Abstract
Background
The prevalence of diabetes mellitus (DM) is increasing globally and its comorbidity with tuberculosis (TB) is re-emerging, especially in low- and middle-income countries.
Objective
The main aim of this study is to determine the prevalence of DM and HIV infection and their associated risk factors among active tuberculosis patients in Northwest Ethiopia.
Methods
This hospital-based cross-sectional study was conducted between February 1st and June 30th, 2017 among active TB patients in two hospitals of Northwest Ethiopia. Two hundred and sixty-seven active TB cases aged 18 years or older were screened for diabetes using fasting blood glucose (FBG) test. Semi-structured questionnaires were used to collect demographic data, lifestyle habits and clinical data. Identification of pre-diabetes or diabetes in TB patients was achieved according to American Diabetes Association guidelines (2016).
Results
Prevalence of DM and TB comorbidity was 11.5% (95% confidence interval, CI 7.8–15.2) compared to 24.9% (95% CI 20.1–30.1) for pre-diabetes. Prevalence of HIV/TB co-infection was 21.9% (95% CI 16.7–26.8). Risk of DM was higher in TB patients from a rural location (adjusted odds ratio, aOR 3.13, 95% CI 1.02–9.62, p = 0.046). Similarly, DM was higher in TB patients who have a family history of DM (aOR 4.54, 95% CI 1.31–15.68, p = 0.017). Furthermore, HIV/TB co-infection was identified as a predictor of DM comorbidity in active TB patients (aOR 5.11, 95% CI 2.01–12.98, p = 0.001).
Conclusion
The magnitude of DM and pre-diabetes in active TB patients in Northwest Ethiopia was high, warranting collaborative efforts to improve screening and adopt better clinical management strategies for DM–TB comorbid patients. Furthermore, being rural residents, family history of DM and HIV/TB co-infection were found to associate with DM among TB patients, highlighting the importance of the above-mentioned risk factors in the clinical management of this comorbidity.
Keywords: Tuberculosis, Comorbidity, Diabetes mellitus, Risk factors, Northwest Ethiopia
Introduction
Diabetes mellitus (DM) is a chronic non-communicable disease which impacts the lives and wellbeing of our global population: the prevalence of DM in 2019 was estimated to be 9.3% (463 million people), which is predicted to rise to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 [1]. In comparison, TB remains a major cause of ill health and is one of the top 10 causes of death worldwide. In 2018, an estimated 10 million people fell ill with TB, resulting in close to 1.2 million deaths among human immunodeficiency virus (HIV) negative individuals and 251,000 deaths among HIV-positive cases [2].
The high burden of TB is driven by risk factors like HIV, diabetes, substance misuse, advanced kidney disease, malnutrition and treatment with corticosteroids or immunosuppressants [3]. Diabetes patients have a three times greater risk of contracting TB compared to non-diabetic individuals [4]. Although the biological association of TB and DM is not fully understood, studies suggest that diabetes depresses immune responses, which in turn facilitates infection with Mycobacterium tuberculosis (M. tuberculosis) and/or progression to symptomatic disease. This is corroborated by the fact that diabetes is generally diagnosed before TB develops [5, 6]. Additionally, poor glycemic control adversely affects TB treatment outcomes with effects such as prolongation of culture conversion, treatment failure, relapse, and death [7].
According to studies conducted in different parts of the world, DM prevalence ranges from 0.1 to 44.5% in TB patients, as reviewed in [8]. In sub-Saharan Africa, including Ethiopia, the pooled prevalence of DM in TB patients was reported to be 9.0% [9] although it is estimated that 50% of the DM patients are not aware of their DM status in developing countries [10].
Epidemiological data on the magnitude of DM–TB comorbidity is important in order to control and prevent both diseases. In this regard, facility-based studies conducted in Ethiopia have indicated an increasing trend from 8.3% in South Eastern Amhara [11] to 13.5% in Eastern Ethiopia [12]. These studies also indicated factors like gender, older age, being pulmonary TB and having known family history with DM were associated with DM–TB comorbidity [11, 12]. Additional studies are required in different regions of Ethiopia in order to identify high-risk regions and promote proper patient management.
