Abstract
Background
The aim of this study was to evaluate long‐term survival and freedom from coronary artery reintervention after the arterial switch operation (ASO).
Methods and Results
This single‐center nationwide retrospective study included consecutive children who underwent ASO between 1990 and 2016 (n=605). Long‐term outcomes were obtained by cross‐mapping individual data with the National Death Registry and the National Registry of Cardiovascular Interventions for adults. A control group was randomly retrieved at a 1:10 ratio from the National Birth and Death Registries. Early mortality was 3.3% and late mortality was 1.7% during a median follow‐up of 10 (interquartile range, 5–16) years. The probability of overall survival at 20 years after ASO was 94.9% compared with 99.5% in the background population (hazard ratio [HR] 15.6; 95% CI, 8.9–27.5, P<0.001). Independent multivariable predictors of worse survival were an intramural coronary artery (HR, 5.2; 95% CI, 1.8–15.2, P=0.002) and period of ASO 1990 to 1999 (HR, 4.6; 95% CI, 1.5–13.6, P<0.001). Fourteen patients (2.3%) required 16 coronary artery reoperations. Freedom from coronary artery reintervention at 20 years after ASO was 96%. The only independent multivariable predictor associated with a higher hazard for coronary artery reintervention was an intramural coronary artery (HR, 33.9; 95% CI, 11.8–97.5, P<0.001).
Conclusions
Long‐term survival after ASO is excellent. Coronary artery reinterventions are rare. An intramural coronary artery was an independent predictor associated with a higher risk for coronary artery reintervention and death, regardless of the surgical period.
Keywords: congenital heart disease, coronary artery anomaly, transposition of great vessels
Subject Categories: Congenital Heart Disease, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- ASO
arterial switch operation
- DORV‐TGA
Taussig‐Bing anomaly
- IMCA
intramural coronary artery
- TGA
transposition of the great arteries
- TGA‐IVS
transposition of the great arteries with intact ventricular septum
- TGA‐VSD
transposition of the great arteries with a ventricular septal defect
Clinical Perspective
What Is New?
Long‐term survival after arterial switch operation is excellent but still inferior to survival of the general population.
Coronary artery reinterventions after arterial switch operation are rare.
An intramural coronary artery was a risk factor for survival and freedom from coronary artery reintervention in this study, regardless of the surgical era.
What Are the Clinical Implications?
Identification of patients who are at increased risk of development of acquired coronary artery stenoses is crucial.
More studies are needed to investigate the influence of coronary artery patterns and anomalies on the outcomes after arterial switch operation, because published data are inconsistent.
Arterial switch operation (ASO)1 has become the standard surgical treatment for transposition of the great arteries (TGA). High‐volume centers have reported excellent early and long‐term results.2, 3, 4, 5, 6, 7
Transfer of the coronary arteries remains the most challenging part of ASO. It has been suggested that abnormal coronary artery patterns and coronary artery anomalies might increase mortality.3, 4, 8 Some studies have reported the presence of an intramural coronary artery as a risk factor for death,8, 9, 10, 11 whereas others have not.2, 6, 12, 13 The reported rate of coronary artery reinterventions after ASO was <3% in most studies.2, 3, 4, 6, 7, 9, 14 However, identification of risk factors for coronary artery reinterventions was rarely addressed.5, 6
The first patients operated on by the arterial switch technique worldwide are in their middle age and their survival is excellent. The differentiation between myocardial ischemia because of the TGA coronary artery patterns and anomalies in relation to atherosclerosis‐related coronary artery disease will be of utmost importance in some patients with their increasing age.
The aim of this retrospective study was to analyze the risk factors for long‐term survival and freedom from coronary artery reinterventions after ASO with emphasis on coronary artery patterns and anomalies. We also sought to compare overall survival rate after ASO with the background population of sex and age‐matched children.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Design
This study included all consecutive children with TGA or Taussig‐Bing anomaly (DORV‐TGA) born in the Czech Republic who underwent ASO between 1990 and 2016 at a single nationwide pediatric cardiac center. Demographic and clinical data were obtained from the institutional database. Data on coronary artery pattern and anomalies were retrieved from detailed surgical reports. Patients were regularly followed at the study institution until the age of 18 years when they were referred to specialized cardiac centers for adults with congenital heart disease. Besides standard clinical follow‐up, 12‐lead resting ECG, and echocardiography, cardiopulmonary function tests were performed in all patients as soon as they were able to exercise on a bicycle ergometer (8–10 years of age). The test was usually repeated later before patients reached 18 years of age. In cases with suspected myocardial ischemia, coronary artery imaging (coronary angiography or computed tomography coronary angiography) and myocardial perfusion scintigraphy were performed as appropriate. Long‐term outcomes were obtained by cross‐mapping the individual patient data with the National Death Registry and the National Registry of Cardiovascular Interventions for adults using a personal identification number to allow for long‐term follow‐ups during both the pediatric and adult ages. The study was approved by the Motol University Hospital Ethics Committee. Because of the retrospective character of the study and the absence of any impact on clinical patient management and anonymized data presentation, informed consent was not required.
Classification of Coronary Artery Patterns and Anomalies
Patients were divided according to their coronary artery patterns using the Leiden convention15 into groups with a usual pattern (1LCx‐2R), circumflex artery from the right coronary artery (1L‐2CxR), inverted origin (1R‐2LCx), partially inverted origin (1RL‐2Cx), double origin from sinus 1 (1R,LCx); double origin from sinus 2 (2LCx,R), and single origin from sinus 1 or sinus 2 (1RLCx or 2LCxR). Additional coronary artery anomalies recorded separately were intramural coronary artery (IMCA), abnormal ostial origin (abnormally high above sinotubular junction and/or in the proximity of attachments of the valvar leaflets), and accessory coronary artery orifices. Cardiac catheterization and coronary angiography, respectively, were performed after ASO only in cases with global or regional left ventricular dysfunction, ischemic changes on ECG or on 24‐hour ambulatory ECG, and/or on exercise tests in older children.
