Abstract
Background
Blood pressure variability (BPV) in midlife increases risk of late‐life dementia, but the impact of BPV on the cognition of adults who have already reached older ages free of major cognitive deficits is unknown. We examined the risk of incident dementia and cognitive decline associated with long‐term, visit‐to‐visit BPV in a post hoc analysis of the ASPREE (Aspirin in Reducing Events in the Elderly) trial.
Methods and Results
ASPREE participants (N=19 114) were free of dementia and significant cognitive impairment at enrollment. Measurement of BP and administration of a standardized cognitive battery evaluating global cognition, delayed episodic memory, verbal fluency, and processing speed and attention occurred at baseline and follow‐up visits. Time‐to‐event analysis using Cox proportional hazards regression models were used to calculate hazard ratios (HR) and corresponding 95% CI for incident dementia and cognitive decline, according to tertile of SD of systolic BPV. Individuals in the highest BPV tertile compared with the lowest had an increased risk of incident dementia and cognitive decline, independent of average BP and use of antihypertensive drugs. There was evidence that sex modified the association with incident dementia (interaction P=0.02), with increased risk in men (HR, 1.68; 95% CI, 1.19–2.39) but not women (HR, 1.01; 95% CI, 0.72–1.42). For cognitive decline, similar increased risks were observed for men and women (interaction P=0.15; men: HR, 1.36; 95% CI, 1.16–1.59; women: HR, 1.14; 95% CI, 0.98–1.32).
Conclusions
High BPV in older adults without major cognitive impairment, particularly men, is associated with increased risks of dementia and cognitive decline.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01038583; isrctn.com. Identifier: ISRCTN83772183.
Keywords: blood pressure, blood pressure variability, cognitive impairment, dementia
Subject Categories: High Blood Pressure
Nonstandard Abbreviations and Acronyms
- ASPREE
Aspirin in Reducing Events in the Elderly
- BPV
blood pressure variability
Clinical Perspective
What Is New?
We explored the association of long‐term, visit‐to‐visit blood pressure variability with risk of incident dementia and cognitive decline in a generally healthy cohort of older adults who were enrolled in the ASPREE (Aspirin in Reducing Events in the Elderly) trial.
Because ASPREE was not a blood pressure intervention study, and included adults who were normotensive and hypertensive, investigating blood pressure variability and its risks in this cohort potentially increases the generalizability of the findings to a broader population of older adults.
What Are the Clinical Implications?
We observed increased risks of incident dementia and cognitive decline, which were independent of average blood pressure and use of antihypertensive drugs, for individuals in the highest blood pressure variability tertile compared with the lowest.
There was evidence of interaction by sex, with men demonstrating the most increased risk of dementia and cognitive decline; it remains uncertain whether reducing blood pressure variability can protect from the development of cognitive decline.
Sex is emerging as a key biological variable in cerebrovascular and cardiovascular research; our findings highlight the need for further research into the potential mechanisms underlying sex‐specific differences in diseases of aging.
Increased longevity and a decline in fertility in recent decades have led to an aging global population. It is estimated that people aged 60 years and older will outnumber adolescents and youth by 2050.1 As an age‐related disease, dementia has become a major public health concern worldwide, and the lack of disease‐modifying treatments ensures it will indefinitely remain a public health priority.
Hypertension is highly prevalent in midlife and is a strong risk factor for late‐life cognitive decline and dementia.2, 3, 4 Although the duration and severity of hypertension are important etiologies,2, 3, 4, 5, 6, 7 the short‐ and long‐term fluctuation, or variability, of blood pressure (BP) has emerged as a novel risk factor for cognitive impairment and Alzheimer's disease.8, 9, 10, 11, 12, 13, 14, 15, 16 However, previous studies examining high BP variability (BPV) and cognitive outcomes had limitations. These include using a single cognitive assessment or instrument,12 analyzing the relationship within predominantly younger cohorts,6, 7, 9, 10, 11, 16 in cohorts at high risk of cardiovascular disease and receiving antihypertensive drugs,14 or in individuals with established cognitive impairment.16
Short‐term BPV can be ascertained through 24‐hour BP monitoring, but it is an assessment not routinely used in clinical practice.17 Office BPs accumulated over multiple visits are more readily accessible, and an estimate of long‐term BPV can be obtained from these readings.18 In adults who have already reached advanced aged with preserved cognition and in otherwise good health, it is unknown whether long‐term BPV is relevant to predicting their cognitive trajectory during their remaining lifespan. As a possible clinically actionable biomarker, there is a need for research to determine the extent that long‐term BPV, beyond that of routine BP, can identify older adults at increased risk of cognitive impairment. Large cohorts with standardized assessments of BP and cognition conducted in parallel throughout long‐term follow‐up are required to address this research gap. The ASPREE (Aspirin in Reducing Events in the Elderly) study19 is a longitudinal cohort uniquely suited to answer this question.
We examined the risk of incident dementia and cognitive decline associated with long‐term, visit‐to‐visit BPV in participants of ASPREE, a randomized primary prevention trial of daily low‐dose aspirin conducted in 19 114 older adults who were free from dementia, significant cognitive impairment, disability, or prior cardiovascular disease events, at baseline.
Methods
The data (version 3.0) that support the findings of this study are available from the ASPREE Data Coordinating Center, Monash University School of Public Health (Aspree.AMS@monash.edu) upon reasonable request.
Study Participants
The detailed methods of ASPREE, its recruitment, and primary outcomes have been previously reported.19, 20, 21 Briefly, ASPREE enrolled community‐dwelling adults aged 70 years and older from Australia and the United States (65 years and older if US minority) from March 2010 to December 2014 and randomized them to aspirin 100 mg daily or matching placebo. At enrollment, participants were free of documented evidence of dementia, significant physical disability, prior cardiovascular events, as well as any medical condition expected to limit life expectancy to <5 years. Individuals with uncontrolled high BP (systolic BP ≥180 mm Hg and/or diastolic BP ≥105 mm Hg), or a Modified Mini‐Mental State Examination score of <78 were ineligible. All participants provided written informed consent for their participation, and the study was approved by institutional review boards in both countries. After a median follow‐up of 4.7 years, aspirin did not extend disability‐free survival21—a composite of death, incident dementia, or persistent physical disability—nor did it lower the risk of cognitive decline.22
Standard Assessments
After completing baseline visits, participants were contacted quarterly by telephone and seen annually in person for clinical assessments by trained study staff following standard operating procedures. These assessments gathered data on physical function, lifestyle, anthropometrics, cognition, disability, and other health parameters, including medical diagnoses and prescription medications.