In order to curb the global epidemic of DM, TB and HIV, a collaborative framework for care and control of both diseases is important. More specifically, national programs, clinicians, and other care givers should be guided on how to establish effective and collaborative systems for both diseases at the organizational and clinical levels. In this regard, screening for DM and glycemic control in needed, as an essential part of TB patient management [10]. Data on the prevalence of DM and HIV in patients with active TB are important for proper health-care planning and resource allocation. Hence, the aim of this study was to estimate the prevalence of DM and HIV and its associated risk factors, among TB patients in Northwest Ethiopia.
Materials and methods
Study setting
The study was conducted in Felege Hiwot Referral Hospital (FHRH) and Debere Tabor General Hospital (DTGH) of Northwest Ethiopia, between February 1st and June 30th, 2017 (Fig. 1). FHRH is located in Bahir Dar, the capital of Amhara National Regional State, situated approximately 560 km from the capital city, Addis Ababa in the North of Ethiopia, which has a latitude and longitude of 11° 36′ N 37° 23′ E and an elevation of 1840 m above sea level. Based on the 2007 census conducted by the Central Statistical Agency of Ethiopia (CSA), Bahir Dar Special Zone has a total population of 221,991 (108,456 males; 113,535 females); 180,174 (81.16%) are urban inhabitants, the rest of population live in rural districts. Similarly, Debre Tabor Hospital is the main public Hospital in the South Gonder Zone, situated in Debre Tabor City which is located approximately 110 km from Bahir Dar, the capital of Amhara Region in the North East.
Fig. 1.
Map of the study area
Study design, population and sample size
This study was a hospital-based cross-sectional study conducted among active TB patients at FHRH and DTGH. All TB patients, new and those under anti-TB treatments attending both hospitals during the study period were included. Our sample size was determined using a single population formula and estimated by p = 8.3% [11] to obtain maximum sample size:
where Zα/2 = level of significance, at 95% confidence interval = 1.96, d = marginal error 5%. Adding 20% non-response rate, the minimum sample size 140 individuals from both hospitals was considered.
In our two recruitment facilities, TB patients aged ≥ 18 years who were in treatment follow-up and were new active TB attendees, were considered for study inclusion during sample collection time. Diagnosis of TB was made based on national guidelines for the clinical and programmatic management of TB in Ethiopia [13]. Smear-positive pulmonary TB (PTB) was diagnosed in patients with at least two sputum smear examinations that were positive for acid-fast bacilli (AFB), or in patients that had one initial smear examination which was AFB-positive and additionally had radiographic abnormalities consistent with active TB, as determined by a clinician. Smear-negative PTB was diagnosed in patients with symptoms suggestive of TB, with at least three initial smear examinations that were negative for AFB by direct microscopy, and showed no response to a course of broad-spectrum antibiotics, or in patients that had three negative smear examinations by direct microscopy, radiological abnormalities consistent with PTB, and decision by a clinician to treat with a full course of anti-TB chemotherapy. The diagnosis of extra-PTB (EPTB) was made by identifying AFBs in organs other than lung, proven by at least one specimen with confirmed M. tuberculosis, or histological or strong clinical evidence consistent with active EPTB followed by a decision by a clinician to treat with a full course of anti-TB chemotherapy [16].
All patients diagnosed as having active TB were screened for DM through their history, previous medical records, and measurement of fasting plasma glucose (FPG) concentrations. The Mindray BS-200E chemistry analyzer was used for the determination of fasting plasma glucose level. Blood sugar status was classified based on the American Diabetes Association cut-off points. Accordingly, patients were classified into three groups: normal (70–99 m/dL), pre-diabetes (100–125 mg/dL), and diabetes (≥ 126 mg/dL) [19]. HIV sero-diagnosis was conducted according to the national test algorithm for Ethiopia following the manufacturer’s guidelines. For screening purposes, KHB (Shanghai Kehua Bio-engineering Co., Ltd) was used and positive samples were re-tested with STAT-PACK (Chembio HIV 1/2 STAT-PAK™ Assay, Chembio Diagnostic Systems, Inc., Medford, NY, USA). Discordant results were re-examined using the tiebreaker (Uni-Gold HIV, Trinity Biotech PLC, Co. Wicklow, Ireland). All TB patients were tested for HIV serology, which was part of the TB/HIV collaborative national program. Height and weight were measured using a digital weight scale and stadiometer. BMI was calculated, and weight status was classified using the WHO cut-off points.
