Abstract
Background
We assessed the impact of preprocedural plasma levels of MRproANP (midregional N‐terminal pro–atrial natriuretic peptide) and sST2 (soluble suppression of tumorigenicity 2) on recurrence of atrial fibrillation (AF) at 1 year after catheter ablation of AF.
Methods and Results
This was a prospective, multicenter, observational study including patients undergoing catheter ablation of AF. MRproANP and sST2 were measured in a peripheral venous blood preprocedure, and MRproANP was assessed in the right and left atrial blood during ablation. The primary end point was recurrent AF between 3 and 12 months postablation, defined as a documented (>30 seconds) episode of AF, flutter, or atrial tachycardia. We included 106 patients from December 2017 to March 2019; 105 had complete follow‐up, and the mean age was 63 years with 74.2% males. Overall, 34 patients (32.1%) had recurrent AF. In peripheral venous blood, MRproANP was significantly higher in patients with recurrent AF (median, 192.2; [quartile 1–quartile 3, 155.9–263.9] versus 97.1 [60.9–150.7] pmol/L; P<0.0001), as was sST2 (median, 30.3 [quartile 1–quartile 3, 23.3–39.3] versus 23.4 [95% CI, 17.4–33.0] ng/mL; P=0.0033). In the atria, MRproANP was significantly higher than in peripheral blood and was higher during AF than during sinus rhythm. Receiver operating characteristic curve analysis identified a threshold of MRproANP>107.9 pmol/L to predict AF recurrence at 1 year and a threshold of >26.7 ng/mL for sST2. By multivariate analysis, MRproANP>107.9 pmol/L was the only independent predictor of recurrent AF (OR, 24.27; 95% CI, 4.23–139.18). MRproANP<107.9 pmol/L identified subjects at very low risk of recurrence (negative predictive value >95%).
Conclusions
Elevated MRproANP level independently predicts recurrent AF, whereas sST2 levels do not appear to have any prognostic value in assessing the risk of recurrence of AF up to 1 year after catheter ablation.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03351816.
Keywords: atrial fibrillation, biomarkers, catheter ablation, MRproANP, sST2
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator
Nonstandard Abbreviations and Acronyms
- MRproANP
midregional N‐terminal pro–atrial natriuretic peptide
- RA
right atrium
- sST2
soluble suppression of tumorigenicity 2
Clinical Perspective
What Is New?
We show for the first time that MRproANP (midregional N‐terminal pro–atrial natriuretic peptide) is an independent predictor of recurrent atrial fibrillation at 1 year after catheter ablation, whereby patients with preprocedural plasma MRproANP values exceeding 107.9 pmol/L have a 24‐fold increase in risk.
What Are the Clinical Implications?
Patients with elevated MRproANP before catheter ablation of atrial fibrillation warrant closer monitoring up to 1 year after the procedure and an adaptation of medical therapy, notably antiarrhythmics, to take account of the increased risk of recurrence.
Catheter ablation is the cornerstone of therapy for atrial fibrillation (AF).1, 2 The main limitation of this procedure is the success rate, which varies between 50% and 75%.3, 4, 5, 6, 7, 8 It may also lead to potentially serious complications,3, 4, 5, 6, 7, 8, 9 and therefore the careful selection of patients is warranted to identify those likely to yield the greatest benefit, in particular among patients with preserved left ventricular function in whom no reduction in mortality has been reported after catheter ablation of AF.10
Several clinical factors have previously been identified as predictors of AF recurrence after catheter ablation, including left atrium (LA) dilation, type of AF (persistent versus paroxysmal), obesity, diabetes mellitus, hypertension, sleep apnea, or CHA2DS2VASC score.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Although biomarkers are widely used in cardiac disease,24, 25, 26 there is a paucity of data concerning the prognostic impact of biomarkers on the course of AF after catheter ablation. Indeed, in a recent report from a National Heart, Lung, and Blood Institute Virtual Workshop describing research needs and priorities for catheter ablation of atrial fibrillation, Al‐Khatib et al called for the development of biomarkers “with the goal of stratifying individuals for risks of complications and benefit from AF ablation.”27
MRproANP (midregional N‐terminal pro–atrial natriuretic peptide) is a stable peptide that results from the cleavage of proANP (pro–atrial natriuretic peptide) and is elevated in numerous pathologies specifically affecting the LA, such as mitral stenosis.28, 29 Charitakis et al previously suggested that recurrent AF postablation is more frequent in patients with elevated MRproANP.30
Fibrosis is frequent in AF, and the degree of fibrosis may be associated with AF recurrence after catheter ablation.31 ST2 (suppression of tumorigenicity 2) from the family of interleukin‐1 receptors, is a biomarker of fibrosis and cardiac biomechanical constraint.32, 33 Its soluble form, sST2, can be detected using commercially available assays. sST2 has been shown to be of prognostic importance in heart failure,34, 35, 36, 37, 38 but its utility has not been widely investigated in the setting of AF.
In this context, the aim of the present study was to assess the impact of plasma levels of MRproANP and sST2 measured before catheter ablation for AF on the rate of recurrence of AF at 1 year after ablation.
Secondary objectives were to (1) compare MRproANP concentrations in the LA, right atrium (RA), and peripheral venous blood; (2) compare MRproANP concentrations in the LA and RA in sinus rhythm and during AF; and (3) investigate the existence of a link between plasma levels of MRproANP and sST2 and the LA area.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We performed a prospective, multicenter, observational study in 3 university teaching hospitals in Eastern France (Besançon, Nancy, and Dijon) from December 2017 to March 2019. The study protocol was approved by the Ethics Committee Comité de Protection des Personnes (CPP) Ile‐de‐France VI (CPP Ile‐de‐France VI, No. 34–17, approval number: 2017‐A00731‐52, approved on June 9, 2017). The study was registered with ClinicalTrials.gov under the number NCT03351816. All patients provided written informed consent for participation.
