Abstract
Background
Leadless pacemaker is a novel technology, and evidence supporting its use is uncertain. We performed a systematic review and meta‐analysis to examine the safety and efficacy of leadless pacemakers implanted in the right ventricle.
Methods and Results
We searched PubMed and Embase for studies published before June 6, 2020. The primary safety outcome was major complications, whereas the primary efficacy end point was acceptable pacing capture threshold (≤2 V). Pooled estimates were calculated using the Freedman‐Tukey double arcsine transformation. Of 1281 records screened, we identified 36 observational studies of Nanostim and Micra leadless pacemakers, with most (69.4%) reporting outcomes for the Micra. For Micra, the pooled incidence of complications at 90 days (n=1608) was 0.46% (95% CI, 0.08%–1.05%) and at 1 year (n=3194) was 1.77% (95% CI, 0.76%–3.07%). In 5 studies with up to 1‐year follow‐up, Micra was associated with 51% lower odds of complications compared with transvenous pacemakers (3.30% versus 7.43%; odds ratio [OR], 0.49; 95% CI, 0.34–0.70). At 1 year, 98.96% (95% CI, 97.26%–99.94%) of 1376 patients implanted with Micra had good pacing capture thresholds. For Nanostim, the reported complication incidence ranged from 6.06% to 23.54% at 90 days and 5.33% to 6.67% at 1 year, with 90% to 100% having good pacing capture thresholds at 1 year (pooled result not estimated because of the low number of studies).
Conclusions
Most studies report outcomes for the Micra, which is associated with a low risk of complications and good electrical performance up to 1‐year after implantation. Further data from randomized controlled trials are needed to support the widespread adoption of these devices in clinical practice.
Keywords: efficacy, leadless pacemaker, meta‐analysis, safety, systematic review
Subject Categories: Pacemaker, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- LP
leadless pacemaker
- TV
tricuspid valve
- TVP
transvenous pacemaker
Clinical Perspective
What Is New?
We performed the first systematic review and meta‐analysis that comprehensively examines the safety and efficacy of the Micra and Nanostim leadless pacemakers implanted in the right ventricle.
Our results showed leadless pacemakers are associated with a low incidence of complications (0.46% at 90 days and 1.77% at 1 year for Micra) and good electrical performance at 1 year after implantation, with >90% of devices having an acceptable capture threshold.
Micra is associated with 51% lower odds of complications compared with a transvenous pacemaker.
What Are the Clinical Implications?
Based on observational data, leadless pacemakers appear to have a markedly lower incidence of early complications compared with transvenous pacemakers.
Nevertheless, data on battery longevity (beyond 2 years) and clinical outcomes, such as incidence of new‐onset heart failure, are currently lacking.
Robust randomized trials that directly compare the safety and efficacy of leadless and transvenous pacemakers are needed to provide more rigorous data to support the widespread adoption of this novel technology.
Transvenous pacemakers (TVPs), consisting of a subcutaneously implanted pulse generator and one or more transvenous electrodes extending to the heart chamber(s), are a well‐established treatment for bradyarrhythmias.1 Nevertheless, implantation of these devices is not devoid of substantial complications.2, 3, 4, 5 Studies have shown that TVPs are consistently associated with a 7.76% to 12.4% risk of serious complications at 90 days, with nearly half of these attributable to lead‐ and generator‐related complications.2, 3, 4 In the longer term, TVPs have a 1% to 2% risk of complications per year, mainly attributable to lead failure and infection.3 About 1 in 6 patients with a TVP experiences a serious complication by 3 years,3, 4 and these complications are exceedingly costly to treat.4, 5 Strategies to minimize harm and costs associated with permanent pacemakers are therefore highly desirable.
The leadless pacemaker (LP) is a novel alternative consisting of a capsule‐like device containing a generator and electrode system that is implanted into the right ventricle via a percutaneously inserted femoral venous catheter. By omitting the need for a generator pocket and transvenous leads, a LP may avoid many of the lead‐ and generator pocket–related complications typically associated with a TVP. Although the LP was first solely indicated for right ventricular pacing, the emergence of LPs capable of atrioventricular synchronous pacing promises expanding indications for these novel devices.6, 7 Nevertheless, the initial evidence supporting the use of these devices was limited and came from mostly small observational studies,8, 9, 10, 11 with no randomized control trials that have directly compared safety and efficacy of LPs versus TVPs. Furthermore, despite initial promising data, the Nanostim (Abbott Medical, Abbott Park, IL) LP was withdrawn from premarket testing because of premature battery failure,12 raising concerns about the long‐term performance of LPs.
To date, there has been no systematic review and meta‐analysis of LPs beyond narrative reviews13, 14 and limited reviews of LP‐associated cardiac perforation15 and dislodgement.16 Accordingly, we sought to perform a systematic review and meta‐analysis of published studies to evaluate the safety and efficacy of LPs. Specifically, we examined the pooled incidence of early complications up to 3 months after implant as well as the incidence of complications beyond the early postimplantation period. The pooled odds ratio (OR) was drawn from studies that compared complications associated with LPs versus TVPs. We also evaluated the proportion of patients with a successful implant, and the efficacy of LPs focusing on electrical performance and clinical outcomes.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis protocol.17 All data used in this study were extracted from individual studies. The authors declare that all supporting data are available within the article and the supplementary documents.
Literature Search
Two independent reviewers (L.N. and D.N.) performed a comprehensive systematic search across PubMed and Embase databases and included all studies published up to the June 6, 2020. The keyword search terms were leadless, pacemaker, Micra, and Nanostim. Studies were included if they explored either the primary safety or efficacy end point of LP implantation in the right ventricle. The exclusion criteria were (1) sample size of <10 patients; (2) review, survey, abstracts, or conference proceedings without full text, editorial comments, or responses (to ensure reliable data could be extracted); (3) studies with concurrent atrioventricular nodal ablation, defibrillator or resynchronization device implantations, or those conducted on patients with heart block requiring pacemaker implantation after transcatheter aortic valve replacement, because complications could be attributable to these additional interventions; (4) studies that reported different results from the same population or outcomes not relevant to LP safety and efficacy; (5) studies in which an LP was implanted through the jugular vein instead of the conventional femoral vein; (6) studies not conducted on live humans; and (7) studies published in languages other than English.
Included studies were agreed on by both reviewers, with discrepancies resolved by a third reviewer (I.R.). All search keywords used are described in Table S1.
Data Extraction
Data were extracted using a data extraction form with a standard set of variables collected for each publication.
Quality Assessment
We used the National Institute of Health Quality Assessment Tool to evaluate the quality of each included study.18 This tool includes a set of questions (14 for cohort studies and 9 for case‐series studies), with overall quality of the study graded as good, fair, or poor. The first reviewer (L.N.) assessed the quality of included studies, and results were confirmed by the second reviewer (D.N.), with conflicts resolved by consensus.
Primary End Points
The primary safety end point was the occurrence of any major device and procedure‐related complications, defined as events that resulted in death, required intervention, or prolonged the hospital stay, or led to a readmission. For example, a pericardial effusion not requiring drainage or surgery or a groin hematoma not requiring blood transfusion are not considered major complications. Secondary safety end points included the proportion of patients with a successful implant and the incidences of specific complications that could be extracted from each study. We examined the pooled incidence of complications at up to 90 days including those that occurred during implantation, and ≈1 year after implant. These time points were selected because they are the timeframes most commonly used by the studies reporting complications following cardiac device implantation.2, 8, 10, 19, 20 The proportion of patients with a successful implant was calculated as the percentage of patients received a LP among those with an attempted implant.
The primary efficacy end point was good electrical performance indicated by a pacing capture threshold of ≤2 V at 1 year after device implantation. The secondary efficacy endpoints were other clinical outcomes including quality of life and cardiac function.
Statistical Analysis
All analysis was performed using Stata version 16.0 statistical software (StataCorp, College Station, TX).
