Abstract
Introduction
The COVID-19 pandemic reached New York City in early March 2020 resulting in an 11-week lockdown period to mitigate further spread. It has been well documented that cancer care was drastically affected as a result. Given New York City's early involvement, we attempted to identify any stage shift that may have occurred in the diagnoses of non-small cell lung cancer (NSCLC) at our institution as a result of these lockdowns.
Patients and Methods
We conducted a retrospective review of a prospective database of lung cancer patients at our institution from July 1, 2019 until March 31, 2021. Patients were grouped by calendar year quarter in which they received care. Basic demographics and clinical staging were compared across quarters.
Results
Five hundred and fifty four patients were identified that underwent treatment during the time period of interest. During the lockdown period, there was a 50% reduction in the mean number of patients seen (15 ± 3 vs. 28 ± 7, P = .004). In the quarter following easing of restrictions, there was a significant trend towards earlier stage (cStage I/II) disease. In comparison to quarters preceding the pandemic lockdown, there was a significant increase in the proportion of patients with Stage IV disease in the quarters following phased reopening (P = .026).
Conclusion
After a transient but significant increase in Stage I/II disease with easing of restrictions there was a significant increase in patients with Stage IV disease. Extended longitudinal studies must be conducted to determine whether COVID-19 lockdowns will lead to further increases in the proportion of patients with advanced NSCLC.
Keywords: Cancer care delays, Pandemic, SARS-CoV-2, Thoracic oncology, NSCLC
Abbreviations: COVID-19, Coronavirus Disease 2019; NSCLC, non-small cell lung cancer; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization; ANOVA, analysis of variance; CCI, Charlson Comorbidity Index; Q, Quarter; CMS, Centers for Medicare and Medicaid Services; ECOG, Eastern Cooperative Oncology Group Performance Status; CT, computed tomography; VDT, Volume-Doubling Time; SEER, Surveillance, Epidemiology and End Results Database
Introduction
The novel coronavirus responsible for COVID-19, SARS-CoV-2 was first identified in Wuhan, the capital city of China's Hubei province in December 2019.1 It rapidly spread around the globe and by March 11, 2020, the World Health Organization (WHO) declared it a global pandemic.2 New York City recorded its first known case of COVID-19 on March 1 and quickly became the global epicenter of the pandemic.3 In an attempt to divert resources and minimize local transmission, New York State underwent an 11-week “Pause” order starting March 22 lasting until June 8 within New York City, effectively halting all elective care.4 , 5 Many other states followed suit and, as a result, a drastic reduction in surgical volume has been well documented nationwide.6 , 7 More worrisome is literature that has accrued revealing significant decreases in new cancer diagnoses and delays in definitive care across all major cancer types.8, 9, 10 Growing concern exists that these delays in care will result in patients presenting with more advanced malignancies and subsequently worse survival.11 Given that New York City was one of the locations affected earliest by cessation of elective care, we sought to evaluate trends in clinical stage distribution in patients with non-small cell lung cancer (NSCLC) seen at our institution prior to, during and immediately following the COVID-19 lockdown period.
Materials and Methods
We conducted a retrospective review of 2 prospectively assembled databases (medical oncology and thoracic surgery) at a large, academic hospital. Patients were identified for inclusion if they presented for surgical or non-surgical treatment of NSCLC between July 1, 2019 and March 31, 2021. Patient treatment dates were grouped by calendar year quarter. Those who received treatment from both medical oncology and thoracic surgery were assigned their treatment quarter by day of surgery since greater limitations were placed on surgical encounters than clinic visits.
Basic demographic and clinical information were collected which included clinical stage (TNM 8th Edition). Categorical variables were compared across calendar year quarters using Chi-Squared testing. Continuous variables were compared between binary groups using independent Student t test and across multiple groups through 1-way analysis of variance (ANOVA) and are presented as a mean and standard deviation. All statistical analysis was performed using IBM SPSS Statistics version 25.