Study Population
Inclusion criteria were patients aged >18 years with symptomatic paroxysmal or persistent AF2 with preserved left ventricular ejection fraction (≥45%) and in whom a first catheter ablation of AF was to be performed. The decision to perform catheter ablation was made in accordance with the European Society of Cardiology (ESC) guidelines.2, 39
Exclusion criteria were patients aged <18 years or >80 years; patients with significant valvular heart disease, defined as symptomatic aortic regurgitation, severe aortic or mitral stenosis, or mitral or tricuspid insufficiency grade 3/4 or 4/4; presence of pulmonary hypertension on echocardiography (>45 mm Hg); chronic respiratory disease; chronic obstructive pulmonary disease stages 2, 3, or 4; left ventricular ejection fraction <45%; recent (<1 month) decompensation of heart failure; recent (<1 month) acute coronary syndrome; or anemia (hemoglobin <10 g/dL). We also excluded pregnant women, people under judicial or legal protection, people with poor anticipated compliance, people with no social security coverage, and individuals within the exclusion period of a prior clinical trial as per the national register of research volunteers.
Ablation Procedure
Transthoracic and transoesophageal echocardiography were performed by a trained operator before the procedure to ensure the absence of intracardiac thrombus and to record the following data as per current guidelines40: left ventricular ejection fraction, LA area in the 4‐cavity view, and maximum thickness of the interventricular septum. A maximum thickness >13 mm was considered as left ventricular hypertrophy. Usual curative anticoagulant therapy was not interrupted before the procedure. The aim of the procedure was pulmonary vein isolation. Additional lesions were made at the discretion of the operator. A total of 2 types of procedures could be performed, namely, radiofrequency ablation with an irrigated tip ablation catheter with electrophysiological and anatomical mapping of the LA or cryoablation.5 The choice of technique (cryoablation or radiofrequency ablation) and the system used for electroanatomical navigation in case of radiofrequency ablation were at the discretion of the operator. Procedures were performed by qualified and experienced operators having a volume of activity of at least 50 procedures each per year. Transthoracic echocardiography was performed at the end of the procedure to ensure an absence of pericardial effusion. Treatment was administered after the procedure in accordance with guidelines in force at the time of the inclusions.1, 39 Usual anticoagulant therapy was then pursued for 8 weeks postprocedure in patients with CHA2DS2VASC scores of 0 or indefinitely for patients with CHA2DS2VASC scores ≥1.1, 2 Antiarrhythmic agents could be continued at the operator’s discretion. It was recommended that every effort be made to maintain sinus rhythm.
MRproANP and sST2 Measurements
MRproANP and sST2 were measured in peripheral venous blood drawn within 24 hours before the catheter ablation procedure. MRproANP was also measured in a sample of blood from the RA at the start of the procedure and in a sample of blood from the LA drawn immediately after transseptal puncture. Tubes were centrifuged and stored at −20°C for later centralized analysis in the Biochemistry Department of the University Hospital of Besancon. MRproANP was measured using BRAHMS Kryptor Compact Plus (Thermo Fisher Diagnostics SAS, Dardilly, France). sST2 was also centrally analyzed in the Biochemistry Department of the University Hospital of Besancon using the Aspect Plus kit from Eurobio Ingen (Les Ulis, France).
Study End Points
The primary end point was recurrence of AF, defined as occurrence between 3 and 12 months after the procedure of a documented episode (documented by ECG, Holter monitoring, ECG event recorder, or telemetry) of AF, atrial tachycardia, or atrial flutter lasting >30 seconds. All patients were followed up with 24‐hour Holter ECG monitoring and a clinical consultation at 3 months (the end of a blanking period) and 12 months after the procedure. Between the end of the blanking period and the 12‐month follow‐up, patients were followed by their treating cardiologist and/or general practitioner who were informed of the patient’s participation in this study and were requested to inform the coordinating center of any intercurrent events. In case of symptoms suggestive of recurrent arrhythmia, patients were seen in consultation and had an ECG with the possibility of additional Holter ECG monitoring, ECG event recorder, or hospitalization with continuous ECG to identify recurrence.
Statistical Analysis
Quantitative data are described as mean±SD or median (25th percentile [quartile 1]–75th percentile [quartile 3]) and were compared with the Student t test for normally distributed variables or the Mann–Whitney test for nonnormally distributed variables. Paired tests were used as appropriate. Qualitative data are expressed as number (percentage) and were compared using the chi‐square or Fisher exact test as appropriate. Multivariate analysis was performed using multivariable logistic regression. Variables with a P value <0.20 by univariate analysis were included in the model, and the results are expressed as odds ratios (OR) and 95% CI. Receiver operating characteristic (ROC) curves were constructed to identify the threshold of sST2 and MRproANP that best predict recurrence. Survival curves were generated using the Kaplan–Meier method and compared with the log‐rank test. Correlations between quantitative variables were examined using the Spearman correlation coefficient. All tests were 2 sided, and a P value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 106 patients were included from December 2017 to March 2019. One patient withdrew consent, and thus, 105 patients were followed up to 1 year.
Characteristics of Study Population
The baseline characteristics of the study population are displayed in Table 1. Median age was 63 (56–96) years, and 20.1% had diabetes mellitus. The median time since onset of AF was 18 (6.75–48) months; 61 patients (58.1%) had paroxysmal AF, and 44 patients (41.9%) had persistent AF.
Table 1.
