Abstract
Shp-2 is an SH2 domain-containing protein tyrosine phosphatase. Although the mechanism remains to be defined, substantial experimental data suggest that Shp-2 is primarily a positive regulator in cell growth and development. We present evidence here that Shp-2, while acting to promote mitogenic signals, also functions as a negative effector in interferon (IFN)-induced growth-inhibitory and apoptotic pathways. Treatment of mouse fibroblast cells lacking a functional Shp-2 with IFN-α or IFN-γ resulted in an augmented suppression of cell viability compared to that of wild-type cells. To dissect the molecular mechanism, we examined IFN-induced activation of signal transducers and activators of transcription (STATs) by electrophoretic mobility shift assay, using a specific DNA probe (hSIE). The amounts of STAT proteins bound to hSIE upon IFN-α or IFN-γ stimulation were significantly increased in Shp-2−/− cells. Consistently, tyrosine phosphorylation levels of Stat1 upon IFN-γ treatment and, to a lesser extent, upon IFN-α stimulation were markedly elevated in mutant cells. Furthermore, IFN-γ induced a higher level of caspase 1 expression in Shp-2−/− cells than in wild-type cells. Reintroduction of wild-type Shp-2 protein reversed the hypersensitivity of Shp-2−/− fibroblasts to the cytotoxic effect of IFN-α and IFN-γ. Excessive activation of STATs by IFNs was also diminished in mutant cells in which Shp-2 had been reintroduced. Together, these results establish that Shp-2 functions as a negative regulator of the Jak/STAT pathway. We propose that Shp-2 acts to promote cell growth and survival through two mechanisms, i.e., the stimulation of growth factor-initiated mitogenic pathways and the suppression of cytotoxic effect elicited by cytokines, such as IFNs.
The cytotoxic or growth-inhibitory activity of interferons (IFNs) in many cell types has been recognized for decades, although the molecular mechanism is not fully understood (9). A group of SH2-containing transcription factors, collectively called signal transducers and activators of transcription (STATs), play important roles in signal relay downstream of receptors for IFNs as well as other cytokines (4, 21). Binding of IFNs to their specific receptors, which lack intrinsic kinase activity, induces oligomerization of receptor subunits and triggers phosphorylation on tyrosyl residues by associated Janus tyrosine kinases (Jaks). Phosphorylation of receptors results in subsequent recruitment and phosphorylation by Jaks of latent STAT proteins in the cytoplasm, which are then dimerized and translocated to the nucleus. By assembling into different complexes, activated STATs bind to specific DNA sequences and enhance transcription (4, 14, 22, 24). Genetic and biochemical evidence indicates that IFN-α/β induces activation of Jak1 and Tyk2, resulting in tyrosine phosphorylation of Stat1 and Stat2, while binding of IFN-γ to its receptor activates Jak1 and Jak2, which mediate the phosphorylation of Stat1 (4, 13, 20, 28, 43). Apparently, various combinations of STATs by themselves or with other transcription factors activate distinct sets of genes, leading to different biological consequences. More recent data suggested a direct link between activation of the STAT signaling pathway and cell apoptosis through induction of caspase expression (1).
Whereas activation of STATs by Jak kinases has been extensively investigated, relatively little is known regarding the role of protein tyrosine phosphatases (PTPs) in this signal-transducing pathway. Several studies showed that inhibition of PTP activities can interfere with the IFN-induced Jak/STAT pathway in a positive or a negative manner (6, 18, 19). In particular, two mammalian SH2-containing cytoplasmic PTPs, Shp-1 and Shp-2, have been implicated in the regulation of IFN signaling (5, 7). Enhanced tyrosyl phosphorylation of Jak1 but not Tyk2 was observed upon IFN treatment of Shp-1-deficient macrophages isolated from motheaten (me) mutant mice, which was accompanied by a significant increase in the amount of IFN-induced STATs bound to the gamma response region (GRR) DNA probe (5). After IFN stimulation, Shp-1 was reversibly associated with IFN-α receptor complex that contains Jak1 and Tyk2 as well. Thus, Shp-1 appears to participate in a negative control of distinct components in IFN-stimulated Jak/STAT pathways in macrophages, although the biological significance remains to be determined (5). In another study, Shp-2 was shown to bind the IFN-α/β receptor in vitro with a purified glutathione S-transferase fusion protein containing the intracellular part of the receptor. This interaction in vitro was not affected by IFN occupation of the receptor (7). Treatment of cells with IFN induced tyrosyl phosphorylation of Shp-2, and transient expression of a catalytically inactive mutant of Shp-2 had a dominant negative effect on IFN-stimulated luciferase activity under the control of an IFN-stimulated response element (ISRE). These results suggest that Shp-2 might be involved in the IFN-initiated signaling pathway. However, it is not understood whether and how Shp-2 modulates the Jak/STAT activity.