To calculate the pooled proportion (for implant success and efficacy) and pooled incidence of complications, we used the Stata user‐written command Metaprop, with the Freedman‐Tukey double arcsine transformation that allows inclusion of studies with an incidence or proportion of 0% and 100%.21 The meta‐analysis of studies comparing LP with TVP was performed using Stata's in‐built Metan command, with results being reported as OR and 95% CI.22 We chose to report OR because of the lack of reporting of either hazard ratio or statistics to estimate hazard ratio in individual studies. The heterogeneity among studies was evaluated using the I 2 statistic,23 and the sensitivity of the pooled estimates was examined by subgroup analyses of different types of study design or quality. Results were reported for Micra and Nanostim separately because of the stark differences in design and fixation mechanism of these 2 devices. A 2‐tailed P value of <0.05 was considered statistically significant.
Results
Characteristics of Included Studies
A total of 1281 studies were screened, and 36 were included for our analysis8, 9, 10, 11, 19, 20, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 (Figure 1). Table 1 summarizes the characteristics of the included studies, all of which were observational (details of the 36 studies are provided in Tables 2, 3, 4, 5, 6, and Table S2). Eight studies8, 10, 27, 28, 29, 30, 31, 32 used the same cohort as other included studies but reported outcomes at a different follow‐up interval, leaving 28 studies with unique patient cohorts (n=4748 patients; mean age, 83.3 years [95% CI, 80.9–85.6], 61.0% [95% CI, 59.6%–62.4%] were men). Most of the patients had comorbid hypertension (69.7%; 95% CI, 64.2%–75.0%) and atrial fibrillation (66.7%; 95% CI, 59.7%–73.5%). Only 24.3% (95% CI, 18.0%–31.3%) of patients had a history of heart failure at implantation. Median sample size was 66 patients (range, 10–1817 patients), with 64.3% having a sample size <100 patients. Median follow‐up time was 6 months (range, 0–24 months). Among the 36 studies included, 10 were retrospective (27.8%), 22 were prospective (61.1%), and 4 (11.1%) did not clearly state the design. Five studies (13.9%) analyzed the Nanostim LP, whereas 25 (69.4%) explored the Micra LP (Medtronic, Minneapolis, MN), 5 (13.9%) analyzed both, and 1 study did not clearly report the type of LP used.24 Seven studies compared LPs with TVPs, 3 of which used propensity score–matched controls29, 30, 44 and 1 used a historical control group.27
Figure 1. Study selection flow diagram.
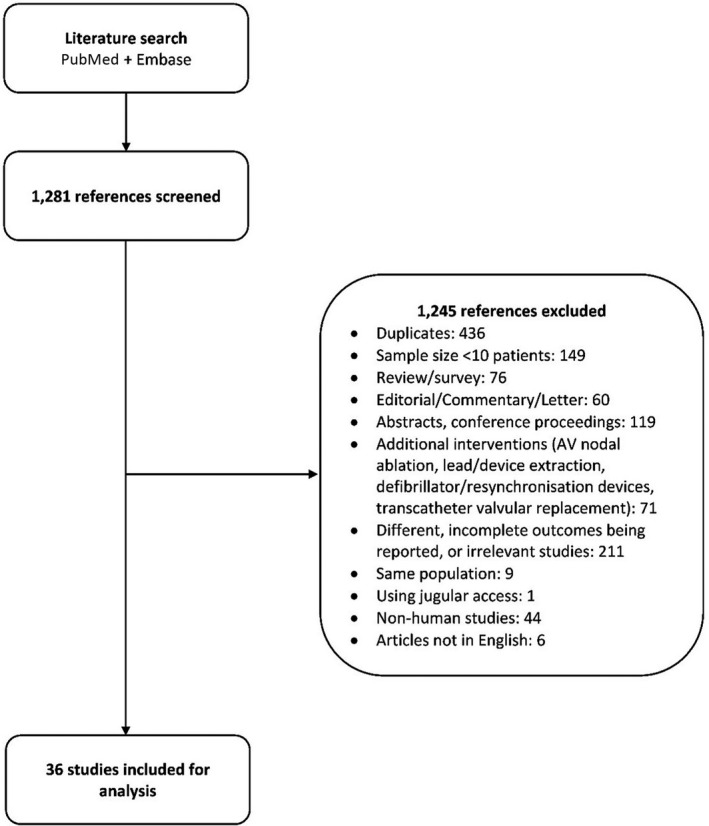
AV indicates atrioventricular.
Table 1.
Characteristics of Included Studies
| Characteristics | No. of Studies (No. of Patients) | Summary Estimate |
|---|---|---|
| Patient demographics | ||
| Age, y, pooled mean (95% CI) | 24 (4335 patients) | 83.3 (80.9–85.6) |
| Men* | 28 (4748 patients) | 61.0% (59.6%–62.4%) |
| Comorbidities | ||
| Heart failure | 18 (4580 patients) | 24.3% (18.0%–31.3%) |
| Hypertension | 23 (4881 patients) | 69.7% (64.2%–75.0%) |
| Coronary artery disease | 20 (4556 patients) | 28.5% (23.1%–34.2%) |
| Atrial fibrillation | 20 (4611 patients) | 66.7% (59.7%–73.5%) |
| Diabetes mellitus | 21 (4695 patients) | 23.3% (20.5%–26.3%) |
| Atrioventricular block | 18 (3786 patients) | 43.5% (28.2%–59.5%) |
| Study design | ||
| Prospective | 22 | 61.1% |
| Retrospective | 10 | 27.8% |
| Not reported | 4 | 11.1% |
| Quality assessment | ||
| Good | 20 | 55.6% |
| Fair | 15 | 41.7% |
| Poor | 1 | 2.8% |
| Device used | ||
| Nanostim | 5 | 13.9% |
| Micra | 25 | 69.4% |
| Both devices | 5 | 13.9% |
| Not reported | 1 | 2.8% |
Eight studies used the same cohort as another included study but reported outcomes at a different follow‐up interval, leaving 28 studies with unique patient cohorts, 24 of which reported mean and standard deviation for age, and all 28 studies reported the percentage of male patients. CI indicates confidence interval; and y, years.
Table 2.
Studies Included for Meta‐Analysis of Incidence of Complications at Up to 90 Days
| Author (y) | Study Design | Study Population | Device | Sample Size | Follow‐Up Time, mo | Major Complications | Dislodgment | Tamponade | Infection | Vascular Injury |
|---|---|---|---|---|---|---|---|---|---|---|
| Reddy (2014)8 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Nanostim | 33 | 3 | 2 | 1 | 1 | 0 | 0 |
| Cantillon (2018)29 | Prospective, 2‐arm, multicenter cohort study | Patients implanted with Nanostim were propensity‐score matched in 1:2 ratio with 1436 patients implanted with TVP. | Nanostim | 718 | 1 | 42 | 7 | 7 | 0 | 8 |
| Vaidya (2019)44 | Retrospective, 2‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Nanostim | 17 | 2.1 | 4 | 0 | 0 | 0 | 0 |
| Ritter (2015)10 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Micra | 140 | 3 | 2 | 0 | 1 | 0 | 1 |
| Pachón (2016)33 | Single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 10 | 1.8 | 0 | 0 | 0 | 0 | 0 |
| Da Costa (2017)34 | Prospective, single‐arm, single‐center cohort study | Consecutive patients with full or relative contraindications of traditional TVP | Micra | 14 | 3 | 0 | 0 | 0 | 0 | 0 |
| Roberts (2017)28 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Micra | 795 | 1 | 12 | 1 | 1 | 1 | 6 |
| Vaidya (2019)44 | Retrospective, 2‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 73 | 2.1 | 0 | 0 | 0 | 0 | 0 |
| Kiani (2019)42 | Retrospective, single‐arm, multicenter cohort study | Patients underwent LP implantations, among which 26 continued oral anticoagulation during implantation and 144 patients did not. | Micra | 170 | 0 | 2 | 0 | 1 | 0 | 1 |
| Grabowski (2020)47 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 10 | 0 | 1 | 0 | 0 | 0 | 1 |
| Mohammed (2020)49 | Retrospective, single‐arm, single‐center cohort study | Patients underwent LP implantations using different types of dilators. | Micra | 84 | 0 | 2 | 0 | 0 | 0 | 0 |
| El Amrani (2020)46 | Prospective, single‐arm, single‐center cohort study | Consecutive patients >70 y with an attempted LP implant, among which 41 were aged ≥90 y | Micra | 129 | 1 | 3 | 0 | 0 | 0 | 1 |
| Pagan (2020)50 | Retrospective, 2‐arm, multicenter cohort study | Patients ≥85 y implanted with a Micra | Micra | 183 | 0 | 2 | 0 | 1 | 0 | 0 |
LP indicates leadless pacemaker; and TVP, transvenous pacemaker.