Data collections and quality assurance
An interview was conducted using structured questionnaire to collect information on socio-demographic characteristics and associated risk factors. Laboratory technologists/technicians in the TB laboratory and nurses in the TB treatment clinic were specifically trained and recruited to collect data.
Approximately 3 mL of venous blood was collected from patients by laboratory professionals. The sample was centrifuged and aspirated serum was stored at – 20 °C until later processing at Amhara Regional Health Research Laboratory Center. To assure good data quality, researchers were trained to collect data using a structured pre-testing questionnaire. Data entry and data checking was conducted using Epidata and was transferred to SPSS (version 21) for data analysis. The questionnaire was pre-tested on 30 randomly selected TB patients to ensure practicability, validity and interpretation of responses.
Statistical analysis
Data entry, cleaning and analysis were performed using Statistical Package for Social Science (SPSS), version 21; New York, IBM Corp. Patients with non-conclusive blood glucose test results for DM diagnosis were excluded from the analysis. The main outcome variables were: proportions of patients with a diagnosis of TB-DM and TB-NDM. Categorical variables were expressed as proportions and Chi-square analysis was performed to compare proportions. Multivariate logistic regression analysis was performed to analyze the association of predictor variables with the outcome variable. A p-value < 0.05 was considered statistically significant. Effectiveness of the screening approach was estimated by the number of TB patients needed to screen (NNS) to get one additional new DM case, calculated by the reciprocal proportion of newly detected DM cases.
Results
Socio-demographic characteristics
Of the 280 TB patients who met inclusion criteria, 269 (96.1%) were included in the final analysis: six (2.1%) patients refused to participate in screening and 5 patients (2.0%) were excluded from the analysis because they did not have a confirmatory test result. Approximately 66.5% of participants were rural dwellers; the male-to-female ratio was 1.15, and the mean age of participants was 36.1 (SD 14.2; median age 35; range X to 62). The majority of the participants were married (59.5%) and 66.2% of participants had a family size of more than five (Table 1).
Table 1.
Socio-demographic characteristics of the study participants