In contrast to the predominant expression of Shp-1 in hematopoietic cells, Shp-2 is an ubiquitously expressed enzyme that appears to be involved in multiple signaling pathways downstream of a variety of growth factors and cytokines (12, 30). In previous studies, we introduced a targeted mutation into the Shp-2 locus in mouse embryonic stem cells that results in a deletion of exon 3, encoding for amino acids 46 to 110 in the N-terminal SH2 (SH2-N) domain (36, 39). Homozygous mutant embryos died at midgestation with severe defects in the mesodermal patterning, and heterozygous animals appeared normal, suggesting that the mutant Shp-2 protein does not function in a dominant negative manner. The Shp-2 mutation suppressed embryonic stem cell proliferation as well as differentiation into hematopoietic, epithelial, and cardiac muscle cells in vitro and in vivo (34–36). To determine the Shp-2 function in cell signaling, we established embryonic fibroblast cells from wild-type, heterozygous, and homozygous Shp-2 mutant embryos (40). Using these cells, we found that Shp-2 tyrosine phosphatase functions as a positive regulator in mitogenic stimulation of extracellular signal-regulated kinase (Erk) and the expression of platelet-derived growth factor receptor-β, while being a negative effector in C-jun N-terminal kinase (Jnk) activation under cellular stress (25, 40). Furthermore, our results indicate that Shp-2 plays a critical role in the control of cell spreading, migration, and focal adhesion, by working in concert with focal adhesion kinase, Fak (48).
In this study, we demonstrate that Shp-2 is involved in protection of cells from the cytotoxic effect of IFNs and that Shp-2 acts as a negative effector in mediating the activation of STATs induced by IFN-α or IFN-γ. We propose a model whereby the function of Shp-2 as a survival factor, in combination with its promoting activity of mitogenic signaling pathways, constitutes the positive regulatory role of the tyrosine phosphatase in cell growth.
MATERIALS AND METHODS
Cell lines and reagents.
Wild-type (Shp-2+/+), heterozygous (Shp-2+/−), and homozygous (Shp-2−/−) mutant embryonic fibroblast cell lines were isolated as described in detail previously (40). Reintroduction of wild-type Shp-2 into Shp-2−/− fibroblast cells was described elsewhere (48). Recombinant mouse IFN-γ was purchased from R&D Systems, and recombinant mouse IFN-α A/D was provided by Hoffmann-LaRoche Inc. Polyclonal anti-Stat1, anti-Stat2, and anti-Jak2 antibodies were gifts from Andrew Larner at the Cleveland Clinic Foundation. Monoclonal antiphosphotyrosine (anti-PY) and anti-tyrosine-phosphorylated Stat1 (anti-PY-Stat1) antibodies were purchased from Upstate Biotechnology, Inc. Polyclonal anti-Shp-2, anti-Jak1, anti-IFN-γ receptor α subunit (Rα), anti-IFN-αR, anti-caspase 1 antibodies were from Santa Cruz Biotechnology, Inc. Buffer A is composed of 50 mM β-glycerophosphate (pH 7.3), 2 mM EDTA, 1 mM EGTA, 5 mM β-mercaptoethanol, 1% Triton X-100, and 0.05 M NaCl. Buffer B contains 20 mM HEPES (pH 7.9), 20 mM NaF, 1 mM EDTA, 1 mM EGTA, and 1 mM dithiothreitol (DTT). Both buffers were also supplemented before use with 0.2 mM Na3VO4, 0.4 μM microcystin, 0.1 mM phenylmethylsulfonyl fluoride, 20 μg of leupeptin/ml, 1 μM pepstatin A, and 1 μg of aprotinin/ml.
Stimulation of cells and preparation of cell extracts.
Cells at approximately 80% confluency were starved in serum-free Dulbecco modified Eagle medium for 24 h before treatment with IFN-α or IFN-γ. Factor stimulation was terminated by washing cells with ice-cold phosphate-buffered saline, and cell extracts were made as follows. Whole cell extracts were made by homogenization of cells in buffer A followed by high-speed centrifugation. For preparation of nuclear extracts, pelleted cells were resuspended in three packed cell volumes of buffer B, swollen for 10 min, and lysed by repeated passage through a 25-gauge needle. Nuclei were collected by centrifugation at 16,000 × g for 20 min and then extracted in 2.5 packed cell volumes of buffer B supplemented with 0.42 M NaCl and 20% glycerol. The supernatant, referred to as the nuclear extract, was cleared by centrifugation at 16,000 × g for 30 min and used for analysis of STAT activity as described below.
EMSA of STATs.
The activation of Stat1,2 was evaluated by assessing the ability of the proteins to form complexes with a specific DNA probe in electrophoretic mobility shift assays (EMSA). In this study, the DNA probe used was the hSIE sequence, which has been widely used for detection of STAT activation induced by a variety of stimuli (2, 38, 44, 47, 49). The probe was prepared by annealing 5′-GTCGACATTTCCCGTAAATC-3′ with 5′-TCGACGATTTACGGGAAATG-3′, and the resulting double-stranded DNA with staggered ends was labeled by the Klenow fragment in the presence of [α-32P]dCTP and [α-32P]dTTP (3,000 mCi/mmol; Amersham). Free nucleotides were removed by using a nucleotide-removing kit from Qiagen. For STAT activity assay, nuclear extracts containing 15 μg of total proteins were preincubated with 2 μg of polydI-dC–polydI-dC (Pharmacia) for 20 min, followed by the addition of 2 fmol of 32P-labeled DNA probe and further incubation for 30 min at room temperature. The buffer used for the reaction was 20 mM HEPES (pH 7.9), 25 mM KCl, 4 mM MgCl2, 0.5 mM DTT, 1 mM EDTA, 10% glycerol. Resulting protein-DNA complexes were resolved on 5% polyacrylamide gels (acrylamide:bisacrylamide = 39:1) containing 2.5% glycerol made in 0.5× Tris-borate-EDTA buffer. Gels were dried and exposed to X-ray films.