Surgical Technique
The operation was conducted with a cardiopulmonary bypass with mild hypothermia at 28°C. Myocardial protection was achieved using cold blood antegrade cardioplegia. The coronary artery neo‐ostia were excised with a generous U‐shaped aortic cuff and extensively mobilized and reimplanted in the corresponding pulmonary sinus with either a trap‐door or button technique, depending on coronary artery anatomy. The Lecompte maneuver was performed. In cases with IMCA, unroofing of the intramural portion was carried out cautiously and the coronary arteries were transferred as 2 separate buttons as described in Fricke and Konstantinov.16 Only in patients in whom the ostia were too close to each other, making this technique high‐risk, the coronary ostia were transferred on a single button.
Statistical Analysis
All tested continuous variables were found to be nonnormally distributed by a Shapiro–Wilk test and are reported as the median and interquartile range (IQR, 25th–75th percentile). The comparisons between groups were performed using a Kruskal–Wallis test. Categorical variables were evaluated by χ2 test or Fisher exact test as appropriate. Early mortality was defined as death within 30 days after ASO. To evaluate the risk of early mortality over time, the study period was divided into quartile groups as Period 1990 to 1999, Period 2000 to 2005, Period 2006 to 2010, and Period 2011 to 2016. Survival and freedom from reintervention were assessed by the Kaplan–Meier method and the differences between groups by the log‐rank test. To compare overall survival after ASO with the background population, a control group of 6050 sex‐matched children who were alive and had the same age as children at ASO was randomly retrieved at a 1:10 ratio from a cohort of 3 269 337 children born between years 1987 and 2016 using anonymous data from the National Birth and Death Registries. Survival time was calculated from the date of ASO to the date of death or to the end of study in December 2016 for patients who were not identified as deceased. The end points for overall survival analyses were death and freedom from the first subsequent coronary artery reintervention. The risk factors for early mortality were assessed by binary logistic regression. Following this multivariable analysis, a receiver operating characteristic curve was plotted for the final model, along with computation of the area under the curve and its 95% CI. The Cox proportional hazard analysis method was used to evaluate factors associated with the outcome interest. All factors from the univariable analyses were included in the multivariable backwards model (Wald) in which a P>0.1 was required for the factor to be removed from the model. Odds and hazard ratios (HRs) were stated with 95% CIs. Statistical analyses were carried out using IBM SPSS Statistic, version 26. A P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 605 patients, 175 female (28.9%), underwent ASO at median age of 7 days (IQR 5–10 days) and median weight of 3.4 kg (IQR, 3.0–3.7 kg). Arterial switch operation was performed in 428 (70.7%) patients with TGA and intact ventricular septum or with small muscular ventricular septal defects that were left open (TGA‐IVS), in 129 (21.3%) patients with a large ventricular septal defect closure (TGA‐VSD) and in 48 (7.9%) patients with DORV‐TGA repair. Previous balloon atrial septostomy was performed in 457 (75.5%) cases. Seventeen patients had previous pulmonary artery banding: 16 for a large VSD and 1 patient with isolated TGA for left ventricular retraining. Previous coarctation of the aorta repair was performed in 23 patients, and 26 additional patients underwent aortic arch repair at ASO (23 patients with coarctation of the aorta, 3 with interrupted aortic arch). Basic characteristics and previous interventions are summarized in Table 1.
Table 1.
Baseline Characteristics
| Patients | All (n=605) | TGA‐IVS (n=428) | TGA‐VSD (n=129) | DORV‐TGA (n=48) | P Value |
|---|---|---|---|---|---|
| Female, n (%) | 175 (28.9) | 111 (25.9) | 46 (35.7) | 18 (37.5) | 0.030 |
| Age, d | 7 (5–10) | 6 (5–8) | 10 (7–28) | 41 (9–166) | <0.001 |
| Weight, kg | 3.4 (3.0–3.7) | 3.3 (3.0–3.7) | 3.4 (3.1–3.8) | 3.5 (3.1–4.9) | 0.005 |
| Height, cm | 50 (49–52) | 50 (49–51) | 51 (49–53) | 53 (50–57) | <0.001 |
| Cardiopulmonary bypass time, min | 186 (166–210) | 176 (160–193) | 207 (191–230) | 257 235–291) | <0.001 |
| Cross‐clamp time, min | 97 (83–117) | 90 (80–102) | 118 (106–134) | 127 (108–150) | <0.001 |
| Previous pulmonary artery banding, n (%) | 17 (2.8) | 1* (0.2) | 1 (0.8) | 15 (31.3) | <0.001 |
| Previous aortic arch repair, n (%) | 23 (3.8) | 3 (0.7) | 11 (8.5) | 9 (18.8) | <0.001 |
| Aortic arch repair at ASO, n (%) | 26 (4.3) | 2 (0.5) | 10 (7.8) | 14 (29.2) | <0.001 |
| Early mortality, n (%) | 20 (3.3) | 12 (2.8) | 5 (3.9) | 3 (6.3) | 0.268 |
| Late mortality, n (%) | 10 (1.7) | 6 (1.4) | 2 (1.6) | 2 (4.2) | 0.325 |
| Overall mortality, n (%) | 30 (5.0) | 18 (4.2) | 7 (5.4) | 5 (10.4) | 0.453 |
| Follow‐up, y | 10 (5–16) | 10 (5–16) | 11 (6–17) | 9 (3–15) | 0.141 |
Variables are presented as median and interquartile range (25th–75th percentile); P, difference between TGA‐IVS, TGA‐VSD, and DORV‐TGA groups. ASO indicates arterial switch operation; DORV‐TGA, Taussig‐Bing anomaly; IVS, intact ventricular septum; TGA, transposition of the great arteries; and VSD, ventricular septal defect.
