Abstract
Background
Acute hematogenous osteomyelitis (AHOM) is an insidious infection of the bone that more frequently affects young males. The etiology, mainly bacterial, is often related to the patient’s age, but it is frequently missed, owing to the low sensitivity of microbiological cultures. Thus, the evaluation of inflammatory biomarkers and imaging usually guide the diagnosis and follow-up of the infection. The antibiotic treatment of uncomplicated AHOM, on the other hand, heavily relies upon the clinician experience, given the current lack of national guidelines for the management of this infection.
Methods
A systematic review of the studies on the empirical treatment of uncomplicated AHOM in children published in English or Italian between January 1, 2009, and March 31, 2020, indexed on Pubmed or Embase search engines, was carried out. All guidelines and studies reporting on non-bacterial or complicated or post-traumatic osteomyelitis affecting newborns or children older than 18 years or with comorbidities were excluded from the review. All other works were included in this study.
Results
Out of 4576 articles, 53 were included in the study. Data on different topics was gathered and outlined: bone penetration of antibiotics; choice of intravenous antibiotic therapy according to the isolated or suspected pathogen; choice of oral antibiotic therapy; length of treatment and switch to oral therapy; surgical treatment.
Conclusions
The therapeutic management of osteomyelitis is still object of controversy. This study reports the first Italian consensus on the management of uncomplicated AHOM in children of pediatric osteomyelitis, based on expert opinions and a vast literature review.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13052-021-01130-4.
Keywords: Paediatric infectious diseases, Paediatric osteomyelitis, Bone infections, Antibiotic therapy, Children, Paediatrics
Background
Osteomyelitis (OM) is an acute or chronic infection of the bone that more frequently affects preschool children, with a male-to-female ratio of 2:1. Long bones and vertebrae are the most freque ntly affected skeletal segments [1–5]. The etiology of the infection is bacterial in most cases [1]. The most common type of bone infection in children is Acute Hematogenic Osteomyelitis (AHOM) [5], whose pathogens may diverge based on children’s age (Table 1).
Table 1.
Age distribution of most frequently involved pathogens in pediatric AHOM
| Age | Pathogens |
|---|---|
| < 3 months |
S. aureus E. coli H. influenzae N. gonorrhoeae (in case of congenital infection) Streptococcus β haemolyticus group B C. albicans |
| 3 months – 5 years |
S. aureus K. kingae Streptococcus β haemolyticus group A S. pneumoniae (especially under 2 years-old) H. influenzae type B (rare in fully vaccinated immunocompetent patients) |
| > 5 years |
S. aureus Streptococcous β haemolyticus group A N. gonorrhoeae (in sexually active adolescents) |
Clinical presentation of AHOM is highly variable and depends on multiple factors, including age, causative organism, anatomical site, and presence of an underlying disease [3, 4]. Symptoms’ onset is often insidious, especially in newborns and younger patients [6, 7]. Moreover, while the lower extremities are more frequently affected compared to the upper ones, the involvement of a single bone segment is much more frequent than multifocal infections [2, 3].
The AHOM diagnostic work-up includes the evaluation of several inflammatory markers such as white blood cell count, erythrocyte sedimentation rate, and C-reactive protein level. The combination of those biomarkers can be helpful for AHOM diagnosis as well as for the evaluation of treatment response and monitoring, while procalcitonin is less useful due to its low sensitivity and high cost [1–3, 8, 9].
Overall, microbial cultures show lower capability to diagnose AHOM depending on patient age, location of the infection, techniques used, as well as laboratory experience. Since the hematogenous dissemination during AHOM has been documented as a common occurrence in children, blood cultures represent a valuable diagnostic tool to establish the etiology, even if in a high percentage of children with AHOM (30–50%), cultures do not allow the isolation of the germ responsible for the infection [1–4, 10]. On the other hand, the microbial culture of the material derived from the infected bone constitutes the diagnostic gold standard [1, 3]. In recent years, the introduction of new diagnostic techniques, i.e. molecular tests on bone biopsy, and/or mass spectrometry with MALDI-TOF increased the overall diagnostic performance and ensured a rapid identification of the pathogen [1].
The initial diagnostic approach when AHOM is suspected is the imaging because of its role in excluding other possible differential diagnoses, such as traumatic or non-infectious lesions. X-rays own a reasonably high specificity (75–83%), while its sensitivity remains lower (43–75%) than other techniques [11]. Magnetic Resonance Imaging (MRI) is the gold standard in the diagnosis of osteomyelitis, due to its higher sensitivity and specificity (respectively of 82–100% and 75–99%) [12]. MRI is also able to document bone edema, the first non-specific sign of osteomyelitis, within 24–48 h from the infection onset. MRI is also increasingly used in evaluating multifocality [13], and it has almost completely replaced bone scintigraphy, being more sensitive and avoiding exposure to radiations. Ultrasound instead is useful in identifying joint effusion, soft tissue abscess, and sub-periosteal collection, findings that may be associated with AHOM [11].
Methods
A systematic literature review on the empirical treatment of uncomplicated AHOM in children was carried out in order to evaluate the available data from the studies published between 2009 and March 2021.
A multidisciplinary Italian panel of experts was set up to address the following scientific issues:
antibiotic molecules for intravenous (iv) empiric therapy
duration of intravenous antibiotic therapy
factors influencing the switch from iv therapy to oral (os) therapy
duration of os therapy
total duration of antibiotic treatment
The characteristics and results of the selected studies are summarized in Supplementary Table 1, and the panel analyzed the results through frontal and online discussions. Consent was obtained by using the Delphi method.
All articles in English and Italian published from January 1, 2009, to March 31, 2021, concerning the empirical antibiotic therapy of uncomplicated AHOMs in children aged between 28 days and 18 years were selected using Pubmed and Embase search engines.