Determination of cell viability.
Cell viability was measured by crystal violet or trypan blue staining assay. Cells cultured in 96-well plates at 8,000 cells/well were treated with or without IFN-α and IFN-γ. After 72 or 96 h of treatment, the media were decanted, plates were submerged in 50% crystal violet in 50% methanol for 30 min and rinsed with water, and the bound dye was solubilized by incubation at 37°C for 1 h with 200 μl of 0.5% sodium dodecyl sulfate (SDS) in 50% ethanol. The plates were then scanned at 595 nm.
Immunoprecipitation and immunoblot analysis.
Cell extracts were incubated with antibodies prebound to protein A-Sepharose beads overnight. The beads were washed three times with buffer A with 0.15 M NaCl. For immunoblot analysis, samples were separated by SDS–10% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was first probed with a specific antibody and then detected by using the ECL system with horseradish peroxidase-conjugated secondary antibodies (Amersham).
RESULTS
Shp-2−/− fibroblast cells were hypersensitive to the cytotoxic effect of IFNs.
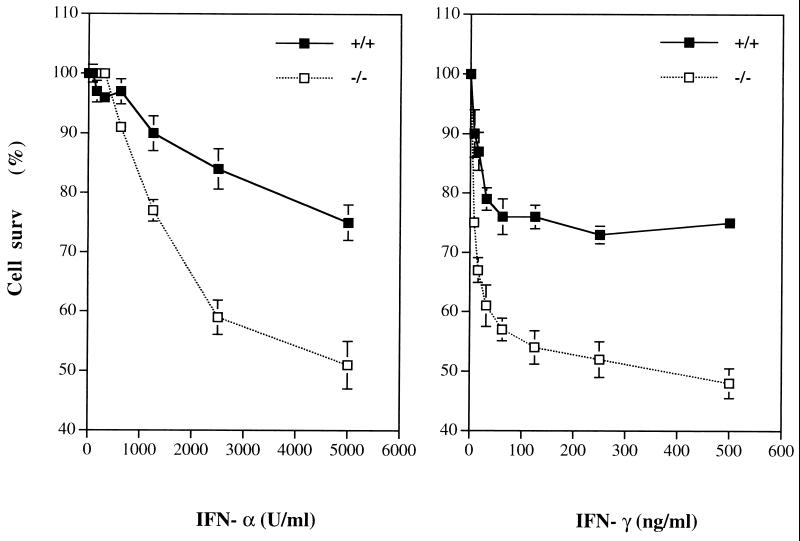
To assess the putative role of Shp-2 in mediating IFN-induced cytotoxicity, we compared the growth rate of wild-type and Shp-2−/− fibroblast cells in the presence or absence of IFN-α or IFN-γ. Cells were incubated with different amounts of IFNs for 96 h, and cell numbers were measured after crystal violet staining. As shown in Fig. 1, wild-type fibroblast cells were sensitive to the growth-inhibitory effect of either IFN-α or IFN-γ in a dose-dependent manner. Notably, the sensitivity of Shp-2−/− cells to the cytotoxicity of IFN-α and IFN-γ was significantly enhanced compared to that of wild-type cells. This result suggests that Shp-2 might be involved in cellular protective events against growth-inhibitory factors such as IFNs. Enhanced cell death and decreased growth rate of Shp-2−/− cells upon IFN-α or IFN-γ treatment were also observed by counting viable cells with trypan blue staining (data not shown). All the experiments described in this study were repeated with reproducible results for at least two cell lines of wild-type and Shp-2−/− origins, which were originally derived from different embryos.
FIG. 1.
Hypersensitivity to IFN cytotoxicity of Shp-2−/− fibroblast cells. Wild-type and Shp-2−/− cells were treated with various concentrations of IFN-α or IFN-γ for 96 h. Surviving cells were quantitated by crystal violet staining assay. Data shown are the means of three independent experiments ± standard deviations. The percentage of cell survival (surv) was defined as the relative number of treated versus untreated cells.
Activation of STATs by IFNs was substantially enhanced in Shp-2−/− cells.
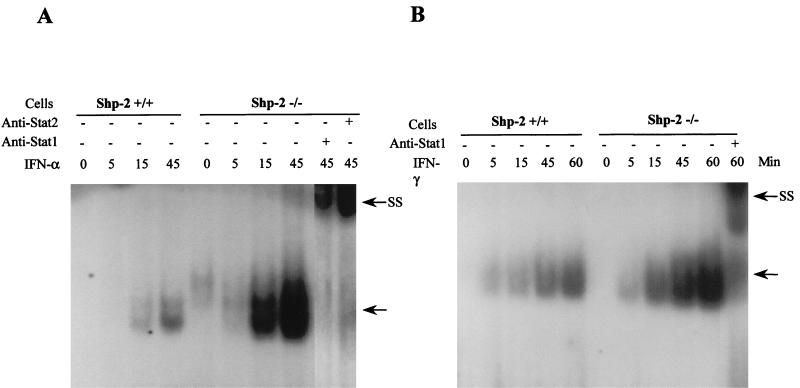
To explore the molecular mechanism for the Shp-2 involvement in IFN-initiated cell signaling, we examined Stat1 and Stat2 activation in wild-type as well as Shp-2−/− fibroblast cells. STAT activity in nuclear extracts was measured by EMSA with a specific DNA probe, the hSIE sequence, which is a high-affinity mutant of ISRE in the c-fos promoter (44). Serum-starved cells were treated with IFN-α or IFN-γ for 5, 15, 45, and 60 min, and nuclear extracts were made to assess STAT activity. As shown in Fig. 2, treatment with IFNs resulted in a time-dependent induction of STAT-binding activity toward hSIE in wild-type cells. However, an increased amount of DNA-binding activity was observed in Shp-2−/− cells after treatment with either IFN-α or IFN-γ. Dose-dependent effects were also analyzed in a concentration range of 15.6 to 1,000 U/ml with IFN-α and 12.5 to 100 ng/ml with IFN-γ after 45 min of treatment. Significantly more IFN-induced DNA binding proteins were detected in Shp-2−/− cells than in wild-type cells in a dose-dependent manner (data not shown).
