Abstract
Background
Systemic lupus erythematosus (SLE) is a systemic autoimmune inflammatory disorder associated with premature atherosclerosis and increased cardiovascular risk. Systemic inflammation is an emerging risk factor for coronary microvascular dysfunction (CMD). We aimed to test whether CMD, defined as abnormal myocardial flow reserve (MFR) by positron emission tomography‐computed tomography, would be independently associated with SLE after adjusting for nonobstructive atherosclerotic burden and common cardiovascular risk factors.
Methods and Results
Consecutive patients with SLE who underwent symptom‐prompted stress cardiac positron emission tomography‐computed tomography were included (n=42). Obstructive coronary artery disease and systolic dysfunction were excluded. MFR was quantified by positron emission tomography‐computed tomography, and CMD was defined as MFR <2. We frequency matched patients who did not have SLE and had symptom‐prompted positron emission tomography studies on age, sex, and key cardiovascular risk factors (n=69). The attenuation correction computed tomography scans were reviewed for qualitative assessment of coronary artery calcium. Patients with SLE had a more severe reduction in global MFR compared with controls and a higher prevalence of CMD, despite a similar degree of nonobstructive atherosclerotic burden (1.91±0.5 versus 2.4±0.7, respectively, P<0.0001; CMD, 57.1% versus 33.3%, respectively, P=0.017).
Conclusions
We demonstrated that patients with SLE with cardiac symptoms without obstructive coronary artery disease have a high prevalence of coronary vasomotor abnormalities. In comparison with symptomatic matched controls, patients with SLE have a more severe reduction in MFR that is not accounted for by common cardiovascular factors or atherosclerotic burden.
Keywords: coronary microvascular dysfunction, inflammation, systemic lupus erythematosus
Subject Categories: Inflammatory Heart Disease, Cardiovascular Disease, Nuclear Cardiology and PET
Nonstandard Abbreviations and Acronyms
- aPL
antiphospholipid antibodies
- CMD
coronary microvasculature dysfunction
- MBF
myocardial blood flow
- MFR
myocardial flow reserve
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
Clinical Perspective
What Is New?
Coronary microvascular dysfunction is prevalent in patients with systemic lupus erythematosus (SLE) and independent of atherosclerotic burden and common cardiovascular risk factors.
SLE is associated with a high prevalence of coronary vasomotor dysfunction, reflecting the presence of diffuse atherosclerosis and coronary microvascular dysfunction.
This finding is correlated with disease activity and not entirely accounted for by coronary risk factors, renal dysfunction, or atherosclerotic burden.
What Are the Clinical Implications?
Coronary vascular dysfunction in patients with SLE supports a possible role for inflammation in driving coronary vasomotor abnormalities that might contribute to excess cardiovascular risk in patients with SLE.
Future prospective studies are needed to address the spectrum of coronary vasomotor dysfunction across clinical severities of SLE and whether reducing systemic inflammation with disease‐modifying antirheumatic drugs, immunosuppressants, and/or the newer novel biologics in SLE can improve abnormalities in coronary vascular function and cardiovascular outcomes.