Table 3.
Studies Included for Meta‐Analysis of Incidence of Complications at ≈1 Year After Implant
| Author (y) | Study Design | Study Population | Device | Sample Size | Follow‐Up Time, mo | Major Complications |
|---|---|---|---|---|---|---|
| Reddy (2015)9 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Nanostim | 300 | 6 | 20 |
| Knops (2015)19 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Nanostim | 33 | 12 | 2 |
| Sperzel (2018)36 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Nanostim | 300 | 6 | 16 |
| Reynolds (2016)11 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Micra | 725 | 6 | 25 |
| Martínez‐Sande (2017)35 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 30 | 5.3 | 0 |
| El‐Chami (2018)20 | Prospective, single‐arm, multicenter cohort study | Consecutive patients implanted with Micra devices after approval | Micra | 1817 | 12 | 41 |
| Bongiorni (2018)37 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 52 | 13 | 0 |
| Kaczmarek (2019)41 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 133 | 13.9 | 0 |
| Valiton (2019)45 | Retrospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Micra | 92 | 12 | 8 |
| Roberts (2019)43 | Retrospective, single‐arm, multicenter cohort study | Patients implanted with Micra LP for cardioinhibitory vasovagal syncope | Micra | 32 | 13.5 | 1 |
| Garweg (2019)39 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 10 | 13 | 1 |
| Denman (2019)38 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 79 | 11.8 | 1 |
| Hai (2019)40 | Prospective, single‐arm, single‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 51 | 7.3 | 1 |
| Haeberlin (2020)48 | Prospective, single‐arm, 2‐center cohort study | Consecutive patients undergoing LP implantations | Micra | 111 | 13 | 3 |
| Turagam (2020)53 | Retrospective, 2‐arm, multicenter cohort study | Patients with cardio inhibitory vasovagal syncope implanted with LP | Micra | 21 | 12 | 1 |
| Tachibana (2020)51 | Retrospective, 2‐arm, single‐center cohort study | Consecutive patients ≥85 y underwent LP implantation | Micra | 27 | 6 | 2 |
LP indicates leadless pacemaker.
Table 4.
Studies Comparing Incidence of Complications Between Leadless Pacemakers and Transvenous Pacemakers
| Author (y) | Study Design | Study Population | Device | Sample Size of the LP Group | Follow‐Up Time, mo | Major Complications in the LP Group | Sample Size of the TVP Group | Major Complications in the TVP Group | Reported Hazard Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Tjong (2018)30 | Retrospective, 2‐arm, multicenter cohort study | Both | 220 | 26.7 | 9 | 220 | 21 |
HR, 0.20 (0.04–0.89; P=0.02) excluding PM advisory‐related events* HR, 2.09 (0.94–4.62; P=0.06) including PM advisory‐related events |
|
| Cantillon (2018)29 | Prospective, 2‐arm, multicenter cohort study |
|
Nanostim | 718 | 1 | 42 | 1436 | 165 | Adjusted HR, 0.44 (0.32–0.60); P<0.001 |
| Vaidya (2019)44, † | Retrospective, 2‐arm, single‐center cohort study |
|
Nanostim | 17 | 2.1 | 4 | 90 | 5 | Not reported |
| Vaidya (2019)44, † | Retrospective, 2‐arm, single‐center cohort study |
|
Micra | 73 | 2.1 | 0 | 90 | 5 | Not reported |
| Duray (2017)27 |
Prospective, 2‐arm, multicenter cohort study |
|
Micra | 726 | 12 | 29 | 2667 | 209 | HR, 0.52 (0.35–0.77); P=0.001 |
| Tachibana (2020)51 | Retrospective, 2‐arm, single‐center cohort study |
|
Micra | 27 | 6 | 1 | 35 | 4 | Not reported |
| Pagan (2020)50 | Retrospective, 2‐arm, multicenter cohort study |
|
Micra | 183 | 0 | 2 | 119 | 4 | Not reported |
| Turagam (2020)53 | Retrospective, 2‐arm, multicenter cohort study |
|
Micra | 21 | 12 | 1 | 48 | 5 | Not reported |
HR indicates hazard ratio; LP, leadless pacemaker; MICRA TPS, Micra transcatheter pacing study; and TVP, transvenous pacemaker.
During the study, a pacemaker advisory was issued for the Nanostim LP on the occurrence of device failures because of abrupt battery failure. The authors performed separate analyses including and excluding pacemaker advisory‐related complications to examine the differences in performance with and without the effects of this advisory.
Vaidya et al44 reported complications for both Nanostim (17 patients) and Micra LPs (73 patients) compared with those associated with TVPs (90 patients).
Table 5.
Studies Reporting Efficacy Outcome of Acceptable Capture Threshold
| Author (y) | Study Design | Study Population | Device | Follow‐Up Time, mo | No. of Patients at Follow‐Up | No. of Patients With Acceptable Capture Threshold |
|---|---|---|---|---|---|---|
| Knops (2015)19 | Prospective, single‐arm, multicenter cohort study | Consecutive patients undergoing LP implantations | Nanostim | 12 | 31 | 31 |
| Reddy (2015)9 | Prospective, single‐arm, multicenter cohort study | Consecutive patients implanted with LP | Nanostim | 6 | 300 | 270 |
| Sperzel (2018)36 | Prospective, single‐arm, multicenter cohort study | Consecutive patients with an attempted implantation | Nanostim | 6 | 390 | 390 |
| Reynolds (2016)11 | Prospective, single‐arm, multicenter cohort study | Consecutive patients implanted with LP | Micra | 6 | 297 | 292 |
| Pachón (2016)33 | Single‐arm, single‐center cohort study | Consecutive patients underwent LP implantation attempts | Micra | 1.8 | 10 | 10 |
| Da Costa (2017)34 | Prospective, single‐arm, single‐center cohort study | Consecutive patients with full or relative contraindications of traditional TVP | Micra | 3 | 14 | 14 |
| El‐Chami (2018)20 | Prospective, single‐arm, multicenter cohort study | Consecutive patients implanted with Micra devices after approval | Micra | 12 | 566 | 549 |
| Kiani (2019)31 | Retrospective, single‐arm, multicenter cohort study | Patients underwent LP implantations, among which 25 patients were discharged on the same day of implantation | Micra | 125 | 1.5 | 125 |
| Deman (2019)38 | Prospective, single‐arm, single‐center cohort study | Consecutive patients underwent LP implantations | Micra | 11.8 | 74 | 74 |
| Kaczmarek (2019)41 | Prospective, single‐arm, single‐center cohort study | Consecutive patients underwent LP implantation | Micra | 13.9 | 23 | 23 |
| Valiton (2019)45 | Retrospective, single‐arm, multicenter cohort study | Patients with an attempted LP implantation | Micra | 12 | 30 | 27 |
| Garweg (2019)39 | Prospective, single‐arm, single‐center cohort study | Consecutive patients underwent LP implantations | Micra | 10.4 | 66 | 66 |
| Hai (2019)40 | Prospective, single‐arm, single‐center cohort study | Consecutive patients underwent LP implantation | Micra | 7.3 | 45 | 45 |
| Turagam (2020)53 | Retrospective, 2‐arm, multicenter cohort study | Consecutive patients with an attempted implantation | Micra | 12 | 24 | 21 |
| Tachibana (2020)51 | Retrospective, 2‐arm, single‐center cohort study | Consecutive patients ≥85 y underwent LP implantation | Micra | 6 | 23 | 20 |
LP indicates leadless pacemaker; and TVP, transvenous pacemaker.