FIG. 2.
Kinetics of STAT activation by IFN-α or IFN-γ. Serum-starved wild-type and Shp-2−/− cells were treated with 1,000 U of IFN-α/ml (A) or 100 ng/ml IFN-γ (B) for the indicated time periods. Nuclear extracts were prepared for detection of STAT activity by EMSA with 32P-labeled hSIE probe. The arrows denote the specific STATs-DNA complexes. In supershift assays (SS) shown in panels A and B, anti-Stat1 or anti-Stat2 antibodies were added to the reaction mixture before incubation with the DNA probe.
The identity of the proteins involved in hSIE DNA binding was confirmed by preincubating the cell lysates with anti-Stat1 or anti-Stat2 antibodies. Consistent with previous observations (4), the addition of anti-Stat1 antibody resulted a supershift in response to both IFN-α and IFN-γ, and the Stat2 antibody produced a supershift upon IFN-α treatment (Fig. 2).
The results shown above point to an enhanced activation of Stat1 and Stat2 induced by IFN-α and IFN-γ, which correlates with increased sensitivity to IFN cytotoxicity in Shp-2 mutant cells. To corroborate this observation, we examined the tyrosine phosphorylation status of Stat1 and Stat2. It has been documented that Stat1 and Stat2 are phosphorylated on tyrosyl residue in response to IFN-α, and that Stat1, but not Stat2, is activated via tyrosine phosphorylation by IFN-γ (4). Using a specific antibody against tyrosine-phosphorylated Stat1 (anti-PY-Stat1), we found that upon IFN-γ stimulation, tyrosine phosphorylation of Stat1 was markedly increased in Shp-2−/− cells compared to that in wild-type cells, whereas a modestly enhanced tyrosine phosphorylation of Stat1 was observed in response to IFN-α (Fig. 3A and B). To examine tyrosine phosphorylation of Stat2, the protein was immunoprecipitated by using anti-Stat2 antibody and analyzed by immunoblot analysis with anti-PY antibody. As depicted in Fig. 3C, tyrosine phosphorylation of Stat2 was also modestly increased in Shp-2−/− cells in response to IFN-α.
FIG. 3.
Increased tyrosine phosphorylation of Stat1,2 and Jak1 by IFNs in Shp-2−/− cells. Serum-starved wild-type and Shp-2−/− cells were treated with 100 ng of IFN-γ/ml (A) or 1,000 U of IFN-α/ml (B and C) for the indicated time periods. Whole cell extracts (A and B) or anti-Stat2 immunoprecipitates (C) were subjected to SDS-polyacrylamide gel electrophoresis and subsequently to immunoblot analyses with anti-PY, anti-PY-Stat1, anti-Stat1, or anti-Stat2 antibodies as indicated. (D) Serum-starved wild-type and Shp-2−/− cells were treated with 100 ng of IFN-γ/ml for the indicated time periods. Whole cell extracts were immunoprecipitated with anti-Jak1 antibody and subjected to immunoblot analyses with anti-PY and anti-Jak1 antibodies as indicated.
IFN-γ-induced Jak1 phosphorylation was increased in Shp-2−/− cells.
Both Jak1 and Jak2 are known to be involved in mediating IFN-γ activation of Stat1 (4). To examine whether activation of Jak1 or Jak2 is altered by the Shp-2 mutation, wild-type and mutant cells were lysed at various time points after IFN-γ treatment and then subjected to anti-Jak1 or Jak2 immunoprecipitation, followed by anti-PY immunoblotting. As shown in Fig. 3D, in comparison with that in wild-type cells, the phosphorylation level of Jak1 in Shp-2−/− cells was significantly increased, although similar amounts of Jak1 were precipitated from wild-type and mutant cells. The tyrosine phosphorylation level of Jak2 was not significantly changed in mutant cells upon IFN-γ treatment under the same conditions (data not shown). We also examined Jak1 and Tyk2 kinases upon IFN-α treatment. The expression levels of Jak1 and Tyk2 were not changed in Shp-2 mutant cells as demonstrated by anti-Jak1 or anti-Tyk2 immunoblotting, and IFN-α stimulated tyrosine phosphorylation of Jak1 or Tyk2 was hardly detectable in these cells under similar conditions (data not shown).
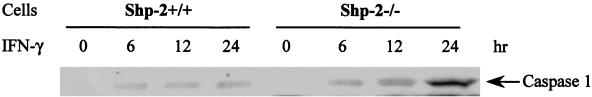
The Shp-2 mutation promoted caspase 1 induction by IFN-γ.