Left ventricle retraining.
Coronary Artery Patterns and Anomalies
Coronary artery patterns and anomalies for each TGA group are listed in Table 2. Overall unusual coronary artery patterns were more common in patients with DORV‐TGA than in those with TGA‐VSD or TGA‐IVS (52.1% versus 34.9% versus 26.2%, respectively, P=0.001). The relationship between coronary artery patterns and the occurrence of additional coronary anomalies is shown in Table 3. IMCA was present in 21 (3.5%) cases, abnormal ostial origin in 46 (7.6%), and accessory coronary artery orifices in 46 (7.6%) cases. Double coronary origin from sinus 2 (2LCx,R) was strongly associated with IMCA (15/19 cases, 78.9%, P<0.001 ) and with abnormal ostial origin (6/19 cases, 31.6%, P=0.007). During ASO, the coronary arteries were transferred individually as 2 buttons in 18 of 21 cases with IMCA and as a single button in the remaining 3.
Table 2.
Coronary Artery Patterns and Additional Anomalies
| Patients | All (n=605) | TGA‐IVS (n=428) | TGA‐VSD (n=129) | DORV‐TGA (n=48) | P Value |
|---|---|---|---|---|---|
| Coronary artery patterns | |||||
| Usual (1LCx‐2R) | 423 (69.9) | 316 (73.8) | 84 (65.1) | 23 (47.9) | 0.001 |
| Circumflex from right coronary artery (1L‐2CxR) | 101 (16.7) | 72 (16.8) | 23 (17.8) | 6 (12.5) | 0.722 |
| Partially inverted origin (1RL‐2Cx) | 35 (5.8) | 15 (3.5) | 11 (8.5) | 9 (18.8) | <0.001 |
| Inverted origin (1R‐2LCx) | 12 (2.0) | 2 (0.5) | 3 (2.3) | 7 (14.6) | <0.001 |
| Double origin from sinus 2 (2LCx,R) | 19 (3.1) | 13 (3.0) | 5 (3.9) | 1 (2.1) | 0.856 |
| Double origin from sinus 1 (1R,LCx) | 8 (1.3) | 5 (1.2) | 3 (2.3) | 0 | 0.460 |
| Single origin from sinus 1 or 2 (1RLCx or 2LCxR) | 7 (1.2) | 5 (1.2) | 0 | 2 (4.2) | 0.079 |
| Additional coronary artery anomalies | |||||
| Intramural course (IMCA) | 21 (3.5) | 14 (3.3) | 6 (4.7) | 1 (2.1) | 0.650 |
| Abnormal ostial origin | 46 (7.6) | 36 (8.4) | 8 (6.2) | 2 (4.2) | 0.523 |
| Accessory coronary artery orifices | 46 (7.6) | 30 (7.0) | 11 (8.5) | 5 (10.4) | 0.503 |
Coding of coronary arteries according to the Leiden convention: 1, sinus 1; 2, sinus 2; L, left anterior descending; Cx, left circumflex; R, right coronary artery. Data are presented as numbers and percentages (parentheses) in each group; P, difference between TGA‐IVS, TGA‐VSD, and DORV‐TGA groups. DORV‐TGA indicates Taussig‐Bing anomaly; IMCA, intramural coronary artery; IVS, intact ventricular septum; TGA, transposition of the great arteries; and VSD, ventricular septal defect.
Table 3.
Occurrence of Additional Coronary Artery Anomalies in Relation to Coronary Artery Patterns
| Usual (1LCx‐2R) | Circumflex from the right coronary artery (1L‐2CxR) | Partially Inverted (1RL‐2Cx) | Inverted (1R‐2LCx) |
Double Orifice Sinus 2 (2LCx,R) |
Double Orifice Sinus 1 (1R,LCx) |
Single Orifice (1RLCx) (2LCxR) |
P Value | |
|---|---|---|---|---|---|---|---|---|
| n=423 | n=101 | n=35 | n=12 | n=19 | n=8 | n=7 | ||
| Intramural course (IMCA) | 2 (0.5) | 3 (3.0) | 0 | 1 (8.3) | 15 (78.9) | 0 | 0 | <0.001 |
| Abnormal ostial origin | 34 (8.0) | 2 (2.0) | 3 (8.6) | 1 (8.3) | 6 (31.6) | 0 | 0 | 0.007 |
| Accessory orifice | 19 (4.5) | 13 (12.9) | 10 (28.6) | 1 (8.3) | 0 | 2 (25.0) | 1 (14.3) | <0.001 |
Coding of coronary arteries according to the Leiden convention: 1, sinus 1; 2, sinus 2; L, left anterior descending; Cx, left circumflex; R, right coronary artery. Data are presented as numbers and percentages in each pattern group (parentheses). IMCA indicates intramural coronary artery.
Early Mortality
There were 20/605 (3.3%) early deaths. Early mortality rate decreased significantly over periods of ASO, from 8.8% (13/147) in Period 1990 to 1999, 1.3% (2/157) in Period 2000 to 2005, 2.0% (3/150) in Period 2006 to 2010 to 1.3% (2/151) in Period 2011 to 2016 (P=0.001). There was no difference in early mortality between the TGA‐IVS, TGA‐VSD, and DORV‐TGA groups (Table 1). There was also no difference in early mortality between a subgroup of patients with primary aortic arch repair at ASO (3.8%; 1/26 patients) and other patients (3.3%, 19/579 patients); P=0.591. Using multivariable binary logistic regression, independent risk factors for early mortality were the early period of ASO in 1990 to 1999 (odds ratio [OR], 7.8; 95% CI, 1.7–35.9; P=0.008) and IMCA (OR, 7.8; 95% CI, 1.9–32.7; P=0.005), (area under the curve=0.83; 95% CI, 0.74–0.93). Neither TGA‐VSD type of repair (OR, 1.3; 95% CI, 0.4–3.9; P=0.633) nor DORV‐TGA repair (OR, 2.7; 95% CI, 0.7–10.6; P=0.147), nor associated aortic arch repair (OR, 1.3; 95% CI, 0.1–15.4; P=0.843) had influence on early mortality. Early mortality also was not influenced by the coronary artery pattern.