Table 6.
Studies Reporting Clinical Outcomes as Efficacy End Points
| Author (y) | Study Design | Study Population | Follow‐Up Time | Efficacy End Point | Results |
|---|---|---|---|---|---|
| Cabanas‐Grandío (2020)24 | 2‐arm, multicenter cohort study | One hundred six patients (64 patients implanted with TVP and 42 patients implanted with LP). The choice of TVP or LP was based on clinical criteria and operator availability. | 6 mo | Quality of life evaluated by the SF‐36 questionnaire | LP is associated with significantly higher scores on physical function (63 vs 42; P<0.001), physical role (64 vs 36; P=0.004), and mental health (75 vs 65; P=0.017) compared with TVP. LP is also associated with lower discomfort and physical restrictions compared with TVP. Hazard/odds ratio not reported. |
| Tjong (2018)32 |
Prospective, multicenter, single‐arm cohort study |
Seven hundred twenty patients. Number of patients who completed the SF‐36 questionnaire at baseline, 3, and 12 mo was 702, 681, and 635, respectively. | 3 and 12 mo | Health‐related quality of life evaluated using the SF‐36 questionnaire | Health‐related quality of life was improved at 3 and 12 mo after LP implantation (mental component score improved by 28.4% at 3 mo and 26.9% at 12 mo; increases in physical component score were 26.8% and 25.3%, respectively). At 3 mo, most patient were satisfied with the treatment. |
| Beurskens (2019)25 | Retrospective, 2‐arm, single‐center cohort study | Fifty‐six consecutive patients underwent LP implantations, but only 53 patients (28 Nanostim and 25 Micra) with quality echocardiography images were included. | 12 mo | Tricuspid valve regurgitation grade evaluated by echocardiography | Tricuspid valve regurgitation worsened in 23 (43%) patients but comparable to that (38%) in those with TVP (P=0.39) and was unrelated to pacing rates. Hazard/odds ratio not reported. |
| Salaun (2018)26 | Single‐arm, single‐center cohort study | Twenty‐nine consecutive patients implanted with LP, but only 23 were included for analysis (14 with Nanostim and 9 with Micra). Three patients were excluded because of lack of echocardiography images, and 3 refused to participate. | 2 mo | Right ventricular and tricuspid valve function evaluated by echocardiography | No significant change in right ventricular function was observed. One patient experienced significantly deteriorating tricuspid valve regurgitation that was related to pulmonary hypertension caused by chronic obstructive lung disease. |
LP indicates leadless pacemaker; SF‐36, Short Form‐36; and TVP, transvenous pacemaker.
Quality Assessment
Of the 36 studies, most (n=20, 55.6%) were evaluated as having good quality, whereas 15 (41.7%) and 1 (2.8%) were graded as fair and poor, respectively. Detailed assessments are provided in Table S3.
Proportion of Patients With a Successful Implant
A total of 23 studies (n=4769 patients) reported the proportion of patients with a successful implant. The pooled proportion was 99.85% (95% CI, 99.59%–99.99%; I 2=0.00%) for Micra and 97.12% (95% CI, 95.86%–98.20%; I 2=0.00%) for Nanostim (Table S2 and Figure 2).
Figure 2. Pooled proportion of patients with a successful implant.
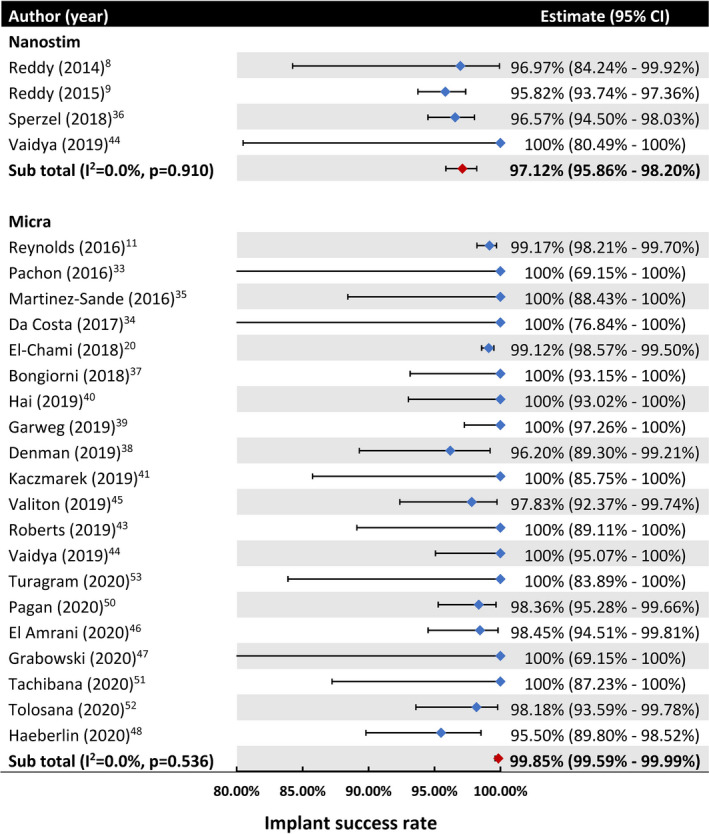
Catillon et al (2018)29 and Roberts et al (2017)28 both reported the proportion of patients with a successful implant, but they used the same population as the Reddy et al (2015)9 study (LEADLESS II trial) and El‐Chami et al (2018)20 study (Micra Post‐Approval Registry), and therefore were not included in this meta‐analysis.
Safety of LPs
Overall, 12 studies (n=2376 patients) reported safety end points at up to 90 days after implant (Table 2 and Figure 3A). Three (n=768 patients) used the Nanostim LP and reported a 90‐day complication incidence of 5.85% to 23.5%, with pooled estimates not drawn because of the small number of studies. The pooled incidence of complications of the Micra LP (n=1608 patients) was 0.46% (95% CI, 0.08%–1.05%; I 2=0.00%). When individual complications associated with Micra devices were considered, incidences of device dislodgement (0.00%; 95% CI, 0.00%–0.00%; I 2=0.00%), tamponade/cardiac perforation (0.00%; 95% CI, 0.00%–0.26%; I 2=0.00%), infection (0.00%; 95% CI, 0.00%–0.00%; I 2=0.00%), and vascular injury (0.05%; 95% CI, 0.00%–0.77%; I 2=0.00%) at 90 days were low. Incidences of other complications, such as minor vascular injury or pericardial effusion that did not require intervention, could not be reliably extracted from the included studies.
Figure 3. Meta‐analysis of the safety of the leadless pacemaker (LP).