The induction of caspase 1 expression was shown to be involved in IFN-mediated cell apoptosis through activation of the STAT signaling pathway (1). To examine caspase 1 expression, immunoblot analysis of whole cell extracts from wild-type and Shp-2−/− cells was performed with anti-caspase 1 antibody. IFN-γ treatment promoted expression of p45 caspase 1 in wild-type and Shp-2−/− cells (Fig. 4). Interestingly, a substantially increased amount of caspase 1 enzyme was detected in Shp-2−/− cells after IFN-γ treatment for 24 h compared with that in wild-type cells. It should be noted that a proteolytically cleaved form of caspase 1, p10, was unstable and difficult to detect in these cell lines, as has been reported previously (1). Following IFN-α stimulation, there was a very weak induction of caspase 1 expression in these cells (data not shown).
FIG. 4.
The Shp-2 mutation enhanced IFN-γ-induced caspase 1 expression. Wild-type and Shp-2−/− cells were treated with or without 100 ng/ml IFN-γ for indicated time periods. Whole cell extracts were resolved on SDS-polyacrylamide gel and then immunoblotted with anti-caspase 1 antibody.
Shp-2 constitutively interacts with IFN-α/β and IFN-γ receptors.
To examine the physical association of Shp-2 with IFN-γR or IFN-αR, we prepared cell lysates by treating cells with detergent that solubilizes membrane proteins. Cell lysates were first subjected to immunoprecipitation with anti-Shp-2 antibody that recognizes both the wild-type and mutant Shp-2 proteins (36, 40, 48). Subsequent blotting with anti-IFN-γRα (Fig. 5A) or anti-IFN-αR antibodies (Fig. 5B) revealed a constitutive association of Shp-2 with IFN-γRα or IFN-αR, since the immune complexes were detected in the absence or presence of treatment with IFN-γ or IFN-α. This is consistent with a previous observation that Shp-2 constitutively interacts with IFN-α receptor in vitro (7). In a reciprocal experiment, Shp-2 was also detected in anti-IFN-γRα or anti-IFN-αR immunoprecipitates (data not shown). The association of Shp-2 with IFN receptors was apparently not changed by the deletion mutation in the SH2-N domain of Shp-2, since the mutant protein was also detected in a complex with IFN receptors.
FIG. 5.
Interaction of Shp-2 with IFN receptors. Serum-starved wild-type and Shp-2−/− cells were treated with 100 ng of IFN-γ/ml (A, C), or 1,000 U of IFN-α/ml (B) for the indicated time periods. (A, B) Whole cell extracts were immunoprecipitated with anti-Shp-2 antibody or preimmune antiserum (Pre.). The resulting immunoprecipitates were resolved on SDS-polyacrylamide gel and immunoblotted with anti-IFN-γRα, anti-IFN-αR, or anti-Shp-2 antibodies as indicated. (C) Cell lysates were immunoprecipitated with anti-IFN-γRα and subjected to immunoblot analysis with anti-PY or anti-IFN-γRα antibodies.
To determine whether Shp-2 directly acts on IFN receptors, we assessed tyrosine phosphorylation levels of IFN-γRα. The receptor was immunoprecipitated with its specific antibody and subjected to immunoblot analysis with anti-IFN-γRα or anti-PY antibodies. As revealed by anti-IFN-γRα blotting (Fig. 5C), the expression level of the receptor was not altered in Shp-2−/− cells. Stimulation with IFN-γ for 15 min induced similar levels of tyrosine phosphorylation of the receptor in wild-type and mutant cells (Fig. 5C). This result suggests that Shp-2 may not act directly on the IFN receptor but, rather, on downstream signaling components. In parallel experiments, we failed to detect a significant tyrosine phosphorylation of IFN-αR upon IFN-α treatment in wild-type or mutant cells under similar conditions.
Rescue of the abnormal phenotype by reintroduction of wild-type Shp-2.
To establish the role of Shp-2 in cell signaling, wild-type Shp-2 cDNA was transfected into Shp-2−/− cells and cell clones expressing different levels of Shp-2 were isolated (48). In previous experiments, we showed a rescue of defective cell migration upon restoring Shp-2 expression (48). We have now reexamined the modulation of IFN signaling by Shp-2 in these cells. In agreement with the data shown in Fig. 1, Shp-2−/− cells and the vector-transfected Shp-2−/− cells had an increased sensitivity to the cytotoxic effect of IFNs compared to wild-type and Shp-2+/− cells (Fig. 6). However, ectopic expression of wild-type Shp-2 in Shp-2−/− cells decreased their sensitivity to the growth-inhibitory effect of both IFN-α and IFN-γ. This result indicates that the absence of a functional Shp-2 sensitizes fibroblasts to IFN cytotoxicity. Consistent with the increased sensitivity of Shp-2−/− cells to IFNs, we observed enhanced activation of STATs by IFNs (Fig. 2). The levels of activated STATs were reexamined in Shp-2−/− cells transfected with the vector or wild-type Shp-2. As shown in Fig. 7, upon reintroduction of wild-type Shp-2 protein, a reduced DNA binding activity was observed in response to IFN-α or IFN-γ stimulation. More importantly, the reduction of STAT activity correlated with the expression levels of wild-type Shp-2 in two rescued cell lines. We further examined tyrosine phosphorylation levels of Stat1 in rescued cells. Indeed, reintroduction of Shp-2 into Shp-2−/− cells decreased IFN-stimulated Stat1 phosphorylation to the wild-type level (Fig. 7). Taken together, these data strongly suggest that Shp-2 functions as a negative regulator in IFN-mediated STAT activation and growth arrest.