Long‐Term Survival
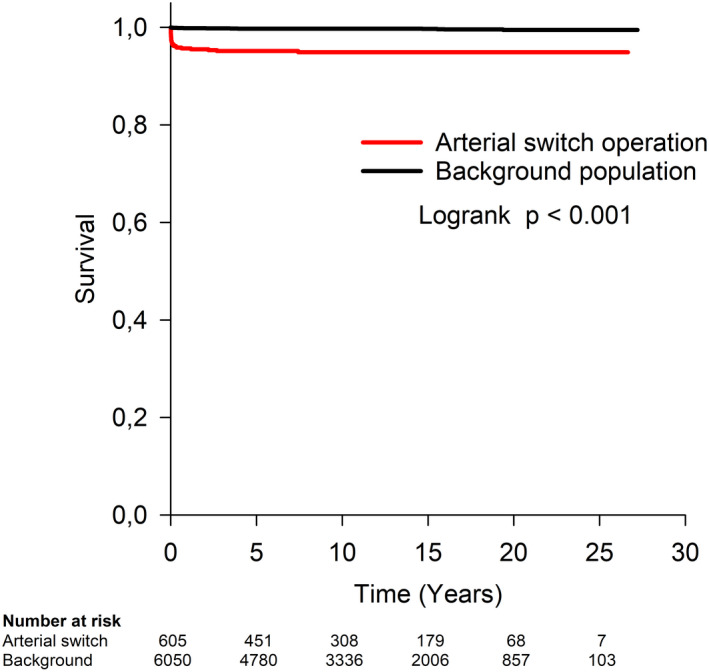
During a median follow‐up of 10 years (IQR, 5–16 years), late death occurred in 10 (1.7%) of 605 patients at a median interval from ASO of 5.7 months (IQR, 1.9–27.8 months). The probability of overall survival at 10, 15, 20, and 25 years after ASO was 94.9%, 94.9%, 94.9%, and 94.9% as compared with 99.7%, 99.7%, 99.5%, and 99.5% survival in the background population (HR, 15.6; 95% CI, 8.9–27.5, P<0.001) (Figure 1).
Figure 1. Overall survival after arterial switch operation compared with background population of children matched for sex and age.
The type of ASO (Figure 2A) or associated aortic arch repair (Figure 2B) had no significant influence on long‐term survival.
Figure 2. Overall survival after arterial switch operation was not influenced by the type of surgery (P=0.162) (A) or concomitant aortic arch repair (P=0.801) (B), but it was significantly lower in children operated in period between 1990 and 1999 (P=0.007) (C).
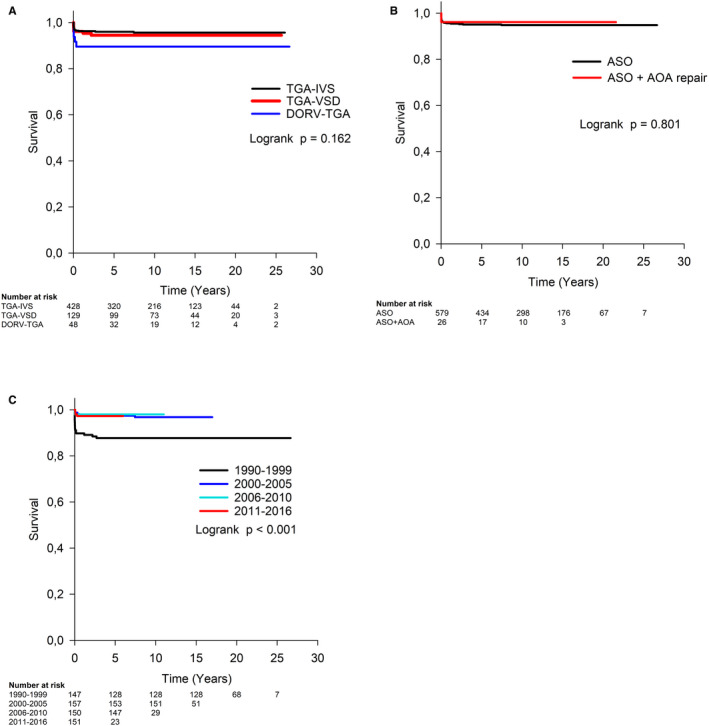
AOA indicates aortic arch; ASO, arterial switch operation; DORV‐TGA, Taussig‐Bing anomaly; IVS, intact interventricular septum; TGA, transposition of the great arteries; and VSD, ventricular septal defect. P value refers to Cox regression analysis.
Overall survival was influenced by mortality in the early period of ASO (Figure 2C). In a univariable analysis, compared with period 1990 to 1999, survival improved significantly, with a HR ratio for death of 0.25 (95% CI, 0.09–0.66; P=0.005) in Period 2000 to 2005, 0.16 (0.05–0.53; P=0.003) in Period 2006 to 2010, and 0.24 (0.08–0.66; P=0.007) in Period 2011 to 2016.
Compared with the usual coronary artery pattern, the only univariable independent risk factor for death was double coronary origin from sinus 2 (2LCx,R) (HR, 4.6; 95% CI, 1.3 to 15.7, P=0.016) (Figure 3A). Coronary artery pattern had no effect on overall survival when cases with IMCA were excluded from the analysis to eliminate association between the 2LCx,R pattern and IMCA (P=0.550).