Baseline Characteristics of the Study Population
| Total, N=105 | No Recurrence, n=71 | Recurrence, n=34 | P Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y, median (Q1–Q3) | 63 (56–69) | 63 (54–68) | 65 (58–69) | 0.25* |
| Male sex, n (%) | 78 (74.2) | 55 (77.5) | 23 (67.6) | 0.28† |
| BMI, kg/m², mean±SD | 28.7±5.2 | 28.7±5.1 | 28.8±5.3 | 0.96* |
| Medical history, n (%) | ||||
| Hypertension | 56 (53.3) | 33 (46.5) | 23 (67.6) | 0.04† |
| Diabetes mellitus | 22 (20.1) | 11 (15.5) | 11 (32.4) | 0.05† |
| LVH | 8 (7.6) | 3 (4.2) | 5 (14.7) | 0.06† |
| Sleep apnea | 12 (11.4) | 8 (11.3) | 4 (11.8) | 0.94† |
| Coronary artery disease | 12 (11.4) | 3 (4.2) | 9 (26.5) | <0.01† |
| LVEF, %, median (Q1–Q3) | 60 (55–65) | 60 (55–65) | 60 (50–65) | 0.19* |
| Prior stroke or TIA | 7 (6.7) | 4 (5.6) | 3 (8.8) | 0.54† |
| CHA2DS2VASC, median (Q1–Q3) | 2 (0–3) | 1 (0–3) | 3 (1–4) | <0.01* |
| 0, n (%) | 30 (28.6) | 26 (36.6) | 4 (11.8) | <0.01‡ |
| 1, n (%) | 18 (17.1) | 13 (18.3) | 5 (14.7) | 0.65‡ |
| 2, n (%) | 15 (14.3) | 9 (12.7) | 6 (17.6) | 0.50† |
| 3, n (%) | 21 (20.0) | 16 (22.5) | 5 (14.7) | 0.35‡ |
| 4, n (%) | 17 (16.2) | 6 (8.5) | 11 (32.4) | <0.01† |
| ≥5, n (%) | 4 (3.8) | 1 (1.4) | 3 (3.8) | 0.06‡ |
| Arrhythmia history | ||||
| Median time since onset, months, median (Q1–Q3) | 18 (6.75–48) | 16 (6.25–48) | 24 (7–48) | 0.53* |
| Type of AF at inclusion, n (%) | 0.17† | |||
| Paroxysmal | 61 (58.1) | 45 (63.4) | 16 (47.1) | 0.11† |
| Persistent | 44 (41.9) | 26 (36.6) | 18 (52.9) | 0.57† |
| Prior electrical cardioversion | 37 (35.2) | 23 (32.4) | 14 (42.1) | 0.38† |
| Prior ablation for atrial flutter | 11 (10.5) | 9 (12.7) | 2 (5.9) | 0.29‡ |
| Rhythm control therapy at inclusion | 74 (71.2) | 53 (75.7) | 21 (61.8) | 0.14† |
| LA size | ||||
| LA area, cm2, median§ (Q1–Q3) | 22 (18–26) | 21 (17–23.75) | 24.5 (19–29) | <0.01* |
AF indicates atrial fibrillation; BMI, body mass index; LA, left atrium; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; Q1, quartile 1; Q3, quartile 3; and TIA, transient ischemic attack.
Mann–Whitney test.
Chi‐square test.
Fisher exact test.
LA area measured by transthoracic echocardiography in the 4‐chamber view.
Catheter Ablation Procedures
A total of 68 patients (64.7%) were in sinus rhythm at the beginning of the ablation procedure. Overall, 38 (36.2%) underwent cryoablation, and 67 (63.8%) had radiofrequency ablation. Of the 105 patients included, all pulmonary veins were successfully isolated in 104 patients (99%). A total of 14 patients (13.3%) had complex fractionated atrial electrogram ablation in addition to pulmonary vein isolation, 11 patients (10.6%) had a linear ablation across the roof of the LA, 10 patients (9.5%) had cavotricuspid isthmus ablation, and 3 (2.8%) had a mitral line (Table S1).
Regarding complications, 2 patients had transient ischemic attack or ischemic stroke, there was no cardiac tamponade, one patient had persistent phrenic paralysis at discharge after cryoablation; 1 patient had an access point hematoma, 1 patient had postprocedural pulmonary edema, and 1 patient had perforation of the mitral valve with significant mitral insufficiency (Table S1).
In total, 62 patients (59.0%) received rhythm‐control therapy at discharge (Table S1).
Primary Outcome
Overall, 34 patients (32.3%) had recurrent AF at 1 year (recurrence group). There were significantly more patients with hypertension (67.6% versus 46.5%; P=0.04) and ischemic heart disease (26.5% versus 4.2%; P<0.01) in the recurrence group. In this group, there was also a higher CHA2DS2VASC score (median, 3 [quartile 1–quartile 3, 1–4] versus 1 [0–3]; P<0.01) and more dilation of the LA in terms of the area measured by transthoracic echocardiography (median, 21 [quartile 1–quartile 3, 17–23.75] versus 24.5 [19–29] cm2; P=0.02).
There was no difference in the rate of antiarrhythmics use at 1 year after the procedure between groups (47.1% versus 35.2%, recurrence versus no recurrence, respectively; P=0.25; Table S1).
Biomarker Levels and AF Recurrence
MRproANP measured in peripheral venous blood drawn preprocedure was significantly higher in patients with recurrence versus those without recurrence (median, 192.2 [quartile 1–quartile 3, 155.9–263.9] versus 97.1 [60.9–150.7] pmol/L; P<0.01). MRproANP levels measured in blood drawn from the LA and RA were also significantly higher in patients in the recurrence group (Table 2). Similarly, sST2 levels were significantly higher in the recurrence group (median30.3 [quartile 1–quartile 3, 23.3–39.3] versus 23.4 [17.4–33.0] ng/mL; P<0.01).
Table 2.
Results of MRproANP and sST2 Assessments
| Total, N=105 | No Recurrence, n=71 | Recurrence, n=34 | P Value* | |
|---|---|---|---|---|
| MRproANP PV, pmol/L | 131.3 (73.5–200.6) | 97.1 (60.9–150.7) | 192.2 (155.9–263.9) | <0.01 |
| MRproANP LA, pmol/L | 143.5 (89.8–208.3) | 110.7 (79.3–172.1) | 234.3 (163.4–334.2) | <0.01 |
| MRproANP RA, pmol/L | 142.2 (90.3–210.3) | 108.8 (81.8–168.1) | 207.8 (159.1–347.2) | <0.01 |
| sST2 PV, ng/mL | 26.4 (19.5–36.4) | 23.4 (17.4–33.0) | 30.3 (23.3–39.8) | <0.01 |
Values are provided as median (quartile 1–quartile 3). MRproANP indicates midregional N‐terminal pro–atrial natriuretic peptide; MRproANP LA, level of midregional N‐terminal pro–atrial natriuretic peptide measured in blood drawn from the left atrium; MRproANP PV, midregional N‐terminal pro–atrial natriuretic peptide measured in peripheral venous blood; MRproANP RA, level of midregional N‐terminal pro–atrial natriuretic peptide measured in blood drawn from the right atrium; sST2, level of soluble suppression of tumorigenicity 2; and sST2 PV, soluble suppression of tumorigenicity 2 measured in peripheral venous blood.
P values from Mann–Whitney tests.