FIG. 6.
Rescue of the mutant phenotype by reintroduction of wild-type Shp-2. Shp-2−/− cells were transfected with an expression construct for wild-type Shp-2 and selected in hygromycin B. Cell lines stably expressing wild-type Shp-2 were established from isolated clones (48). Shp-2+/+, +/−, −/− cells and Shp-2−/− cells expressing wild-type Shp-2 (R3, R4) or the vector only (V) were treated with various concentrations of IFN-α or IFN-γ for 72 h. Surviving cells were quantitated by crystal violet staining assay. Data shown are the means of three independent experiments ± standard deviations, and the percentage of cell survival (surv) was defined as the relative number of treated versus untreated cells.
FIG. 7.
Modulation of IFN-stimulated STAT activity by Shp-2. (A and B) Serum-starved cells of Shp-2+/+, +/−, and −/− origins and Shp-2−/− cells expressing the vector only (Vector), lower level of wild-type Shp-2 (R3) or higher level of wild-type Shp-2 (R4) were treated with 1,000 U of IFN-α/ml (A) or 100 ng of IFN-γ/ml (B) for the indicated time periods. Nuclear extracts were prepared for EMSA as described in the legend for Fig. 2. The arrows denote the specific STATs-DNA complexes. (C and D) Shp-2−/− cells expressing the vector only (Vector) or wild-type Shp-2 (R4) were treated with 100 ng of IFN-γ/ml (C) or 1,000 U of IFN-α/ml (D) for the indicated time periods. Whole cell extracts were subjected to SDS-polyacrylamide gel electrophoresis and then to immunoblot analyses with anti-PY-Stat1 and anti-Stat1 antibodies as indicated.
DISCUSSION
We have investigated the involvement of Shp-2 tyrosine phosphatase in mediating cellular responses to IFNs. Our results indicate a negative regulatory role of Shp-2 in the IFN-initiated STAT signaling pathway that leads to cell growth arrest. Fibroblast cells in which a functional Shp-2 molecule is absent were hypersensitive to the cytotoxic effect of IFN-α and IFN-γ. Consistently, IFN-stimulated Stat1 and Stat2 activities were significantly increased in Shp-2−/− cells, as revealed by elevated tyrosine phosphorylation and enhanced DNA-binding activity. Increased sensitivity to IFN cytotoxicity and enhanced STAT activation upon IFN-α or IFN-γ stimulation were reduced by reintroduction of wild-type Shp-2 into Shp-2−/− cells. These results suggest that Shp-2 functions to protect cells against IFN toxicity and that Shp-2 is negatively involved in IFN-induced STAT activation.
It was shown previously that Shp-1 is negatively involved in the modulation of IFN-α/β-stimulated Jak1 and Stat1 activities in macrophages by a naturally-occurring mutation in the Shp-1 gene in me/me mice, which results in an enhanced tyrosyl phosphorylation of Jak1 but not Tyk2 in response to IFN-α (5). Concomitantly, IFN-induced Stat1 tyrosine phosphorylation was dramatically increased, while the phosphorylation status of Stat2 was not significantly changed in response to IFN-α in Shp-1-deficient cells. As a consequence, the amount of IFN-α-stimulated factors bound to GRR sequence in the high-affinity FcγR1 gene promoter was markedly increased. Therefore, it was proposed that Shp-1 selectively regulates distinct components in the IFN-stimulated Jak/STAT pathway, which was speculated to account for the abnormal inflammatory behavior of macrophages in me/me mice (5). Due to the restricted expression pattern of the Shp-1 gene, primarily in hematopoietic cells, it is reasonable to imagine that other tyrosine phosphatases might be involved in IFN signaling in other cell types. We have now provided evidence that Shp-2, another cytoplasmic SH2-containing phosphatase closely related to Shp-1, has a similar role in negative control of the IFN-stimulated Jak/STAT pathway in fibroblast cells. We have further revealed the biological significance of this finding by demonstrating that the enhanced Jak/STAT activity in Shp-2 mutant cells is responsible for hypersensitivity to the cytotoxic effect of IFNs. Although a similar negative effect of Shp-1 and Shp-2 in IFN-initiated Jak/STAT signaling was observed, different biochemical mechanisms might be involved. In previous work, Shp-1 was found to be complexed with IFN-α receptor in untreated cells (5). IFN stimulation induced a transient dissociation of Shp-1 from the receptor, followed by a reassociation of the complex. In contrast, we found that Shp-2 was constitutively associated with the IFN-α and IFN-γ receptors, consistent with a previous observation (7).
One possible, simple explanation of the negative effect of Shp-2 in IFN signaling is that Shp-2 tyrosine phosphatase can work directly on IFN receptors. However, the data shown in this study argue against this possibility, since the Shp-2 mutation did not significantly affect the tyrosine phosphorylation level of IFN-γRα. It seems more likely that Shp-2 interferes with IFN-mediated signaling by negatively regulating cellular events downstream of IFN receptors. Indeed, we observed that the tyrosine phosphorylation of Jak1 was significantly increased in Shp-2−/− cells upon IFN-γ stimulation. This enhanced phosphorylation may lead to enhanced Jak1 activation that promotes Stat1 activation in Shp-2−/− cells. It is not known yet whether Shp-2 directly acts on and inactivates the Jak1 kinase, although we have previously observed physical interaction between Shp-2 and Jak1, Jak2 kinases (45). In any case, it is interesting that the mutant Shp-2 protein with a deletion in the SH2-N domain was also detected in complexes with IFN-α and IFN-γ receptors, suggesting that the SH2-N domain is required for its physiological function in cells but not for association with IFN receptors. Consistent with this notion, biochemical and structural data suggest that the SH2-N domain is engaged in recognition of and interaction with phosphatase targets (12, 15, 17, 30, 32).