Figure 3. Overall survival after arterial switch operation was lower in cases with double coronary origin from sinus 2 (2LCx,R) compared with usual coronary artery pattern (P=0.016) (A) and in patients with IMCA (P=0.004) (B).
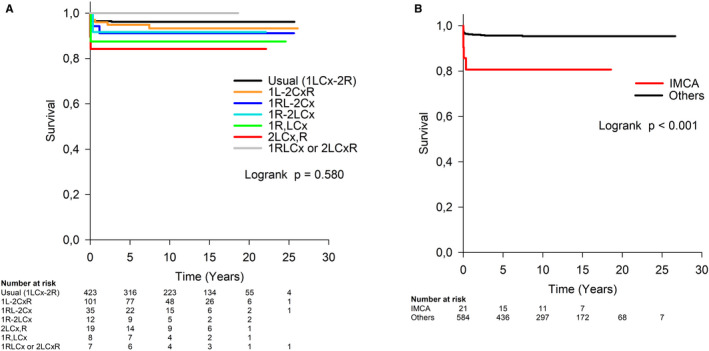
Coding according to the Leiden convention: 1LCx‐2R, usual pattern; 1L‐2CxR, circumflex artery from the right coronary artery; 1RL‐2Cx, partially inverted origin; 1R‐2LCx, inverted origin; 2LCx,R, double origin from sinus 2; 1R,LCx, double origin from sinus 1; 1RLCx or 2LCxR, single origin from sinus 1 or sinus 2. P value refers to Cox regression analysis. IMCA indicates intramural coronary artery.
The presence of IMCA was the only coronary artery anomaly that presented a univariable independent risk factor for death (HR, 4.7; 95% CI, 1.6–13.4, P=0.004) (Figure 3B). Neither abnormal coronary ostial origin nor accessory coronary artery orifice affected long‐term survival.
The only independent multivariable predictors of worse overall survival were IMCA (HR, 5.2; 95% CI, 1.8–15.2, P=0.002) and period of ASO 1990 to 1999 (HR, 4.6; 95% CI, 1.5–13.6, P<0.001). There was no interaction between period of ASO and IMCA (P=0.385); in other words, the HR of survival in the presence of IMCA was not related to periods of ASO.
Coronary Artery Reinterventions
Fourteen patients (2.3%) required 16 coronary artery reoperations. The diagnosis of severe coronary artery stenosis or occlusion was confirmed in all cases by selective coronary angiography. Coronary artery reimplantation was performed in 6 cases, coronary artery ostial reconstruction in 2 cases, and coronary artery bypass graft in 8 cases. There were no catheter‐based coronary artery reinterventions. Seven patients underwent early coronary artery reimplantation or reconstruction for an acute ischemic left ventricular failure between 1 and 19 days after ASO. One patient died early and one 4 months after reoperation. Two patients from this subgroup required subsequent coronary artery bypass graft for residual coronary artery stenosis at 4 months and 15 years after ASO. Late surgical reinterventions were performed in 7 patients at a median interval from ASO of 11 years (range, 2–17 years): 6 patients underwent coronary artery bypass graft using internal mammary artery graft and 1 patient using coronary artery reconstruction. All of these patients survived and had normal left ventricular function at the end of the study period.
Freedom from coronary artery reintervention at 10, 15, 20, and 25 years after ASO was 98%, 97%, 96%, and 96%, respectively (Figure 4). Neither the type of ASO nor the period of ASO had an influence on freedom from coronary artery reintervention. In a univariable analysis, the hazard for coronary artery reintervention was higher in cases with double coronary origin from sinus 2 compared with usual pattern (HR, 18.9; 95% CI, 5.3–67.6, P<0.001), in cases with IMCA (HR, 33.9; 95% CI, 11.8–97.5, P<0.001), and in those with an abnormal coronary artery ostial origin (HR, 9.9; 95% CI, 3.4–28.5, P<0.001) (Figure 5A through 5C).
Figure 4. Freedom from coronary artery reintervention after arterial switch operation.
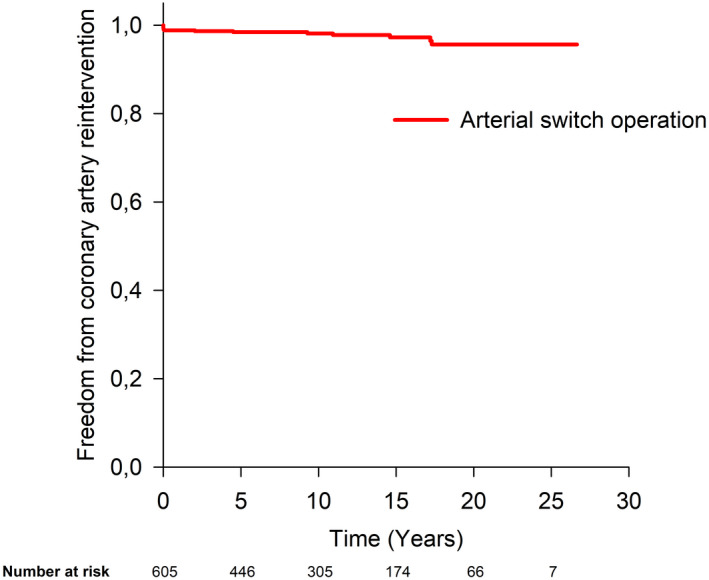
Figure 5. Probability of coronary artery reintervention after arterial switch operation was significantly higher in patients with double coronary origin from sinus 2 (2LCx,R) compared with usual pattern (P<0.001) (A), in patients with IMCA (P<0.001) (B), and in patients with an abnormal coronary artery ostial origin (P<0.001) (C).