| Characteristics | Total (%) | DM | HIV | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Address | |||||
| Urban | 90 (33.5) | 5 (16.1) | 85 (35.7) | 31 (52.5) | 59 (28.1) |
| Rural | 179 (66.5) | 26 (83.6) | 153 (64.3) | 28 (47.5) | 151 (71.9) |
| Gender | |||||
| Male | 144 (53.5) | 19 (61.3) | 125 (52.5) | 29 (49.2) | 115 (54.8) |
| Female | 125 (46.5) | 12 (38.7) | 113 (47.5) | 30 (50.8) | 95 (45.2) |
| Age category | |||||
| 18–30 | 126 (46.8) | 9 (25.7) | 117 (50.0) | 28 (47.5) | 98 (46.7) |
| 31–40 | 61 (22.7) | 13 (37.1) | 48 (20.5) | 13 (22.0) | 48 (22.9) |
| > 40 | 82 (30.5) | 13 (37.1) | 69 (29.5) | 18 (30.5) | 64 (30.5) |
| Marital status | |||||
| Single | 62 (23.0) | 7 (22.6) | 55 (23.1) | 17 (28.8) | 45 (21.4) |
| Married | 160 (59.5) | 19 (61.3) | 141 (59.2) | 31 (52.5) | 129 (61.4) |
| Widowed or divorced | 47 (17.5) | 5 (16.1) | 42 (17.6) | 11 (18.6) | 36 (17.1) |
| Family size | |||||
| < 5 | 178 (66.2) | 15 (48.4) | 163 (68.5) | 42 (71.2) | 136 (64.8) |
| ≥ 5 | 91 (33.8) | 16 (51.6) | 75 (31.5) | 17 (28.8) | 74 (35.2) |
| Educational status | |||||
| No formal education | 152 (56.5) | 17 (54.8) | 135 (56.7) | 27 (45.8) | 125 (59.5) |
| Primary (grade 1–8) | 60 (22.3) | 7 (22.6) | 53 (22.3) | 16 (27.1) | 44 (21.0) |
| Secondary (grade 9–12) | 26 (9.7) | 3 (9.7) | 23 (9.7) | 5 (8.5) | 21 (10.0) |
| College or University | 31 (11.5) | 4 (12.9) | 27 (11.3) | 11 (18.6) | 20 (9.5) |
| Occupation | |||||
| Employee | 31 (11.5) | 4 (12.9) | 27 (11.3) | 11 (18.6) | 20 (9.5) |
| Farmer | 145 (53.9) | 18 (58.1) | 127 (53.4) | 21 (35.6) | 124 (59.0) |
| Merchant | 23 (8.6) | 2 (6.5) | 21 (8.8) | 9 (15.3) | 14 (6.7) |
| Students | 22 (8.2) | 4 (12.9) | 18 (7.6) | 8 (13.6) | 14 (6.7) |
| No job | 48 (17.8) | 3 (9.7) | 45 (18.9) | 10 (16.9) | 38 (18.1) |
| Income per month in ETB | |||||
| < 1000.0ETB | 113 (42.0) | 15 (48.4) | 98 (41.2) | 23 (39.0) | 90 (42.9) |
| 1000.00–1999.00ETB | 62 (23.0) | 9 (29.0) | 53 (22.3) | 21 (35.6) | 67 (31.9) |
| > 2000.00ETB | 94 (34.9) | 7 (22.6) | 87 (36.6) | 15 (25.4) | 53 (25.2) |
ETB: Ethiopian Birr, the exchange rate 1USD = 27.00ETB; DM: diabetes mellitus; HIV: human immunodeficiency virus
Prevalence of DM and HIV among active TB patients
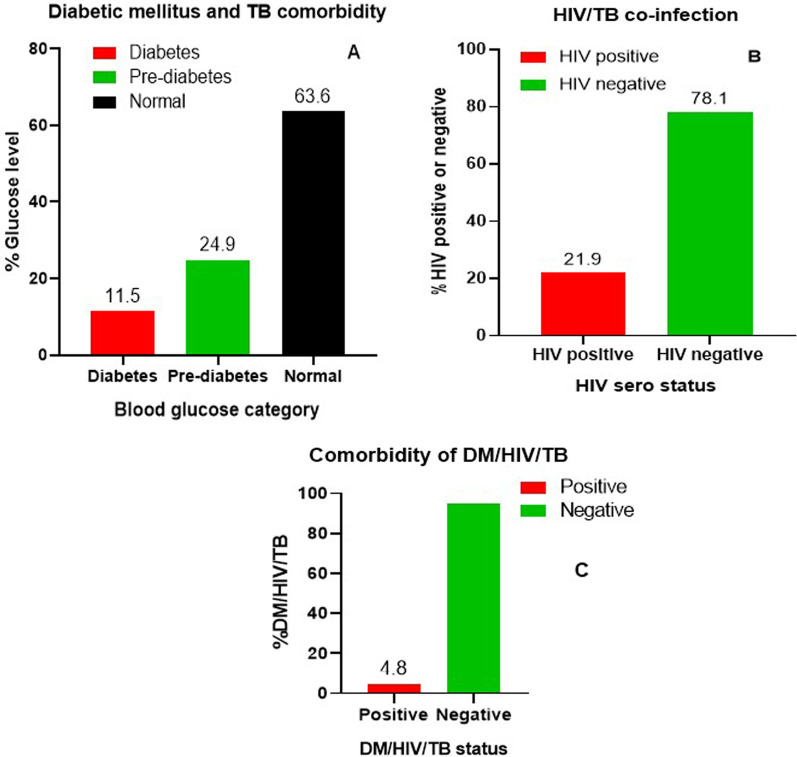
Based on the FPG analysis, prevalence of DM comorbidity (FPG ≥ 126 mg/dL) and impaired fasting glucose (FPG = 100–125 mg/dL) among active TB patients was 11.5% (31/269) and 24.9% (67/269), respectively. Prevalence of HIV/TB co-infection was 21.9% (59/269), and prevalence of DM/HIV/TB triple-infection was 4.8% (Fig. 2).
