF, and 29 [29%] in HF with preserved EF groups). During the follow‐up duration, 397 patients had new‐onset AF. The incidence of new‐onset AF was significantly higher in the stroke group (32.0% versus 15.5%, P<0.001). Moreover, LAGLS was significantly lower in patients with stroke than in patients without stroke (14.5%±8.8% versus 17.3%±10.5%, P=0.010). However, there were no differences between cardiovascular risk factors and LV systolic parameters between the 2 groups. The LA volume index was higher in patients with stroke than that in patients without stroke. However, the difference was not statistically significant between the 2 groups (56.6±28.9 mL/m2 versus 49.8±24.8 mL/m2, P=0.059).
The univariate and multivariate analyses are listed in Table 2. In the univariate analysis, age (hazard ratio [HR], 1.022; 95% CI, 1.006–1.038; P=0.005), diabetes mellitus (HR, 1.552; 95% CI, 1.044–2.307; P=0.030), LA diameter (HR, 1.032; 95% CI, 1.007–1.056; P=0.010), LA volume index (HR, 1.007; 95% CI, 1.002–1.012; P=0.007), new‐onset AF (HR, 2.494; 95% CI, 1.635–3.805; P<0.001), and LAGLS (per 1% decrease, HR, 1.040; 95% CI, 1.017–1.064; P=0.001) were significant determinants of stroke. Because LA diameter showed a higher hazard ratio than that of LA volume index (1.032 versus 1.007), we included LA diameter instead of using LA volume index in the multivariate model. In the multivariate analysis, LAGLS (HR, 1.038; 95% CI, 1.012–1.064; P=0.003) remained statistically significant after the adjustment of age, diabetes mellitus, LA diameter, and new‐onset AF.
Table 2.
Univariate and Multivariate Analysis of the Prediction of Stroke
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Age, y | 1.022 | 1.006–1.038 | 0.005 |
| Male sex | 0.838 | 0.565–1.243 | 0.380 |
| Body mass index, kg/m2 | 1.001 | 0.954–1.050 | 0.963 |
| New York Heart Association functional class IV | 1.534 | 0.995–2.365 | 0.053 |
| Systolic blood pressure, mm Hg | 1.004 | 0.997–1.011 | 0.267 |
| Diastolic blood pressure, mm Hg | 1.001 | 0.989–1.013 | 0.864 |
| Heart rate, /min | 1.001 | 0.992–1.010 | 0.881 |
| Hypertension | 1.285 | 0.857–1.927 | 0.225 |
| Diabetes mellitus | 1.552 | 1.044–2.307 | 0.030 |
| Ischemic heart disease | 1.275 | 0.853–1.905 | 0.236 |
| Total cholesterol, mg/dL | 0.996 | 0.991–1.001 | 0.088 |
| Triglyceride, mg/dL | 1.000 | 0.996–1.003 | 0.835 |
| High‐density lipoprotein‐cholesterol, mg/dL | 0.993 | 0.974–1.013 | 0.492 |
| Hemoglobin, g/dL | 1.002 | 0.895–1.122 | 0.975 |
| Serum urea nitrogen, mg/dL | 1.004 | 0.992–1.015 | 0.517 |
| Creatinine, mg/dL | 1.041 | 0.971–1.116 | 0.261 |
| Glucose, mg/dL | 1.002 | 0.999–1.004 | 0.140 |
| LV end‐diastolic dimension, mm | 1.017 | 0.996–1.038 | 0.115 |
| LV end‐systolic dimension, mm | 1.007 | 0.989–1.026 | 0.434 |
| LV end‐diastolic volume, mL | 1.003 | 1.000–1.006 | 0.053 |
| LV end‐systolic volume, mL | 1.003 | 1.000–1.007 | 0.058 |
| LVEF, % | 0.996 | 0.983–1.008 | 0.496 |
| LA diameter, mm | 1.032 | 1.007–1.056 | 0.010 |
| LA volume index, mL/m2 | 1.007 | 1.002–1.012 | 0.007 |
| Mitral E/Eʹ ratio | 1.001 | 0.982–1.021 | 0.907 |
| Tricuspid regurgitation velocity, m/s | 1.223 | 0.780–1.919 | 0.380 |
| New‐onset AF | 2.494 | 1.635–3.805 | <0.001 |
| LAGLS (per 1% decrease) | 1.040 | 1.017–1.064 | 0.001 |
| Multivariate analysis | |||
| Age, y | 1.024 | 1.007–1.041 | 0.006 |
| Diabetes mellitus | 1.655 | 1.102–2.486 | 0.015 |
| LA diameter, mm | 1.015 | 0.989–1.043 | 0.253 |
| New‐onset AF | 1.557 | 0.737–3.290 | 0.246 |
| LAGLS (per 1% decrease) | 1.038 | 1.012–1.064 | 0.003 |
AF indicates atrial fibrillation; EF, ejection fraction; HR, hazard ratio; LA, left atrial; LV, left ventricular; and LAGLS, left atrial global peak systolic longitudinal strain.