The search was conducted exploiting the two strings shown below:
Pubmed search string
((((osteomyelitis) OR ((bone* OR osteoarticular OR musculoskeletal) AND infection*)) AND (manag* OR therap* OR antibiotic* OR treatment*)) AND (child* OR pediatric* OR paediatric* OR kid* OR infant))
Embase search string
(((‘osteomyelitis’/exp. OR osteomyelitis:ti,ab) AND acute:ti,ab) OR ((bone:ti,ab OR osteoarticular:ti,ab OR musculoskeletal:ti,ab) AND infection:ti,ab)) AND (manag*:ti,ab OR therap*:ti,ab OR antibiotic*:ti,ab OR treatment*:ti,ab) AND ([infant]/lim OR [child]/lim OR [preschool]/lim OR [school]/lim OR [adolescent]/lim) AND [2009–2020]/py AND ([english]/lim OR [italian]/lim)
Criteria used to select articles are shown in Table 2.
Table 2.
Inclusion and exclusion criteria of selected studies
| Inclusion criteria | Exclusion criteria |
|---|---|
| Subacute or acute infectious osteomyelitis due to bacterial etiology | Subacute or chronic non-infectious osteomyelitis or articles related to non-bacterial (e.g., fungal, or mycobacterial) osteomyelitis |
| Osteomyelitis in children aged 28 days to 18 years-old | Osteomyelitis in patients aged < 28 days and > 18 years-old |
| Uncomplicated osteomyelitis | Complicated osteomyelitis |
| Osteomyelitis not caused by surgery or trauma | Osteomyelitis caused by surgery or trauma |
| Osteomyelitis onset in healthy children | Osteomyelitis in children with underlying chronic, onco-hematological or immunodeficiency disorders |
| Cohort studies or case reports including more of 10 patients | Guidelines |
Results
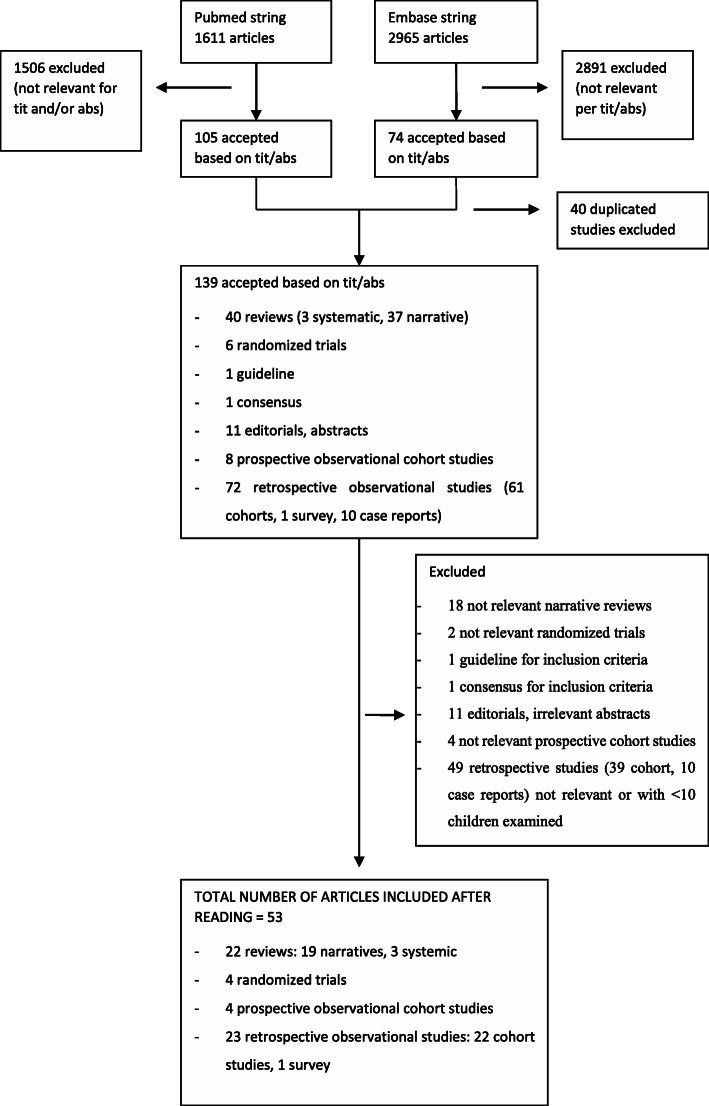
Out of 4576 articles, 139 were selected based on title and abstract and 53 were considered relevant as they satisfied the established inclusion/exclusion criteria (Fig. 1).
Fig. 1.
Articles selection’s search tree algorithm
Bone penetration of antibiotics
The bone penetration of the different classes of antibiotics was evaluated by pharmacokinetic studies using different methodologies [14–16]. According to the available data, bone penetration of the main antibiotics used for treating AHOM is reported in Table 3.
Table 3.
Percentage of bone penetration of the main antibiotics used in AHOM
| Antibiotic | Percentage of bone penetration | ||
|---|---|---|---|
| Boselli 1999 [14] | Landrsdorfer 2009 [15] | Thabit 2019 [16] | |
| BETA-LACTAMS | |||
| Amoxicillin | 17–31% | 18–20% | 10% (amoxi-clavulanate) |
| Clavulanic | – | 10–15% | – |
| Ampicillin | 16% | 11–71% | – |
| Sulbactam | – | 17–71% | – |
| Piperacillin | 18–23% | 18–23% or 15% | 15% (piperacillin-tazobactam) |
| Tazobactam | 22–26% | 22–26% | – |
| Flucloxacillin | 8–15% | 5–15% | 65% |
| Oxacillin | – | 11% | 21% |
| CARBAPENEMS | |||
| Ertapenem | – | 10–20% | 35% |
| Meropenem | – | – | 50% |
| CEPHALOSPORINS | |||
| Ceftriaxone | – | 7–17% | – |
| Cefazolin | 18% | 18% | 25% |
| Cefepime | – | 46–76% | – |
| Cefuroxime | 14–23% | – | – |
| Cefotaxime | 8,8% | – | – |
| Ceftazidime | 20–35% | 54% | 49% |
| MACROLIDES | |||
| Erythromycin | 28.5–39% | 18–28% | – |
| Azithromycin | – | 250–630% | – |
| GLYCOPEPTIDES | |||
| Vancomycin | 60.8% | 5–67% | 20–40% |
| Teicoplanin | 14–290% | 50–64% | – |
| AMINOGLYCOSIDES | |||
| Gentamicin | 14–55% | 16–33% | – |
| Amikacin | 15–30% | – | – |
| OTHERS | |||
| Metronidazole | – | – | 50% |
| Linezolida | – | 23–51% | 44% |
| Daptomycin | – | 12–55% or 108% | 20% |
| TMP-SMX | 11–60% | 15–50% | 25% |
| Rifampicin | 17–41% | 20–25% | 40% |
| Tigecyclinea | – | 35–195% or 47% | – |
| Clindamycin | 98.3% | 21–45% | 26% |
aNot registered for pediatric use
However, it should be considered that the minimum inhibitory concentration (MIC) of the isolated bacteria [14] is also determinant for the choice of the best antibiotic.