ROC curve analysis of plasma MRproANP measured in peripheral venous blood identified a threshold value of >107.9 pmol/L as having the best predictive value for recurrent AF at 1 year after ablation, with a sensitivity of 94.1%, a specificity of 63.4%, and an area under the ROC curve of 0.838 (P<0.01; Figure 1). The associated positive predictive value and negative predictive value (NPV) were, respectively, 55.2% and 95.7% (Table 3). For sST2, the threshold identified by ROC curve analysis was >26.7 ng/mL, with a sensitivity and a specificity of 73.5% and 64.8%, respectively. The area under the ROC curve for sST2 was 0.678 and was significantly lower than the area under the ROC curve for MRproANP (P<0.01; Figure 1). The associated positive predictive value and NPV were, respectively, 49.0% and 83.3% (Table 3). The proportion of recurrence and nonrecurrence per quartiles of MRproANP and sST2 are presented in Figure 2. There was a significant difference in AF recurrence between groups when classified according to whether MRproANP measured in peripheral venous blood was above or below the threshold of 107.9 pmol/L (Figure 3).
Figure 1. Receiver operating characteristic curves showing the levels of MRproANP and sST2 measured before the procedure in peripheral venous blood that best predict the recurrence of atrial fibrillation up to 1 year after catheter ablation.
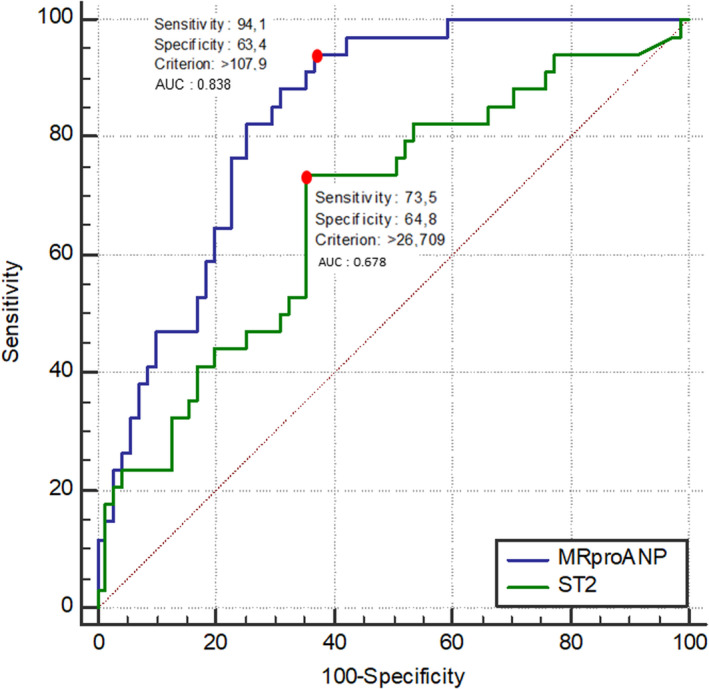
AUC indicates area under the curve; MRproANP, midregional N‐terminal pro–atrial natriuretic peptide; and sST2, soluble suppression of tumorigenicity 2.
Table 3.
Sensitivity, Specificity, and Positive and Negative Predictive Values of MRproANP and sST2 for the Prediction of Recurrent Atrial Fibrillation After Catheter Ablation
| Sensitivity, % | Specificity,% | PPV, % | NPV, % | |
|---|---|---|---|---|
| MRproANP PV>107.9 pmol/L | 94.1 | 63.4 | 55.2 | 95.7 |
| MRproANP LA>143.5 pmol/L | 88.2 | 69.0 | 57.7 | 92.5 |
| MRproANP RA>122.1 pmol/L | 84.1 | 59.2 | 52.5 | 95.5 |
| sST2 PV>26.7 ng/mL | 73.5 | 64.8 | 49.0 | 83.3 |
MRproANP indicates midregional N‐terminal pro–atrial natriuretic peptide; MRproANP LA, level of midregional N‐terminal pro–atrial natriuretic peptide measured in blood drawn from the left atrium; MRproANP PV, midregional N‐terminal pro–atrial natriuretic peptide measured in peripheral venous blood; MRproANP RA, level of midregional N‐terminal pro–atrial natriuretic peptide measured in blood drawn from the right atrium; NPV, negative predictive value; PPV, positive predictive value; sST2, level of soluble suppression of tumorigenicity 2; and sST2 PV, soluble suppression of tumorigenicity 2 measured in peripheral venous blood.
Figure 2. Distribution of atrial fibrillation recurrence per quartile (Q1–Q4) of MRproANP and sST2 concentration as measured in peripheral venous blood before the catheter ablation procedure.
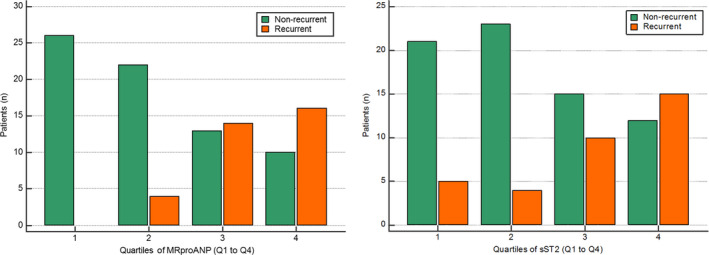
MRproANP indicates midregional N‐terminal pro–atrial natriuretic peptide; Q1, quartile 1; Q4, quartile 4; and sST2, soluble suppression of tumorigenicity 2.
Figure 3. Kaplan–Meier curves of AF recurrence‐free survival according to whether MRproANP level was above or below the threshold of 107.9 pmol/L.
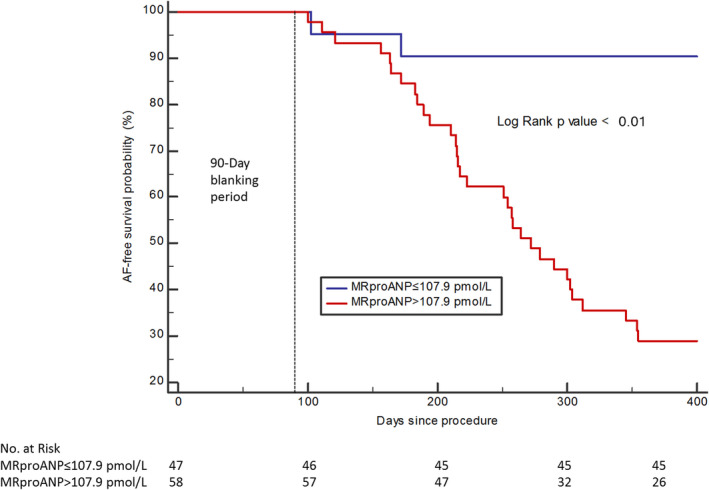
AF indicates atrial fibrillation; and MRproANP, midregional N‐terminal pro–atrial natriuretic peptide.