Results
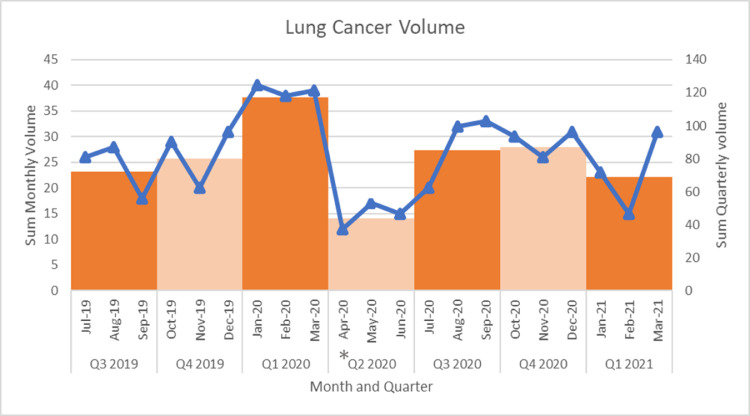
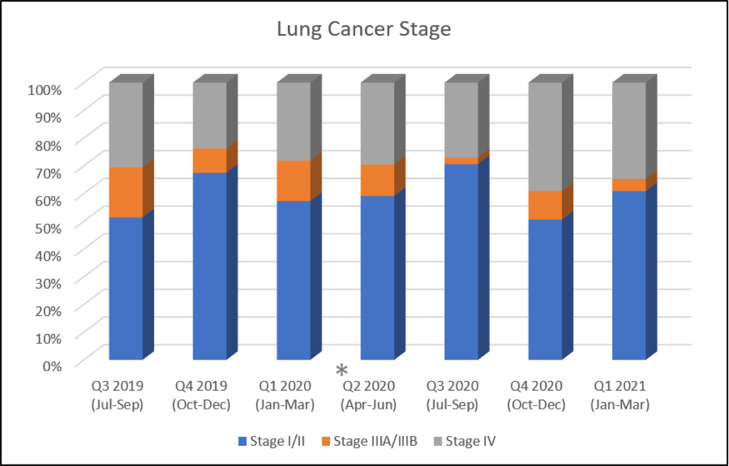
The total number of patients undergoing evaluation and/or treatment for NSCLC during the time period of interest was 554 patients. Baseline demographics are presented by calendar year quarter in Table 1 . Age, sex, race and Charlson Comorbidity (CCI) Index were relatively consistent across the time period. During the second quarter of 2020 which included the lockdown period, patients had more advanced ECOG status than those in other quarters likely reflecting prioritization of care for patients in whom delayed treatment was deemed inappropriate. The monthly and quarterly number of patients is presented in Figure 1 . The mean number of patients during Q2 of 2020 was 15 ± 3 which represents a nearly 50% reduction in the overall mean number of patients (excluding Q2 2020) of 28 ± 7, P = .004 (Supplementary appendix file). After initiation of Phase 1 of reopening on June 8, 2020, it took approximately 2 months to restore baseline monthly volume. Clinical staging during Q2 2020 was similar to prior quarters (Table 1 and Figure 2 ). However, the first quarter following phased reopening (Q3 2020) showed a higher proportion of clinical Stage I/II disease, likely reflecting a back log of surgical cases from the prior quarter. However, in the following quarters there was an increase in the proportion of patients with Stage IV disease, 39.1% and 34.8% respectively. When the stage distribution 9 months prior to the lockdown quarter of Q2 2020 were compared to the 9 months following lockdown, again we see higher incidence of Stage IV disease, (74/269, 27.5% vs. 81/241, 33.6%), with lower incidence of Stage III (37/269, 13.8% vs. 14/241, 5.8%), P = .008 (Table 1.)
Table 1.