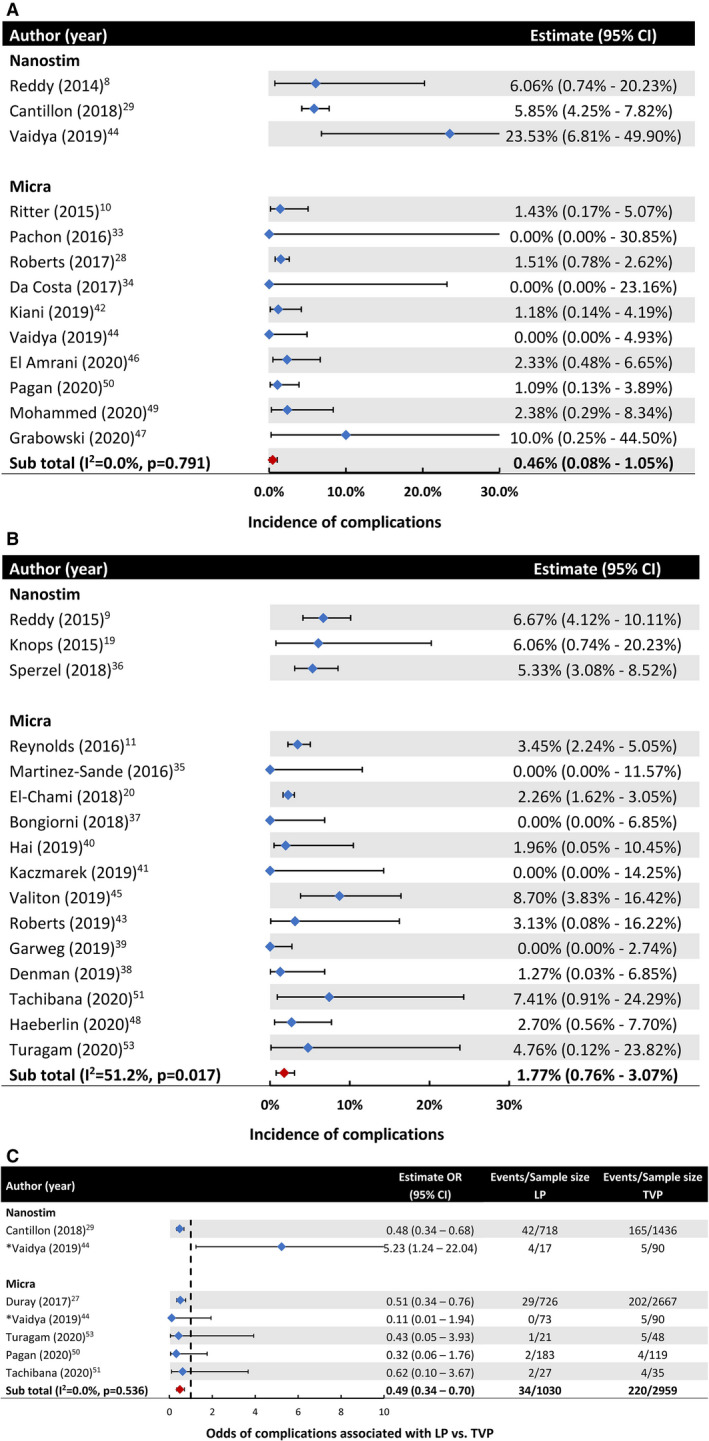
A, Pooled incidence of overall complications at up to 90 days after LP implantation. B, Pooled incidence of overall complications at ≈1 year after LP implantation in studies that reported safety outcomes beyond 90 days. C, Incidence of overall complications in studies that compared the LP with the transvenous pacemaker (TVP) implantation. *Vaidya et al44 reported complications for both Nanostim (17 patients) and Micra LPs (73 patients) compared with those associated with a TVP (90 patients). OR indicates odds ratio.
Sixteen studies (n=3827 patients) with follow‐up times beyond 90 days reported safety endpoints at ≈12 months after implantation (Table 3 and Figure 3B). A pooled estimate for Nanostim was also not drawn because of the low number of studies that reported a complication incidence ranging from 5.33% to 6.67%. The pooled incidence of complications for the Micra LP (n=3194 patients) was 1.77% (95% CI, 0.76%–3.07%; I 2=51.2%). There was a lack of safety data beyond 2 years, with only 1 report of a complication incidence of 1.82% at 24 months after implantation.52
Seven studies compared outcomes of patients implanted with a LP versus TVP (Table 4 and Figure 3C). The follow‐up period in these studies varied from 0 to 26.7 months. In 5 studies with follow‐up time of up to 1 year, Micra was associated with 51% lower odds of complications compared with a TVP (3.30% versus 7.43%; OR, 0.49 [95% CI, 0.34–0.70; I 2=0.00%]) (Figure 3C). Only 2 studies compared the safety of Nanostim versus TVP, and therefore, the pooled OR for this comparison was not estimated. One study did not report the numbers for Nanostim and Micra separately.30
Efficacy of LPs
The proportion of patients having a pacing capture threshold ≤2 V at 1 year reported for the Nanostim LP ranged from 90% to 100% in 3 studies (Table 5 and Figure 4, pooled estimate not drawn). For the Micra LP, among 12 studies (n=1376 patients), the pooled proportion of patients with a pacing capture threshold ≤2 V at 1 year was 98.96% (95% CI, 97.26%–99.94%) (Figure 4). Only 2 studies, all with Micra implantation, reported an efficacy endpoint beyond 1 year, with 100% (41) and 91.53% (52) of patients having pacing threshold ≤2 V at 13 and 24 months, respectively. Examination of electrical performance beyond 2 years was lacking.
Figure 4. Pooled proportion of patients having a pacing capture threshold ≤2 V at 1 year after implantation.
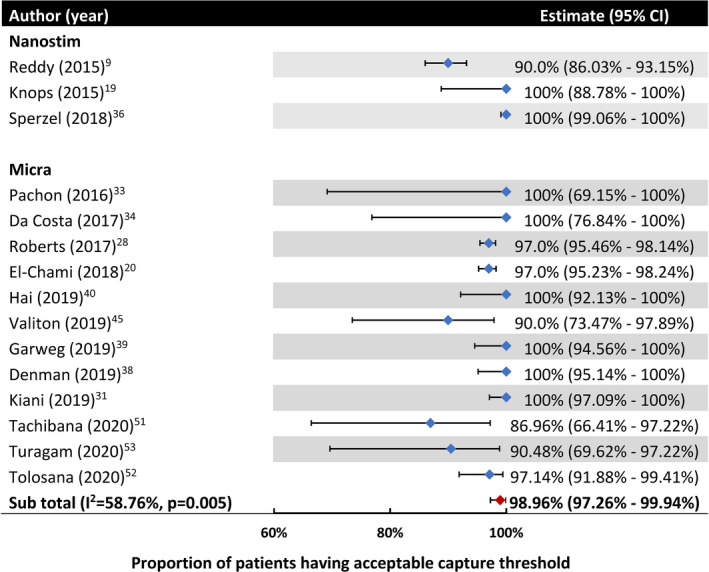
Four studies reported clinical outcomes as their efficacy end point, among which 2 showed improved quality of life and good patient satisfaction (Table 6). The other 2 examined right ventricular and tricuspid valve (TV) function, with 1 reporting that 43% of 53 patients experienced worsening TV regurgitation, whereas the other found 1 out of 23 patients (4.35%) experienced significantly deteriorated TV function.
Sensitivity Analysis
Given the low number of studies investigating the Nanostim system, all sensitivity analyses were performed using studies that reported data for the Micra LP. When we examined good‐quality studies only (12 studies, n=3270 patients), the pooled proportion of patients with a successful implant (99.85%; 95% CI, 99.56%–99.99%; I 2=0.00%) and the pooled incidence of complications at 90 days (0.50%; 95% CI, 0.00%–1.78%; I 2=0.00%) were comparable to the overall results. However, the complication incidence at 1 year was higher, with an estimated incidence of 2.39% (95% CI, 1.14%–3.99%; I 2=55.10%). The proportion of patients meeting the efficacy end point of adequate capture threshold at 1 year was 98.77% (95% CI, 97.16%–99.81%; I 2=37.96%), similar to the overall results. We also compared results when only prospective or retrospective studies were included in the meta‐analysis. Results from analysis of prospective studies showed pooled estimates of patients experiencing complications and meeting the efficacy end point at 1 year after implant were 1.77% (95% CI, 0.76%–3.07%; I 2=51.20%) and 98.98% (95% CI, 97.66%–99.83%; I 2=26.37%), respectively. On the other hand, retrospective studies reported a slightly lower proportion of patients meeting the efficacy end point of 94.36% (95% CI, 80.54%–100%; I 2=83.86%) and higher incidence of complications at 1 year of 6.52% (95% CI, 2.97%–11.06%; I 2=0.00%).