MRproANP levels were significantly higher in blood drawn from the LA and RA than in peripheral venous blood (median, 143.5 [quartile 1–quartile 3, 89.8–208.3] versus 131.5 [73.5–200.6] pmol/L [P<0.01]; and median, 142.2 [quartile 1–quartile 3, 90.3–210.3] versus 131.5 [73.5–200.6] pmol/L [P<0.01]; respectively). There was a significant correlation between MRproANP levels in the atria (left and right) and peripheral venous blood (P<0.01; Figure S1). There was no significant difference in MRproANP levels between the LA and RA (Figure S2). MRproANP levels were higher in the LA and RA when patients were in AF at the time of the blood draw compared with blood samples performed in sinus rhythm (median, 187.9 [quartile 1–quartile 3, 135.1–288.4] versus 115.2 [81.9–168.6] [P<0.01]; and median, 191.0 [quartile 1–quartile 3, 127.0–276.9] versus 109.4 [82.3–169.8] pmol/L [P<0.01]; respectively; Figure S3). The predictive value of MRproANP was the same, regardless of the site of blood draw, as illustrated by the lack of difference between ROC curves (Figure 4) and the sensitivity, specificity, positive predictive value, and NPV (using the best predictive value of MRproANP as identified by ROC curve analysis) measured in the LA and RA as well as in peripheral venous blood (Table 3).
Figure 4. Receiver operating characteristic curves showing the levels of MRproANP measured before the procedure in PV blood, the LA blood, and RA blood predicting the recurrence of atrial fibrillation up to 1 year after catheter ablation.
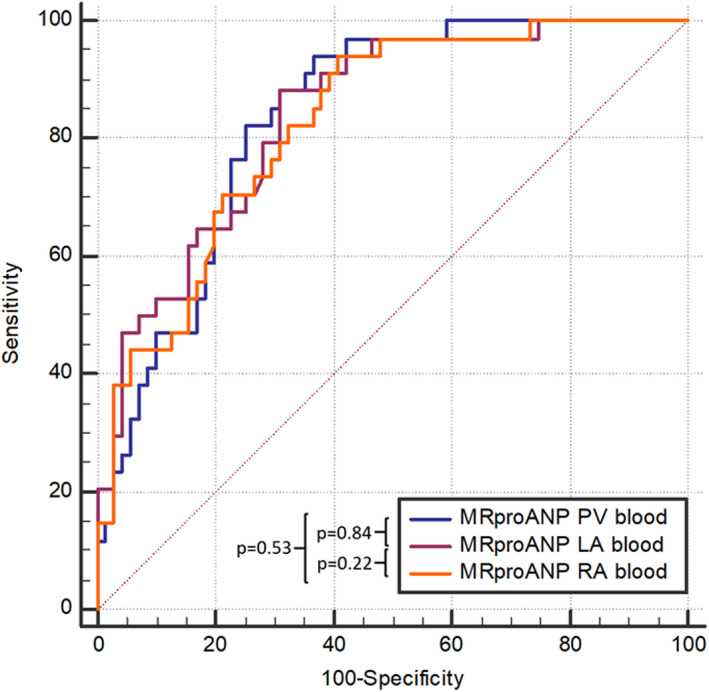
LA indicates left atrium; MRproANP, midregional N‐terminal pro–atrial natriuretic peptide; PV, peripheral venous; and RA, right atrium.
There was a significant correlation between both MRproANP (r=0.465; P<0.01) and sST2 (r=0.256; P<0.01) levels and LA area (Figure S4).
By multivariate analysis, MRproANP measured in peripheral venous blood was the only factor independently associated with recurrent AF at 1 year postablation (OR, 1.01; 95% CI, 1.01–1.02; Table 4). When including MRproANP as a binary variable (using the ROC‐identified threshold of 107.9 pmol/L), an elevated MRproANP value (>107.9 pmol/L) was associated with an OR of 24.27 for recurrence of AF at 1 year (95% CI, 4.23–139.18), after adjustment for hypertension, ischemic heart disease, CHA2DS2VASC≥2, sST2>26.7 ng/mL, LA area>22 cm2, diabetes mellitus, left ventricular hypertrophy, type of AF and rhythm control therapy at inclusion, and left ventricular ejection fraction.
Table 4.
Independent Predictors of Recurrent AF After Catheter Ablation by Multivariate Logistic Regression
| OR | 95% CI | |
|---|---|---|
| MRproANP*, per unit | 1.01 | 1.01–1.02 |
| sST2, per unit | 1.01 | 0.98–1.05 |
| Hypertension | 1.78 | 0.47–6.71 |
| Coronary artery disease | 5.40 | 0.76–38.17 |
| CHA2DS2VASC≥2 | 0.98 | 0.21–4.45 |
| LA area>22 cm2 | 2.69 | 0.71–10.14 |
| Diabetes mellitus | 1.28 | 0.28–5.92 |
| LVH | 0.35 | 0.02–6.70 |
| Type of AF at inclusion | 2.00 | 0.93–4.26 |
| Rhythm control therapy at inclusion | 0.45 | 0.14–1.46 |
| LVEF, per unit | 1.03 | 0.96–1.11 |
AF indicates atrial fibrillation; LA, left atrium; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MRproANP, midregional N‐terminal pro–atrial natriuretic peptide; OR, odds ratio; and sST2, soluble suppression of tumorigenicity 2.
Measured in peripheral venous blood.
DISCUSSION
To the best of our knowledge, this is the first prospective, multicenter study to investigate the impact of plasma levels of MRproANP and sST2 before catheter ablation of AF on the risk of recurrence of AF at 1 year after the procedure. Preprocedural levels of MRproANP and sST2 were higher in patients with recurrent AF at 1 year. By multivariate analysis, plasma MRproANP measured preprocedure was found to be a significant independent predictor of recurrent AF postablation. We observed a strong linear relation between the level of MRproANP before the procedure and recurrence of AF up to 1 year after ablation. In patients with preserved left ventricular ejection fraction, our results show that MRproANP >107.9 pmol/L before the procedure is indicative of a significant, 24‐fold increase in the risk of AF recurrence at 1 year. Conversely, MRproANP levels <107.9 pmol/L can identify individuals who will almost certainly not experience recurrence, with a NPV of >95%. sST2 was not found to be an independent predictor of AF recurrence after catheter ablation in our study.