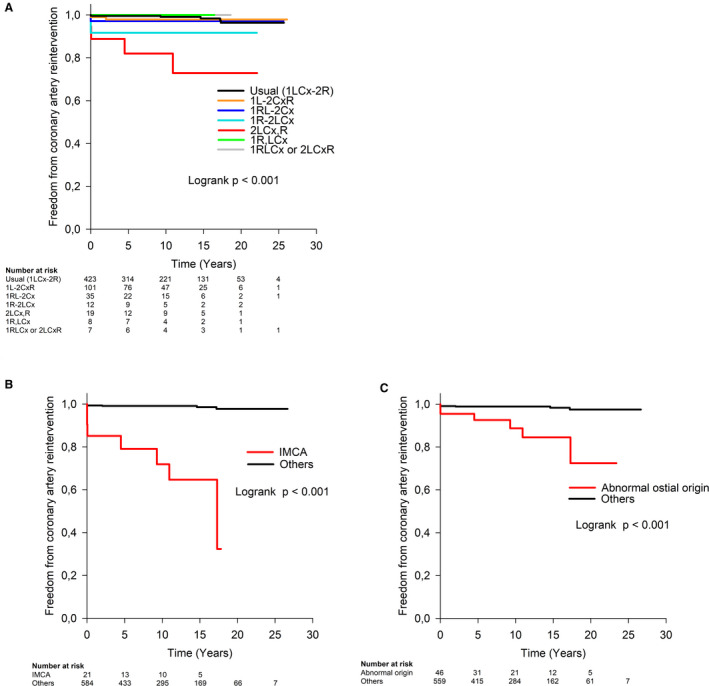
Coding according to the Leiden convention: 1LCx‐2R, usual pattern; 1L‐2CxR, circumflex artery from the right coronary artery; 1RL‐2Cx, partially inverted origin; 1R‐2LCx, inverted origin; 2LCx,R, double origin from sinus 2; 1R,LCx, double origin from sinus 1; 1RLCx or 2LCxR, single origin from sinus 1 or sinus 2. P value refers to Cox regression analysis. IMCA indicates intramural coronary artery.
In a multivariable analysis, the only independent predictor associated with a higher hazard for coronary artery reintervention was IMCA (HR, 33.9; 95% CI, 11.8–97.5, P<0.001).
Discussion
An overall survival rate of 94.5% at 25 years after ASO in our study was excellent, and it was not influenced by the type of ASO. Published data from other high‐volume centers are in agreement with our results.2, 3, 4, 6, 7 Survival after ASO compared with the background population was mentioned only in a few large population‐based studies.17, 18, 19 Compared with the background population of 6 050 children resident in the Czech Republic matched for sex and age, the HR for death in patients after ASO was 15.6‐fold higher. Long‐term survival was mainly determined by early mortality as in other high‐volume cardiac centers. In our study, early mortality decreased from 8.8% in Period 1990 to 1999 to <2% in recent decades. Patients with concomitant aortic arch repair at ASO did not have worse early mortality or long‐term survival, contrary to poorer outcomes in this subgroup reported in the literature.20
We have decided to analyze the influence of coronary artery pattern on the long‐term outcome after ASO separately from associated coronary artery anomalies. The Leiden coronary artery classification was applied, because it is used in our institutional database and recommended by The International Society for Nomenclature of Paediatric and Congenital Heart Disease.21 We realized that it might be difficult to compare our results with other high‐volume centers' studies because of different nomenclature, such as Yacoub22 or Marie‐Lannelongue.23
The occurrence of coronary artery patterns in our patients with TGA‐IVS, TGA‐VSD, and DORV‐TGA was similar to previously published data.3, 4, 6 Cross‐mapping of coronary artery patterns with other coronary anomalies revealed that 78.9% of cases with the double origin from sinus 2 (2LCx,R) were associated with IMCA and 31.6% with an abnormal ostial orifice. A similar association was described in other studies11, 24 and mentioned in the Gittenberger‐de Groot editorial.15 The reason for this phenomenon is not known, but it was confirmed that both coronary arteries have separate developmental processes and their ability to reach and penetrate the programmed sites on the aorta depends on the transcription of several genes.25, 26, 27
Published data regarding the influence of coronary artery patterns on outcomes after ASO are not consistent. The described independent risk factors for mortality were, for instance: single ostium patterns,8 two ostia arising from one sinus4 and a single right coronary artery.3 In our study as in others, coronary artery pattern per se was not associated with overall survival.2, 5, 13, 28
The presence of IMCA was the most significant predictor of worse survival in this study. Increased HR for death in cases with double coronary origin from sinus 2 (2LCx,R) was attributed to the strong association of this pattern with IMCA. A higher mortality in patients with IMCA was reported in previous studies.8, 10, 14, 29 Several reports did not identify IMCA as a risk factor for mortality, explaining this disparity by improving surgical technique over time.6, 13, 30 However, in this study, it was a risk factor that was independent from the surgical period.
Only a small proportion of our patients (2.3%) required a surgical reintervention for residual coronary artery stenoses similar to reported rates of 0.5% to 2.8% in other studies.3, 4, 6, 7, 9, 14 Seven patients with an acute ischemic left ventricular dysfunction underwent early coronary artery reintervention within days after ASO with 2 subsequent early deaths, and 7 patients underwent late coronary artery revascularization for chronic ischemic changes and decreased myocardial reserve with good results.
Freedom from coronary artery reintervention was 96% at 25 years after ASO and it was independent from the type of ASO and period of surgery. In a multivariable logistic regression analysis, the presence of IMCA was the only independent risk factor for coronary artery reintervention.