General Demographics and Clinical Stage (TNM 8th Ed) Grouped by Yearly Quarter
| General Demographics n = 554 | Q 3 2019 (Jul to Sep) n = 72 | Q 4 2019 (Oct to Dec) n = 80 | Q 1 2020 (Jan to Mar) n = 117 | Q 2 2020 (Apr to Jun) n = 44 | Q 3 2020 (Jul to Sep) n = 85 | Q 4 2020 (Oct to Dec) n = 87 | Q 1 2021 (Jan to Mar) n = 69 | P Value |
|---|---|---|---|---|---|---|---|---|
| Mean age, SD | 68.6 ± 11.5 | 71.7 ± 10.5 | 68.9 ± 10.9 | 71.4 ± 9.9 | 71.0 ± 9.6 | 70.8 ± 9.9 | 69.4 ± 10.7 | .347 |
| Sex | .256 | |||||||
| Female | 37 (51.4%) | 43 (53.8%) | 64 (54.7%) | 22 (50%) | 54 (63.5%) | 38 (43.7%) | 40 (58.0%) | |
| Male | 35 (48.6%) | 37 (46.3%) | 53 (45.3%) | 22 (50%) | 31 (36.5%) | 49 (56.3%) | 29 (42.0%) | |
| Race | .743 | |||||||
| White | 45 (62.5%) | 45 (56.3%) | 80 (68.4%) | 30 (68.2%) | 49 (57.6%) | 56 (64.4%) | 40 (58.0%) | |
| Black | 7 (9.7%) | 7 (8.8%) | 8 (6.8%) | 4 (9.1%) | 10 (11.8%) | 8 (9.2%) | 5 (7.2%) | |
| Asian | 8 (11.1%) | 10 (12.5%) | 14 (12.0%) | 7 (15.9%) | 16 (18.8%) | 13 (14.9%) | 11 (15.9%) | |
| Other/Unkn. | 12 (16.7%) | 18 (22.5%) | 15 (12.8%) | 3 (6.8%) | 10 (11.8%) | 0 | 13 (18.8%) | |
| CCI | .174 | |||||||
| 0 | 4 (5.6%) | 2 (2.5%) | 5 (4.3%) | 1 (2.3%) | 0 | 9 (10.3%) | 3 (4.3%) | |
| 1 | 4 (5.6%) | 4 (5.0%) | 2 (1.7%) | 2 (4.5%) | 7 (8.2%) | 78 (89.7%) | 4 (5.8%) | |
| ≥2 | 64 (88.9%) | 74 (92.5%) | 110 (94.0%) | 41 (93.2%) | 78 (91.8%) | 10 (11.5%) | 62 (89.9%) | |
| ECOG | .041 | |||||||
| 0 | 42 (59.2%) | 57 (71.3%) | 84 (71.8%) | 25 (56.8%) | 61 (72.6%) | 53 (60.9%) | 39 (57.4%) | |
| 1 | 18 (25.4%) | 14 (17.5%) | 10 (8.5%) | 8 (18.2%) | 13 (15.5%) | 20 (23.0%) | 19 (27.9%) | |
| ≥2 | 11 (15.5%) | 9 (11.3%) | 23 (19.7%) | 11 (25.0%) | 10 (11.9%) | 14 (16.1%) | 10 (14.7%) | |
| Primary payer | .708 | |||||||
| CMS | 28 (38.9%) | 39 (48.8%) | 55 (47.0%) | 24 (54.5%) | 43 (50.6%) | 37 (42.5%) | 27 (39.7%) | |
| Commercial | 22 (30.6%) | 14 (17.5%) | 30 (25.6%) | 6 (13.6%) | 19 (22.4%) | 19 (21.8%) | 15 (22.1%) | |
| CMS Managed Care |
21 (29.2%) | 25 (31.3%) | 30 (25.6%) | 13 (29.5%) | 23 (27.1%) | 27 (31.0%) | 24 (35.3%) | |
| Other/None | 1 (1.4%) | 2 (2.5%) | 2 (1.7%) | 1 (2.3%) | 0 | 4 (4.6%) | 2 (2.9%) | |
| Clinical stage | .026 | |||||||
| cStage I/II | 37 (51.4%) | 54 (67.5%) | 67 (57.3%) | 26 (59.1%) | 60 (70.6%) | 44 (50.6%) | 42 (60.9%) | |
| cStage III | 13 (18.1%) | 7 (8.8%) | 17 (14.5%) | 5 (11.4%) | 2 (2.4%) | 9 (10.3%) | 3 (4.3%) | |
| cStage IV | 22 (30.6%) | 19 (23.8%) | 33 (28.2%) | 13 (29.5%) | 23 (27.1%) | 34 (39.1%) | 24 (34.8%) | |
| Clinical stage | n = 269 | n = 241 | .008 | |||||
| cStage I/II | 158 (58.7%) | 146 (60.6%) | ||||||
| cStage III | 37 (13.8%) | 14 (5.8%) | ||||||
| cStage IV | 74 (27.5%) | 81 (33.6%) | ||||||
Abbreviations: CCI = Charlson Comorbidity Index; CMS = Centers for Medicare and Medicaid Services; ECOG = Eastern Cooperative Oncology Group Performance Status; SD = standard deviation.