Fig. 2.
Prevalence of diabetes mellitus and HIV among TB patients. A Fasting plasma glucose level; B HIV–TB co-infection and C the triplets’ comorbidity (DM/HIV/TB) among TB patients. DM diabetes mellitus, TB tuberculosis, HIV human immunodeficiency virus
Clinical characteristics and factors associated with DM, among TB patients
The majority (90.3%) of the DM–TB comorbid patients were new TB cases. Clinically, the majority of participants were EPTB (54.8%), followed by smear negative (41.9%). A total of 6.7% of TB patients reported that they had known family history of DM, of which 16.1% were found to be comorbid with DM. Furthermore, 64.5% of the DM–TB comorbid patients were found to be underweight (BMI < 18.5%) according to the WHO cut-off point (Table 2).
Table 2.
Clinical characteristics and life style of TB patients
| Characteristics | Total (%) | DMTB | NDMTB | HIV/TB | NHIV/TB |
|---|---|---|---|---|---|
| Type of TB patient | |||||
| New | 243 (90.3) | 28 (90.3) | 215 (90.3) | 53 (89.8) | 190 (90.5) |
| Retreated | 26 (9.7) | 3 (9.7) | 23 (9.7) | 6 (10.2) | 20 (9.5) |
| TB category | |||||
| Smear positive | 33 (12.3) | 1 (3.2) | 32 (13.4) | 13 (22.0) | 20 (9.5) |
| EPTB | 165 (61.3) | 17 (54.8) | 148 (62.2) | 33 (55.9) | 132 (62.9) |
| Smear negative | 71 (26.4) | 13 (41.9) | 58 (24.4) | 13 (22.0) | 58 (27.6) |
| Known family history of DM | |||||
| Yes | 18 (6.7) | 5 (16.1) | 13 (5.5) | 3 (5.1) | 15 (7.1) |
| No | 251 (93.3) | 26 (83.9) | 225 (94.5) | 56 (94.9) | 195 (92.9) |
| BMI | |||||
| < 18.5 | 137 (50.9) | 20 (64.5) | 117 (49.2) | 30 (50.8) | 107 (51.0) |
| 18.5–25.0 | 132 (49.1) | 11 (35.5) | 121 (50.8) | 29 (49.2) | 103 (49.0) |
| Any chronic diseases | |||||
| Yes | 38 (14.1) | 6 (19.4) | 32 (13.4) | 23 (39.0) | 15 (7.1) |
| No | 231 (85.9) | 25 (80.6) | 206 (86.6) | 36 (61.0) | 195 (92.9) |
| Active smoker | |||||
| Yes | 6 (2.2) | 1 (3.2) | 5 (2.1) | 0 | 6 (2.9) |
| No | 263 (97.8) | 30 (96.8) | 233 (97.9) | 59 (100.0) | 204 (97.1) |
| Drink alcohol regularly | |||||
| Yes | 107 (39.8) | 13 (41.9) | 94 (39.5) | 21 (35.6) | 86 (41.0) |
| No | 162 (60.2) | 18 (58.1) | 144 (60.5) | 38 (64.4) | |
BMI body mass index, EPTB extra-pulmonary TB, DM diabetes mellitus, TB tuberculosis, DMTB DM among tuberculosis, NDMTB negative-DM among TB patients, HIV/TB human immunodeficiency virus infection among TB, NHIV/TB negative HIV infection among TB patients
In order to identify risk factors associated with DM comorbidity among active TB patients, a binary logistic regression analysis was conducted. The univariate analysis showed that rural residence; age greater than 34 years; HIV positivity, and known family history with DM were significantly associated with DM–TB comorbidity (p < 0.05).
In multivariable logistic regression analysis, residence, HIV co-infection, and known family history of DM were the independent predictors of DM–TB comorbidity (p < 0.05). TB patients who were rural residents were found to be 3 times more likely to be positive for DM (aOR 3.13, 95% CI 1.02–9.62, p = 0.046), compared to urban residents. HIV-positive TB patients were found to be 5 times more likely to be DM positive (aOR 5.14, 95% CI 2.00–13.25, p = 0.001), compared to HIV-negative patients. TB patients who had a known family history of DM were found to be more than 4 times more likely to be DM positive, compared to TB patients with no known family history of DM (aOR 4.54, 95% CI 1.31–15.68, p = 0.017) (Table 3).