We assessed the best cutoff point of LAGLS for the prediction of stroke by performing the receiver operating curve analysis with the Youden index and found that 14.5% was the best cutoff point. The annual incidences of stroke in patients with LAGLS <14.5% and ≥14.5% were 2.38/100 person‐year and 1.08/100‐person‐year (P=0.008), respectively. After the adjustment of age, sex, diabetes mellitus, cholesterol concentration, LV end‐diastolic volume, LA diameter, and new‐onset AF, patients with LAGLS <14.5% still had a higher incidence of stroke than patients with LAGLS ≥14.5% (HR, 1.940; 95% CI, 1.269–2.965; P=0.002).
In the survival analysis with the Kaplan‐Meier method, patients with LAGLS ≥14.5% had significantly higher 5‐year stroke‐free survival than patients with LAGLS <14.5% (96.0%±0.7% versus 90.4%±1.4%, P<0.001, Figure 2).
Figure 2. Event‐free survival curve according to a left atrial global longitudinal strain (LAGLS) of 14.5%.
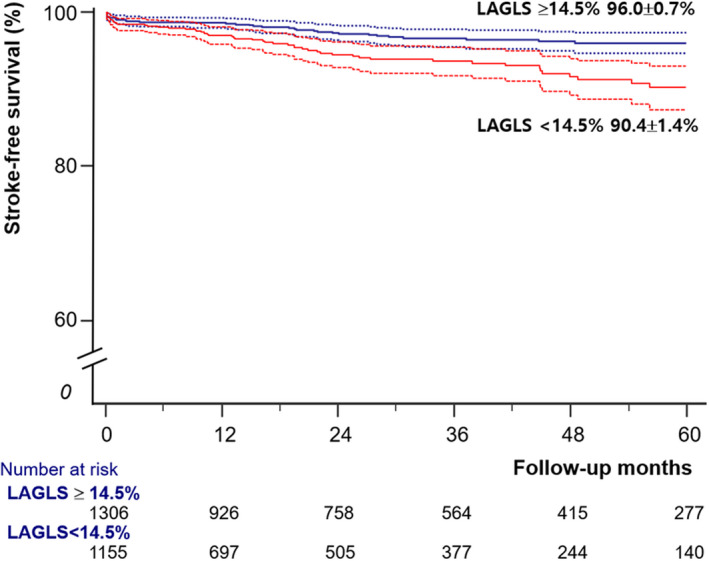
In patients with acute heart failure and sinus rhythm, LAGLS <14.5% had significantly higher stroke risk (P<0.001 by log‐rank test).
Variability of LAGLS
The intraobserver variability of the intraclass correlation coefficient of LAGLS was 0.957 (95% CI, 0.889–0.982), and interobserver variabilities of the intraclass correlation coefficient was 0.938 (95% CI, 0.844–0.976).
Discussion
In this study, we showed that LAGLS was an independent predictor of stroke in AHF patients with sinus rhythm and that decreased LAGLS was associated with an increased risk of stroke after the adjustment of other clinically important variables.