A standardized cognitive battery was administered at the baseline and at regular intervals over follow‐up visits. The battery consisted of the Modified Mini‐Mental State Examination for global cognition,23 the Hopkins Verbal Learning Test‐Revised for delayed episodic memory,24 the single letter (F) Controlled Oral Word Association Test for verbal fluency,25 and the Symbol Digit Modalities Test for processing speed and attention.26 The 10‐item Center for Epidemiologic Studies‐Depression scale was administered before the cognitive battery to account for the possible confounding effect of depression on cognitive function.
Assessment of Visit‐to‐Visit BPV
Blood pressure was measured at each study visit according to American Heart Association guidelines, in the seated position after at least 5 minutes of rest using a validated automated oscillometric device with an occluding cuff of appropriate size for the upper arm circumference.27 Three separate and consecutive BP readings 1 minute apart were taken in a single arm. Consistent with the main ASPREE study reports, the average of all 3 measurements in each participant was recorded as the BP for that visit.
Several methods to estimate BPV have been used to characterize both short‐term and long‐term variability, with no universal consensus on the best measure.18 Long‐term, visit‐to‐visit BPV is usually estimated using the SD18; therefore, we estimated BPV initially using the within‐individual SD of mean systolic BP obtained from the baseline, first‐, and second‐year annual visits. Sensitivity analyses were also undertaken to assess the robustness of the results, using the coefficient of variation and average real variability to estimate BPV, as well as expanding the SD estimate of BPV to 4 measures (baseline, first‐, second‐, and third‐year annual visits), and then analyzing events occurring after the third annual visit. Lastly, we also explored the relationship between diastolic BPV and incident dementia and cognitive decline, estimating diastolic BPV in a similar manner as our primary BPV estimate.
Cognitive Outcomes
The protocol for clinical adjudication of incident dementia in ASPREE has been reported previously.22 Suspected dementia “triggers” were identified by a Modified Mini‐Mental State Examination score <78 or a drop of >10.15 points from baseline (adjusted for age and education), or medical record report of dementia or memory problems, or prescription for cholinesterase inhibitor. Following a trigger, additional standardized cognitive and functional assessments were conducted whenever possible, which included the Alzheimer's Disease Assessment Scale—Cognitive subscale,28 Color Trails,29 Lurian overlapping figures,30 and the Alzheimer Disease Cooperative Study Activities of Daily Living Scale.31 Clinical case notes and other supporting documentation were also obtained for these dementia triggers. All information was reviewed by an expert panel, blinded to treatment arm, who adjudicated the dementia end point using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria.32
Because individuals could experience deterioration in cognitive function without meeting the threshold for referral for further testing, we also separately examined a broader outcome of incident cognitive decline. This was defined as a >1.5 SD decline in score from an individual's baseline on any of the cognitive tests (Modified Mini‐Mental State Examination, Symbol Digit Modalities Test, Hopkins Verbal Learning Test‐Revised delayed recall, and/or Controlled Oral Word Association Test) during follow‐up and sustained over 2 testing time points.22
Statistical Analysis
Cox proportional hazards regression models with time‐to‐event analysis were used to calculate hazard ratios (HR) and corresponding 95% CI for incident dementia, using BPV as a continuous variable, and then according to tertile of BPV. The proportional hazards assumption was checked using Schoenfeld residuals and found to be appropriate. Year 2 was used as the new baseline. Tertiles were defined initially for the entire sample and then separately for men and women for planned a priori, sex‐specific analyses. Initial models were adjusted for age and sex, followed by additional adjustment for diabetes mellitus, depression (10‐item Center for Epidemiologic Studies‐Depression scale score ≥8), body mass index, statin use, smoking, dyslipidemia, ethnicity, education, and living situation. Analyses were repeated similarly for incident cognitive decline. To minimize immortal time bias during the period used to estimate BPV,33 participants with incident dementia or cognitive decline in the first 2 years were excluded from the analysis. The study design is shown in Figure S1.
We investigated potential effect modification by sex, and antihypertensive drug use,3, 34 by including a multiplicative interaction term in the models. When significance was found, BPV tertiles were redefined separately within the subgroups and stratified analyses performed. Sensitivity analyses were also conducted restricting the analysis to only those participants who remained consistently on or off antihypertensive drugs during the BPV estimation period, to account for potential variability in BP that could be linked to initiation or discontinuation of antihypertensives.35 A 2‐sided P value of <0.05 was used as the cutoff for statistical significance. All analyses were conducted using Stata version 16 (StataCorp, College Station, TX).
Results
Among 19 114 individuals originally randomized into ASPREE, 16 758 participants remained in the study and had mean BP recorded at baseline, year 1, and year 2 visits, for estimation of BPV (Figure 1). Of these participants, 16 600 were free of dementia at 2 years, and 396 cases of incident dementia subsequently occurred over a median follow‐up of 2.7 years (95% CI, 1.6–3.6). At 2 years, 14 105 participants were free of cognitive decline, and 1993 events subsequently occurred over a median follow‐up of 2.0 years (95% CI, 1.0–3.0). In the initial age and sex‐adjusted Cox models, being in the highest tertile of BPV compared with the lowest tertile was associated with an increased risk of both incident dementia (HR, 1.33; 95% CI, 1.05–1.70) and cognitive decline (HR, 1.21; 95% CI, 1.09–1.35) (Figure S2). However, there was evidence that sex modified this association (interaction P=0.02 for incident dementia and P=0.15 for cognitive decline). As such, all subsequent analyses are presented separately for men and women.
Figure 1. Consort flow diagram of participants included in the analysis.