Intravenous antibiotic therapy
The scientific literature review revealed heterogeneous data on the empiric antibiotic therapy of AHOM.
The most commonly administered antibiotics are anti-staphylococcal penicillins (oxacillin, nafcillin, cloxacillin, and flucloxacillin) and cephalosporins. Among the latter, the most commonly used first generation molecules are cefazolin (the only one available in Italy) [1–4, 17–26], cephalothin [4, 20, 21, 23], and cefradine [20, 21, 23], while the most used second generation one is cefuroxime [18, 22]. Third generation cephalosporins such as ceftriaxone and cefotaxime [3, 27] are less frequently used.
The antibiotic choice should consider several factors, including age, drug toxicity, bone penetration, and local prevalence of methicillin-resistant S. aureus (MRSA) and Extended-Spectrum Beta-Lactamases (ESBL) bacteria. According to age and etiology, intravenous treatment options for uncomplicated AHOM are shown in Tables 4 and 5.
Table 4.
Intravenous treatment of non-complicated AHOM according to age
| Age | Empiric treatment (I choice) | Empiric treatment (II choice) |
|---|---|---|
| < 3 months |
Ampicillin-sulbactam OR Cephazolin + Gentamycin |
Oxacillin + Gentamycin OR Amoxicillin/clavulanate + Gentamycin OR Cefotaxime + Oxacillin (if low prevalence of ESBL) |
| 3 months- 5 years | Cephazolin |
Amoxicillin/clavulanate OR Ampicillin/sulbactam OR Ceftriaxone + Clindamycin or Glycopeptides (if MRSA prevalence > 10%) |
| > 5 years |
Oxacillin OR Cephazolin OR Clindamycin |
Amoxicillin/clavulanate OR Ampicillin/sulbactam OR Ceftriaxone OR Ceftazidime + Clindamycin or Glycopeptides (if MRSA prevalence > 10%) |
Table 5.
Intravenous antibiotic dosage
| Antibiotic | Recommended dose |
|---|---|
| Amoxicillin/clavulanate | 75–100 mg/kg daily of amoxicillin in 3–4 divided doses (max 1 g/dose) |
| Ampicillin/sulbactam | 100–200 mg/kg daily of ampicillin in 4 divided doses (max 2 g/dose) |
| Cephazolin | 150 mg/kg daily in 3–4 divided doses (max 2 g/dose) |
| Ceftazidime | 150 mg/kg daily in 3 divided doses (max 2 g/dose) |
| Ceftriaxone | 50–100 mg/kg daily (max 2 g) |
| Clindamycin | 45 mg/kg daily in 3 divided doses (max 900 mg/dose) |
| Oxacillin | 150–200 mg/kg daily in 4 divided doses (max 2 g/dose) |
| Gentamycin |
neonates ≥35 weeks of gestational age: 4 mg/kg daily during the first week of life, then 5 mg/kg daily > 1 month-10 years: 8 mg/kg the first day, then 6 mg/kg daily > 10 years: 7 mg/kg daily the first day, then 5 mg/kg daily |
| Linezolida |
< 12 years: 30 mg/kg daily in 3 divided doses (max 600 mg/dose) > 12 years: 600 mg twice a day |
| Vancomycin | 45 mg/kg daily in 3 divided doses |
aNot registered for pediatric use
Methicillin-resistant S. aureus
In western countries, the overall prevalence of community-acquired MRSA has increased in recent years and, more specifically, Italian data show high but stable rates of oxacillin-resistant strains [28]. Thus, knowledge of local epidemiology is crucial in establishing the empirical therapy of AHOM. In this regard, data are often little or difficult to analyze because usually not homogeneous and frequently not discriminating between adults and children [5, 7, 29].
There is a broad debate on the empirical use of antibiotics active against MRSA. In fact, according to the European Society of Pediatric Infectious Diseases (ESPID) 2017 guidelines, such drugs should be reserved for areas with MRSA prevalence rates > 10% [7].
In cases of strong clinical suspicion of MRSA osteomyelitis or areas where the local prevalence of MRSA is greater than 10%, the drugs of choice are clindamycin, vancomycin, and linezolid [1–4, 17, 25, 26, 30–32], bearing in mind that the use of the linezolid is off-label in the pediatric age; the use of daptomycin is indicated in case of first-line therapeutic failure [33].
Panton-valentine Leukocidin–producing S. aureus
In recent years, the pathogenic role of Panton-Valentine Leukocidin-producing S. aureus (PVL-SA) has been highlighted, with a reported prevalence in Italy up to 10% of all pediatric AHOM cases [34]. PVL is a toxin causing leukocyte lysis by forming pores in the membrane, with the consequent risk of severe lung, bone, skin, and soft tissues infections [35].