Stroke is a major cardiovascular disease with significant disability and mortality. The identification of patients at high risk of developing stroke is important to prevent stroke and to reduce individual and social burden. Control of hypertension is a well‐known method to lower stroke risk and prevention.21 AF is a well‐known powerful risk factor for stroke, and anticoagulation therapy in patients with AF can reduce stroke risk.22 Even without AF, HF itself can increase the risk of stroke because HF patients have body fluid imbalances, endothelial dysfunction, proinflammatory and prothrombotic responses.23, 24 A recent population‐based study comprising HF patients showed that HF patients had a higher risk of stroke compared with the general population.25 There are arguments on antiplatelet or anticoagulation therapy in HF with sinus rhythm. In the Warfarin and Antiplatelet Therapy in Chronic Heart Failure trial, warfarin treatment had more favorable effect in reducing all strokes compared with aspirin (P=0.0163) or clopidogrel (P=0.0164).26 In the Warfarin Versus Aspirin in Reduced Cardiac Ejection Fraction trial, the incidence of ischemic stroke was significantly lower with warfarin treatment than that with aspirin treatment.27 However, the use of warfarin can increase the risk of bleeding.27 Therefore, with the proper identification of patients with high risk for stroke, the unnecessary use of anticoagulants and the bleeding complications associated with the anticoagulants can be reduced. In a previous meta‐analysis, the annual incidence of stroke in patients with HF and sinus rhythm was 1.2%. In our study, the annual incidence of stroke was 1.60%. In patients with LAGLS <14.5%, the annual stroke incidence was 2.38%, which corresponds to a CHA2DS2‐VASc score of 2 to 3. Therefore, further studies are needed to verify the effectiveness of anticoagulation therapy in this group.
LA diameter and LA volume index were also significant predictors of stroke in our study. Although increased LA size and volume were associated with an increased risk of ischemic stroke,28, 29 the mechanisms related to LA enlargement and stroke events have not been fully evaluated. There are several possible hypotheses. First, LA enlargement may precede AF development.30 In our previous study, we demonstrated that reduced LAGLS (<18.0%) and increased LA volume index were associated with increased risk of new‐onset AF in patients with AHF.15 Second, LA enlargement can be a marker of reduced LV diastolic filling and may promote blood stasis and thrombus formation.31 Patients with HF have several risk factors of developing AF, and approximately 40% of people with either HF or AF will have the other disease category.32 Furthermore, HF meets all the criteria of Virchow’s triad, which leads to increased risk of thrombus formation. Third, increased LA size and volume can be associated with other risk factors including hypertension and atherosclerosis, which have increased risk of stroke.33
Fibrosis of the LA wall can result in LA dysfunction and is influenced by several biological factors, including energy metabolism, neurohumoral factors, and renin‐angiotensin‐aldosterone system.34 Increased fibrosis of the LA can provoke AF, correlates with both persistence and burden of AF, and increase subsequent embolic risk. The decreased LA function and blood stasis in LA can increase the risk of thrombus and subsequent risk of embolic infarction.35 Gadolinium‐enhanced magnetic resonance imaging can be used for the detection and quantification of LA fibrosis, but methodological challenges limit its use.36 Strain analysis can estimate the extent of LA fibrosis, and the lower LAGLS can represent a higher extent of fibrosis.37 Decreased LAGLS can be used as a poor prognostic factor in several cardiovascular diseases.14, 38 Because LAGLS can detect subclinical LA dysfunction before the development of LA enlargement and may offer unique insights into LA pathophysiology, it is a more sensitive and reproducible marker of LA function than other echocardiographic volumetric indices.38, 39 Therefore, LA strain can be used as a prognostic marker for cryptogenic stroke.40, 41, 42
Limitations
This study has several limitations. First, this study was limited by the inherent limitations of its retrospective nature. Thus, residual confounding variables may have affected our findings due to the observational nature of this study. Second, the incidence of stroke may have been underestimated in our study. In our study, we had 100 patients with stroke (4.1%) during the study period (mean 30.3±25.4 months). This result was similar to that of the previous study performed in Denmark. They found that the incidence of ischemic stroke in patients with HF and sinus rhythm was 1.54% during the first year after HF.25 Therefore, we might identify nearly all episodes of stroke.