Systemic lupus erythematosus (SLE) is a multiorgan, systemic inflammatory autoimmune disease that predominantly affects young women. Increased prevalence of traditional cardiovascular risk factors including hypertension, hyperlipidemia, and obesity, as well as nontraditional SLE‐specific factors, including systemic inflammation, glucocorticoids, and antiphospholipid antibodies, contribute to high cardiovascular event rates among patients with SLE.1, 2, 3, 4, 5, 6 The excess risk of a major adverse cardiovascular event among patients with SLE has been linked to an enhanced proinflammatory state.7 There is ample laboratory and clinical evidence that systemic inflammation plays a major role in all stages of atherothrombosis,8, 9 including diffuse atherosclerosis and the early functional abnormalities in vascular endothelial and smooth muscle cell function leading to coronary vascular dysfunction. This may help explain symptoms of chest pain and dyspnea, which are common in patients with SLE, even in the absence of obstructive coronary artery disease (CAD).10 Our objective was to test the hypothesis that coronary microvascular dysfunction (CMD) is a common feature in symptomatic high‐risk patients with SLE, independent of common cardiovascular risk factors or atherosclerotic burden.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We studied consecutive patients diagnosed with SLE who underwent stress positron emission tomography (PET) myocardial perfusion imaging for the evaluation of suspected CAD, on the basis of the presence of chest pain and/or dyspnea between January 1, 2006 and December 31, 2018 at Brigham and Women’s Hospital. SLE diagnosis was confirmed on the basis of the Systemic Lupus International Collaborating Clinics criteria and further validated by cross‐reference to the Brigham and Women’s Hospital Rheumatology SLE Registry. The SLE disease activity index was calculated according to the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) criteria.11, 12 Control patients were identified on the basis of age, sex, obesity, hyperlipidemia, hypertension, diabetes mellitus, and history of CAD, defined as prior myocardial infarction or percutaneous coronary intervention obtained from the cardiology radiology database, which includes >6000 patients and linked electronic health records. Included were patients aged >18 years. Patients with left ventricular ejection fraction <50%, an abnormal myocardial perfusion study (summed stress score ≥3), prior coronary artery bypass surgery, heart transplantation, or other systemic inflammatory disorder were excluded. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines, and all patients signed informed consent for this procedure. Women of childbearing age were required to have a negative blood pregnancy test on the morning of the exam.
Quantification of Coronary Vascular Function
Myocardial blood flow (MBF) and myocardial flow reserve (MFR), reflecting large and small coronary vessel function, were quantified with PET myocardial perfusion imaging performed on a whole‐body PET‐computed tomography scanner (Discovery RX or STE Lightspeed 64; GE Healthcare, Milwaukee, WI) in 2‐dimensional mode using 82rubidium or 13N‐ammonia as previously described.13, 14 Computed tomography was used for attenuation correction. Coronary vasodilation was achieved using regadenoson or dipyridamole as per standard care. PET images were evaluated semiquantitatively by a 17‐segment visual assessment of gated myocardial perfusion images with a standard 5‐point scoring system. Rest left ventricular ejection fractions were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; INVIA Medical Imaging Solutions, Ann Arbor, MI). Summed stress score <3 was considered to be normal and reflect absence of overt obstructive CAD. Rest and stress MBF, in milliliters per minute per gram, was quantified using a validated tracer kinetic model as previously described.13, 14 In our laboratory, the intraclass correlation coefficient for MFR among 4 readers is 0.94 (95% CI, 0.88–0.98),15 reflecting high reproducibility. CMD was defined as an MFR <2.0,15 that is, a failure to augment MBF at least 2.0‐fold from baseline during maximal hyperemia. All reported MBF and MFR values reflect global measures. Quantitative measures of MBF and MFR were recorded by a single experienced operator blinded to patient data.
Semiquantitative Assessment of Atherosclerotic Burden
The presence and extent of coronary artery calcium (CAC) was assessed using semiquantitative visual analysis of the low‐dose, noncontrast computed tomography scan obtained for attenuation correction of the PET images.16 Semiquantitative assessment of CAC was performed by a cardiologist with advanced cardiovascular imaging training for each of the available noncontrast computed tomography scans in a blinded fashion (n=101, SLE=41, and controls=60). The degree of CAC was determined to be none, mild, moderate, or severe as previously described by the National Lung Screening Trial investigators.17 This approach was previously deemed comparable to Agatston scoring and strongly associated with cardiovascular death.
Laboratory Measurements
Renal function (estimated glomerular filtration rate [eGFR]) was calculated with the Chronic Kidney Disease (CKD) Epidemiology Collaboration formula18 and obtained for all patients with available data within 90 days of the cardiac PET scan. Renal function was defined as normal (eGFR >60), mild (eGFR 30–59), moderate (eGFR 15–29), and severe (eGFR <15).