Stat1 is apparently a critical signaling component mediating cellular responses to IFNs. Ablation of the Stat1 gene in mice leads to a block to cellular responses to IFN-α and IFN-γ (10, 26). One recent report described a role of Stat1 activation in the induction of caspase 1 expression and cell apoptosis under IFN treatment (1). Another study demonstrated that Stat1 is required for the expression of caspases and also for tumor necrosis factor-α-induced cell apoptosis (23). To this body of knowledge, we now add that the Shp-2 tyrosine phosphatase negatively regulates IFN-induced Stat1,2 activities which mediate the cytotoxic effect of IFNs. Our data also suggest that the enhanced expression of caspase 1 accounts at least in part for the increased sensitivity to IFN-γ in Shp-2−/− cells. On the other hand, increased caspase 1 induction was not observed in Shp-2−/− cells in response to IFN-α. Thus, other mechanisms might also be involved, for example, through the expression of cyclin-dependent kinase inhibitors or other caspases (2, 8).
Several groups have recently isolated putative inhibitors for the Jak/STAT pathways by functional or molecular screening of cDNA libraries (3, 11, 29, 41). An SH2 domain-containing protein, variously named SOCS-1 (for suppressor-1 of cytokine signaling), JAB (for Jak-binding protein), or SSI-1 (for STAT-induced STAT inhibitor 1), was identified as a member of cytokine-inducible family of proteins that includes a previously described molecule, CIS (46). Overexpression of SOCS-1 in murine monocytic leukemic M1 cell line suppressed interleukin-6-induced macrophage differentiation and phosphorylation of Stat3 (41). Physical interaction of JAB with Jak kinases inhibited their kinase activity (11). Cytokine induction of SSI-1 gene expression was blocked by transfection of a dominant negative mutant of Stat3, suggesting that SSI-1 might be involved in a negative feedback mechanism for the control of cytokine-induced Jak/STAT pathways (29). Another group identified a family of proteins with a putative zinc-binding motif that was named PIAS, for protein inhibitor of activated STAT (3). It was observed that PIAS3 specifically interacts with activated Stat3, thereby inhibiting its DNA-binding activity and induction of gene expression. Although further investigation is required for elucidation of the molecular mechanism for functions of these inhibitors, it is conceivable that cytokine-induced Jak/STAT pathways might be controlled at multiple levels.
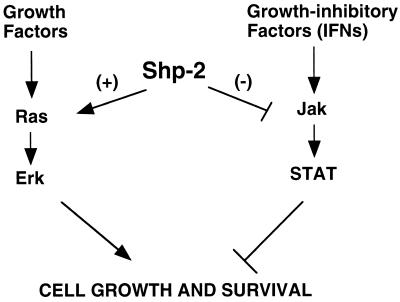
Genetic analysis in Drosophila provided the first clue that Csw, the Drosophila homologue of Shp-2, functions as a positive signal transducer downstream of Torso (33). Subsequently, substantial genetic and biochemical data point to a positively regulatory role of Shp-2 in cell growth and differentiation as well as animal development, and Shp-2 appears to act upstream of Erk (16, 27, 31, 34, 36, 37, 40, 42). In this regard, the most interesting part of our results presented here is that Shp-2 apparently operates as a negative regulator in IFN-mediated activation of STAT transcription factors. This finding, together with our previous result that Shp-2 is a suppressor in the Jnk pathway (40), challenges a conventional view that Shp-1 plays a negative role in cell signaling, whereas Shp-2 always acts as a positive regulator to promote signal transmission from cytokine receptors. As illustrated in Fig. 8, this new result for Shp-2 allows us to propose a model that Shp-2 can play either a positive or a negative role in the modulation of multiple signaling pathways, depending on its cellular compartmentalization and on the molecules with which it interacts. The negative effect of Shp-2 in the Jnk and Jak/STAT pathways might guard cells against various damages induced by stress or growth-inhibitory cytokines. Overall, Shp-2 functions seem to promote cell growth and survival.
FIG. 8.
A model for Shp-2 functions in promoting cell growth and survival. We and others have shown previously that protein tyrosine phosphatase Shp-2 promotes mitogenic signaling pathways. Evidence is presented in this study that Shp-2 also acts as a survival factor in protecting cells against the cytotoxic effect of IFNs through modulation of Jak/STAT activities. Therefore, we propose a model in which the positive role of Shp-2 in cell growth and survival is contributed by its promotion of mitogenic signals and suppression of Jak/STAT activities.
ACKNOWLEDGMENTS
We thank Andrew Larner for antibodies and Mark Kaplan, Lawrence Quilliam, and Rebecca Chan for helpful discussions and for critically reading the manuscript.
This work was supported in part by grants from the National Institutes of Health (R29GM53660) and the Showalter Trust to G.S.F. G.S.F. received a career development award from the American Diabetes Association.