Third, whether a noncardiac source of emboli was identified in all patients with stroke was not confirmed. However, because the main etiology of stroke in HF patients with stroke is an ischemic etiology from the heart,10 the proportion of nonischemic stroke may be low in our patients. Fourth, we identified the presence of stroke from their medical records during the regular clinical follow‐up. Thus, there might be missed cases of stroke in this study. However, because most stroke patients are treated at nearby regional cardiocerebrovascular disease centers, and 3 hospitals in this registry are regional cardiocerebrovascular disease centers, the proportion of missed patients with stroke in this study might be small. Fifth, because this study cohort included only East Asian patients admitted for AHF, the results may not be generalizable in different clinical settings or to those with other ethnicities.
Conclusions
LAGLS was a good predictor of developing stroke despite other risk factors including age, diabetes mellitus, cholesterol concentration, LV volume, and LA volume index in AHF patients with sinus rhythms. In patients with LAGLS <14.5%, the annual stroke incidence was 2.38%.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 7.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving BO, Shiue I, Ng M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. DOI: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 3.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short‐term risk of stroke: case‐crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. DOI: 10.1161/CIRCEP.114.003057. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new‐onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. DOI: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 5.Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982. DOI: 10.1161/STROKEAHA.111.628479. [DOI] [PubMed] [Google Scholar]
- 6.Pullicino PM, Halperin JL, Thompson JL. Stroke in patients with heart failure and reduced left ventricular ejection fraction. Neurology. 2000;54:288–294. DOI: 10.1212/WNL.54.2.288. [DOI] [PubMed] [Google Scholar]
- 7.Lip GYH, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow's triad revisited. J Am Coll Cardiol. 1999;33:1424–1426. [DOI] [PubMed] [Google Scholar]
- 8.Dunkman WB, Johnson GR, Carson PE, Bhat G, Farrell L, Cohn JN. Incidence of thromboembolic events in congestive‐heart‐failure. Circulation. 1993;87:94–101. [PubMed] [Google Scholar]
- 9.Fanola CL, Norby FL, Shah AM, Chang PP, Lutsey PL, Rosamond WD, Cushman M, Folsom AR. Incident heart failure and long‐term risk for venous thromboembolism. J Am Coll Cardiol. 2020;75:148–158. DOI: 10.1016/j.jacc.2019.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherbakov N, Haeusler KG, Doehner W. Ischemic stroke and heart failure: facts and numbers. ESC Heart Fail. 2015;2:1–4. DOI: 10.1002/ehf2.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107:85–91. DOI: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy‐Dicey A, Harris TB, Pencina MJ, D’Agostino RB, Levy D, Kannel WB, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. DOI: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JH. Two‐dimensional echocardiographic assessment of myocardial strain: important echocardiographic parameter readily useful in clinical field. Korean Circ J. 2019;49:908–931. DOI: 10.4070/kcj.2019.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JH, Hwang IC, Park JJ, Park JB, Cho GY. Prognostic power of left atrial strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging. 2021;22:210–219. DOI: 10.1093/ehjci/jeaa013. [DOI] [PubMed] [Google Scholar]
- 15.Park JJ, Park JH, Hwang IC, Park JB, Cho GY, Marwick TH. Left atrial strain as a predictor of new‐onset atrial fibrillation in patients with heart failure. JACC Cardiovasc Imaging. 2020;13:2071–2081. DOI: 10.1016/j.jcmg.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. DOI: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. DOI: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. DOI: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle‐tracking echocardiography: a systematic review and meta‐analysis. J Am Soc Echocardiogr. 2017;30:59–70.e8. DOI: 10.1016/j.echo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR 3rd, Polonsky T, Thompson‐Paul AM, Vupputuri S. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138:e595–e616. DOI: 10.1161/CIR.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 22.Xian Y, O’Brien EC, Liang LI, Xu H, Schwamm LH, Fonarow GC, Bhatt DL, Smith EE, Olson DM, Maisch L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in‐hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317:1057–1067. DOI: 10.1001/jama.2017.1371. [DOI] [PubMed] [Google Scholar]