Antibiotic therapy of PVL-SA must aim at inhibiting toxin production: thus, antibiotics inhibiting protein synthesis such as clindamycin, linezolid, or rifampicin are indicated [35].
However, for uncomplicated pediatric AHOM, indications on empirical administration of anti-PVL antibiotics are currently lacking [2, 7].
K. kingae
K. kingae is among the most frequently isolated pathogens in AHOM of children between 3 months and five years of age [1, 2, 7]. A few studies show age stratification of empirically used antibiotics [1, 2]. In these studies, the empirical use of a cephalosporin, or ampicillin/ampicillin-sulbactam, is suggested in this age group to ensure coverage of K. kingae, as anti-staphylococcal penicillins, clindamycin, and glycopeptides are ineffective against this pathogen [36].
Oral antibiotic therapy
In most studies, oral therapy of AHOM is done with high-dose cephalosporin, clindamycin, or amoxicillin-clavulanic acid, alone or in combination with rifampicin [1–5, 10, 19, 21–24, 37–41].
Cephalexin is the oral drug of choice after parenteral therapy with first-generation cephalosporins, as its action profile against penicillin-resistant S.aureus and S.pyogenes is adequate in vitro, with a good absorption and tolerance profile in pediatric patients [19–24].
Clindamycin is a safe, inexpensive, and effective against MSSA and MRSA, available both for intravenous and oral administration [23]. The clinical and bacteriological response to clindamycin is generally excellent when the pathogen is susceptible, with optimal serum and tissue concentrations [19–24]. Clindamycin may therefore be a valid choice when the S.aureus strain is fully susceptible.
According to the literature, amoxicillin-clavulanate is the most widely used antibiotic in European observational studies, either as monotherapy or combined with rifampicin, in settings with a low prevalence of MRSA [10, 40]. In a recent survey on empirical oral therapy of AHOM sent to 31 Italian pediatric centers, amoxicillin-clavulanate was found to be the first choice in all age groups [42]. However, only limited data are available in the literature regarding its effectiveness, and this regimen is associated with a higher rate of side effects when compared with narrower spectrum molecules [43–45]. Nonetheless, the ESPID guidelines emphasize that these side effects are usually non-severe and transient [7].
Trimethoprim/sulfamethoxazole could be used to manage osteomyelitis, even as monotherapy, when the clinical condition is stable [10, 21, 38, 39, 46–49]. This drug represents an attractive option due to its anti-MRSA activity: time-kill kinetic studies have demonstrated bactericidal action at concentrations four times higher than the MIC. Its bone penetration profile is satisfactory (approximately 50% of serum levels for trimethoprim and 15% for sulfamethoxazole). Both oral and parenteral formulations are available. However, a recent study suggested limiting the use of trimethoprim/sulfamethoxazole to severe cases, especially when associated with bacteremia [50].
Rifampicin may also play a role in the combination therapy for S.aureus driven bone infections. Both oral and intravenous formulations are available, with excellent oral bioavailability. Its efficacy is still a matter of debate, and much of its use is based on clinical practice, above all for orthopedic prostheses-related staphylococcal infections [37, 51].
In clindamycin-resistant MRSA infections, linezolid may play an important role. It is considered by many authors as an alternative in MRSA bone and joint infections, first intravenous and then as an oral therapy, particularly in patients with systemic reactions to intravenous vancomycin [52–54], such as the red-man syndrome. It is a good option for managing serious infections that may require long-term therapy, including osteomyelitis, due to its excellent bone penetration. It has an oral bioavailability of 99–100%, so it can easily be switched from parenteral to oral treatment. Neuropathic signs are described among irreversible severe adverse events, especially after prolonged courses of therapy in adults [55]. At present this drug is not registered by European Medicine Agency for pediatric use, even if US-Food and Drug Administration has approved linezolid for several infections in pediatrics.
According to age group, oral treatment options for the therapy of uncomplicated AHOM according to etiology are suggested and shown in Tables 6 and 7.
Table 6.
Suggested oral therapy in uncomplicated AHOM by age group
| Age | Oral therapy |
|---|---|
| Unknown aetiological agent (age < 5 years)a |
Cephalexin OR Amoxicillin-clavulanate +/− Rifampicin |
| Unknown aetiological agent (age > 5 years) |
Cephalexin OR Flucloxacillin OR Clindamycin |
| S. aureus |
Cephalexin OR Flucloxacillin OR Clindamycinb OR TMX-SMX + Rifampicinb OR Linezolidbc |
| K. kingae |
Amoxicillin-clavulanate OR Cefixime OR Cefpodoxime OR Cefazolin OR Trimethoprim/sulphamethoxazole |
| S. pyogenes |
Cephalexin OR Flucloxacillin OR Amoxicillin |
aOral antibiotic therapy is not indicated in infants < 3 months old
bIf MRSA or PVL-SA is suspected or confirmed
cNot registered for pediatric use
Table 7.
Oral antibiotic dosage
| Antibiotic | Recommended dose |
|---|---|
| Cephalexin | 100 mg/kg daily in 4 divided doses (max daily dosage 4 g) |
| Amoxicillin-clavulanate | 80 mg/kg daily in 3 divided doses (max daily dosage 2 g) |
| Amoxicillin | 75–100 mg/kg daily in 3 divided doses (max daily dosage 3 g) |
| Clindamycin | 30–40 mg/kg daily in 3–4 divided doses (max daily dosage 1.8 g) |
| TMP-SMX | 8 mg/kg daily of TMP in 2 divided doses (max daily dosage 320 mg of TMP) |
| Rifampicin | 10–20 mg/kg daily in 1–2 divided doses (max daily dosage 600 mg) |
Length of treatment and switch to oral therapy in AHOM
The total duration (intravenous and oral) of AHOM therapy is widely debated in the scientific literature. The mean total length of treatment of uncomplicated AHOM is approximately four weeks, ranging from 3 to 6 weeks [1–5, 19, 21–26, 46, 47, 56].