There is a compelling need for simple and objective criteria that can identify patients likely to respond well to catheter ablation of AF, to facilitate selection of candidates for ablation, and to guide postablation management. Certain clinical variables, such as LA dilation, type of AF (persistent versus paroxysmal), obesity, diabetes mellitus, hypertension, sleep apnea, or CHA2DS2VASC score have been shown to predict recurrent AF postablation.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 However, to date no reliable biological parameter has been identified that can identify patients at risk of AF recurrence after catheter ablation. In a previous single‐center study, recurrent AF was found to be more frequent in patients with an MRproANP value in the fourth quartile compared with the first quartile.30 This is line with our findings, as in our study no recurrence was observed in the first quartile of MRproANP versus a recurrence rate of >60% in the fourth quartile. In terms of quantitative thresholds, this means that patients with MRproANP<73.5 pmol/L preprocedure have no recurrence, whereas among those with values >200 pmol/L, >60% experienced recurrent AF. Furthermore, our results also illustrate the linear nature of the relation between preprocedural MRproANP levels and postablation recurrence rates and identified MRproANP as an independent predictor of the risk of recurrence, which is a novel finding. Regardless of the site from which the blood was drawn (peripheral blood, LA, or RA), the predictive capacity of MRproANP for recurrent AF was the same. Thus, it would seem that assessing MRproANP from peripheral venous blood is sufficient to identify patients who will respond well or those who may be at increased risk of AF recurrence after the procedure.
proANP is stored and released by atrial cardiomyocytes and is cleaved into mature ANP, and its N‐terminal fragment, NTproANP.41 Within this fragment is the mid‐regional section, MRproANP, which has a longer half‐life than mature ANP (atrial natriuretic peptide), making its assessment more reliable.42, 43 In stable clinical conditions, which was the case of the patients included in our study, the variability of MRproANP is low.44 It was previously shown that MRproANP is increased in patients with AF, independently of hemodynamic conditions, and also that there is a link between AF burden and MRproANP levels.45, 46 Other studies have also reported that catheter ablation for AF significantly reduced plasma concentrations of ANP and MRproANP in the long term.47, 48 In our study, MRproANP levels were higher in the RA and LA than in peripheral venous blood, with a strong correlation between the levels observed in the atria and those in the peripheral circulation, and the levels in the atria were higher during AF than in sinus rhythm. These data complement previous knowledge and suggest that there is an increased release of ANPs by the myocytes of the LA and RA during AF. Taken together, these findings suggest that MRproANP may be a good marker of AF progression and prognosis. We purport that MRproANP could be preferred over BNP (B‐type natriuretic peptide) in the context of AF, especially because it is established that ANP and BNP levels are discordant in patients with AF.49
sST2 is a marker of fibrosis, particularly cardiac fibrosis.32 The clinical course of AF is marked by the development of atrial fibrosis.31, 50, 51, 52, 53 In clinical practice, sST2 is an important prognostic marker in several domains, such as heart failure and renal insufficiency.34, 35, 36, 37, 38, 54, 55 A previous study investigating sST2 found that it was an independent predictor of AF recurrence.56 However, this was a single‐center study performed in patients with nonvalvular paroxysmal AF undergoing cryoablation of AF.56 In our study, we did not find sST2 to be an independent predictor of AF recurrence after catheter ablation. It would seem that sST2 is less determinant than MRproANP in light of our multivariate analysis and the comparison of the ROC curves comparing the predictive capacity of each marker. However, we did observe a correlation between sST2 and LA dilation, corroborating the findings of Okar et al.56 Taken together, the data regarding sST2 suggest that it is an important biomarker for the prognosis and overall follow‐up of cardiac disease and progression toward heart failure, but not specifically useful in the setting of AF.
Our study has some limitations. First, it was an observational study with a relatively small sample size (N=105), and certain known predictors of recurrence (body mass index, sleep apnea) were not found to be significant in our study, likely because of a lack of statistical power. Second, the methods of assessing AF recurrence may have led to an underestimation of actual recurrence rates. Indeed, patients did not have implanted Holter devices (implantable cardiac monitors), and the duration of systematic ECG Holter monitoring was only 24 hours, without the performance of longer duration monitoring. Nevertheless, the likelihood of underestimation of AF recurrence is low given than the recurrence rate at 1 year observed in our study was similar to, if not higher than that reported in other prospective studies.5, 10, 57 This may be explained by the fact that all patients were symptomatic, making the detection of recurrence easier. Indeed, patients were instructed to consult the cardiologist or general practitioner immediately in case of symptoms. In addition, if underestimation occurred, it likely affected both groups equivalently. We therefore believe that the monitoring modalities likely had little effect on the results. In addition, multiple factors affect the success rate of AF ablation and may confound the predictive role of the markers. Nevertheless, MRproANP was found to be an independent predictor even after adjustment for potential confounders in multivariate analysis, thus supporting the robustness of our findings. Finally, our data deserve confirmation in a larger population to confirm the prognostic value of MRproANP to identify patients at high risk of AF recurrence up to 1 year after catheter ablation of AF on top of previously identified predictors.11, 12, 13, 18, 19, 20, 21, 22, 23
CONCLUSIONS
An elevated MRproANP level is an independent predictor of recurrent AF at 1 year after catheter ablation, whereas sST2 levels do not appear to have any prognostic value in assessing the risk of recurrence of AF up to 1 year. An MRproANP level >107.9 pmol/L before the procedure is associated with a 24‐fold increase in the risk of recurrent AF within 1 year. Conversely, an MRproANP value <107.9 pmol/L identifies patients unlikely to experience recurrent AF, with a high NPV. Larger prospective studies are warranted to confirm the utility of assessing MRproANP in this setting.
Sources of Funding
This study was partially funded by an unrestricted grant from Abbott Medical France.