Discussion
In this systematic review and meta‐analysis, we found that a LP, especially the Micra, is associated with a high proportion of patients having a successful implant and a low incidence of complications at 90 days and 1 year after implantation. In the few studies that compared LPs with TVPs, Micra devices were associated with 51% lower odds of complications. Furthermore, the combined data suggested that LPs have good electrical performance up to 1 year after implantation, with >90% of devices having an adequate pacing capture threshold. However, the current literature is predominantly based on the Micra LP and includes only observational data with limited follow‐up time, with electrical performance and clinical outcomes rarely being reported beyond the second year. Although the available data are promising, robust randomized trials with longer‐term clinical outcome data are required to confirm these findings.
This study represents the first systematic evaluation of the safety and efficacy of LPs implanted in the right ventricle. There are 2 systematic reviews related to LPs that examined the incidences of cardiac perforation15 and device dislodgement,16 respectively, although neither reported pooled estimates because they included only 2 and 3 LP studies, respectively. We extend the literature by providing pooled estimates of overall as well as specific complications. Notably, the pooled complication incidence associated with Micra is considerably lower than the 7.76% to 12.4% incidence of early complications2, 3, 4 (within 3 months) or the 15% to 16% incidence of long‐term complications3, 4 that are typically associated with TVPs. Our meta‐analysis of studies comparing LPs and TVPs confirmed this observation, with the Micra LP having half the odds of TVPs. Collectively, these findings suggest that LP implantation is safe and associated with less harm than TVPs.
Besides the good safety profile, the implant success and the short‐term efficacy of LPs were high. However, there was a lack of efficacy data beyond 2 years, which leaves uncertainty about the longevity of the device performance. The unexpected premature battery failure of the Nanostim LP occurred at 2.3 to 4.0 years after implantation.12 Although no such concern has been reported with the Micra LP, the only LP currently approved by the Food and Drug Administration, whether it can match the battery life of contemporary TVPs remains uncertain. More data are also needed about the long‐term management of these devices such as pacemaker retrieval and how additional devices are implanted when LPs reach the end of battery life.
Uncertainties also exist about the clinical outcomes associated with LPs, because most studies only report acceptable pacing capture threshold as the primary efficacy end point. Although the electrical performance is easy to measure, clinical outcomes like mortality, syncope, heart failure, TV function and quality of life are equally relevant to patients and clinicians. Only a few studies have evaluated quality of life, all of which reported improved quality of life,24, 32 and 1 showed better physical activity, physical role, and mental health associated with LP compared with conventional devices.24 On the other hand, TV function was evaluated in 2 small studies, with 1 study suggesting worsening TV regurgitation in up to 45% of patients, which is comparable to the 38% incidence associated with TVPs (P=0.39).25 The other recorded only 1 out of 23 patients experiencing increased TV regurgitation,26 which was thought to result from pulmonary hypertension rather than pacing. Nevertheless, neither studies reported new onset of heart failure as a clinical endpoint. Theoretically, without a lead crossing the valve, TV regurgitation should occur less frequently with LPs, because studies have shown that worsening TV function associated with traditional cardiac devices are likely attributable to lead‐related damages to TV leaflets or subvalvular structures, or impairment of leaflet mobility and coaptation.54, 55, 56 Future investigations are needed to examine the underlying mechanisms of this phenomenon as well as evaluate other clinical outcomes.
Several limitations should be considered when interpreting our results. Although our findings are promising, the data were entirely observational, and most studies had a small sample size and short follow‐up time of <1 year. Because there are essential differences in sizes, fixation and pacing mechanisms between the Nanostim and Micra devices, a meta‐analysis was performed separately for each device. However, because of the low number of studies that used the Nanostim LP, pooled estimates for this device were not drawn, and most of the pooled estimates reflect the performance of the Micra LP only. The inconsistency about which complications are reported makes estimating pooled incidences for specific complications challenging. Similarly, efficacy endpoints about pacing capture threshold were defined differently among studies, with some considering ≤2 V acceptable, whereas others used the 1.5‐V threshold. The exclusion of abstracts and conference proceedings may increase the risk of publication bias, although data using only fully published articles are considered more reliable and generally necessarily provide all required information.
Conclusions
Based on pooled observational data, leadless pacemakers have a low incidence of complications (0.46% at 3 months and 1.77% at 1 year for the Micra LP) and good short‐term electrical performance, with >90% of LPs having acceptable pacing threshold at 1 year. A Micra is also associated with 51% lower odds of complications when compared with a TVP. Further data from well‐designed randomized controlled trials with longer follow‐up time are still required to determine longer‐term safety and efficacy of LPs to support the widespread adoption of these novel devices in clinical practice.
Sources of Funding
Dr Ngo is supported by a Research Training Program Scholarship from The University of Queensland. Dr Ranasinghe is supported by National Heart Foundation of Australia Future Leader Fellowships (ID 101186).
Disclosures
Dr Denman has delivered talks for Medtronic on LPs, has run 4 training courses for Medtronic at The Prince Charles Hospital to train other physicians in how to implant the Micra LP. Dr Denman is also a local principal investigator for the St Jude Nanostim study. Dr Haqqani has received speaking and proctoring honoraria from Medtronic and has served on the scientific advisory board of Medtronic. Dr Haqqani has also received speaking honoraria from Abbott. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
References 8–11, 20, 24–53
(J Am Heart Assoc. 2021;10:e019212. DOI: 10.1161/JAHA.120.019212.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019212
For Sources of Funding and Disclosures, see page 16.