Previously, children with osteomyelitis were switched to oral therapy after several weeks of iv treatment (usually 2–4 weeks) and often close to recovery [5]. However, prolonged intravenous antibiotic treatment is associated with longer hospitalization, higher costs, and a central venous catheter placement, with the risk of mechanical complications (i.e., occlusion, rupture, dislocation), venous thrombosis, and catheter-related infection.
In the last decade, several studies were conducted to evaluate the possibility of an early switch to oral therapy (within 2–7 days of starting iv treatment) [21–26, 40, 57, 58]. No difference in terms of treatment failure was observed. However, these studies were conducted in settings with low MRSA prevalence.
The leading indicators for switching from intravenous to oral therapy are still debated in the literature [1–5, 21–25, 31, 59]. In more detail, to guide the switch from intravenous antibiotics to oral therapy, several qualitative and quantitative variables need to be verified, such as good clinical status, improvement of local signs, apyrexia for more than 48 h, and reduction of C-Reactive Protein (CRP) values by at least 50% (< 2–3 mg/dl) [22, 58].
The 2017 ESPID Guidelines recommend to switch to oral therapy after 2–4 days of intravenous antibiotics when the patient shows:
clinical improvement (afebrile or decreasing body temperature for 24–48 h);
improvement of local symptoms;
lack of signs related to complication;
30–50% decrease of CRP (compared to the peak value in the course of the infection);
negative culture tests:
absence of pathogens such as MRSA or PVL-SA, or other antibiotic-resistant pathogens [7].
The duration of oral therapy in uncomplicated AHOM is usually 3–4 weeks, with close monitoring of clinical manifestations, inflammatory markers, and drug tolerability [1, 2, 22].
There is insufficient data to support short iv therapy with a subsequent switch to oral treatment in infants under 3 months of age. According to some studies, infants should receive no less than 4 weeks of parenteral antibiotic therapy exclusively [6, 31]. Switching to oral therapy can only be considered in infants without severe complications who are able to take oral medications.
Suggestions for switching to oral therapy according to the intravenous treatment previously performed are given in Table 8.
Table 8.
Proposed switch to oral therapy on the basis of intravenous therapy
| Intravenous therapy | Proposed oral therapy |
|---|---|
| Cefazolin | Cephalexin |
| Amoxicillin-clavulanate | Amoxicillin-clavulanate |
| Ampicillin | Amoxicillin |
| Ampicillin-sulbactam | Amoxicillin-clavulanate |
| Oxacillin |
Flucloxacillin OR Cephalexina |
| Clindamycin |
Clindamycin OR Trimethoprim/sulfamethoxazolea |
| Ceftriaxone | Amoxicillin-clavulanate |
|
Ceftriaxone + Clindamycin or glycopeptides |
Trimethoprim/sulfamethoxazole + Rifampicin |
| Vancomycin |
Trimethoprim/sulfamethoxazole + Rifampicin OR Linezolidb |
aIf ingestion of tablets is difficult/compromised
bNot registered for pediatric use
Surgical treatment
According to the latest ESPID Guidelines, the approach to osteoarticular infections must include, when possible, the drainage of purulent material and the collection for culture samples, in order to isolate the infectious agent and verify its antimicrobial susceptibility [7].
Nonetheless, the most common approach in treating AHOM is medical therapy [1], since conservative intervention is effective in 90% of cases [5]. Surgery is reserved for those cases where antibiotic therapy alone is insufficient for clinical and laboratory improvement.
Discussion and suggestions for recommendations
The present review on uncomplicated AHOM in children shows that data regarding the epidemiology as well as the type and duration of antibiotic therapy are discordant and not homogeneous.
Thus, the recommendations that the Italian panel suggest for empirical therapy in uncomplicated AHOMs in children between 28 days and 18 years are the following:
infants < 3 months of age: initial empiric use of ampicillin/sulbactam + gentamicin or cefazolin + gentamicin;
- if the prevalence of MRSA is less than 10%
- Infants and children aged 3 months to 5 years: initial empiric use of a first- or second-generation iv cephalosporin;
- Children > 5 years of age: initial empiric use of an iv anti-staphylococcal penicillin or a first- or second-generation cephalosporin or clindamycin, if the prevalence of MRSA is less than 10%;
in case of therapeutic failure demonstrated by clinical and/or laboratory data, switch to second-line therapy (see Table 4);
in infants and children > 3 months of age, switch to oral therapy within 5–7 days of iv therapy, after verifying the compliance of the child and the family;
when switching from iv to oral therapy, prioritize the use of cephalexin or amoxicillin-clavulanic acid, possibly associated with rifampicin; among the anti-staphylococcal penicillin, although difficult to use due to the type of formulation that reduces its compliance, favor the use of flucloxacillin, well tolerated and with good bone penetration;
monitor clinical signs and inflammatory biomarkers 48 to 72 h after the start of iv therapy and before switching to oral therapy (avoid switching in case of worsening of clinical conditions or increase of inflammatory biomarkers);
in case of clinical worsening, modify iv therapy to ensure adequate coverage against resistant pathogens;
favor the use of rifampicin and trimethoprim-sulfamethoxazole, given the good bone penetration and the optimal cost/benefit ratio; administer rifampicin in 2 daily doses, always in combination with other antibiotics, in order to avoid development of resistant strains;
clindamycin should be used with caution due to the high prevalence of resistance in Italy (> 25%), the type of capsule formulation, and frequent gastrointestinal side effects that may reduce compliance with treatment;
discontinue oral therapy 3 to 5 weeks after the switch if there are no complications;
establish close clinical, laboratory, and instrumental follow-up during the administration of oral therapy and in the weeks following the complete discontinuation of therapy, possibly by a multidisciplinary team including pediatricians, an infectious disease specialist, and an orthopedics.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AHOM
Acute Hematogenic Osteomyelitis
- IV
Intravenous
- MALDI-TOF
Matrix Assisted Laser Desorption Ionization – Time of Flight
- MIC
Minimum Inhibitory Concentration
- MRI
Magnetic Resonance Imaging
- MRSA
Methicillin-resistant S. aureus
- OM
Osteomyelitis
- PVL-SA
Panton-Valentine Leukocidin-producing S. aureus.