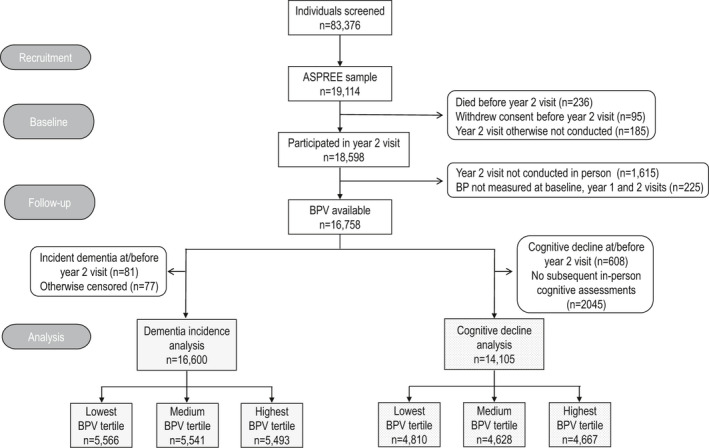
ASPREE indicates Aspirin in Reducing Events in the Elderly; BP, blood pressure; and BPV, blood pressure variability.
Table 1 shows the baseline characteristics according to tertiles of BPV of the men and women included in the analysis of incident dementia. On average, women had higher BPV than men. As tertiles progressed from lowest to highest, mean age increased as did baseline and average systolic BP, and participants had more comorbidities such as diabetes mellitus, chronic kidney disease, and pack years of smoking. Baseline cognitive scores were similar across all BPV tertiles for both men and women. Characteristics of participants included in the analysis of incident cognitive decline demonstrated mostly similar patterns (Table S1).
Table 1.
Baseline Characteristics of ASPREE Participants Included in the Dementia Incidence Analysis, by Sex and Tertiles of BPV (n=16 600)
| Characteristic | Men | Women | ||||
|---|---|---|---|---|---|---|
| T1, n=2503 | T2, n=2401 | T3, n=2414 | T1, n=3117 | T2, n=3083 | T3, n=3082 | |
| SD of SBP, mean (SD) | 4.2 (1.6) | 9.0 (1.4) | 16.6 (4.4) | 4.4 (1.7) | 9.4 (1.4) | 17.4 (5.0) |
| Baseline SBP, mm Hg, mean (SD) | 137.9 (13.8) | 140.5 (14.8) | 145.0 (17.8) | 133.6 (14.5) | 136.4 (15.6) | 142.6 (18.7) |
| Baseline diastolic blood pressure, mm Hg, mean (SD) | 77.2 (8.9) | 77.9 (9.3) | 79.2 (10.3) | 75.3 (9.5) | 76.3 (9.8) | 78.2 (11.0) |
| Baseline antihypertensive medications, n (%) | 1116 (44.6) | 1090 (45.4) | 1360 (56.3) | 1509 (48.4) | 1665 (54.0) | 1887 (61.2) |
| Average SBP* over blood pressure variability period, mean (SD) | 137.7 (13.4) | 139.4 (13.1) | 141.9 (13.0) | 133.3 (14.0) | 135.6 (13.6) | 139.7 (13.5) |
| Ethno‐racial group, n (%) | ||||||
| Australian White | 2240 (89.5) | 2152 (89.6) | 2141 (88.7) | 2666 (85.5) | 2616 (84.9) | 2592 (84.1) |
| US White | 114 (4.6) | 101 (4.2) | 87 (3.6) | 204 (6.5) | 225 (7.3) | 208 (6.8) |
| Black | 69 (2.8) | 65 (2.7) | 71 (2.9) | 131 (4.2) | 143 (4.6) | 169 (5.5) |
| Hispanic/Latino | 52 (2.1) | 43 (1.8) | 61 (2.5) | 82 (2.6) | 67 (2.2) | 70 (2.3) |
| Other | 28 (1.1) | 40 (1.7) | 54 (2.2) | 34 (1.1) | 32 (1.0) | 43 (1.4) |
| Age, y, n (%) | ||||||
| 65–73 | 1366 (54.6) | 1263 (52.6) | 1175 (48.7) | 1701 (54.6) | 1540 (50.0) | 1392 (45.2) |
| ≥74 | 1137 (45.4) | 1138 (47.4) | 1239 (51.3) | 1416 (45.4) | 1543 (50.1) | 1690 (54.8) |
| Education y, n (%) | ||||||
| <12 | 1096 (43.8) | 1034 (43.1) | 1038 (43.0) | 1460 (46.8) | 1385 (44.9) | 1433 (46.5) |
| 12–15 | 657 (26.3) | 657 (27.4) | 671 (27.8) | 899 (28.8) | 967 (31.4) | 922 (29.9) |
| 16+ | 750 (30.0) | 710 (29.6) | 705 (29.2) | 758 (24.3) | 731 (23.7) | 727 (23.6) |
| Alcohol, n (%) | ||||||
| Current | 2096 (83.7) | 2044 (85.1) | 2013 (83.4) | 2264 (72.6) | 2229 (72.3) | 2226 (72.2) |
| Former | 173 (6.9) | 153 (6.4) | 167 (6.9) | 150 (4.8) | 122 (4.0) | 150 (4.9) |
| Never | 234 (9.4) | 204 (8.5) | 234 (9.7) | 703 (22.6) | 732 (23.7) | 706 (22.9) |
| Body mass index, kg/m2, mean (SD) | 27.9 (3.9) | 27.9 (3.9) | 28.1 (4.0) | 28.1 (5.0) | 28.1 (5.2) | 28.4 (5.3) |
| Living alone, n (%) | 482 (19.3) | 456 (19.0) | 519 (21.5) | 1246 (40.0) | 1275 (41.4) | 1323 (42.9) |
| Current or past smoker, n (%) | 1372 (54.8) | 1330 (55.4) | 1415 (58.6) | 1080 (34.7) | 1040 (33.7) | 1067 (34.6) |
| Smoking pack years, mean (SD) | 24.3 (27.7) | 24.5 (25.5) | 25.2 (25.4) | 17.7 (20.2) | 18.0 (18.9) | 18.8 (21.5) |
| Diabetes mellitus, n (%) | 265 (10.6) | 291 (12.1) | 331 (13.7) | 270 (8.7) | 246 (8.0) | 304 (9.9) |
| Depression, n (%) | 181 (7.2) | 176 (7.3) | 181 (7.5) | 358 (11.5) | 320 (10.4) | 348 (11.3) |
| Dyslipidemia, n (%) | 1392 (55.6) | 1328 (55.3) | 1326 (54.9) | 2275 (73.0) | 2233 (72.4) | 2282 (74.0) |
| Chronic kidney disease, n (%) | 507 (21.9) | 545 (24.4) | 643 (28.6) | 702 (24.1) | 704 (24.5) | 878 (30.6) |
| Statin medications, n (%) | 712 (28.5) | 663 (27.6) | 675 (28.0) | 1025 (32.9) | 1010 (32.8) | 1084 (35.2) |