REFERENCES
- 1.Chin Y E, Kitagawa M, Kuida K, Flavell R A, Fu X Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin Y E, Kitagawa M, Su W C, You Z H, Iwamoto Y, Fu X Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 3.Chung C D, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.David M, Chen H E, Goelz S, Larner A C, Neel B G. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–7058. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David M, Romero G, Zhang Z Y, Dixon J E, Larner A C. In vitro activation of the transcription factor ISGF3 by interferon alpha involves a membrane-associated tyrosine phosphatase and tyrosine kinase. J Biol Chem. 1993;268:6593–6599. [PubMed] [Google Scholar]
- 7.David M, Zhou G, Pine R, Dixon J E, Larner A C. The SH2 domain-containing tyrosine phosphatase PTP1D is required for interferon alpha/beta-induced gene expression. J Biol Chem. 1996;271:15862–15865. doi: 10.1074/jbc.271.27.15862. [DOI] [PubMed] [Google Scholar]
- 8.Deiss L P, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 9.DeMaeyer E, DeMaeyer-Guignard J. Interferons and other regulatory cytokines. New York, N.Y: John Wiley and Sons; 1988. [Google Scholar]
- 10.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 11.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 12.Feng G S, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 13.Fu X Y. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s) Cell. 1992;70:323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- 14.Fu X Y, Kessler D S, Veals S A, Levy D E, Darnell J E., Jr ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci USA. 1990;87:8555–8559. doi: 10.1073/pnas.87.21.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 17.Hof P, Pluskey S, Dhe-Paganon S, Eck M J, Shoelson S E. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi K, David M, Finbloom D S, Larner A C. In vitro activation of the transcription factor gamma interferon activation factor by gamma interferon: evidence for a tyrosine phosphatase/kinase signaling cascade. Mol Cell Biol. 1993;13:1634–1640. doi: 10.1128/mcb.13.3.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igarashi K, David M, Larner A C, Finbloom D S. In vitro activation of a transcription factor by gamma interferon requires a membrane-associated tyrosine kinase and is mimicked by vanadate. Mol Cell Biol. 1993;13:3984–3989. doi: 10.1128/mcb.13.7.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ihle J N, Kerr I M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 21.Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Thierfelder W E, Kreider B, Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 22.Kessler D S, Veals S A, Fu X Y, Levy D E. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990;4:1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Commane M, Flickinger T W, Horvath C M, Stark G R. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 24.Levy D E, Lew D J, Decker T, Kessler D S, Darnell J E., Jr Synergistic interaction between interferon-alpha and interferon-gamma through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J. 1990;9:1105–1111. doi: 10.1002/j.1460-2075.1990.tb08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Qu C K, Shi Z Q, Feng G S. Downregulation of platelet-derived growth factor receptor-β in Shp-2 mutant fibroblast cell lines. Oncogene. 1998;17:441–448. doi: 10.1038/sj.onc.1201988. [DOI] [PubMed] [Google Scholar]
- 26.Meraz M A, White J M, Sheehan K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, Carver-Moore K, DuBois R N, Clark R, Aguet M, Schreiber R D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 27.Milarski K L, Saltiel A R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 28.Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell J E, Jr, Stark G R, Kerr I M. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 1993;12:4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 30.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly A M, Neel B G. Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction. Mol Cell Biol. 1998;18:161–177. doi: 10.1128/mcb.18.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins L A, Larsen I, Perrimon N. Corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 34.Qu C K, Feng G S. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–440. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- 35.Qu C K, Yu W M, Azzarelli B, Cooper S, Broxmeyer H E, Feng G S. Biased suppression of hematopoiesis and multiple developmental defects in chimeric mice containing Shp-2 mutant cells. Mol Cell Biol. 1988;18:6075–6082. doi: 10.1128/mcb.18.10.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu C K, Shi Z Q, Shen R, Tsai F Y, Orkin S H, Feng G S. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 38.Ruff-Jamison S, Chen K, Cohen S. Epidermal growth factor induces the tyrosine phosphorylation and nuclear translocation of Stat 5 in mouse liver. Proc Natl Acad Sci USA. 1995;92:4215–4218. doi: 10.1073/pnas.92.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxton T M, Henkemeyer M, Gasca S, Shen R, Shalaby F, Feng G S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Z Q, Lu W, Feng G S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 41.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 42.Tang T L, Freeman R, Jr, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 43.Velazquez L, Fellous M, Stark G R, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 44.Wagner B J, Hayes T E, Hoban C J, Cochran B H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990;9:4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin T, Shen R, Feng G S, Yang Y C. Molecular characterization of domains required for the specific interactions between Shp-2 phosphatase and JAK tyrosine kinase. J Biol Chem. 1997;272:1032–1037. doi: 10.1074/jbc.272.2.1032. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins N A, Gilbert D J, Copeland N G, Hara T, Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.You M, Zhao Z. Positive effects of SH2 domain-containing tyrosine phosphatase SHP-1 on epidermal growth factor- and interferon-gamma-stimulated activation of STAT transcription factors in HeLa cells. J Biol Chem. 1997;272:23376–23381. doi: 10.1074/jbc.272.37.23376. [DOI] [PubMed] [Google Scholar]
- 48.Yu D H, Qu C K, Henegariu O, Lu X, Feng G S. Protein tyrosine phosphatase Shp-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 49.Zhong Z, Wen Z, Darnell J E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]