Authors’ contributions
AK, EC: Conceptualized and designed the study. MC, FC, GB, RMT, SB, DD, SB, AG: Literature review and data collection. PM, ES, EC, DT, AF, GMR, AN, MC, DC, CT, BB, GT: Data analysis. EC, AK, EV, LG, MR, CM: Drafted and wrote the manuscript. DD, SG, LP, TM, PB, CM, EB, DV, GN, ALV, AG, PM, ES: Interpretation of data. AV, LG, GCG, SE, PM: Revised the manuscript for important intellectual content. All authors reviewed and accepted the final manuscript.
Funding
No funding was secured for this study.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Scientific societies represented:
Italian society of pediatrics (SIP)
Italian society of pediatrics infectious disease (SITIP)
Italian society of traumatology and pediatric orthopedics (SITOP)
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McNeil JC. Acute Hematogenous osteomyelitis in children: clinical presentation and management. Infect Drug Resist. 2020;13:4459–4473. doi: 10.2147/IDR.S257517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Congedi S, Minotti C, Giaquinto C, et al. Acute infectious osteomyelitis in children: new treatment strategies for an old enemy. World J Pediatr. 2020;16:446–455. doi: 10.1007/s12519-020-00359-z. [DOI] [PubMed] [Google Scholar]
- 3.Autore G, Bernardi L, Esposito S. Update on acute bone and joint infection in paediatrics: a narrative review on the most recent evidence-based recommendations and appropriate antinfective therapy. Antibiotics. 2020;9(8):486. doi: 10.3390/antibiotics9080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiappini E, Mastrangelo G, Lazzeri S. A case of acute osteomyelitis: an update on diagnosis and treatment. Int J Environ Res Public Health. 2016;13:1–10. doi: 10.3390/ijerph13060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med. 2014;370(4):352–360. doi: 10.1056/NEJMra1213956. [DOI] [PubMed] [Google Scholar]
- 6.Roversi M, Chiappini E, Toniolo R, et al. Neonatal osteomyelitis: an Italian multicentre report of 22 cases and comparison with the inherent literature. J Perinatol. 2021;41(6):1293–1303. doi: 10.1038/s41372-021-00956-4. [DOI] [PubMed] [Google Scholar]
- 7.Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, Girschick H, Hartwig N, Kaplan S, Lorrot M, Mantadakis E, Peltola H, Rojo P, Zaoutis T, LeMair A. Bone and joint infections. Pediatr Infect Dis J. 2017;36(8):788–799. doi: 10.1097/INF.0000000000001635. [DOI] [PubMed] [Google Scholar]
- 8.Pääkkönen M, Kallio MJT, Kallio PEK, et al. Sensity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res. 2010;468(3):861–866. doi: 10.1007/s11999-009-0936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butbul-Aviel Y, Koren A, Halevy R, Sakran W. Procalcitonin as a diagnostic aid in osteomyelitis and septic arthritis. Pediatr Emerg Care. 2005;21(12):828–832. doi: 10.1097/01.pec.0000190226.12610.24. [DOI] [PubMed] [Google Scholar]
- 10.Chiappini E, Krzysztofiak A, Bozzola E, Gabiano C, Esposito S, Lo Vecchio A, Govoni MR, Vallongo C, Dodi I, Castagnola E, Rossi N, Valentini P, Cardinale F, Salvini F, Bona G, Bossi G, Olivieri AN, Russo F, Fossali E, Bottone G, Dellepiane M, de Martino M, Villani A, Galli L. Risk factors associated with complications/sequelae of acute and subacute haematogenous osteomyelitis: an Italian multicenter study. Expert Rev Anti-Infect Ther. 2018;16(4):351–358. doi: 10.1080/14787210.2018.1453357. [DOI] [PubMed] [Google Scholar]
- 11.Bartoloni A, Aparisi Gómez MP, Cirillo M, Allen G, Battista G, Guglielmi G, Tomà P, Bazzocchi A. Imaging of the limping child. Eur J Radiol. 2018;109:155–170. doi: 10.1016/j.ejrad.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Guillerman RP. Osteomyelitis and beyond. Pediatr Radiol. 2013;43(Suppl 1):S193–S203. doi: 10.1007/s00247-012-2594-9. [DOI] [PubMed] [Google Scholar]
- 13.Jaramillo D, Dormans JP, Delgado J, Laor T, St Geme JW., III Hematogenous osteomyelitis in infants and children: imaging of a changing disease. Radiology. 2017;283(3):629–643. doi: 10.1148/radiol.2017151929. [DOI] [PubMed] [Google Scholar]
- 14.Boselli E, Allaouchiche B. Diffusion in bone tissue of antibiotics. Presse Med. 1999;28:2265–2276. [PubMed] [Google Scholar]
- 15.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48(2):89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–136. doi: 10.1016/j.ijid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti-Infect Ther. 2010;8(2):175–181. doi: 10.1586/eri.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Saux N. Diagnosis and management of acute osteoarticular infections in children. Paediatr Child Health. 2018;23(5):336–343. doi: 10.1093/pch/pxy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pääkkönen M, Peltola H. Antibiotic treatment for acute haematogenous osteomyelitis of childhood: moving towards shorter courses and oral administration. Int J Antimicrob Agents. 2011;38(4):273–280. doi: 10.1016/j.ijantimicag.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Pääkkönen M, Kallio MJ, Peltola H, et al. Antibiotic treatment and surgery for acute Hematogenous calcaneal osteomyelitis of childhood. J Foot and Ankle Surg. 2015;54(5):840–843. doi: 10.1053/j.jfas.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Peltola H, Pääkkönen M, Kallio P, Kallio MJT. Osteomyelitis-septic arthritis study group. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128. doi: 10.1097/INF.0b013e3181f55a89. [DOI] [PubMed] [Google Scholar]