Disclosures
Dr de Chillou reports personal fees from Biosense Webster, Medtronic, Stereotaxis, and Mylan and nonfinancial support from Abbott and Microport outside the submitted work. Dr Sellal reports personal fees from Microport, Bayer, Mylan, Pfizer, and Boeringher outside the submitted work. Dr Meneveau reports personal fees from Abbott, Bayer, Boston Scientific, Bristol Myers Squibb, Edwards Lifesciences, and Terumo outside the submitted work. Dr Seronde reports personal fees from Astra Zeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Novartis, and Servier outside the submitted work. Dr Mebazaa reports personal fees from Orion, Servier, Otsuka, Philips, Sanofi, Epygon, and Fire 1; grants and personal fees from Roche, Adrenomed, and 4TEEN4; personal fees from grants from Abbott; and grants and personal fees from Sphyngotec outside the submitted work. Dr Schiele reports grants and personal fees from Bayer, Sanofi Aventis, MSD, and Amgen; grants from St Jude Medical and Daiichi Sankyo; and personal fees from Boehringer Ingelheim, Pfizer, Astra Zeneca, Mylan, and Servier outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figures S1‐S4
(J Am Heart Assoc. 2021;10:e020917. DOI: 10.1161/JAHA.121.020917.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020917.
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. DOI: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. DOI: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.Dukkipati SR, Cuoco F, Kutinsky I, Aryana A, Bahnson TD, Lakkireddy D, Woollett I, Issa ZF, Natale A, Reddy VY. Pulmonary vein isolation using the visually guided laser balloon: a prospective, multicenter, and randomized comparison to standard radiofrequency ablation. J Am Coll Cardiol. 2015;66:1350–1360. DOI: 10.1016/j.jacc.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta‐analysis. Europace. 2015;17:370–378. DOI: 10.1093/europace/euu376. [DOI] [PubMed] [Google Scholar]
- 5.Kuck K‐H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ, Elvan A, Arentz T, Bestehorn K, Pocock SJ, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. DOI: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 6.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2): a randomized trial. JAMA. 2014;311:692–700. DOI: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 7.Verma A, Jiang C‐Y, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. DOI: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 8.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. DOI: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 9.Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, Laroche C, Anselme F, Inama G, Jais P, et al. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35:1466–1478. DOI: 10.1093/eurheartj/ehu001. [DOI] [PubMed] [Google Scholar]
- 10.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. DOI: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balk EM, Garlitski AC, Alsheikh‐Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. DOI: 10.1111/j.1540-8167.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 12.Chao T‐F, Cheng C‐C, Lin W‐S, Tsao H‐M, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, Tuan T‐C, Suenari K, et al. Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2011;8:1155–1159. DOI: 10.1016/j.hrthm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Winkle RA, Jarman JW, Mead RH, Engel G, Kong MH, Fleming W, Patrawala RA. Predicting atrial fibrillation ablation outcome: the CAAP‐AF score. Heart Rhythm. 2016;13:2119–2125. DOI: 10.1016/j.hrthm.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, Rolf S, Richter S, Huo Y, Piorkowski C, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2‐VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281–287. DOI: 10.1161/CIRCEP.113.001182. [DOI] [PubMed] [Google Scholar]
- 15.Cai L, Yin Y, Ling Z, Su LI, Liu Z, Wu J, Du H, Lan X, Fan J, Chen W, et al. Predictors of late recurrence of atrial fibrillation after catheter ablation. Int J Cardiol. 2013;164:82–87. DOI: 10.1016/j.ijcard.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 16.Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta‐analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108:47–51. DOI: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 17.Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S, Bernstein N, Chinitz L. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta‐analysis. JACC Clin Electrophysiol. 2015;1:41–51. DOI: 10.1016/j.jacep.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Abecasis J, Dourado R, Ferreira A, Saraiva C, Cavaco D, Santos KR, Morgado FB, Adragão P, Silva A. Left atrial volume calculated by multi‐detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace. 2009;11:1289–1294. DOI: 10.1093/europace/eup198. [DOI] [PubMed] [Google Scholar]
- 19.Costa FM, Ferreira AM, Oliveira S, Santos PG, Durazzo A, Carmo P, Santos KR, Cavaco D, Parreira L, Morgado F, et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long‐term success of catheter ablation. Int J Cardiol. 2015;184:56–61. DOI: 10.1016/j.ijcard.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 20.Nedios S, Kosiuk J, Koutalas E, Kornej J, Sommer P, Arya A, Richter S, Rolf S, Husser D, Hindricks G, et al. Comparison of left atrial dimensions in CT and echocardiography as predictors of long‐term success after catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2015;43:237–244. DOI: 10.1007/s10840-015-0010-8. [DOI] [PubMed] [Google Scholar]
- 21.Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, Dominic P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta‐analysis. Europace. 2018;20:33–42. DOI: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 22.Tang R‐B, Yan X‐L, Dong J‐Z, Kalifa J, Long D‐Y, Yu R‐H, Bai R, Kang J‐P, Wu J‐H, Sang C‐H, et al. Predictors of recurrence after a repeat ablation procedure for paroxysmal atrial fibrillation: role of left atrial enlargement. Europace. 2014;16:1569–1574. DOI: 10.1093/europace/euu013. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C, Xu Y. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta‐analysis of observational studies. Europace. 2012;14:638–645. DOI: 10.1093/europace/eur364. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. DOI: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 25.Troughton R, Michael Felker G, Januzzi JL Jr. Natriuretic peptide‐guided heart failure management. Eur Heart J. 2014;35:16–24. DOI: 10.1093/eurheartj/eht463. [DOI] [PubMed] [Google Scholar]