Figure 1.
Graphical representation of the monthly and quarterly sum of patient encounters during the study time-period. The blue line represents monthly sums, and the orange boxes represent quarterly sums. The asterisk highlights the yearly quarter most affected by the New York State lockdown.
Figure 2.
Quarterly clinical stage distribution as a percentage of the total diagnoses across the study time-period. The asterisk highlights the yearly quarter with patient volume most affected by the New York State lockdown.
Discussion
Significant concern exists within the medical community regarding delays in cancer care as a result of COVID-19 lockdowns. Within the US, weekly cancer diagnoses fell 46% during the pandemic which is consistent with other countries like the UK which experienced 350,000 less cancer referrals than the prior year.8 , 12 Historically, much research has been conducted in attempts to understand the chronologic window in which progression of cancer occurs. Within thoracic oncology, computed tomographic (CT) quantification of volume-doubling time (VDT) has been used as a means to measure the presumed exponential nature of lung cancer growth grossly, in order to better understand time to progression.13 , 14 VDT varies by methodology and histologic type but a recent study by Hong et al places the VDT of squamous cell carcinoma at 149 days (∼21 weeks) and adenocarcinoma ranging by subtype from 232 days or ∼33 weeks (solid/micropapillary) to 1140 days or ∼ 163 weeks (lepidic).15 While 21 weeks is likely much longer than any experienced delay during the lockdown, it certainly puts context to the minimum of 11 weeks delay that many New Yorkers may have experienced. VDT does not directly predict when patients will progress but how quickly tumors grow. Putting this timeline into further context, Yuan et al evaluated the adjusted average age between stages as a rough surrogate for time to progression.16 In their retrospective analysis of the Surveillance, Epidemiology and End Results (SEER) database, they found that patients with stage IA NSCLC were on average 0.25 (3mos) adjusted years younger than patients with Stage IIA disease, 0.58 (∼7mos) adjusted years younger than those with Stage IIIA disease and 0.83 (∼10mos) adjusted years younger than those with Stage IIIB disease.16 Importantly, this study only included those with squamous cell or adenocarcinoma. Taken together, these findings certainly raise concern that even 11 weeks of delay could potentially result in progression of disease. More direct evidence indicating worse survival following delays is indicated by a systematic review by Hanna et al suggesting that a 4-week delay in cancer care can result in increased mortality across a variety of cancer types.17 Based upon their findings, a 12-week delay can increase the risk of death by 26% in certain malignancies. Although they were unable to find a significant change in mortality following delays for NSCLC, likely due to low sample size, the analysis is still concerning given their overwhelming findings related to other malignancies. Regarding thoracic oncology, a recent retrospective analysis of the US Veterans Administration data evaluating the effects of delayed surgical treatment in clinical Stage I NSCLC found worse survival and increased risk of recurrence if definitive surgical resection was delayed beyond 12 weeks of radiographic diagnosis.18 Significant delays in care due to COVID-19 are likely to result in worse survival and more advanced NSCLC.