- 22.Jagodzinski NA, Kanwar R, Graham K, Bache CE. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop. 2009;29(5):518–525. doi: 10.1097/BPO.0b013e3181ab472d. [DOI] [PubMed] [Google Scholar]
- 23.Peltola H, Pääkkönen M, Kallio P, Kallio MJ, OM-SA Study Group OM-SA study group. Clindamycin vs. first-generation cephalosporins for acute osteoarticular infections of childhood--a prospective quasi-randomized controlled trial. Clin Microbiol Infect. 2012;18(6):582–589. doi: 10.1111/j.1469-0691.2011.03643.x. [DOI] [PubMed] [Google Scholar]
- 24.Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636–642. doi: 10.1542/peds.2008-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeRonde KJ, Girotto JE, Nicolau DP. Management of Pediatric Acute Hematogenous Osteomyelitis, part II: a focus on methicillin-resistant Staphylococcus aureus, current and emerging therapies. Pharmacotherapy. 2018;38(10):1021–1037. doi: 10.1002/phar.2164. [DOI] [PubMed] [Google Scholar]
- 26.DeRonde KJ, Girotto JE, Nicolau DP. Management of Pediatric Acute Hematogenous Osteomyelitis, part I: antimicrobial stewardship approach and review of therapies for methicillin-susceptible Staphylococcus aureus, Streptococcus pyogenes, and Kingella kingae. Pharmacotherapy. 2018;38(9):947–966. doi: 10.1002/phar.2160. [DOI] [PubMed] [Google Scholar]
- 27.McBride S, Thurm C, Gouripeddi R, Stone B, Jaggard P, Shah SS, Tieder JS, Butcher R, Weiser J, Hall M, Keren R, Landrigan CP. Comparison of empiric antibiotics for acute osteomyelitis in children. Hosp Pediatr. 2018;8(5):280–287. doi: 10.1542/hpeds.2017-0079. [DOI] [PubMed] [Google Scholar]
- 28.Campanile F, Bongiorno D, Perez M, Mongelli G, Sessa L, Benvenuto S, Gona F, AMCLI – S. aureus Survey Participants. Varaldo PE, Stefani S. Epidemiology of Staphylococcus aureus in Italy: first nationwide survey, 2012. J Global Antimicrobial Resistance. 2015;3(4):247–254. doi: 10.1016/j.jgar.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Pääkkönen M, Peltola H. Bone and joint infections. Pediatr Clin N Am. 2013;60(2):425–436. doi: 10.1016/j.pcl.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Spyridis N, Syridou G, Goossens H, Versporten A, Kopsidas J, Kourlaba G, Bielicki J, Drapier N, Zaoutis T, Tsolia M, Sharland M, ARPEC Project Group Members. ARPEC project group members Variation in paediatric hospital antibiotic guidelines in Europe. Arch Dis Child. 2016;101(1):72–76. doi: 10.1136/archdischild-2015-308255. [DOI] [PubMed] [Google Scholar]
- 31.Howard-Jones AR, Isaacs D. Systematic review of duration and choise of systemic antibiotic therapy for acute haematogenous bacterial osteomyelitis in children. J Pediatr Child Health. 2013;49(9):760–768. doi: 10.1111/jpc.12251. [DOI] [PubMed] [Google Scholar]
- 32.McNeil JC, Kaplan SL, Vallejo JG. The influence of the route antibiotic administration, methicillin-susceptibility, vancomycn duration and serum trough concentration on outcome of pediatric Staphylococcus aureus bacteriemic osteoarticular infection. Pediatr Infect Dis J. 2017;36(6):572–577. doi: 10.1097/INF.0000000000001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley JS, Arrieta AC, Digtyar VA, Popejoy MW, Grandhi A, Bokesch P, Hershberger E, Dorr MB, Tan CM, Murata Y, Wolf DJ, Bensaci M. Daptomycin for pediatric gram-positive acute Hematogenous osteomyelitis. Pediatr Infect Dis J. 2020;39(9):814–823. doi: 10.1097/INF.0000000000002790. [DOI] [PubMed] [Google Scholar]
- 34.Chiappini E, Camposampiero C, Lazzeri S, et al. Epidemiology and Management of Acute Haematogenous Osteomyelitis in a tertiary Paediatric center. Int J Environ Res Public Health. 2017;14(5). [DOI] [PMC free article] [PubMed]
- 35.Montagnani C, Cocchi P, Bianchi L, Resti M, de Martino M, Galli L. Severe infections cause by Panton-valentine leucocidin-positive Staphylococcus aureus in infants: report of three cases and review of literature. Acta Paediatr. 2013;102(6):e284–e287. doi: 10.1111/apa.12225. [DOI] [PubMed] [Google Scholar]
- 36.Alcobendas R, Murias S, Remesal A, et al. Oral treatment of osteoarticular infections caused by Kingella kingae in children. Eur J Rheumatol. 2018;5:147–148. doi: 10.5152/eurjrheum.2017.17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sendi P, Zimmerli W. The use of rifampin in staphylococcal orthopaedic-device-related infections. Clin Microbiol Infect. 2017;23(6):349–350. doi: 10.1016/j.cmi.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Kargel JS, Sammer DM, Pezeshk RA, Cheng J. Oral Antibiotics Are Effective for the Treatment of Hand Osteomyelitis in Children. Hand (N Y) 2018;3:1558944718788666. doi: 10.1177/1558944718788666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alcobendas R, Remesal A, Murias S, Nuñez E, Calvo C. Outpatients with acute osteoarticular infections had favourable outcomes when they received just oral antibiotics without intravenous antibiotics. Acta Paediatr. 2018;107(10):1792–1797. doi: 10.1111/apa.14373. [DOI] [PubMed] [Google Scholar]