Although coronary reinterventions late after ASO were rare in our study, the real prevalence of acquired coronary artery abnormalities remains uncertain. Most studies report 3% to 8% incidence of coronary stenoses in patients who undergo coronary angiography,5, 9, 28 but it has been proposed that as many as 25% to 50% of patients may have coronary abnormalities despite being asymptomatic.31
Regular imaging of coronary arteries after ASO is not performed during follow‐up in our center. Detailed investigation in cases with suspected myocardial ischemia includes imaging (coronary angiography or computed tomography angiography), cardiopulmonary functional tests, 24‐hour ECG monitoring, and myocardial perfusion scintigraphy. Routine coronary artery imaging in patients after ASO was discussed in a meta‐analysis and was not considered justified.32
Limitations
Small samples of rare coronary artery patterns as well as small number of coronary artery reinterventions might influence the statistical significance.
Conclusions
Long‐term survival at 25 years after ASO is excellent. Independent predictors associated with worse survival were an early surgical period and the presence of IMCA. Coronary artery reinterventions are rare at 25 years after ASO. IMCA was the only independent predictor associated with a higher risk of coronary artery reintervention regardless of the surgical period.
Sources of Funding
This work was supported by the Ministry of Health, Czech Republic—conceptual development of research organization, Motol University Hospital, Prague, Czech Republic 00064203.
Disclosures
None.
Acknowledgments
We would like to thank Marek Mojzisek for invaluable help with reviewing surgical reports.
(J Am Heart Assoc. 2021;10:e020479. DOI: 10.1161/JAHA.120.020479.)
For Sources of Funding and Disclosures, see page 10.
References
- 1.Jatene AD, Fontes VF, Paulista PP, de Souza LC, Neger F, Galantier M, Souza JE. Successful anatomic correction of transposition of the great vessels. A preliminary report. Arq Bras Cardiol. 1975;28:461–464. [PubMed] [Google Scholar]
- 2.Fricke TA, D’Udekem Y, Richardson M, Thuys C, Dronavalli M, Ramsay JM, Wheaton G, Grigg LE, Brizard CP, Konstantinov IE. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg. 2012;94:139–145. DOI: 10.1016/j.athoracsur.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW, Landzberg MJ, Mayer JE. Cardiovascular outcomes after the arterial switch operation for D‐transposition of the great arteries. Circulation. 2013;127:331–339. DOI: 10.1161/CIRCULATIONAHA.112.135046. [DOI] [PubMed] [Google Scholar]
- 4.Moll M, Michalak KW, Sobczak‐Budlewska K, Moll JA, Kopala M, Szymczyk K, Dryżek P, Moll JJ. Coronary artery anomalies in patients with transposition of the great arteries and their impact on postoperative outcomes. Ann Thorac Surg. 2017;104:1620–1628. DOI: 10.1016/j.athoracsur.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 5.Vida VL, Zanotto L, Zanotto L, Triglia LT, Bellanti E, Castaldi B, Padalino MA, Gasperetti A, Battista F, Varnier M, et al. Arterial switch operation for transposition of the great arteries: a single‐centre 32‐year experience. J Card Surg. 2019;34:1154–1161. DOI: 10.1111/jocs.14045. [DOI] [PubMed] [Google Scholar]
- 6.Fricke TA, Bell D, Daley M, D’Udekem Y, Brizard CP, Alphonso N, Konstantinov IE, D’Udekem Y, Brizard CP, Alphonso N, et al. The influence of coronary artery anatomy on mortality after the arterial switch operation. J Thorac Cardiovasc Surg. 2020;160:191–199.e1. DOI: 10.1016/j.jtcvs.2019.11.146. [DOI] [PubMed] [Google Scholar]
- 7.Fraser CD, Chacon‐Portillo MA, Well A, Zea‐Vera R, Binsalamah Z, Adachi I, Mery CM, Heinle JS. Twenty‐three‐year experience with the arterial switch operation: expectations and long‐term outcomes. Semin Thorac Cardiovasc Surg. 2020;32:292–299. DOI: 10.1053/j.semtcvs.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Pasquali SK, Hasselblad V, Li JS, Kong DF, Sanders SP. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries. Circulation. 2002;106:2575–2580. DOI: 10.1161/01.CIR.0000036745.19310.BB. [DOI] [PubMed] [Google Scholar]
- 9.Losay J, Touchot A, Serraf A, Litvinova A, Lambert V, Piot JD, Lacour‐Gayet F, Capderou A, Planche C. Late outcome after arterial switch operation for transposition of the great arteries. Circulation. 2001;104:I121–I126. DOI: 10.1161/hc37t1.094716. [DOI] [PubMed] [Google Scholar]
- 10.Metton O, Calvaruso D, Gaudin R, Mussa S, Raisky O, Bonnet D, Sidi D, Vouhé PR. Intramural coronary arteries and outcome of neonatal arterial switch operation. Eur J Cardiothorac Surg. 2010;37:1246–1253. DOI: 10.1016/j.ejcts.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Moll M, Moll JA, Moll JJ, Łubisz M, Michalak KW. Intramural coronary pattern in patients with transposition: incidence and impact on follow‐up. Eur J Cardiothorac Surg. 2020;58:145–152. DOI: 10.1093/ejcts/ezaa021. [DOI] [PubMed] [Google Scholar]
- 12.Vida VL, Zanotto L, Zanotto L, Stellin G, Padalino M, Sarris G, Protopapas E, Prospero C, Pizarro C, Woodford E, et al. Left‐sided reoperations after arterial switch operation: a European multicenter study. Ann Thorac Surg. 2017;104:899–906. DOI: 10.1016/j.athoracsur.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Lalezari S, Bruggemans EF, Blom NA, Hazekamp MG. Thirty‐year experience with the arterial switch operation. Ann Thorac Surg. 2011;92:973–979. DOI: 10.1016/j.athoracsur.2011.04.086. [DOI] [PubMed] [Google Scholar]