- 23.Kim W, Kim EJ. Heart failure as a risk factor for stroke. J Stroke. 2018;20:33–45. DOI: 10.5853/jos.2017.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira JP, Girerd N, Gregson J, Latar I, Sharma A, Pfeffer MA, McMurray JJV, Abdul‐Rahim AH, Pitt B, Dickstein K, et al. Stroke risk in patients with reduced ejection fraction after myocardial infarction without atrial fibrillation. J Am Coll Cardiol. 2018;71:727–735. DOI: 10.1016/j.jacc.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Adelborg K, Szepligeti S, Sundboll J, Horvath‐Puho E, Henderson VW, Ording A, Pedersen L, Sorensen HT. Risk of stroke in patients with heart failure a population‐based 30‐year cohort study. Stroke. 2017;48:1161. DOI: 10.1161/STROKEAHA.116.016022. [DOI] [PubMed] [Google Scholar]
- 26.Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JGF, Ezekowitz M, Jafri SM, Krol WF, O'Connor CM, Schulman KA, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. DOI: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 27.Homma S, Thompson JLP, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. DOI: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biteker M, Kayatas K, Basaran O, Dogan V, Ozlek E, Ozlek B. The role of left atrial volume index in patients with a first‐ever acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:321–326. DOI: 10.1016/j.jstrokecerebrovasdis.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. DOI: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 30.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. DOI: 10.1161/01.CIR.89.2.724. [DOI] [PubMed] [Google Scholar]
- 31.Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, Chun Y‐H, Tsuji H, Wada H, Hasegawa K, et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non‐valvular atrial fibrillation. Sci Rep. 2016;6:31042. DOI: 10.1038/srep31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. DOI: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 33.Pierdomenico SD, Pierdomenico AM, Di Carlo S, Di Tommaso R, Cuccurullo F. Left atrial enlargement and risk of ischemic stroke in elderly treated hypertensive patients. Am J Hypertens. 2014;27:1179–1184. DOI: 10.1093/ajh/hpu042. [DOI] [PubMed] [Google Scholar]
- 34.Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10:65–77. DOI: 10.1016/j.jcmg.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Kang SH, Kim J, Park JJ, Oh IY, Yoon CH, Kim HJ, Kim K, Choi DJ. Risk of stroke in congestive heart failure with and without atrial fibrillation. Int J Cardiol. 2017;248:182–187. DOI: 10.1016/j.ijcard.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 36.Hirsh BJ, Copeland‐Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. DOI: 10.1016/j.jacc.2015.03.557. [DOI] [PubMed] [Google Scholar]
- 37.Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. DOI: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 38.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen‐Torvik LJ, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9:e003754. DOI: 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:1961–1977. DOI: 10.1016/j.jacc.2019.01.059. [DOI] [PubMed] [Google Scholar]
- 40.Pirinen J, Jarvinen V, Martinez‐Majander N, Sinisalo J, Poyhonen P, Putaala J. Left atrial dynamics is altered in young adults with cryptogenic ischemic stroke: a case‐control study utilizing advanced echocardiography. J Am Heart Assoc. 2020;9:e014578. DOI: 10.1161/JAHA.119.014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leong DP, Joyce E, Debonnaire P, Katsanos S, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Left atrial dysfunction in the pathogenesis of cryptogenic stroke: novel insights from speckle‐tracking echocardiography. J Am Soc Echocardiogr. 2017;30:71–79.e1. DOI: 10.1016/j.echo.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Witt BJ, Gami AS, Ballman KV, Brown RD, Meverden RA, Jacobsen SJ, Roger VL. The incidence of ischemic stroke in chronic heart failure: a meta‐analysis. J Card Fail. 2007;13:489–496. DOI: 10.1016/j.cardfail.2007.01.009. [DOI] [PubMed] [Google Scholar]