Statistical Analysis
Patient baseline characteristics were reported as frequency with percent and mean with SD where appropriate. χ2 and Fisher exact tests for binary variables and 2‐sided t test for continuous variables were used to demonstrate the matching between patients with SLE and control patients and to compare the MFR and prevalence of CMD. The Fisher exact test was used when sample size in an outcome had <5 patients. The primary analysis compared patients with SLE versus patients without SLE (exposure) by cardiac PET‐assessed MBF and MFR, as well as prevalence of CMD, defined as MFR <2 (primary outcome). To determine the effect of qualitative grading of CAC on MFR, a χ2 analysis was performed. To determine the relationship between MFR and SLE disease activity, SLEDAI scores were calculated at the time of the cardiac PET. Pearson correlation was used to compare the SLE disease activity index and MFR. For univariate and multivariable modeling, all analyses, α <0.05 was considered statistically significant. Bivariable correlations between study variables were calculated using Spearman rank correlation coefficients. We conducted a subgroup analysis among those with eGFR <60 mL/min per 1.73 m2. All analyses were performed by using SAS University Edition 9.4 (SAS Institute, Cary, NC).
Results
The SLE cohort was predominantly women, with a mean age of 61 years (Table 1). Patients with SLE had a high prevalence of hypertension (71%) and otherwise low prevalence of other cardiovascular risk factors. The mean SLE disease duration was 15.7 (±10.5 SD) years. The median SLEDAI score was 4 (interquartile range, 0–6), suggesting low‐to‐moderate disease activity. Approximately half of the SLE cohort was taking hydroxychloroquine (23/42, 55%) and/or prednisone (20/42, 48%) at the time of the cardiac PET exam. Within the year of the PET scan, there were 4 patients (9.5%) who had received high‐dose steroids (defined as >50 mg/d for 15 days), and 9 patients (21.4%) with a history of concomitant immunosuppressant therapy. Medication history further revealed a similar prevalence of statin and aspirin use between the SLE cohort and control group, although the SLE cohort had a higher prevalence of antithrombotic therapy (11/42 [26.2%] versus 4/69 [5.8%], P=0.004). Given this difference, we next evaluated the presence of antiphospholipid syndrome and antiphospholipid antibodies (aPL), which can drive microvascular thrombosis.19, 20 Among the SLE cohort, 4 of 42 patients (9.5%) had evidence of antiphospholipid syndrome, and 18 of 42 (42.9%) had evidence of at least 1 positive aPL antibody.
Table 1.
Baseline Characteristics of the Study Cohort
| Clinical Characteristics | SLE, n=42 | Control, n=69 | P Value |
|---|---|---|---|
| Age, y, mean (SD) | 61.2 (0.5) | 61.7 (11.9) | 0.8 |
| Women, n (%) | 41 (97) | 66 (95) | 0.59 |
| Cardiovascular risk factors | |||
| BMI >30 kg/m2, n (%) | 20 (29) | 13 (31) | 0.83 |
| Diabetes mellitus, n (%) | 7 (16.7) | 14 (20.3) | 0.64 |
| Hypertension, n (%) | 30 (71) | 49 (71) | 0.96 |
| Dyslipidemia, n (%) | 15 (35) | 27 (39) | 0.72 |
| Smoking, n (%) | 0 (0) | 4 (5.8) | 0.3 |
| Known CAD, n (%) | 3 (7.1) | 6 (8.7) | 1.0 |
| Medication history | |||
| Statin therapy, n (%) | 18 (42.9) | 27 (39.1) | 0.7 |
| Aspirin therapy, n (%) | 18 (42.9) | 29 (42) | 0.93 |
| Antithrombotic therapy, n (%) | 11 (26.2) | 4 (5.8) | 0.004 |
| SLE disease characteristics | |||
| Disease duration, mean (SD) | 15.7 (10.5) | — | — |
| SLE Disease Activity Index, mean (IQR) | 4 (0–6) | — | — |
| Hydroxychloroquine use, n (%) | 23 (55) | — | — |
| Prednisone use, n (%) | 20 (48) | — | — |
| Antiphospholipid syndrome, n (%) | 4 (9.5) | — | — |
| Antiphospholipid antibodies, n (%) | 18 (42.9) | — | — |
BMI indicates body mass index, CAD, coronary artery disease; IQR, interquartile range; and SLE, systemic lupus erythematosus.