- 40.Roul-Levy A, Looten V, Bachy M, Grimprel E, Carbajal R, Vialle R. Oral ambulatory treatment of acute osteomyelitis in children: a case-control study. Pediatr Emerg Care. 2016;32(3):154–156. doi: 10.1097/PEC.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 41.Yi J, Wood JB, Creech CB, et al. Clinical Epidemiology and Outcomes of Pediatric Musculoskeletal Infections. J Pediatr. 2021;S0022–3476(21):00266–00263. doi: 10.1016/j.jpeds.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiappini E, Serrano E, Galli L, Villani A, Krzysztofiak A, Italian Paediatric Collaborative Osteomyelitis Study Group Practical issues in early switching from intravenous to Oral antibiotic therapy in children with uncomplicated acute Hematogenous osteomyelitis: results from an Italian survey. Int J Environ Res Public Health. 2019;16(19):3557. doi: 10.3390/ijerph16193557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimprel E, Lorrot M, Haas H, Pinquier D, Parez N, Ferroni A, Cohen R, Paediatric Infectious Diseases Group of the French Society of Paediatricis (GPIP) Osteoarticular infections: therapeutic proposals of the Paediatric infectious diseases Group of the French Society of Paediatrics (GPIP) Arch Pediatr. 2008;15:S74–S80. doi: 10.1016/S0929-693X(08)74220-9. [DOI] [PubMed] [Google Scholar]
- 44.Lorrot M, Doit C, Ilharreborde B, Vitoux C, Le Henaff L, Sebag G, Pennecot G, Grimprel E, Bingen É. Antibiotic therapy of bone and joint infections in children: recent changes. Arch Pediatr. 2011;18(9):1016–1018. doi: 10.1016/j.arcped.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, Barkman D, Odeniyi F, Conaboy K, Bell L, Zaoutis TE, Fiks AG. Association of Broad- vs narrow-Spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325–2336. doi: 10.1001/jama.2017.18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferroni A, Al Khoury H, Dana C, et al. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect. 2013;19(9):822–828. doi: 10.1111/clm.12031. [DOI] [PubMed] [Google Scholar]
- 47.Islam S, Biary N, Wrotniak B. Favorable outcomes with early transition to Oral antibiotics for Paediatric Osteoarticular infections. Clin Pediatr (Phila) 2019;58(6):696–699. doi: 10.1177/0009922819829041. [DOI] [PubMed] [Google Scholar]
- 48.Adra M, Lawrence KR. Trimethoprim/sulfamethoxazole for treatment of severe Staphylococcus aureus infections. Ann Pharmacother. 2004;38(2):338–341. doi: 10.1345/aph.1D156. [DOI] [PubMed] [Google Scholar]
- 49.Kim BN, Kim ES, Oh MD. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J Antimicrob Chemother. 2014;69(2):309–322. doi: 10.1093/jac/dkt374. [DOI] [PubMed] [Google Scholar]
- 50.Duvall L, Platt B, Kussin M, et al. Outcomes of children with osteoarticular infections treated with trimethoprim-sulfamethoxazole. Pediatr Infect Dis J. 2021;1:S7–S8. doi: 10.1093/jpids/piaa170.023. [DOI] [Google Scholar]
- 51.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-body infection (FBI) study group. JAMA. 1998;279(19):1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 52.Saphyakhajon P. Empiric antibiotic therapy for acute osteoarticular infections with suspected methicillin-resistant staphylococcus aureus or kingella. Pediatr Infect Dis J. 2008;27(8):765–767. doi: 10.1097/INF.0b013e31816fc34c. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan SL. Osteomyelitis in children. Infect Dis Clin N Am. 2005;19(4):787–797. doi: 10.1016/j.idc.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C-J, Chiu C-H, Lin T-Y, Lee ZL, Yang WE, Huang YC. Experience with linezolid therapy in children with osteoarticular infections. Pediatr Infect Dis J. 2007;26(11):985–988. doi: 10.1097/INF.0b013e31812e62dc. [DOI] [PubMed] [Google Scholar]
- 55.Chiappini E, Conti C, Galli L, de Martino M. Clinical efficacy and tolerability of linezolid in paediatric patients: a systematic review. Clin Ther. 2010;32(1):66–88. doi: 10.1016/j.clinthera.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Liu RW, Abaza H, Mehta P, Bauer J, Cooperman DR, Gilmore A. Intravenous versus oral outpatient antibiotic therapy for pediatric acute osteomyelitis. Iowa Orthop J. 2013;33:208–212. [PMC free article] [PubMed] [Google Scholar]
- 57.Keren R, Shah SS, Srivastava R, Rangel S, Bendel-Stenzel M, Harik N, Hartley J, Lopez M, Seguias L, Tieder J, Bryan M, Gong W, Hall M, Localio R, Luan X, deBerardinis R, Parker A, Pediatric Research in Inpatient Settings Network Comparative effectiveness of intravenous vs. oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128. doi: 10.1001/jamapediatrics.2014.2822. [DOI] [PubMed] [Google Scholar]
- 58.Arnold JC, Cannavino CR, Ross MK, Westley B, Miller TC, Riffenburgh RH, Bradley J. Acute bacterial osteoarticular infections: eight-year analysis of C-reactive protein for oral step-down therapy. Pediatrics. 2012;130(4):e821–e828. doi: 10.1542/peds.2012-0220. [DOI] [PubMed] [Google Scholar]
- 59.de Graaf H, Sukhtankar P, Arch B, Ahmad N, Lees A, Bennett A, Spowart C, Hickey H, Jeanes A, Armon K, Riordan A, Herberg J, Hackett S, Gamble C, Shingadia D, Pallett A, Clarke SC, Henman P, Emonts M, Sharland M, Finn A, Pollard AJ, Powell C, Marsh P, Ballinger C, Williamson PR, Clarke NMP, Faust SN. Duration of intravenous antibiotic therapy for children with acute osteomyelitis or septic arthritis: a feasibility study. Health Technol Assess. 2017;21(48):1–164. doi: 10.3310/hta21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.