References
- 1.Kusumoto Fred M, Schoenfeld Mark H, Barrett C, Edgerton James R, Ellenbogen Kenneth A, Gold Michael R, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–e482. [DOI] [PubMed] [Google Scholar]
- 2.Ranasinghe I, Labrosciano C, Horton D, Ganesan A, Curtis JP, Krumholz HM, McGavigan A, Hossain S, Air T, Hariharaputhiran S, et al. Institutional variation in quality of cardiovascular implantable electronic device implantation: a cohort study. Ann Intern Med. 2019;171:309–317. DOI: 10.7326/M18-2810. [DOI] [PubMed] [Google Scholar]
- 3.Udo EO, Zuithoff NPA, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KGM. Incidence and predictors of short‐ and long‐term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;5:728–735. DOI: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Cantillon DJ, Exner DV, Badie N, Davis K, Gu NY, Nabutovsky Y, Doshi R. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. 2017;3:1296–1305. DOI: 10.1016/j.jacep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig S, Theis C, Wolff C, Nicolle E, Witthohn A, Götte A. Complications and associated healthcare costs of transvenous cardiac pacemakers in Germany. J Comp Eff Res. 2019;8:589–597. DOI: 10.2217/cer-2018-0114. [DOI] [PubMed] [Google Scholar]
- 6.Chinitz L, Ritter P, Khelae SK, Iacopino S, Garweg C, Grazia‐Bongiorni M, Neuzil P, Johansen JB, Mont L, Gonzalez E, et al. Accelerometer‐based atrioventricular synchronous pacing with a ventricular leadless pacemaker: results from the Micra atrioventricular feasibility studies. Heart Rhythm. 2018;15:1363–1371. DOI: 10.1016/j.hrthm.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Steinwender C, Khelae SK, Garweg C, Chan JYS, Ritter P, Johansen JB, Sagi V, Epstein LM, Piccini JP, Pascual M, et al. Atrioventricular synchronous pacing using a leadless ventricular pacemaker: results from the MARVEL 2 study. JACC Clin Electrophysiol. 2020;6:94–106. DOI: 10.1016/j.jacep.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Reddy VY, Knops RE, Sperzel J, Miller MA, Petru J, Simon J, Sediva L, de Groot JR, Tjong FVY, Jacobson P, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129:1466–1471. DOI: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 9.Reddy VY, Exner DV, Cantillon DJ, Doshi R, Bunch TJ, Tomassoni GF, Friedman PA, Estes NAM, Ip J, Niazi I, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. DOI: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 10.Ritter P, Duray GZ, Steinwender C, Soejima K, Omar R, Mont L, Boersma LVA, Knops RE, Chinitz L, Zhang S, et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J. 2015;36:2510–2519. DOI: 10.1093/eurheartj/ehv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, Narasimhan C, Steinwender C, Brugada J, Lloyd M, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. DOI: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 12.Lakkireddy D, Knops R, Atwater B, Neuzil P, Ip J, Gonzalez E, Friedman P, Defaye P, Exner D, Aonuma K, et al. A worldwide experience of the management of battery failures and chronic device retrieval of the Nanostim leadless pacemaker. Heart Rhythm. 2017;14:1756–1763. DOI: 10.1016/j.hrthm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Steinwender C, Lercher P, Schukro C, Blessberger H, Prenner G, Andreas M, Kraus J, Ammer M, Stühlinger M. State of the art: leadless ventricular pacing: a national expert consensus of the Austrian Society of Cardiology. J Interv Card Electrophysiol. 2020;57:27–37. DOI: 10.1007/s10840-019-00680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccini JP, Stromberg K, Jackson KP, Kowal RC, Duray GZ, El‐Chami MF, Crossley GH, Hummel JD, Narasimhan C, Omar R, et al. Patient selection, pacing indications, and subsequent outcomes with de novo leadless single‐chamber VVI pacing. Europace. 2019;21:1686–1693. DOI: 10.1093/europace/euz230. [DOI] [PubMed] [Google Scholar]
- 15.Vamos M, Erath JW, Benz AP, Bari Z, Duray GZ, Hohnloser SH. Incidence of cardiac perforation with conventional and with leadless pacemaker systems: a systematic review and meta‐analysis. J Cardiovasc Electrophysiol. 2017;8:336–346. DOI: 10.1111/jce.13140. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Hou W, Zhou C, Yin Y, Lu S, Liu G, Duan C, Cao M, Li M, Toft ES, et al. Meta‐analysis of the incidence of lead dislodgement with conventional and leadless pacemaker systems. Pacing Clin Electrophysiol. 2018;41:1365–1371. DOI: 10.1111/pace.13458. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. DOI: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.National Heart LaBI . Study Quality Assessment Tool 2014. Available at: HYPERLINK "sps:urlprefix::https" https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. Accessed 17/06/2020.
- 19.Knops RE, Tjong FVY, Neuzil P, Sperzel J, Miller MA, Petru J, Simon J, Sediva L, de Groot JR, Dukkipati SR, et al. Chronic performance of a leadless cardiac pacemaker: 1‐year follow‐up of the LEADLESS trial. J Am Coll Cardiol. 2015;65:1497–1504. [DOI] [PubMed] [Google Scholar]
- 20.El‐Chami MF, Al‐Samadi F, Clementy N, Garweg C, Martinez‐Sande JL, Piccini JP, Iacopino S, Lloyd M, Viñolas Prat X, Jacobsen MD, et al. Updated performance of the Micra transcatheter pacemaker in the real‐world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15:1800–1807. DOI: 10.1016/j.hrthm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72:39. DOI: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. Metan: fixed‐ and random‐effects meta‐analysis. Stata J. 2008;8:3–28. DOI: 10.1177/1536867X0800800102. [DOI] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. DOI: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Cabanas‐Grandío P, García Campo E, Bisbal F, García‐Seara J, Pachón M, Juan‐Salvadores P, Paredes E, Molinero A, Martínez‐Sande JL, Arias MÁ, et al. Quality of life of patients undergoing conventional vs leadless pacemaker implantation: a multicenter observational study. J Cardiovasc Electrophysiol. 2020;31:330–336. DOI: 10.1111/jce.14322. [DOI] [PubMed] [Google Scholar]
- 25.Beurskens NEG, Tjong FVY, De Bruin‐Bon RHA, Dasselaar KJ, Kuijt WJ, Wilde AAM, Knops RE. Impact of leadless pacemaker therapy on cardiac and atrioventricular valve function through 12 months of follow‐up. Circ Arrhythm Electrophysiol. 2019;12:e007124. [DOI] [PubMed] [Google Scholar]
- 26.Salaun E, Tovmassian L, Simonnet B, Giorgi R, Franceschi F, Koutbi‐Franceschi L, Hourdain J, Habib G, Deharo J‐C. Right ventricular and tricuspid valve function in patients chronically implanted with leadless pacemakers. Europace. 2018;20:823–828. DOI: 10.1093/europace/eux101. [DOI] [PubMed] [Google Scholar]
- 27.Duray GZ, Ritter P, El‐Chami M, Narasimhan C, Omar R, Tolosana JM, Zhang S, Soejima K, Steinwender C, Rapallini L, et al. Long‐term performance of a transcatheter pacing system: 12‐month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017;14:702–709. DOI: 10.1016/j.hrthm.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Roberts PR, Clementy N, Al Samadi F, Garweg C, Martinez‐Sande JL, Iacopino S, Johansen JB, Vinolas Prat X, Kowal RC, Klug D, et al. A leadless pacemaker in the real‐world setting: the Micra Transcatheter Pacing System Post‐Approval Registry. Heart Rhythm. 2017;14:1375–1379. DOI: 10.1016/j.hrthm.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Cantillon DJ, Dukkipati SR, Ip JH, Exner DV, Niazi IK, Banker RS, Rashtian M, Plunkitt K, Tomassoni GF, Nabutovsky Y, et al. Comparative study of acute and mid‐term complications with leadless and transvenous cardiac pacemakers. Heart Rhythm. 2018;15:1023–1030. DOI: 10.1016/j.hrthm.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Tjong FVY, Knops RE, Udo EO, Brouwer TF, Dukkipati SR, Koruth JS, Petru J, Sediva L, van Hemel NM, Neuzil P, et al. Leadless pacemaker versus transvenous single‐chamber pacemaker therapy: a propensity score‐matched analysis. Heart Rhythm. 2018;15:1387–1393. DOI: 10.1016/j.hrthm.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Kiani S, Black GB, Rao B, Thakkar N, Massad C, Patel AV, Lu MLR, Merchant FM, Hoskins MH, Lurgio DBD, et al. The safety and feasibility of same‐day discharge after implantation of MICRA transcatheter leadless pacemaker system. J Atr Fibrillation. 2019;12:2153. DOI: 10.4022/jafib.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tjong FVY, Beurskens NEG, Groot JR, Waweru C, Liu S, Ritter P, Reynolds D, Wilde AAM, Knops RE. Health‐related quality of life impact of a transcatheter pacing system. J Cardiovasc Electrophysiol. 2018;29:1697–1704. DOI: 10.1111/jce.13726. [DOI] [PubMed] [Google Scholar]