MFR, Diffuse Atherosclerosis, and SLE Disease Activity
Compared with controls, patients with SLE had similar MBF at rest but lower MBF augmentation during maximal stress (2.5±0.8 versus 2.14±0.72 mL/min per gram, respectively, P=0.01). Consequently, MFR was lower in patients with SLE compared with controls (1.91±0.5 versus 2.4±0.7, respectively, P<0.0001; Figure 1).
Figure 1. Myocardial flow reserve (MFR) and atherosclerotic burden in patients with systemic lupus erythematosus (SLE).
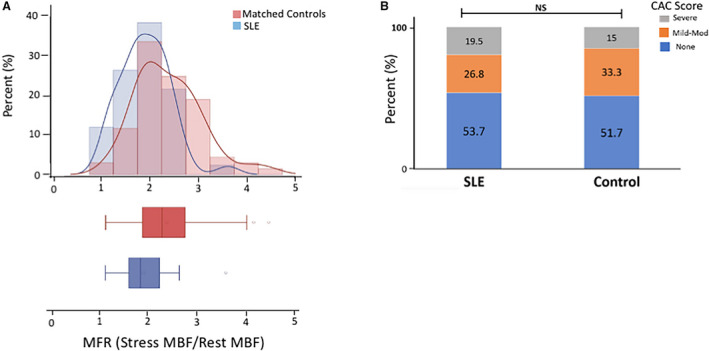
A, Histogram and box plot represent the distribution of MFR in patients with SLE and controls (1.91±0.5 vs 2.4±0.7, respectively, P<0.0001). MFR represents stress/rest myocardial blood flow (MBF). Rest and stress MBF are presented in milliliters per minute per gram. B, The degree of coronary artery calcium (CAC) was semiquantitatively determined to be none, mild‐to‐moderate, or severe as described in the Methods. Stacked bar plots demonstrate the frequency of each CAC category between SLE (n=41) and controls (n=60). Results were not significantly different on the basis of a Fisher exact test (P=0.7).
To account for the confounding of diffuse atherosclerosis on MFR, we assessed the presence and severity of CAC. Approximately half of the patients with SLE and controls had no CAC, and the proportion of patients with mild‐to‐moderate and severe CAC were similar in both groups (Figure 1B). These results suggest that patients with SLE compared with similarly matched patients did not have evidence of a higher atherosclerotic burden. To further determine if the patients with SLE with no evidence of atherosclerosis, reflective of endothelial dysfunction, had impaired MFR compared with the control population, we examined the subgroup of patients without CAC (SLE [n=22], 2.04±0.59 versus control [n=31], 2.54±0.59, P=0.004). These data suggest that even in patients with SLE without evidence of overt atheroma, there is a lower MFR, suggestive of coronary endothelial dysfunction.
The frequency of MFR <2.0, reflecting CMD, was higher in patients with SLE compared with controls (24/42 [57.1%] versus 23/69 [33.3%], P=0.017; Table 2). To determine the relationship between MFR and SLE disease activity, SLEDAI scores were calculated at the time of the cardiac PET. MFR was inversely related to SLEDAI (Pearson r=−0.44, P=0.003; Figure 2). No significant association was found between MFR and SLE disease duration (r=−0.15, P=0.9). Additionally, there was no difference in the MFR between the patients with SLE with or without documented evidence of positive aPL antibodies (SLE with aPL antibodies [n=18], 1.98±0.59 versus SLE without aPL antibodies [n=24], 1.86±0.46, P=0.47).
Table 2.