| Aspirin treatment assignment, n (%) | 1249 (49.9) | 1186 (49.4) | 1189 (49.3) | 1572 (50.4) | 1516 (49.2) | 1523 (49.4) |
| Pulse pressure, mean (SD) | 60.7 (12.1) | 62.6 (12.4) | 65.9 (14.3) | 58.2 (12.9) | 60.1 (13.6) | 64.3 (15.2) |
| Heart rate, mean (SD) | 69.2 (10.4) | 69.0 (11.0) | 68.5 (11.1) | 72.6 (9.9) | 72.0 (10.2) | 71.2 (10.6) |
| SD of heart rate variability, mean (SD) | 5.4 (3.5) | 5.7 (3.9) | 6.2 (4.2) | 5.0 (3.4) | 5.3 (3.6) | 5.7 (3.8) |
| Cognitive performance | ||||||
| Modified Mini‐Mental State Examination, mean (SD) | 93.2 (4.5) | 92.8 (4.7) | 92.9 (4.6) | 94.3 (4.3) | 94.2 (4.2) | 94.2 (4.3) |
| Hopkins Verbal Learning Test‐Revised delayed recall, mean (SD) | 7.3 (2.8) | 7.3 (2.9) | 7.3 (2.8) | 8.3 (2.7) | 8.4 (2.7) | 8.3 (2.7) |
| Symbol Digit Modalities Test, mean (SD) | 36.4 (9.6) | 35.9 (9.9) | 35.1 (9.9) | 38.8 (9.8) | 38.7 (10.0) | 37.6 (10.1) |
| Controlled Oral Word Association Test, mean (SD) | 11.7 (4.5) | 11.5 (4.5) | 11.6 (4.5) | 12.6 (4.6) | 12.7 (4.5) | 12.7 (4.5) |
Age was categorized based on the median age of participants, which was 74 years. Ethno‐racial group was based on self‐report. Other ethno‐racial group was defined as any category with less than 200 participants overall, which included Aboriginal or Torres Strait Islander, Native American, multiple races or ethnic groups, Native Hawaiian or Pacific Islander, and those who indicated that they were not Hispanic but did not state another race or ethnic group. Diabetes mellitus was defined as a participants' report of diabetes mellitus or a fasting glucose level of ≥126 mg per deciliter (≥7 mmol per liter) or receipt of treatment for diabetes mellitus. Depression was defined as a 10‐item Center for Epidemiological Studies Depression Scale score of ≥8. Dyslipidemia was defined as serum cholesterol level of ≥212 mg per deciliter (≥5.5 mmol per liter) in Australia and ≥240 mg per deciliter (≥6.2 mmol per liter) in the United States or as a low‐density lipoprotein level of >160 mg per deciliter (>4.1 mmol per liter); or taking cholesterol‐lowering medication. Chronic kidney disease was defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2 or albumin to creatinine ratio ≥3 mg/mmol. ASPREE indicates Aspirin in Reducing Events in the Elderly; BPV, blood pressure variability; SBP, systolic blood pressure; and T, tertile.
Over the period in which BPV was measured, thus baseline to the second annual visit.
Following the year 2 visit, incident dementia occurred in 2.6% (188/7318) of men and 2.2% (208/9282) of women, whereas incident cognitive decline occurred in 14.7% (915/6204) and 13.6% (1078/7901) of men and women, respectively (Figure 2). In the multivariate‐adjusted Cox model, men in the highest tertile of BPV were at significantly increased risk of both incident dementia (HR, 1.68; 95% CI, 1.19–2.39; P=0.004) and cognitive decline (HR, 1.36; 95% CI, 1.16–1.59; P<0.0001) compared with those in the lowest tertile (Table 2). The HRs were minimally changed after further adjustment for additional covariates including ethnicity, living status, smoking, dyslipidemia, or chronic kidney disease. Furthermore, these associations remained consistent after exclusion of men with a stroke or myocardial infarction during the trial (data not shown). In women, there was no significant association between tertile of BPV and incident dementia (HR, 1.01; 95% CI, 0.72–1.42; P=0.96) or cognitive decline (HR, 1.14; 95% CI, 0.98–1.32; P=0.09). When BPV was treated as a continuous variable, the findings in men and women remained consistent (Table 2).
Figure 2. Cumulative incidence of events according to blood pressure variability tertile; dementia incidence in men (A); dementia incidence in women (B); incidence of cognitive decline in men (C); and incidence of cognitive decline in women (D).
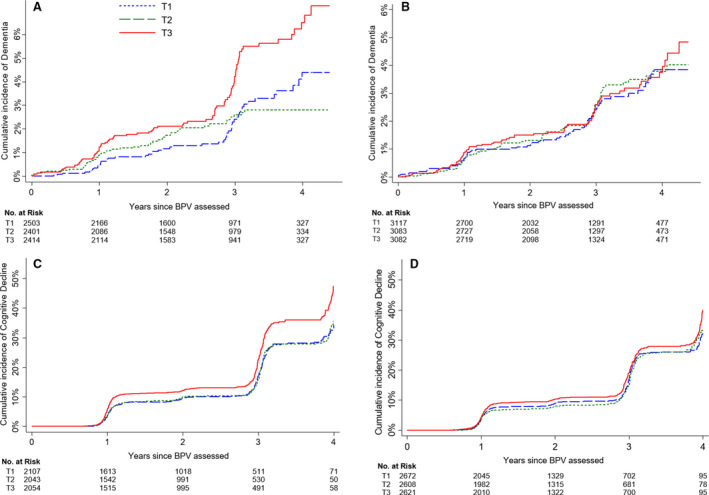
BPV indicates blood pressure variability; and T, tertile.
Table 2.