As of March 2021, we have observed a small but significant increase in the number of patients with more advanced lung cancer. Whether this trend will persist, worsen or reverts to the mean will need to be determined in extended longitudinal studies. A corollary of our findings is whether the delay in surgical therapy due to the lockdown may result in significantly worse patient survival.17 , 18 An important limitation of our study is that our analysis ends in March 2021 while New York City was still recovering from the winter's second wave, which presumably resulted in further delays in care. Ultimately, longitudinal studies are needed to best analyze the long-term effects of the COVID-19 pandemic on cancer care including stage shift and survival.
Authors’ contributions
Nathan Mynard: Conceptualization, Data curation, Formal Analysis, Writing - Original draft. Ashish Saxena: Data curation, Writing – Review & Editing. Alexandra Mavracick: Data curation. Jeffrey Port: Methodology, Resources, Writing – Review & Editing. Benjamin Lee: Methodology, Resources, Writing – Review & Editing. Sebron Harrison: Methodology, Resources, Writing – Review & Editing. Oliver Chow: Methodology, Resources, Writing – Review & Editing. Jonathan Villena-Vargas: Methodology, Resources, Writing – Review & Editing. Ronald Scheff: Resources, Writing - review & editing. Giuseppe Giaccone: Resources, Writing - review & editing. Nasser Altorki: Conceptualization, Supervision, Resources, Writing – Review & Editing.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Dr. Altorki has ownership interest in Angiocrine Bioscience and Viewpoint Medical and conducts supported research for AstraZeneca, Roche/Genentech. Dr. Port has ownership interest in Angiocrine Bioscience, TMRW, and Viewpoint Medical. Dr. Saxena receives consulting fees from Takeda, Medtronic, Genentech, and AstraZeneca.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cllc.2021.08.010.
Appendix. Supplementary materials
References
- 1.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in china. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. Accessed: May 20, 2021.
- 3.Manhattan Woman Is First Confirmed Coronavirus Case in State - The New York Times. Available at: https://www.nytimes.com/2020/03/01/nyregion/new-york-coronvirus-confirmed.html. Accessed: May 20, 2021.
- 4.Governor Cuomo Signs the “New York State on PAUSE” executive order. Available at: https://www.governor.ny.gov/news/governor-cuomo-signs-new-york-state-pause-executive-order. Accessed: May 20, 2021.
- 5.Governor Cuomo Announces New York City to Enter Phase 1 of Reopening on June 8 and Five Regions Enter Phase 2 of Reopening Today. Available at: https://www.governor.ny.gov/news/governor-cuomo-announces-new-york-city-enter-phase-1-reopening-june-8-and-five-regions-enter. Accessed: May 20, 2021.
- 6.Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181:272–274. doi: 10.1001/jamainternmed.2020.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020;39:2010–2017. doi: 10.1377/hlthaff.2020.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epic Health Research Network. Available at: https://ehrn.org/articles/delays-in-preventive-cancer-screenings-during-covid-19-pandemic/. Accessed: May 20, 2021.
- 11.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Lancet Oncology COVID-19 and cancer: 1 year on. Lancet Oncol. 2021;22:411. doi: 10.1016/S1470-2045(21)00148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuvelmans MA, Vliegenthart R, de Koning HJ, et al. Quantification of growth patterns of screen-detected lung cancers: the NELSON study. Lung Cancer. 2017;108:48–54. doi: 10.1016/j.lungcan.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Mets OM, Chung K, Zanen P, et al. In vivo growth of 60 non-screening detected lung cancers: a computed tomography study. Eur Respir J. 2018;51 doi: 10.1183/13993003.02183-2017. [DOI] [PubMed] [Google Scholar]
- 15.Hong JH, Park S, Kim H, et al. Volume and mass doubling time of lung adenocarcinoma according to WHO histologic classification. Korean J Radiol. 2021;22:464–475. doi: 10.3348/kjr.2020.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan P, Cao JL, Rustam A, et al. Time-to-progression of NSCLC from early to advanced stages: an analysis of data from SEER registry and a single institute. Sci Rep. 2016;6:28477. doi: 10.1038/srep28477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heiden BT, Eaton DB, Engelhardt KE, et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I non-small cell lung cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.