Abstract
The PROfound trial evaluated the PARP inhibitor olaparib in metastatic castration-resistant prostate cancers harboring alterations in BRCA1/2 and ATM (cohort A) and in 12 other homologous recombination repair genes (cohort B). Olaparib led to more objective responses and longer radiographic progression-free survival (rPFS) than the control in cohort A and when cohorts A and B were combined. The efficacy of olaparib in cohort B was a secondary objective prespecified in the trial protocol but was not reported. Reconstructing patient-level data for cohort B, two of 54 patients (4%) in the olaparib arm and two of 24 patients (8%) in the control arm had a radiographic response, and there was no evidence that olaparib prolonged rPFS in cohort B (hazard ratio 0.88, 95% confidence interval 0.58–1.34). These results are in strong contrast to cohort A.
Patient summary:
A large clinical study concluded that treatment with the PARP inhibitor olaparib benefits men with metastatic castration-resistant prostate cancer whose tumors harbor alterations in 15 different DNA repair genes. In contrast to the group dominated by BRCA alterations, any potential benefit from olaparib was considerably less, if present at all, for men with prostate cancers harboring one of the 12 other, non-BRCA DNA repair alterations.
Keywords: PARP inhibitor, Olaparib, Tumor sequencing, Homologous recombination repair, Phase 3 trial
A subset of advanced prostate cancers harbor genomic alterations in DNA repair genes. Synthetic lethality exploits the resulting functional deficiency in repairing DNA by further inhibiting homologous recombination repair (HRR) with PARP inhibitors, which induce tumor cell death. In 2015, the phase 2 TOPARP-A trial suggested that the PARP inhibitor olaparib has clinical antitumor activity in pretreated metastatic castration-resistant prostate cancer (mCRPC) [1]. On the basis of these encouraging results, the phase 3 PROfound trial of olaparib in mCRPC was conceived [2].
When PROfound was designed it was not completely understood which HRR alterations are useful predictive biomarkers for sensitivity to PARP inhibition. Prior studies had suggested that alterations in three genes, the HRR genes BRCA2 and BRCA1 and the DNA damage sensor ATM, were most common and potentially were the strongest predictors of sensitivity to PARP inhibition [1,3]. Consequently, PROfound enrolled patients into two separate cohorts based on tumor genomics. Those with alterations in the genes BRCA2, BRCA1, or ATM, were eligible for cohort A. Those with alterations in 12 other HRR-related genes were enrolled in cohort B.
PROfound was a landmark biomarker-driven trial in advanced prostate cancer. In a herculean undertaking, the investigators attempted a clinical-grade tumor DNA sequencing test for HRR alterations in as many as 4425 men in 20 countries. Of the 2792 men for whom sequencing was successful, 778 had tumor HRR defects (28% of those with sequencing results; 18% of the initial population), which made them eligible to receive olaparib. Thus, results from PROfound are relevant for many patients early in their disease course, because eligibility criteria required only one line of prior therapy with abiraterone or enzalutamide. Because PROfound was the main basis for US Food and Drug Administration approval of olaparib, largely following the PROfound eligibility criteria, its results are critical for guiding therapy for thousands of men with prostate cancer.
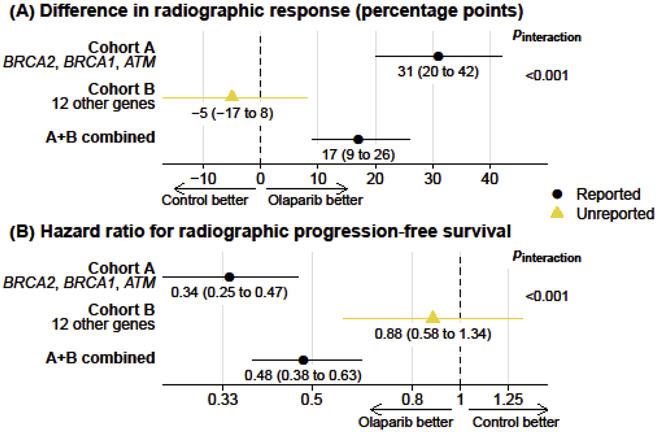
The main publication for PROfound [2] reported efficacy results for olaparib in cohort A and in cohorts A and B combined, following the statistical analysis plan. In cohort A, olaparib led to more confirmed radiographic responses than control treatment (odds ratio for response, 21.0, 95% confidence interval [CI] 4.18–379; Table 1) and prolonged imaging progression-free survival (PFS; hazard ratio 0.34, 95% CI 0.25–0.47; Fig. 1). When cohorts A and B were combined for analyses, treatment effects were considerably attenuated, with a reported odds ratio for radiographic response of 5.9 (95% CI 2.0–25.4; Table 1) and a hazard ratio for radiographic PFS of 0.49 (95% CI 0.38–0.63; Fig. 1).
Table 1 –
Confirmed radiographic responses in the phase 3 PROfound trial
| Cohorts A + B | Cohort A a | Cohort B b | |
|---|---|---|---|
| Olaparib arm (n) | 138 | 84 | 54 * |
| With radiographic response, n (%) | 30 (22) | 28 (33) | 2 (4) * |
| Control arm (n) | 67 | 43 | 24 * |
| With radiographic response, n (%) | 3 (4) | 1 (2) | 2 (8) * |
| Response to olaparib vs control | |||
| Adjusted OR (95% CI)c | 5.93 (2.01–25.4) | 20.9 (4.18–379) | |
| Unadjusted OR (95% CI)c | 5.93 (1.72–31.3) * | 21.0 (3.15–879)* | 0.42 (0.03–6.25) * |
| RR (95% CI)c | 4.86 (1.54–15.3)* | 14.3 (2.02–101) * | 0.44 (0.07–2.97) * |
| RD in nercentage noints (95% CI) d | 17 (9–26) * | 31 (20–42) * | −5 (−17 to 8) * |
CI = confidence interval; OR = odds ratio; RD = risk difference; RR = risk ratio.