Positron Emission Tomography Myocardial Blood Flow, MFR, and Myocardial Function in SLE and Matched Controls
| Imaging Findings | SLE, n=42 | Controls, n=69 | P Value |
|---|---|---|---|
| Rest myocardial blood flow, mL/min/g | 1.2 (0.4) | 1.1 (0.5) | 0.8 |
| Stress myocardial blood flow, mL/min/g | 2.1 (0.8) | 2.5 (0.9) | 0.01 |
| Myocardial flow reserve, mL/min/g | 1.91 (0.5) | 2.4 (0.7) | <0.0001 |
| Coronary microvascular dysfunction (MFR <2), n (%) | 24/42 (57.1% | 23/69 (33.3) | 0.017 |
| Rest LVEF, % | 58.3 (8.2) | 63.4 (7.3) | 0.002 |
| Stress LVEF, % | 64.7 (9.6) | 68 (8.9) | 0.07 |
| Resting HR, beats/min | 70.7 (11.1) | 70.1 (13) | 0.78 |
| Peak HR, beats/min | 87.4 (16) | 94 (19.2) | 0.06 |
| Resting SBP, mm Hg | 146.4 (26.3) | 143.8 (19) | 0.57 |
| Peak SBP, mm Hg | 137.9 (25.6) | 138.4 (23.3) | 0.9 |
| Resting MAP, mm Hg | 97.5 (16.7) | 96.7 (11.9) | 0.78 |
| Peak MAP, mm Hg | 91.6 (14.7) | 92.3 (13.5) | 0.81 |
Mean and SD are shown, except where noted otherwise. HR indicates heart rate; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MFR, myocardial flow reserve; SBP, systolic blood pressure; and SLE, systemic lupus erythematosus.
Figure 2. Correlation between systemic lupus erythematosus disease activity and myocardial flow reserve (MFR).
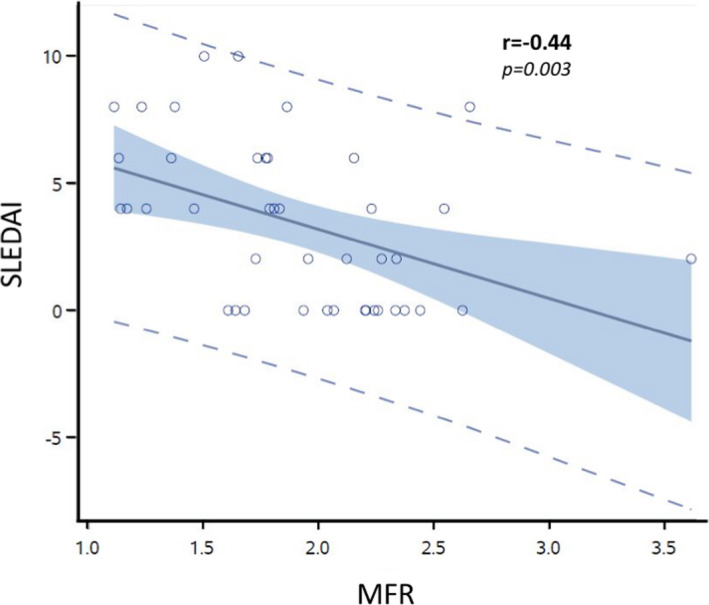
Disease activity was calculated on the basis of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score at the time of the cardiac positron emission tomography scan. Shown is the linear relationship with Pearson correlation (r=−0.44, P=0.003).
Subgroup Analysis Based on Renal Function
Lupus nephritis can lead to CKD. Because CKD is associated with CMD in the absence of SLE, we performed additional sensitivity analyses to account for the potential confounding of CKD (ie, eGFR <60 mL/min) on measures of MFR. Only patients who had an eGFR within 90 days of the cardiac PET scan were included (SLE=32 and controls=52). In patients with normal renal function, patients with SLE had lower MFR than controls (2.15±0.5 versus 2.5±0.65, respectively, P=0.01; Figure 3). Likewise, MFR was also lower in patients with SLE compared with controls among those with CKD (ie, eGFR <60 mL/min) (1.68±0.43 versus 2.4±0.86, respectively, P=0.02; Figure 3).
Figure 3. Subgroup analysis based on chronic kidney disease.