Table 3.
Factors associated with DM among TB patients in North Western Ethiopia
| Characteristics | Total (%) | DMTB (%) | Crude OR (95% CI) | p value | aOR (95% CI) | p value |
|---|---|---|---|---|---|---|
| Address | ||||||
| Rural | 179 (66.5) | 26 (83.9) | 2.89 [1.07–7.79] | 0.036 | 3.13 [1.02–9.62] | 0.046 |
| Urban | 90 (33.5) | 5 (16.1) | 1 | 1 | ||
| Age in years | ||||||
| 18–34 | 134 (49.8) | 10 (32.3) | 1 | – | – | |
| > 34 | 135 (50.2) | 21 (67.7) | 2.28 [1.3–5.06] | 0.042 | – | – |
| TB category | ||||||
| Smear positive | 33 (12.3) | 1 (3.2) | 0.27 [0.04–2.12] | 0.214 | – | – |
| EPTB | 165 (61.3) | 17 (54.8) | 1.95 [0.89–4.27] | 0.094 | – | – |
| Smear negative | 71 (26.4) | 13 (41.9) | 1 | – | – | |
| Known family history of DM | ||||||
| Yes | 18 (6.7) | 5 (16.1) | 3.33 [1.09–10.08] | 0.033 | 4.54 [1.31–15.68] | 0.017 |
| No | 251 (93.3) | 26 (83.9) | 1 | 1 | ||
| HIV test result | ||||||
| Positive | 59 (21.9) | 12 (38.7) | 2.57 [1.16–5.65] | 0.019 | 5.11 [2.01–12.98] | 0.001 |
| Negative | 210 (78.1) | 19 (61.3) | 1 | 1 | ||
| BMI | ||||||
| < 18.5 | 137 (50.9) | 20 (64.5) | 1.88 [0.86–4.09] | 0.112 | – | – |
| 18.5–25 | 132 (49.1) | 11 (35.5) | 1 | – | – | |
aOR adjusted odds ratio, CI confidence interval, OR odds ratio, BMI body mass index, EPTB extra-pulmonary TB, DM diabetes mellitus, TB tuberculosis, DMTB diabetic mellitus among tuberculosis
Discussion
In this study, the prevalence of the DM and HIV infection and its associated risk factors were determined among active TB patients in Northwest Ethiopia. The prevalence of DM–TB comorbidity (11.5%) was higher than the national (8.5%) and the regional (9.0%) estimates [9], but lower than the global estimate (15.3%) [8]. Similarly conducted studies in Mali [14] and Serbia [15] have reported lower prevalences of DM–TB comorbidity (5.5% and 9.9%, respectively) than that of the present study, however there are reports of prevalences in excess of that we report: studies conducted in Ethiopia (13.5%) [12], Vietnam (13.7%) [16], South Korea (24.2%) [17], Mexico (25.2%) [18], Pakistan (39.6%) [19] and India (54.1%) [20]. The difference in socio-demographic characteristics of source populations studied, and the screening methods used could be responsible for such a wide range of DM prevalence among TB patients.
Pre-diabetes is characterized by an abnormally high blood glucose level, but one that is below the diabetic range. Pre-diabetic individuals have a higher risk of developing DM: each year approximately 5 to 10% of pre-diabetic individuals develop DM [21]. In the present study, pre-diabetes among TB patients was 24.9% lower than the prevalence reported in a previous study form Eastern Ethiopia (29.7%) [12]. The observed DM and pre-diabetes prevalence in the studied group deserves an integrated health service approach to address the burden of both DM and TB diseases.
There is a well-established association between advancing age and increasing risk of Type II DM [11, 12, 19]. Similarly, our univariate logistic regression analysis showed that those aged over 34 years had a significantly higher risk of DM–TB comorbidity. This could be explained by reports that advancing age is linked to immune impairment, which is a recognized risk factor for both DM and TB [22].