- 33.Pachón M, Puchol A, Akerström F, Rodríguez‐Padial L, Arias MA. Implantation of the Micra transcatheter pacing system: initial experience in a single Spanish center. Rev Esp Cardiol (Engl Ed). 2016;69:346–349. DOI: 10.1016/j.rec.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Da Costa A, Axiotis A, Romeyer‐Bouchard C, Abdellaoui L, Afif Z, Guichard JB, Gerbay A, Isaaz K. Transcatheter leadless cardiac pacing: the new alternative solution. Int J Cardiol. 2017;227:122–126. DOI: 10.1016/j.ijcard.2016.11.196. [DOI] [PubMed] [Google Scholar]
- 35.Martínez‐Sande JL, García‐Seara J, Rodríguez‐Mañero M, Fernández‐López XA, González‐Melchor L, Redondo‐Diéguez A, González‐Ferreiro R, González‐Juanatey JR. The Micra leadless transcatheter pacemaker. Implantation and mid‐term follow‐up results in a single center. Rev Esp Cardiol. 2017;70:275–281. [DOI] [PubMed] [Google Scholar]
- 36.Sperzel J, Defaye P, Delnoy P‐P, Garcia Guerrero JJ, Knops RE, Tondo C, Deharo J‐C, Wong T, Neuzil P. Primary safety results from the LEADLESS Observational Study. Europace. 2018;20:1491–1497. DOI: 10.1093/europace/eux359. [DOI] [PubMed] [Google Scholar]
- 37.Bongiorni MG, Della Tommasina V, Barletta V, Di Cori A, Rogani S, Viani S, Segreti L, Paperini L, Soldati E, De Lucia R, et al. Feasibility and long‐term effectiveness of a non‐apical Micra pacemaker implantation in a referral centre for lead extraction. Europace. 2019;21:114–120. DOI: 10.1093/europace/euy116. [DOI] [PubMed] [Google Scholar]
- 38.Denman RA, Lee AC, Mengel C, Townsend S, Betts J, Bovey N, Wright D, Davison O, Haqqani HM. Leadless permanent pacing: a single centre Australian experience. Heart Lung Circ. 2019;28:1677–1682. DOI: 10.1016/j.hlc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Garweg C, Vandenberk B, Foulon S, Haemers P, Ector J, Willems R. Leadless pacing with Micra TPS: a comparison between right ventricular outflow tract, mid‐septal, and apical implant sites. J Cardiovasc Electrophysiol. 2019;30:2002–2011. DOI: 10.1111/jce.14083. [DOI] [PubMed] [Google Scholar]
- 40.Hai J‐J, Fang J, Tam C‐C, Wong C‐K, Un K‐C, Siu C‐W, Lau C‐P, Tse H‐F. Safety and feasibility of a midseptal implantation technique of a leadless pacemaker. Heart Rhythm. 2019;16:896–902. DOI: 10.1016/j.hrthm.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Kaczmarek K, Cygankiewicz I, Czarniak B, Jakubowski P, Strzelecki A, Wranicz JK, Drożdż J, Ptaszyński P. Septal implantation of the Micra transcatheter pacing system guided by intraprocedural transesophageal echocardiography. Kardiol Pol. 2019;77:1190–1192. DOI: 10.33963/KP.15043. [DOI] [PubMed] [Google Scholar]
- 42.Kiani S, Black GB, Rao B, Thakkar N, Massad C, Patel AV, Merchant FM, Hoskins MH, Lurgio DB, Patel AM, et al. Outcomes of Micra leadless pacemaker implantation with uninterrupted anticoagulation. J Cardiovasc Electrophysiol. 2019;30:1313–1318. DOI: 10.1111/jce.13965. [DOI] [PubMed] [Google Scholar]
- 43.Roberts PR, Pepper C, Rinaldi CA, Bates MGD, Thornley A, Somani R, Abozguia K, Harris S, Rao A, Pedersen M, et al. The use of a single chamber leadless pacemaker for the treatment of cardioinhibitory vasovagal syncope. Int J Cardiol Heart Vasc. 2019;23:100349. DOI: 10.1016/j.ijcha.2019.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaidya VR, Dai M, Asirvatham SJ, Rea RF, Thome TM, Srivathsan K, Mulpuru SK, Kusumoto F, Venkatachalam KL, Ryan JD, et al. Real‐world experience with leadless cardiac pacing. Pacing Clin Electrophysiol. 2019;42:366–373. DOI: 10.1111/pace.13601. [DOI] [PubMed] [Google Scholar]
- 45.Valiton V, Graf D, Pruvot E, Carroz P, Fromer M, Bisch L, Tran VN, Cook S, Scharf C, Burri H, et al. Leadless pacing using the transcatheter pacing system (Micra TPS) in the real world: initial Swiss experience from the Romandie region. Europace. 2019;21:275–280. DOI: 10.1093/europace/euy195. [DOI] [PubMed] [Google Scholar]
- 46.El Amrani A, Campos B, Alonso‐Martín C, Guerra‐Ramos JM, Rodríguez‐Font E, Moreno‐Weidmann Z, Alcalde‐Rodríguez Ó, Méndez‐Zurita FJ, Santaló M, Espinosa‐Viamonte H, et al. Performance of the Micra cardiac pacemaker in nonagenarians. Rev Esp Cardiol (Engl Ed). 2020;73:307–312. [DOI] [PubMed] [Google Scholar]
- 47.Grabowski M, Michalak M, Gawałko M, Gajda S, Cacko A, Januszkiewicz Ł, Kołodzińska A, Mitkowski PP, Duray GZ, Opolski G, et al. Implantation of the Micra transcatheter pacing system: Single Polish center experience with the real costs of hospitalization analysis. Cardiol J. 2020;27:47–53. DOI: 10.5603/CJ.a2018.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haeberlin A, Kozhuharov N, Knecht S, Tanner H, Schaer B, Noti F, Osswald S, Servatius H, Baldinger S, Seiler J, et al. Leadless pacemaker implantation quality: importance of the operator's experience. Europace. 2020;22:939–946. DOI: 10.1093/europace/euaa097. [DOI] [PubMed] [Google Scholar]
- 49.Mohammed M, Arshi J, Ramza BM, Wimmer AP, Steinhaus DA, Giocondo MJ, Gupta SK, Yousuf SK. Outcomes using a single tapered dilator for Micra leadless pacemaker implant. Indian Pacing Electrophysiol J. 2020;20:105–111. DOI: 10.1016/j.ipej.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagan E, Gabriels J, Khodak A, Chang D, Beldner S, Epstein LM, Willner J. Safety of leadless pacemaker implantation in the very elderly. Heart Rhythm. 2020;17:2023–2028. DOI: 10.1016/j.hrthm.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Tachibana M, Banba K, Matsumoto K, Ohara M. The feasibility of leadless pacemaker implantation for superelderly patients. Pacing Clin Electrophysiol. 2020;43:374–381. DOI: 10.1111/pace.13894. [DOI] [PubMed] [Google Scholar]
- 52.Tolosana JM, Guasch E, San Antonio R, Apolo J, Pujol‐López M, Chipa‐Ccasani F, Trucco E, Roca‐Luque I, Brugada J, Mont L, et al. Very high pacing thresholds during long‐term follow‐up predicted by a combination of implant pacing threshold and impedance in leadless transcatheter pacemakers. J Cardiovasc Electrophysiol. 2020;31:868–874. DOI: 10.1111/jce.14360. [DOI] [PubMed] [Google Scholar]
- 53.Turagam MK, Gopinathannair R, Park PH, Tummala RV, Vasamreddy C, Shah A, Koerber S, Krauthammer Y, Di Biase L, Murtaza G, et al. Safety and efficacy of leadless pacemaker for cardio inhibitory vasovagal syncope. Heart Rhythm. 2020;17:1575–1581. DOI: 10.1016/j.hrthm.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Al‐bawardy R, Krishnaswamy A, Rajeswaran J, Bhargava M, Wazni O, Wilkoff B, Tuzcu EM, Martin D, Thomas J, Blackstone E, et al. Tricuspid regurgitation and implantable devices. Pacing Clin Electrophysiol. 2015;38:259–266. DOI: 10.1111/pace.12530. [DOI] [PubMed] [Google Scholar]
- 55.Lee RC, Friedman SE, Kono AT, Greenberg ML, Palac RT. Tricuspid regurgitation following implantation of endocardial leads: incidence and predictors. Pacing Clin Electrophysiol. 2015;38:1267–1274. DOI: 10.1111/pace.12701. [DOI] [PubMed] [Google Scholar]
- 56.Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid valve dysfunction following pacemaker or cardioverter‐defibrillator implantation. J Am Coll Cardiol. 2017;69:2331. DOI: 10.1016/j.jacc.2017.02.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
References 8–11, 20, 24–53