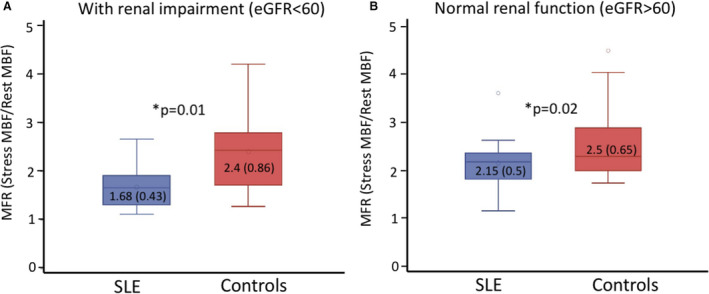
Subjects were only included in the analysis if a serum creatinine was performed within 90 days of cardiac positron emission tomography (40/42 systemic lupus erythematosus [SLE] and 52/69 control group). A, Box and whisker plot of the distribution of myocardial flow reserve (MFR) in SLE and controls with renal impairment (estimated glomerular filtration rate [eGFR] <60). B, Distribution in patients with normal renal function. Mean and SD are shown within box plots. MBF indicates myocardial blood flow.
In unadjusted models, both renal impairment and SLE were univariate predictors of lower MFR. We also performed multivariable modeling adjusting for age, sex, CAC, diabetes mellitus, obesity, and hypertension as additional predictors of MFR. CKD and SLE both remained significant predictors of MFR (P=0.007, β=−0.18 and P<0.001, β=−0.49, respectively) after controlling for these factors. Together, these results demonstrate that both the presence of SLE and renal dysfunction are associated with MFR; however, as demonstrated in the subgroup analyses, patients with SLE have a further reduction of MFR that is not entirely explained by the reduced renal function.
Discussion
Systemic inflammatory disorders such as SLE are not static, and patients exhibit periods of increased and decreased inflammation over the course of the disease. SLE provides an ideal disease model to further understand the vascular changes that occur as a result of longstanding systemic inflammation. Our findings are novel and extend the observations of prior studies of microvascular dysfunction in SLE.21, 22 Our results demonstrate a high prevalence of coronary vasomotor abnormalities in symptomatic high‐risk patients with SLE without obstructive CAD, and also demonstrate that the severity of these abnormalities is not accounted for by the burden of atherosclerosis, by commonly associated coronary risk factors, or the presence of CKD. These data demonstrate that patients with SLE have a reduced MFR even in the presence of normal or reduced renal function. Renal involvement is not uncommon in SLE, and lupus nephritis is linked to increased morbidity and mortality in SLE.23, 24 For this study, we defined renal impairment on the basis of eGFR alone, and not on the presence of other findings seen in active nephritis that might not be associated with a low eGFR (eg, proteinuria, active sediment). In addition, low GFR in an SLE patient may not necessarily be due entirely to lupus nephritis, because other common conditions, such as hypertension and drug effects, could result in CKD. Kidney involvement in SLE is heralded by the presence of an active urinary sediment, presence of proteinuria, or an unexplained rise in the serum creatinine. A kidney biopsy is the gold standard for diagnosis. Whether there is organ‐specific SLE involvement that is directly associated with the coronary microvasculature preferentially, or whether it is the overall degree of systemic inflammatory burden, is not known and is an important question to further address.
Overall, these data support a role for systemic inflammation in driving coronary vasomotor abnormalities that might contribute to the excess cardiovascular risk. These results further suggest a relationship between cardiovascular risk and the severity of SLE disease activity on MFR. CMD was not associated with SLE disease duration, suggesting that control of inflammation and disease activity is an important factor in driving excess cardiovascular risk. The prevalence of documented antiphospholipid syndrome in this cohort was low, although the prevalence of aPL antibodies was relatively high, because almost 50% of this population had documentation of at least 1 positive aPL antibody. A possible role of aPL antibodies, which are associated with microvascular thrombosis, resulting in a reduced MFR, was examined and not associated with a difference in MFR. However, given the small sample size of this cohort and the power to detect a difference, future studies should include more subjects with aPL antibodies and antiphospholipid syndrome to examine this relationship further. Furthermore, in patients with SLE with aPL antibodies, the role of antithrombotic therapy, in addition to the traditional and biologic disease‐modifying antirheumatic drugs, the armamentarium of therapy should be investigated to understand the role in microvascular thrombosis and CMD in this patient population.