In this study, the majority of the participants were from rural areas and consequently, TB patients living in rural residences were found to be 3 times more likely to be positive for DM, compared to those living in urban residences. This finding is in contrast to a previous report [23], where participants in urban residences were found to be at higher risk of DM due to lifestyle differences. However, the higher risk of DM among participants living in rural residences in this study warrants further investigation using a longitudinal study design.
Having a family history of DM is a known risk factor associated with DM–TB comorbidity [11, 12, 16, 24]. Consistent with previous findings, the present study found the risk of DM to be 4 times higher among TB patients who had a family history of DM.
In this study, the prevalence of HIV among TB patients (21.9%) was higher than the prevalence of DM (11.5%) in TB patients. This finding is consistent with a previous study conducted in Ethiopia [11], but conflicts with a study conducted in India which reported the prevalence of HIV among TB patients to be 12.3% [25]. This may be due to the high burden of HIV in our study area. Similarly, HIV sero-positivity was a predictor of DM among TB patients in this study. The high prevalence of DM cases among TB/HIV cases may be linked to changes in quality of life and the adverse effects of anti-retroviral therapy (ART) drugs among HIV-positive patients. In order to enhance early detection and improve management of DM and HIV among TB patients, routine screening and a collaborative framework in existing health-care settings is recommended.
The main limitations of this study include: using random blood sugar and fasting blood sugar for DM diagnosis have lower sensitivity and specificity compared to an oral glucose tolerance test (OGTT). Furthermore, glycated hemoglobin (HbA1c) [26, 27] may underestimate the prevalence of DM. In addition, social desirability bias due to denial of reporting smoking, alcohol drinking and related habits may affect study findings related to behavioral risk factors for DM and TB comorbidity.
Conclusion
In conclusion, this study reported a high prevalence of DM and HIV co-infection among active TB patients in Northwest Ethiopia. Thus, a collaborative approach should be implemented to improve the screening and management of DM among TB patients in TB clinics. Furthermore, rural residential status; a family history of DM, and HIV/TB co-infection were found to associate with DM–TB comorbidity, highlighting the importance of the above-mentioned risk factors in the clinical management of this comorbidity.
Acknowledgements
We thank all the members of the TB clinic and laboratory teams at Debre Tabor General and Felegehiwot Referral hospitals. We also would like to thank the study participants from both health facilities. Finally, we would like to appreciate Dr. David Jolliffe for editing the English grammar and syntax of the manuscript.
Authors’ contributions
BT conceived the study. BT EA, YZ and GA contributed to the study design and development of laboratory assays. BT, EA, YZ, MA, YF, MA, AA, KP and TB contributed to the implementation of the study and data acquisition. BT did statistical analyses, wrote the first draft of the manuscript, and had final responsibility for the decision to submit for publication. All authors reviewed the final draft and agreed with its content and conclusions. All authors read and approved the final manuscript.
Funding
This work was supported by a research grant from College of Medicine and Health Sciences, Bahir Dar University, Ethiopia.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from Bahir Dar University College of Medicine and Health Science Research and Community Service Ethical Review Committee. Additionally, the study was also ethically approved by Amhara Regional Public Health and separate permissions were obtained from respective hospital management. Informed written consent was obtained from all study participants. Confidentiality of results was maintained and positive results were reported to the appropriate physician/nurse for proper management.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global tuberculosis report 2019. Lyon: World Health Organization; 2019. [Google Scholar]
- 3.Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013. 828939. [DOI] [PMC free article] [PubMed]
- 4.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152–e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid MJA, McFadden N, Tsima BM. Clinical challenges in the co-management of diabetes mellitus and tuberculosis in southern Africa. JEMDSA. 2013;18(3):135–140. [Google Scholar]
- 6.Nathella PK, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152:13–24. doi: 10.1111/imm.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahishale V, Avuthu S, Patil B, Lolly M, Eti A, Khan S. Effect of Poor glycemic control in newly diagnosed patients with smear-positive pulmonary tuberculosis and type-2 Diabetes Mellitus. Iran J Med Sci. 2017;42(2):144–151. [PMC free article] [PubMed] [Google Scholar]
- 8.Noubiap JJ, Nansseu JR, Nyaga UF, Nkeck JR, Endomba FT, Kaze AD, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob Health. 2019;7(4):e448–e460. doi: 10.1016/S2214-109X(18)30487-X. [DOI] [PubMed] [Google Scholar]
- 9.Alebel A, Wondemagegn AT, Tesema C, Kibret GD, Wagnew F, Petrucka P, et al. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2019;19(1):254. doi: 10.1186/s12879-019-3892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Restrepo BI. Diabetes and tuberculosis. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.TNMI1127-0023-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PLoS ONE. 2016;11(1):e0147621. doi: 10.1371/journal.pone.0147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenaye L, Mengiste B, Baraki N, Mulu E. Diabetes Mellitus among adult tuberculosis patients attending tuberculosis clinics in Eastern Ethiopia. BioMed Res Int. 2019;2019:7640836. doi: 10.1155/2019/7640836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ethiopia Federal Minstry of Health. Guidelines for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. In: Federal Ministry of Health Addis Ababa; 2012.