Not reported by de Bono et al. (2020) [2] but calculable from the data provided.
Patients with tumor DNA alterations in the three main homologous recombination repair genes (BRCA2, BRCA1, or ATM).
Patients with tumor DNA alterations in 12 additional genes (BRIP1, BARD1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, or RAD54L).
OR and RR values >1 favor olaparib. ORs in the main article were adjusted for stratification factors; however, differences between adjusted and unadjusted point estimates are negligible. RRs are also presented here because ORs always exaggerate results when an outcome is common [8]: compare the OR of 21.0 to the RR of 14.3 for cohort A.
RD values >0 percentage points favor olaparib.
Fig. 1 –
(A) Confirmed radiographic responses and (B) radiographic progression-free survival with olaparib versus control treatment in the phase 3 PROfound trial. Bars denote 95% confidence intervals. Wald tests for effect measure modification (Pinteraction) indicate whether treatment effects differ between cohort A and cohort B.
The observation that olaparib appears less efficacious in cohorts A and B combined than in cohort A begs a question about the efficacy of olaparib in cohort B by itself. PROfound investigators anticipated this question given the different weight of prior evidence for genes defining cohort B. Not only did they conduct randomization to olaparib or control treatment separately in cohorts A and B but they also prespecified separate efficacy analyses for cohort B as secondary objectives of the trial. Surprisingly, separate results for cohort B were not mentioned in the main publication [2].
The unreported results for radiographic response in cohort B can be reconstructed from count data provided in the article (Table 1). Only two of 54 evaluable patients (4%) had a confirmed radiographic response to olaparib, numerically fewer than the two of 24 patients (8%) who had a response in the control arm. These data are in strong contrast to cohort A, as underscored by a comparison of absolute treatment effects: In cohort A, a radiographic response was 31 percentage points more common among patients treated with olaparib than with control (95% CI 20–42 percentage points), corresponding to a number needed to treat for one radiographic response of approximately three patients. By contrast, in cohort B, the difference in absolute risks was −5 percentage points, with a 95% CI of −17 to 8, incompatible with a clinically meaningful treatment effect size.
What about the primary trial endpoint of radiographic PFS for cohort B? The publication [2] included a Kaplan-Meier plot for cohort B as Supplementary Figure 4A that was not referenced in the article. This figure reported an adjusted hazard ratio of 0.88 but without quantification of statistical uncertainty. After reconstructing patient-level data from the Kaplan-Meier curve (Supplementary Fig. 1) [4], an unadjusted hazard ratio could be calculated as 0.88 (95% CI 0.58–1.34), which is a null association but is not incompatible with a clinically meaningful effect. However, for both radiographic responses and radiographic PFS the treatment effects differed strongly and statistically significantly between cohort A and cohort B (Fig. 1).
Taken together, separate analyses of cohorts A and B from PROfound suggest that olaparib improves clinical outcomes among men eligible for cohort A. However, PROfound provides phase 3 trial evidence that in tumors with alterations in one of the 12 other HRR genes (cohort B), olaparib is much less effective, if at all. Importantly, the data retrieved here were generated for predefined endpoints with full benefit of randomization; they are not exploratory, post hoc subgroup analyses. Nevertheless, exploratory analyses, as presented in Figure 2B of the main PROfound publication [2] and elsewhere [5-7], are informative. They suggest that even for tumors with ATM alterations, olaparib may not be effective (hazard ratio for radiographic PFS 1.04, 95% CI 0.64–1.87) [2]. These disappointing results for tumors fitting the criteria for cohort B, as well as ATM, are consistent with phase 2 trials showing a general lack of response to olaparib and rucaparib, another PARP inhibitor under development, in tumors with non-BRCA DNA repair alterations including ATM [6,7]. Collectively, these data do not preclude the possibility that additional biomarkers beyond BRCA alterations may predict benefit from PARP inhibition, including some of the genes that led to eligibility for cohort B [5-7]. Such additional biomarkers will require validation in randomized controlled trials. For the treatment of men with prostate cancer today, PROfound provides strong evidence that BRCA alterations, but not non-BRCA DNA repair alterations, are predictive of a potential benefit from PARP inhibition.
Supplementary Material
Funding/Support and role of the sponsor:
This research was supported by the Department of Defense (Early-Investigator Research Award W81XWH-18-1-0330), the Prostate Cancer Foundation (Young Investigator Award), and the National Institutes of Health (Cancer Center Support Grant P30CA008748). The sponsors had no influence on the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: Konrad H. Stopsack certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;382:2091–102. [DOI] [PubMed] [Google Scholar]
- 3.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J 2017;17:786–802. [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce AH, Sartor O, de Bono J. DNA repair and prostate cancer: a field ripe for harvest. Eur Urol. In press. 10.1016/j.eururo.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 6.Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2020;21:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin Cancer Res 2020;26:2487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. Can Med Assoc J 2012;184:895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.