Limitations of the current study include the study population, which were older symptomatic patients with long‐standing SLE, which represents a higher‐risk cohort. Whether MFR is impaired in younger patients with SLE is not known, but it is important to address in the future given that age of onset is most often between the ages of 15 to 45. Whether CMD is more prevalent in patients who were diagnosed with child‐onset SLE compared with adult‐onset SLE is also not known; although it has been demonstrated that the clinical course and disease activity is worse in child‐onset SLE.25, 26 The small sample size limited the power to ascertain the impact on long‐term cardiovascular outcomes and mortality. Because of the unavailability of unique matching identification in the analysis data set, it was not possible to use statistical techniques that account for the correlation introduced by matching in the control population, which could result in selection bias. Regardless, it is anticipated that this analysis limitation had a marginal effect on reported inference. Previous work from our group and others have established the importance of MFR and CMD on risk stratification and long‐term outcomes in the general population27; however, whether this holds true in systemic inflammatory conditions characterized by flares of inflammation is not known. Given the heterogeneous clinical manifestations of SLE, future prospective studies are needed to address CMD across clinical severities of SLE and organ involvement. Furthermore, studies are needed to address whether reducing systemic inflammation with frequently prescribed disease‐modifying antirheumatic drugs (eg, hydroxychloroquine, methotrexate), systemic immunosuppressants (eg, mycophenolate mofetil), and/or newer targeted biologics (eg, anti‐CD19 [rituximab] and anti‐B‐lymphocyte stimulator protein [belimumab]) can improve coronary microvascular function and cardiovascular outcomes.28, 29
Conclusions
These findings demonstrate that older patients with long‐standing SLE have a much higher prevalence and more severe coronary vasomotor dysfunction than age‐, sex‐, and cardiovascular risk factor–matched controls in the absence of obstructive CAD. Importantly, the similar prevalence and severity of CAC in SLE and control subjects suggest that reduced MFR is not simply a result of diffuse atherosclerosis, and support a potentially earlier abnormality in coronary microvascular response to stress. MFR correlated with SLE disease activity, highlighting the role of systemic inflammation, and was more severely reduced in patients with SLE than controls with both preserved and reduced renal function. The identification of impaired MFR in SLE may be a sensitive marker of excess cardiovascular risk in this population and could necessitate aggressive cardiovascular risk reduction strategies.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute (T32 HL094301, Weber, Sanjay Divarkaran and K24 AR011609, Karen Costenbader).
Disclosures
Dr Di Carli reports grants from Gilead Sciences and Spectrum Dynamics and personal consulting fees from Janssen and Bayer, outside the submitted work. Dr Dorbala reports grants from Pfizer and GE Healthcare and personal consulting fees from GE Healthcare and Pfizer, outside the submitted work. Dr Blankstein reports grants from Amgen Inc. and Astellas and personal consulting fees from Amgen Inc., outside of the submitted work. Dr Massarotti reports grants from BMS and personal consulting fees from UCB and Exagen. Dr Massarotti serves on the Data and Safety Monitoring Board of EMD Serono. The remaining authors have no disclosures to report.
Acknowledgments
The authors thank the patients and clinicians who contributed to this study.
(J Am Heart Assoc. 2021;10:e018555. DOI: 10.1161/JAHA.120.018555.)
For Sources of Funding and Disclosures, see page 7.