Cox Proportional Hazards Analysis for the Association Between BPV and Incident Dementia and Cognitive Decline
| No. | No. of Events | Age Adjusted | Multivariate Adjusted* | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |||
| Dementia | ||||||||
| Men | 7318 | 188 | ||||||
| BPV (continuous) | 1.33 | 1.12–1.60 | 0.002 | 1.33 | 1.11–1.60 | 0.002 | ||
| BPV tertile | ||||||||
| Tertile 1 | 2503 | 51 | Reference | Reference | ||||
| Tertile 2 | 2401 | 48 | 0.94 | 0.64–1.40 | 0.77 | 0.96 | 0.65–1.43 | 0.84 |
| Tertile 3 | 2414 | 89 | 1.68 | 1.19–2.38 | 0.003 | 1.68 | 1.19–2.39 | 0.004 |
| Women | 9282 | 208 | ||||||
| BPV (continuous) | 1.00 | 0.85–1.19 | 0.96 | 1.00 | 0.85–1.19 | 0.98 | ||
| BPV tertile | ||||||||
| Tertile 1 | 3117 | 60 | Reference | Reference | ||||
| Tertile 2 | 3083 | 73 | 1.03 | 0.73–1.44 | 0.88 | 1.03 | 0.74–1.45 | 0.85 |
| Tirtile 3 | 3082 | 75 | 1.01 | 0.72–1.41 | 0.96 | 1.01 | 0.72–1.42 | 0.96 |
| Cognitive decline | ||||||||
| Men | 6204 | 915 | ||||||
| BPV (continuous) | 1.02 | 1.00–1.03 | 0.007 | 1.02 | 1.01–1.03 | 0.005 | ||
| BPV tertile | ||||||||
| Tertile 1 | 2107 | 305 | Reference | Reference | ||||
| Tertile 2 | 2043 | 258 | 0.99 | 0.84–1.17 | 0.92 | 1.00 | 0.85–1.19 | 0.97 |
| Tertile 3 | 2054 | 352 | 1.34 | 1.14–1.56 | <0.0001 | 1.36 | 1.16–1.59 | <0.0001 |
| Women | 7901 | 1078 | ||||||
| BPV (continuous) | 1.01 | 1.00–1.02 | 0.06 | 1.01 | 1.00–1.02 | 0.10 | ||
| BPV tertile | ||||||||
| Tertile 1 | 2672 | 336 | Reference | Reference | ||||
| Tertile 2 | 2608 | 327 | 0.98 | 0.84–1.14 | 0.76 | 0.98 | 0.84–1.14 | 0.81 |
| Tertile 3 | 2621 | 415 | 1.15 | 0.99–1.32 | 0.07 | 1.14 | 0.98–1.32 | 0.09 |
BPV indicates blood pressure variability; and HR, hazard ratio.
Adjusted for age, average systolic blood pressure, antihypertensive medications at baseline, education, diabetes mellitus, depression, body mass index, and statin medications.
In sensitivity analysis limiting the cohort to individuals who remained consistently on or off antihypertensive drugs throughout the period when BPV was estimated, an additional 729 men and 752 women from the analysis of incident dementia, and 617 men and 648 women from the analysis of cognitive decline, were excluded. The results, however, remained consistent with the main findings, showing an increased risk of incident dementia and cognitive decline for men in the highest tertile of BPV compared with the lowest tertile but not in women (Table S2).
We further explored the relationship between use of antihypertensive drugs and the cognitive end points. In men but not women, there was a significant interaction between antihypertensive use and BPV for both incident dementia (P=0.01) and cognitive decline (P=0.02). Men in the highest tertile who were not on antihypertensive drugs during the BPV estimation period were not at increased risk of dementia (HR, 0.83; 95% CI, 0.48–1.43; P=0.51), whereas men in the middle and highest tertiles who were consistently on antihypertensive drugs were at increased risk (T2: HR, 2.11; 95% CI, 1.14–3.91; P=0.02; T3: HR, 2.97; 95% CI, 1.65–5.34; P<0.001) (Table 3). Likewise, men using antihypertensives in the highest tertile had an increased risk of cognitive decline relative to those in the lowest tertile (HR, 1.68; 95% CI, 1.34–2.12; P<0.001), but no such association was seen for the second tertile. Treating BPV as a continuous variable resulted in similar findings.
Table 3.
Cumulative Incidence of Dementia and Cognitive Decline According to BPV in Men, Stratified by Antihypertensive Medication Use Over the BPV Assessed Period
| No. | No Antihypertensive Medication | No. | Consistent Antihypertensive Medication | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events | HR* | 95% CI | P Value | No. of Events | HR* | 95% CI | P Value | |||
| Dementia | 3111 | 70 | 3478 | 92 | ||||||
| BPV (continuous) | 0.97 | 0.93–1.02 | 0.29 | 1.06 | 1.03–1.09 | <0.0001 | ||||
| BPV tertile | ||||||||||
| Tertile 1 | 1055 | 28 | Ref. | 1178 | 15 | Ref. | ||||
| Tertile 2 | 1019 | 16 | 0.58 | 0.31–1.08 | 0.09 | 1146 | 31 | 2.11 | 1.14–3.91 | 0.02 |
| Tertile 3 | 1037 | 26 | 0.83 | 0.48–1.43 | 0.51 | 1154 | 46 | 2.97 | 1.65–5.34 | <0.0001 |
| Cognitive decline | 2665 | 377 | 2922 | 439 | ||||||
| BPV (continuous) | 0.99 | 0.97–1.01 | 0.44 | 1.02 | 1.01–1.04 | 0.002 | ||||
| BPV tertile | ||||||||||
| Tertile 1 | 906 | 128 | Ref. | 992 | 124 | Ref. | ||||
| Tertile 2 | 875 | 120 | 1.03 | 0.80–1.32 | 0.84 | 959 | 125 | 1.05 | 0.82–1.35 | 0.71 |
| Tertile 3 | 884 | 129 | 1.02 | 0.79–1.30 | 0.89 | 971 | 190 | 1.68 | 1.34–2.12 | <0.0001 |
BPV indicates blood pressure variability; and HR, hazard ratio.
Adjusted for age, average systolic blood pressure, education, diabetes mellitus, depression, body mass index, and statin medications.