- 26.Writing Committee Members, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. DOI: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 27.Al‐Khatib SM, Benjamin EJ, Buxton AE, Calkins H, Chung MK, Curtis AB, Desvigne‐Nickens P, Jais P, Packer DL, Piccini JP, et al. Research needs and priorities for catheter ablation of atrial fibrillation: a report from a national heart, lung, and blood institute virtual workshop. Circulation. 2020;141:482–492. DOI: 10.1161/CIRCULATIONAHA.119.042706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badoz M, Arrigo M, Iung B, Amioglu G, Yilmaz MB, Meneveau N, Sadoune M, Brunette A, Mebazaa A, Seronde MF. Role of cardiovascular biomarkers for the assessment of mitral stenosis and its complications. Eur J Intern Med. 2016;34:58–62. DOI: 10.1016/j.ejim.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Badoz M, Arrigo M, Mogenet AC, Sadoune M, Meneveau N, Mebazaa A, Seronde MF. Assessment of successful percutaneous mitral commissurotomy by MRproANP and sCD146. BMC Cardiovasc Disord. 2020;20:157. DOI: 10.1186/s12872-020-01435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charitakis E, Karlsson LO, Papageorgiou JM, Walfridsson U, Carlhäll CJ. Echocardiographic and biochemical factors predicting arrhythmia recurrence after catheter ablation of atrial fibrillation‐an observational study. Front Physiol. 2019;10:1215. DOI: 10.3389/fphys.2019.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravassa S, Ballesteros G, Lopez B, Ramos P, Bragard J, Gonzalez A, Moreno MU, Querejeta R, Vives E, Garcia‐Bolao I, et al. Combination of circulating type I collagen‐related biomarkers is associated with atrial fibrillation. J Am Coll Cardiol. 2019;73:1398–1410. DOI: 10.1016/j.jacc.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 32.Vianello E, Dozio E, Tacchini L, Frati L, Corsi Romanelli MM. ST2/IL‐33 signaling in cardiac fibrosis. Int J Biochem Cell Biol. 2019;116:105619. DOI: 10.1016/j.biocel.2019.105619. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. DOI: 10.1161/01.CIR.0000047274.66749.FE. [DOI] [PubMed] [Google Scholar]
- 34.Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, Bayes‐Genis A, Anand I, Januzzi JL, Emdin M. Prognostic value of soluble suppression of tumorigenicity‐2 in chronic heart failure: a meta‐analysis. JACC Heart Fail. 2017;5:280–286. DOI: 10.1016/j.jchf.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Anand IS, Rector TS, Kuskowski M, Snider J, Cohn JN. Prognostic value of soluble ST2 in the valsartan heart failure trial. Circ Heart Fail. 2014;7:418–426. DOI: 10.1161/CIRCHEARTFAILURE.113.001036. [DOI] [PubMed] [Google Scholar]
- 36.Gaggin HK, Szymonifka J, Bhardwaj A, Belcher A, De Berardinis B, Motiwala S, Wang TJ, Januzzi JL Jr. Head‐to‐head comparison of serial soluble ST2, growth differentiation factor‐15, and highly‐sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2014;2:65–72. DOI: 10.1016/j.jchf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WHW, Wu AHB, Fang JC, et al. High‐sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. DOI: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Vark LC, Lesman‐Leegte I, Baart SJ, Postmus D, Pinto YM, Orsel JG, Westenbrink BD, Brunner‐la Rocca HP, van Miltenburg AJM, Boersma E, et al. Prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol. 2017;70:2378–2388. DOI: 10.1016/j.jacc.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 39.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. DOI: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. DOI: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Vesely DL. Atrial natriuretic peptide prohormone gene expression: hormones and diseases that upregulate its expression. IUBMB Life. 2002;53:153–159. DOI: 10.1080/15216540212336. [DOI] [PubMed] [Google Scholar]
- 42.Buckley MG, Marcus NJ, Yacoub MH. Cardiac peptide stability, aprotinin and room temperature: importance for assessing cardiac function in clinical practice. Clin Sci. 1999;97:689–695. DOI: 10.1042/CS19990194. [DOI] [PubMed] [Google Scholar]
- 43.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro‐atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. DOI: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 44.Kistorp C, Bliddal H, Goetze JP, Christensen R, Faber J. Cardiac natriuretic peptides in plasma increase after dietary induced weight loss in obesity. BMC Obes. 2014;1:24. DOI: 10.1186/s40608-014-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darkner S, Goetze JP, Chen X, Henningsen K, Pehrson S, Svendsen JH. Natriuretic propeptides as markers of atrial fibrillation burden and recurrence (from the AMIO‐CAT trial). Am J Cardiol. 2017;120:1309–1315. DOI: 10.1016/j.amjcard.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Sramko M, Wichterle D, Melenovsky V, Franekova J, Clemens M, Fukunaga M, Kautzner J. Independent effect of atrial fibrillation on natriuretic peptide release. Clin Res Cardiol. 2019;108:142–149. DOI: 10.1007/s00392-018-1332-1. [DOI] [PubMed] [Google Scholar]
- 47.Jones DG, Haldar SK, Donovan J, McDonagh TA, Sharma R, Hussain W, Markides V, Wong T. Biomarkers in persistent AF and heart failure: impact of catheter ablation compared with rate control. Pacing Clin Electrophysiol. 2016;39:926–934. DOI: 10.1111/pace.12919. [DOI] [PubMed] [Google Scholar]
- 48.Sacher F, Corcuff J‐B, Schraub P, Le Bouffos V, Georges A, Jones SO, Lafitte S, Bordachar P, Hocini M, Clementy J, et al. Chronic atrial fibrillation ablation impact on endocrine and mechanical cardiac functions. Eur Heart J. 2008;29:1290–1295. DOI: 10.1093/eurheartj/ehm577. [DOI] [PubMed] [Google Scholar]
- 49.Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45:82–86. DOI: 10.1016/j.jacc.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 50.Begg GA, Holden AV, Lip GY, Plein S, Tayebjee MH. Assessment of atrial fibrosis for the rhythm control of atrial fibrillation. Int J Cardiol. 2016;220:155–161. DOI: 10.1016/j.ijcard.2016.06.144. [DOI] [PubMed] [Google Scholar]
- 51.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. 2015;66:943–959. DOI: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 52.Gal P, Marrouche NF. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur Heart J. 2017;38:14–19. DOI: 10.1093/eurheartj/ehv514. [DOI] [PubMed] [Google Scholar]
- 53.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–2678. DOI: 10.1161/01.CIR.0000016826.62813.F5. [DOI] [PubMed] [Google Scholar]
- 54.O'Meara E, Prescott MF, Claggett B, Rouleau JL, Chiang LM, Solomon SD, Packer M, McMurray JJV, Zile MR. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM‐HF Trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018;11:e004446. DOI: 10.1161/CIRCHEARTFAILURE.117.004446. [DOI] [PubMed] [Google Scholar]
- 55.Tuegel C, Katz R, Alam M, Bhat Z, Bellovich K, de Boer I, Brosius F, Gadegbeku C, Gipson D, Hawkins J, et al. GDF‐15, galectin 3, soluble ST2, and risk of mortality and cardiovascular events in CKD. Am J Kidney Dis. 2018;72:519–528. DOI: 10.1053/j.ajkd.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okar S, Kaypakli O, Şahin DY, Koç M. Fibrosis marker soluble ST2 predicts atrial fibrillation recurrence after cryoballoon catheter ablation of nonvalvular paroxysmal atrial fibrillation. Korean Circ J. 2018;48:920–929. DOI: 10.4070/kcj.2018.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. DOI: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1‐S4