References
- 1.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jansen‐McWilliams L, D’Agostino RB, Kuller LH. Age‐specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. DOI: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 2.Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum. 2013;43:77–95. DOI: 10.1016/j.semarthrit.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med. 1994;96:254–259. DOI: 10.1016/0002-9343(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Perez‐Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–519. DOI: 10.1016/0002-9343(92)90578-Y. [DOI] [PubMed] [Google Scholar]
- 5.Urowitz MB, Gladman D, Ibañez D, Bae SC, Sanchez‐Guerrero J, Gordon C, Clarke A, Bernatsky S, Fortin PR, Hanly JG, et al. Systemic lupus international collaborating clinics. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62:881–887. DOI: 10.1002/acr.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urowitz MB, Gladman D, Ibañez D, Fortin P, Sanchez‐Guerrero J, Bae S, Clarke A, Bernatsky S, Gordon C, Hanly J, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus. 2007;16:731–735. DOI: 10.1177/0961203307081113. [DOI] [PubMed] [Google Scholar]
- 7.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015;36:482–489. DOI: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. DOI: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Hansson GK. Leducq transatlantic network on atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. DOI: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pego‐Reigosa JM, Medeiros DA, Isenberg DA. Respiratory manifestations of systemic lupus erythematosus: old and new concepts. Best Pract Res Clin Rheumatol. 2009;23:469–480. DOI: 10.1016/j.berh.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Orbai A‐M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, et al. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. DOI: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Austin A, Bell A, Bloch DA, Corey PN, Decker JL, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–640. DOI: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 13.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi‐Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N‐ammonia PET. J Nucl Med. 2009;50:1062–1071. DOI: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765–1774. DOI: 10.1007/s00259-007-0478-2. [DOI] [PubMed] [Google Scholar]
- 15.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. DOI: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einstein AJ, Johnson LL, Bokhari S, Son J, Thompson RC, Bateman TM, Hayes SW, Berman DS. Agreement of visual estimation of coronary artery calcium from low‐dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard agatston score. J Am Coll Cardiol. 2010;56:1914–1921. DOI: 10.1016/j.jacc.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, DeMello S, Desjardins SS, Munden RF. NLST study team. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. DOI: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolitz T, Shiber S, Sharabi I, Winder A, Zandman‐Goddard G. Cardiac manifestations of antiphospholipid syndrome with focus on its primary form. Front Immunol [Internet]. 2019. [cited 2020 Nov 4];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6522847/. DOI: 10.3389/fimmu.2019.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz‐Irastorza G, Salmon JE, Shoenfeld Y, Shovman O, Hunt BJ. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:17103. DOI: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 21.Recio‐Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. DOI: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 22.Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LEJ, Schapira J, Yang Y, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4:27–33. DOI: 10.1016/j.jcmg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Fanouriakis A, Kostopoulou M, Cheema K, Anders H‐J, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, et al. 2019 Update of the joint European league against rheumatism and European renal association‐European Dialysis and transplant association (EULAR/ERA‐EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–723. DOI: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 24.Mok CC, Kwok RCL, Yip PSF. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65:2154–2160. DOI: 10.1002/art.38006. [DOI] [PubMed] [Google Scholar]
- 25.Joo YB, Park S‐Y, Won S, Bae S‐C. Differences in clinical features and mortality between childhood‐onset and adult‐onset systemic lupus erythematosus: a prospective single‐center study. J Rheumatol. 2016;43:1490–1497. DOI: 10.3899/jrheum.151129. [DOI] [PubMed] [Google Scholar]
- 26.Sassi RH, Hendler JV, Piccoli GF, Gasparin AA, da Silva Chakr RM, Brenol JCT, Monticielo OA. Age of onset influences on clinical and laboratory profile of patients with systemic lupus erythematosus. Clin Rheumatol. 2017;36:89–95. DOI: 10.1007/s10067-016-3478-4. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, et al. Integrated non‐invasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–2336. DOI: 10.1161/CIRCULATIONAHA.117.029992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li E‐M, Thomas M, Kim H‐Y, León MG, et al. BLISS‐52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011;377:721–731. DOI: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 29.Mok CC. Current role of rituximab in systemic lupus erythematosus. Int J Rheum Dis. 2015;18:154–163. DOI: 10.1111/1756-185X.12463. [DOI] [PubMed] [Google Scholar]