In sensitivity analyses using alternate BPV indices, the observed relationships were consistent with our primary analysis (Table S3). Further sensitivity analysis using BPV calculated from 4 measurements (baseline, year 1, 2, and 3 visits) and restricting to events that occurred after the year 3 visit, produced similar results for dementia in men, but cognitive decline was no longer significant (Table S4). For comparative purposes we also examined the association between other BP measures and incident dementia and cognitive decline in the cohort. The results indicated that none of the measures—systolic or diastolic BP, high BP, or use of antihypertensive drug—were associated with either dementia or cognitive decline in men or women (Table S5). Lastly, we did not observe any significant association between diastolic BPV and incident dementia or cognitive decline (Table S6).
Discussion
In this post hoc analysis of ASPREE participants without dementia, those in the highest tertile of BPV compared with the lowest were at significantly increased risk of incident dementia and cognitive decline during follow‐up, independent of mean systolic BP. Sex and use of antihypertensive drugs modified this association, whereby the risk of incident dementia and cognitive decline appeared greatest in men who were receiving antihypertensive treatment. These findings remained consistent in sensitivity analyses, which included restricting the cohort to those without change in antihypertensive drug use during the BPV estimation period and use of alternate estimates of BPV.
Our findings support previous work establishing BPV as an independent risk factor for cognitive impairment.8, 9, 10, 11, 12, 13, 14, 15, 36 However, our work expands this understanding to older, relatively healthy adults (including those without hypertension) who had reached late life without significant cognitive impairment; a group that is not typically considered at high risk for dementia in their remaining lifespan. Furthermore, our results also provide the first evidence of possible sex‐specific effects of BPV on cognition. Although we and others have found that women have higher BPV than men,37, 38 to our knowledge, none considered whether the association between BPV and cognitive impairment differs between men and women.
The pathobiological mechanisms connecting increased BPV with cognitive decline and dementia have not been fully established. Accumulating evidence suggests BPV is associated with structural brain changes, including increased white matter hyperintensities, cerebral microbleeds, and enlarged perivascular spaces.39, 40, 41, 42 The basal forebrain cholinergic neuronal degeneration and associated presynaptic cholinergic denervation in Alzheimer's disease may influence sympathetic and parasympathetic autoregulation of BP, contributing to BPV. The resultant hemodynamic instability can increase shear stress and promote microvascular damage, which may affect permeability of the blood brain barrier and accelerate neuronal injury.43 Although all ASPREE participants were without major cognitive deficits at baseline, we cannot exclude the possibility that subclinical disease was present and contributed via this pathway.
We considered several explanations for why men, but not women, were at increased risk for dementia and cognitive impairment in our analysis. It may reflect higher cumulative burden from traditional midlife vascular risk factors that are the strongest risk for late‐life dementia. These include BP4, 43 and smoking,44 and men in our study did have higher exposures to both, yet our findings persisted after adjustment for these covariates. Increased vascular stiffness is associated with cognitive decline,45 and stroke and myocardial infarction are manifestations of the underlying vascular risk burden. However, we observed similar pulse pressures in men and women, and our associations remained consistent after exclusion of men with a stroke or myocardial infarction during the trial.
The possibility of different underlying pathways for cognitive decline in men and women exists, perhaps based partly on timing of risk factor exposure, as sex‐specific associations with late‐life cognition have been previously reported.46, 47 Furthermore, the cerebrovasculature is affected by sex hormones, which may lead to differences in how cerebral blood vessels function under both physiological and pathological conditions.48 The effect of estrogen exposure, either through longer endogenous estrogen exposure or hormone therapy, is one such possibility. Although hormone therapy did not benefit cognition in the Women's Health Initiative,49 other research suggests that longer endogenous estrogen exposure and hormone therapy are associated with higher cognitive status in late life.50, 51 The age of women in ASPREE would suggest that many went through menopause at a time when hormone therapy was more widespread, and it is possible they had higher lifetime estrogen exposure.
We observed that the association between high BPV and cognitive decline and dementia was strongest in men who reported using antihypertensive drugs during the 2 years in which BPV was assessed. Some possibilities to explain this finding include that antihypertensive use may be a proxy for underlying vascular risk, and men in our cohort could have had longer durations of hypertension or were more poorly controlled. Indeed, it has been shown that intensive blood pressure control with antihypertensives reduces the risk of mild cognitive impairment.52 Poor adherence to antihypertensives influences visit‐to‐visit BPV,35 and men in our cohort could have been less adherent to their treatments. Although we cannot offer a definitive explanation for the sex‐based differences, our results should not be interpreted as to suggest that antihypertensive treatment in men is harmful to their cognitive trajectory. Sex is clearly emerging as a key biological variable in cerebrovascular and cardiovascular research.48, 53 Our findings highlight the need for more research investigating the potential mechanisms underlying sex‐specific differences in diseases of aging and further support the consideration of potential sex‐related differences in clinical practice and in the design of future trials.
Strengths and Limitations
The large sample size, administration of a comprehensive cognitive battery at multiple study visits, standardized BP assessments, and adjudicated dementia diagnoses are key strengths of our study. The cohort's inclusion of individuals with and without hypertension expands its generalizability to older adults not receiving interventions such as aggressive BP lowering that may affect the underlying causal pathway for dementia.52 In contrast to previous studies,36 we chose a more conservative analysis by only considering cognitive decline and dementia end points occurring after the period during which BPV was estimated. Although minimizing immortal time bias, this approach potentially reduces the precision around effect size estimates because it excludes events occurring early in the study. We also conducted multiple sensitivity analyses that further supported the results. Finally, the lack of significant association of the cognitive end points with any of the other traditional BP measures adds further strength to the concept of BPV as an independent risk factor for cognitive decline and dementia.
Our study has important limitations we acknowledge. It is a post hoc analysis whose findings can be subject to unmeasured confounders. We were unable to account for adjustments to antihypertensive regimens or nonadherence, although our sensitivity analyses limiting the cohort to those consistently on or off antihypertensives during the BPV estimation period did not alter the findings. Reverse causation is also a possibility, because greater BPV may stem from dysfunctional autoregulation of BP in those with cortical atrophy, and we cannot exclude the possibility that subclinical disease was present at baseline in some individuals. The sex‐related differences observed could reflect inadequate statistical power, as fewer women experienced dementia or cognitive impairment during the follow‐up period. We were unable to account for lifetime estrogen exposure, which may have influenced the sex‐related differences observed.50 Finally, our findings are associative, and only a well‐designed prospective intervention trial can establish whether high BPV is a viable therapeutic target to lower the risk of cognitive decline and dementia.