- 14.Angeli E, Raisky O, Bonnet D, Sidi D, Vouhé PR. Late reoperations after neonatal arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg. 2008;34:32–36. DOI: 10.1016/j.ejcts.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Gittenberger‐de Groot AC, Koenraadt WMC, Bartelings MM, Bökenkamp R, DeRuiter MC, Hazekamp MG, Bogers AJJC, Quaegebeur JM, Schalij MJ, Vliegen HW, et al. Coding of coronary arterial origin and branching in congenital heart disease: the modified Leiden convention. J Thorac Cardiovasc Surg. 2018;156:2260–2269. DOI: 10.1016/j.jtcvs.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Fricke TA, Konstantinov IE. Translocation of intramural coronary artery in the arterial switch operation: divide and conquer? J Thorac Cardiovasc Surg. 2018;155:e131–e132. DOI: 10.1016/j.jtcvs.2017.12.108. [DOI] [PubMed] [Google Scholar]
- 17.Larsen SH, Olsen M, Emmertsen K, Hjortdal VE. Interventional treatment of patients with congenital heart disease: nationwide Danish experience over 39 years. J Am Coll Cardiol. 2017;69:2725–2732. DOI: 10.1016/j.jacc.2017.03.587. [DOI] [PubMed] [Google Scholar]
- 18.Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas L. Trends in long‐term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71:2434–2446. DOI: 10.1016/j.jacc.2018.03.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raissadati A, Nieminen H, Sairanen H, Jokinen E. Outcomes after the Mustard, Senning and arterial switch operation for treatment of transposition of the great arteries in Finland: a nationwide 4‐decade perspective. Eur J Cardiothorac Surg. 2017;52:573–580. DOI: 10.1093/ejcts/ezx107. [DOI] [PubMed] [Google Scholar]
- 20.Fricke TA, Donaldson S, Schneider JR, Menahem S, d’Udekem Y, Brizard CP, Konstantinov IE. Outcomes of the arterial switch operation in patients with aortic arch obstruction. J Thorac Cardiovasc Surg. 2020;159:592–599. DOI: 10.1016/j.jtcvs.2019.07.103. [DOI] [PubMed] [Google Scholar]
- 21.Franklin RCG, Béland MJ, Colan SD, Walters HL, Aiello VD, Anderson RH, Bailliard F, Boris JR, Cohen MS, Gaynor JW, et al. Nomenclature for congenital and paediatric cardiac disease: the International Paediatric and Congenital Cardiac Code (IPCCC) and the Eleventh Iteration of the International Classification of Diseases (ICD‐11). Cardiol Young. 2017;27:1872–1938. DOI: 10.1017/S1047951117002244. [DOI] [PubMed] [Google Scholar]
- 22.Yacoub MH, Radley‐Smith R. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax. 1978;33:418–424. DOI: 10.1136/thx.33.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planche C, Lacour‐Gayet F, Serraf A. Arterial switch. Pediatr Cardiol. 1998;19:297–307. DOI: 10.1007/s002469900313. [DOI] [PubMed] [Google Scholar]
- 24.Mishra A, Jain A, Hinduja M, Wadhawa V, Patel R, Vaidhya N, Rodricks D, Patel H. Transposition of great arteries with intramural coronary artery: experience with a modified surgical technique. Braz J Cardiovasc Surg. 2016;31:15–21. DOI: 10.5935/1678-9741.20160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomanek R, Angelini P. Embryology of coronary arteries and anatomy/pathophysiology of coronary anomalies. A comprehensive update. Int J Cardiol. 2019;281:28–34. DOI: 10.1016/j.ijcard.2018.11.135. [DOI] [PubMed] [Google Scholar]
- 26.Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z, et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013;23:1075–1090. DOI: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Théveniau‐Ruissy M, Pérez‐Pomares JM, Parisot P, Baldini A, Miquerol L, Kelly RG. Coronary stem development in wild‐type and Tbx1 null mouse hearts. Dev Dyn. 2016;245:445–459. DOI: 10.1002/dvdy.24380. [DOI] [PubMed] [Google Scholar]
- 28.Oda S, Nakano T, Sugiura J, Fusazaki N, Ishikawa S, Kado H. Twenty‐eight years’ experience of arterial switch operation for transposition of the great arteries in a single institution. Eur J Cardiothorac Surg. 2012;42:674–679. DOI: 10.1093/ejcts/ezs033. [DOI] [PubMed] [Google Scholar]
- 29.Legendre A, Losay J, Touchot‐Koné A, Serraf A, Belli E, Piot JD, Lambert V, Capderou A, Planche C. Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003;108:186–190. DOI: 10.1161/01.cir.0000087902.67220.2b. [DOI] [PubMed] [Google Scholar]
- 30.Fricke TA, Bulstra AE, Naimo PS, Bullock A, Robertson T, D’Udekem Y, Brizard CP, Konstantinov IE. Excellent long‐term outcomes of the arterial switch operation in patients with intramural coronary arteries. Ann Thorac Surg. 2016;101:725–729. DOI: 10.1016/j.athoracsur.2015.08.090. [DOI] [PubMed] [Google Scholar]
- 31.Szymczyk K, Moll M, Sobczak‐Budlewska K, Moll JA, Stefańczyk L, Grzelak P, Moll JJ, Michalak KW. Usefulness of routine coronary CT angiography in patients with transposition of the great arteries after an arterial switch operation. Pediatr Cardiol. 2018;39:335–346. DOI: 10.1007/s00246-017-1761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Wijk SWH, van der Stelt F, ter Heide H, Schoof PH, Doevendans PAFM, Meijboom FJ, Breur JMPJ. Sudden death due to coronary artery lesions long‐term after the arterial switch operation: a systematic review. Can J Cardiol. 2017;33:1180–1187. DOI: 10.1016/j.cjca.2017.02.017. [DOI] [PubMed] [Google Scholar]