- 14.Diarra B, Tolofoudie M, Sarro YS, Togo ACG, Bane S, Nientao I, et al. Diabetes Mellitus among new tuberculosis patients in Bamako, Mali. J Clin Tuberc Other Mycobact Dis. 2019;17:100128. doi: 10.1016/j.jctube.2019.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlović JM, Pavlovic AD, Bulajić MV, Pešut DP. Prevalence of diabetes mellitus (DM) in tuberculosis (TB) patients: clinical and radiologic features in the TB-DM association based on a five-year hospital study. Infez Med. 2018;26(1):22–27. [PubMed] [Google Scholar]
- 16.Hoa NB, Phuc PD, Hien NT, Hoa VQ, Thuong PH, Anh PT, et al. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in Hanoi, Vietnam. BMC Infect Dis. 2018;18(1):603. doi: 10.1186/s12879-018-3519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EH, Lee JM, Kang YA, Leem AY, Kim EY, Jung JY, et al. Prevalence and impact of Diabetes Mellitus among patients with active pulmonary tuberculosis in South Korea. Lung. 2017;195(2):209–215. doi: 10.1007/s00408-017-9978-4. [DOI] [PubMed] [Google Scholar]
- 18.Abdelbary BE, Garcia-Viveros M, Ramirez-Oropesa H, Rahbar MH, Restrepo BI. Tuberculosis-diabetes epidemiology in the border and non-border regions of Tamaulipas, Mexico. Tuberculosis. 2016;101s:S124–s134. doi: 10.1016/j.tube.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Aftab H, Ambreen A, Jamil M, Garred P, Petersen JH, Nielsen SD, et al. High prevalence of diabetes and anthropometric heterogeneity among tuberculosis patients in Pakistan. TM & IH. 2017;22(4):465–473. doi: 10.1111/tmi.12842. [DOI] [PubMed] [Google Scholar]
- 20.Kornfeld H, West K, Kane K, Kumpatla S, Zacharias RR, Martinez-Balzano C, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the effects of diabetes on tuberculosis severity (EDOTS) study. Chest. 2016;149(6):1501–1508. doi: 10.1016/j.chest.2016.02.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Pre-diabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skowroński M, Zozulińska-Ziółkiewicz D, Barinow-Wojewódzki A. Tuberculosis and diabetes mellitus - an underappreciated association. Arch Med Sci. 2014;10(5):1019–1027. doi: 10.5114/aoms.2014.46220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS ONE. 2012;7(10):e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viney K, Cavanaugh J, Kienene T, Harley D, Sleigh A. Tuberculosis and diabetes mellitus in the Republic of Kiribati: a case-control study. Trop Med Int Health. 2015;20:650–657. doi: 10.1111/tmi.12462. [DOI] [PubMed] [Google Scholar]
- 25.Manjareeka M, Nanda S. Prevalence of HIV infection among tuberculosis patients in Eastern India. J Infect Public Health. 2013;6(5):358–362. doi: 10.1016/j.jiph.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a World Health Organization/International Diabetes Federation consultation. 2006.
- 27.WHO . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated report of a WHO consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.