Conclusions
In adults who have reached older ages free of evidence of dementia or major cognitive impairment, high BPV was independently associated with increased risk of dementia and cognitive decline, particularly in men. Future research should investigate sex‐specific effects of high BPV on cognition and prospectively test whether reducing BPV preserves late‐life cognitive function.
Sources of Funding
The ASPREE trial was supported by grants (U01AG029824 and U19AG062682) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants (334047 and 1127060) from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency.
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S2
Acknowledgments
A. G. Bayer provided aspirin and matching placebo for ASPREE but had no other role in the study.
(J Am Heart Assoc. 2021;10:e019613. DOI: 10.1161/JAHA.120.019613.)
This manuscript was sent to Sean Savitz, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
A portion of this work was presented as a virtual poster abstract at the American Heart Association Hypertension Scientific Sessions, September 10 to 13, 2020.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019613
For Sources of Funding and Disclosures, see page 10.
See Editorial by McCormick
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division . World Population Ageing 2017—Highlights (ST/ESA/SER.A/397). 2017.
- 2.Gottesman RF, Schneider ALC, Albert M, Alonso A, Bandeen‐Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, et al. Midlife hypertension and 20‐year cognitive change: the Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014;71:1218–1227. DOI: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ou YN, Tan CC, Shen XN, Xu W, Hou XH, Dong Q, Tan L, Yu JT. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta‐analysis of 209 prospective studies. Hypertension. 2020;76:217–225. DOI: 10.1161/HYPERTENSIONAHA.120.14993. [DOI] [PubMed] [Google Scholar]
- 4.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen‐Roche K, Coresh J, Gross AL, Windham BG, et al. Association of midline to late‐life blood pressure patterns with incident dementia. JAMA. 2019;322:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abell JG, Kivimäki M, Dugravot A, Tabak AG, Fayosse A, Shipley M, Sabia S, Singh‐Manoux A. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018;39:3119–3125. DOI: 10.1093/eurheartj/ehy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Cushman M, McClure LA, Safford MM, Wadley VG. Blood pressure and cognitive decline over 8 years in middle‐aged and older black and white Americans. Hypertension. 2019;73:310–318. DOI: 10.1161/HYPERTENSIONAHA.118.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouch L, Cestac P, Hanon O, Ruidavets J‐B, Ehlinger V, Gentil C, Cool C, Helmer C, Dartigues J‐F, Bouhanick B, et al. Blood pressure and cognitive performances in middle‐aged adults: the aging, health and work longitudinal study. J Hypertens. 2019;37:1244–1253. DOI: 10.1097/HJH.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 8.Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, Shibata M, Ohtsubo T, Kitazono T, Kiyohara Y, et al. Day‐to‐day blood pressure variability and risk of dementia in a general Japanese elderly population. Circulation. 2017;136:516–525. DOI: 10.1161/CIRCULATIONAHA.116.025667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou TL, Kroon AA, van Sloten TT, van Boxtel MPJ, Verhey FRJ, Schram MT, Köhler S, Stehouwer CDA, Henry RMA. Greater blood pressure variability is associated with lower cognitive performance. Hypertension. 2019;73:803–811. DOI: 10.1161/HYPERTENSIONAHA.118.12305. [DOI] [PubMed] [Google Scholar]
- 10.Yoo JE, Shin DW, Han K, Kim D, Lee SP, Jeong SM, Lee J, Kim S. Blood pressure variability and the risk of dementia. Hypertension. 2020;75:982–990. DOI: 10.1161/HYPERTENSIONAHA.119.14033. [DOI] [PubMed] [Google Scholar]
- 11.Qin B, Viera AJ, Muntner P, Plassman BL, Edwards LJ, Adair LS, Popkin BM, Mendez MA. Visit‐to‐visit variability in blood pressure is related to late‐life cognitive decline. Hypertension. 2016;68:106–113. DOI: 10.1161/HYPERTENSIONAHA.116.07494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit‐to‐visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. 2012;30:1556–1563. DOI: 10.1097/HJH.0b013e3283552735. [DOI] [PubMed] [Google Scholar]
- 13.Sabayan B, Wijsman LW, Foster‐Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, van Osch MJP, van der Grond J, et al. Association of visit‐to‐visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. DOI: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- 14.Böhm M, Schumacher H, Leong D, Mancia G, Unger T, Schmieder R, Custodis F, Diener H‐C, Laufs U, Lonn E, et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension. 2015;65:651–661. DOI: 10.1161/HYPERTENSIONAHA.114.04568. [DOI] [PubMed] [Google Scholar]
- 15.Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S; Alzheimer’s Disease Neuroimaging Initiative . Cognitive dysfunction and greater visit‐to‐visit systolic blood pressure variability. J Am Geriatr Soc. 2013;61:2168–2173. DOI: 10.1111/jgs.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Classen JAHR; NILVAD Study Group . Blood pressure variability and progression of clinical Alzheimer disease. Hypertension. 2019;74:1172–1180. DOI: 10.1161/HYPERTENSIONAHA.119.13664. [DOI] [PubMed] [Google Scholar]
- 17.Kronish IM, Kent S, Moise N, Shimbo D, Safford MM, Kynerd RE, O’Beirne R, Sullivan A, Muntner P. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens. 2017;11:573–580. DOI: 10.1016/j.jash.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens. 2018;20:1133–1137. DOI: 10.1111/jch.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson MR; ASPREE Investigator Group . Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–564. DOI: 10.1016/j.cct.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil JJ, Woods RL, Nelson MR, Murray AM, Reid CM, Kirpach B, Storey E, Shah RC, Wolfe RS, Tonkin AM, et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci. 2017;72:1586–1593. DOI: 10.1093/gerona/glw342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, Storey E, Shah RC, Lockery JE, Tonkin AM, et al. Effect of aspirin on disability‐free survival in the healthy elderly. N Engl J Med. 2018;379:1499–1508. DOI: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan J, Storey E, Murray AM, Woods RL, Wolfe R, Reid CM, Nelson MR, Chong TTJ, Williamson JD, Ward SA, et al. Randomized placebo‐controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 2020;95:e320–e331. DOI: 10.1212/WNL.0000000000009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan J, Woods RL, Britt C, Murray AM, Shah RC, Reid CM, Kirpach B, Wolfe RS, Nelson MR, Lockery JE, et al. Normative performance of healthy older individuals on the Modified Mini‐Mental State (3MS) examination according to ethno‐racial group, gender, age, and education level. Clin Neuropsychol. 2018;33:779–797. DOI: 10.1080/13854046.2018.1488996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: normative data and analysis of inter‐form and test‐retest reliability. Clin Neuropsychol. 2001;12:43–55. DOI: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 25.Ross TP. The reliability of cluster and switch scores for the Controlled Oral Word Association Test. Arch Clin Neuropsychol. 2003;18:153–164. DOI: 10.1093/arclin/18.2.153. [DOI] [PubMed] [Google Scholar]
- 26.Smith A. Symbol Digit Modalities Test (SDMT) Manual (Revised). Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 27.Pickering TG, Hall JE, Appel LJ, Falkner B, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ; Subcommittee of Professional and Public Education of the American Heart Association on High Blood Pressure Research . Recommendations for blood pressure measurement in humans and experimental animals, part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. DOI: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 28.Graham DP, Cully JA, Snow AL, Massman P, Doody R. The Alzheimer’s Disease Assessment Scale‐Cognitive subscale: normative data for older adult controls. Alzheimer Dis Assoc Disord. 2004;18:236–240. [PubMed] [Google Scholar]
- 29.D’Elia L. Color Trails Test: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- 30.Alegret M, Boada‐Rovira M, Vinyes‐Junqué G, Valero S, Espinosa A, Hernández I, Modinos G, Rosende‐Roca M, Mauleón A, Becker JT, et al. Detection of visuoperceptual deficits in preclinical and mild Alzheimer’s disease. J Clin Exp Neuropsychol. 2009;31:860–867. DOI: 10.1080/13803390802595568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fish J. Alzheimer’s disease cooperative study ADL scale. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2011. DOI: 10.1007/978-0-387-79948-3_1791. [DOI] [Google Scholar]
- 32.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 33.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;e340:b5087. DOI: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 34.Wijsman LW, de Craen AJM, Muller M, Sabayan B, Stott D, Ford I, Trompet S, Jukema JW, Westendorp RGJ, Mooijaart SP. Blood pressure lowering medication, visit‐to‐visit blood pressure variability, and cognitive function in older age. Am J Hypertens. 2016;29:311–318. [DOI] [PubMed] [Google Scholar]
- 35.Kronish IM, Lynch AI, Oparil S, Whittle J, Davis BR, Simpson LM, Krousel‐Wood M, Cushman WC, Chang TI, Muntner P. The association between antihypertensive medication nonadherence and visit‐to‐visit variability of blood pressure. Findings from the Antihypertensive and Lipid‐Lowering to Prevent Heart Attack Trial. Hypertension. 2016;68:39–45. DOI: 10.1161/HYPERTENSIONAHA.115.06960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouch L, Cestac P, Sallerin B, Piccoli M, Benattar‐Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, et al. Visit‐to‐visit blood pressure variability is associated with cognitive decline and incident dementia. Hypertension. 2020;76:1280–1288. DOI: 10.1161/HYPERTENSIONAHA.119.14553. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson J, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. DOI: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 38.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit‐to‐visit variability in systolic blood pressure and all‐cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. DOI: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 39.Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, Claassen JAHR, Lattanzi S, Vincent AD, Tzourio C, et al. Variability in Blood Pressure and Brain Health Consortium. Association between blood pressure variability and cerebral small‐vessel disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9:e013841. DOI: 10.1161/JAHA.119.013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lattanzi S, Brigo F, Vernieri F, Silvestreni M. Visit‐to‐visit variability in blood pressure and Alzheimer’s disease. J Clin Hypertens. 2018;20:918–924. DOI: 10.1111/jch.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y, Yilmaz P, Bos D, Blacker D, Viswanathan A, Ikram MA, Hofman A, Vernooij MW, Ikram MK. Blood pressure variation and subclinical brain disease. J Am Coll Cardiol. 2020;75:2387–2399. DOI: 10.1016/j.jacc.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Heus RAA, Reumers SFI, van der Have A, Tumelaire M, Tulley PJ, Claassen JAHR. Day‐to‐day home blood pressure variability is associated with cerebral small vessel disease burden in a memory clinic population. J Alzheimers Dis. 2020;74:463–472. DOI: 10.3233/JAD-191134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24. DOI: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta‐analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. 2015;10:e0118333. DOI: 10.1371/journal.pone.0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Lyu P, Ren Y, An J, Dong Y. Arterial stiffness and cognitive impairment. J Neurol Sci. 2017;380:1–10. DOI: 10.1016/j.jns.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Altschul DM, Wraw C, Der G, Gale CR, Deary IJ. Hypertension development by midlife and the roles of premorbid cognitive function, sex, and their interaction. Hypertension. 2019;73:812–819. DOI: 10.1161/HYPERTENSIONAHA.118.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, Dean A, Whitmer RA. Female sex, early‐onset hypertension, and risk of dementia. Neurology. 2017;89:1886–1893. DOI: 10.1212/WNL.0000000000004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robison LS, Gannon OJ, Salinero AE, Zuloaga KL. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019;1710:43–60. DOI: 10.1016/j.brainres.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 49.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN III, Assaf AR, Jackson RD, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. DOI: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 50.Matyi JM, Rattinger GB, Schwartz S, Buhusi M, Tschanz JT. Lifetime estrogen exposure and cognition in late life: the Cache County Study. Menopause. 2019;26:1366–1374. DOI: 10.1097/GME.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan J, Carriére I, Scali J, Ritchie K, Ancelin M‐L. Life‐time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34:287–298. DOI: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 52.The SPRINT MIND Investigators for the SPRINT Research Group . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. DOI: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volgman AS, Bairey Merz CN, Aggarwal NT, Bittner V, Bunch TJ, Gorelick PB, Maki P, Patel HN, Poppas A, Ruskin J, et al. Sex differences in cardiovascular disease and cognitive impairment: another health disparity for women? J Am Heart Assoc. 2019;8:e013154. DOI: 10.1161/JAHA.119.013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S2


