Abstract
Introduction:
Emergency department thoracotomy (EDT) must be rapid and well-executed. Currently there are no defined benchmarks for EDT procedural milestones. We hypothesized that trauma video review (TVR) can be used to define the ‘normative EDT’ and generate procedural benchmarks. As a secondary aim, we hypothesized that data collected by TVR would have less missingness and bias than data collected by review of the Electronic Medical Record (EMR).
Methods:
We used continuously recording video to review all EDTs performed at our centre during the study period. Using skin incision as start time, we defined four procedural milestones for EDT: 1. Decompression of the right chest (tube thoracostomy, finger thoracostomy, or clamshell thoracotomy with transverse sternotomy performed in conjunction with left anterolateral thoracotomy) 2. Retractor deployment 3. Pericardiotomy 4. Aortic Cross-clamp. EDTs with any milestone time ≥ 75th percentile of time or during which a milestone was omitted were identified as outliers. We compared rates of missingness in data collected by TVR and EMR using McNemar’s test.
Results:
44 EDTs were included from the study period. Patients had a median age of 30 [IQR 25–44] and were predominantly African-American (95%) males (93%) with penetrating trauma (95%). From skin incision, median times in minutes to milestones were as follows: right chest decompression: 2.11 [IQR 0.68–2.83], retractor deployment 1.35 [IQR 0.96–1.85], pericardiotomy 2.35 [IQR 1.85–3.75], aortic cross-clamp 3.71 [IQR 2.83–5.77]. In total, 28/44 (64%) of EDTs were either high outliers for one or more benchmarks or had milestones that were omitted. For all milestones, rates of missingness for TVR data were lower than EMR data (p < 0.001).
Conclusions:
Video review can be used to define normative times for the procedural milestones of EDT. Steps exceeding the 75th percentile of time were common, with over half of EDTs having at least one milestone as an outlier. Data quality is higher using TVR compared to EMR collection. Future work should seek to determine if minimizing procedural technical outliers improves patient outcomes.
Keywords: Emergency department, thoracotomy, Video review, Resuscitation, Quality improvement
Introduction
Emergency department thoracotomy (EDT) is associated with mortality rates of 75–94% [1,2] but is currently the only widely accepted salvage option available for injured patients in cardiac arrest. Despite the high mortality, EDT has a favourable incremental cost-effectiveness ratio of $16,125/quality adjusted life year secondary to the young average age of EDT patients [3]. Factors associated with mortality have been described in this patient population including mechanism [4–7] and pattern of injury [8,9], prehospital transport time [10–12], and the presence of signs of life [13] but unfortunately none of these factors are subject to modification by treating centres or providers.
According to the ‘three-phase’ model of cardiac arrest proposed by Becker et al time after cardiac arrest can be divided into three phases (electrical: up to 4 min; circulatory: ~4 to ~10min; and metabolic: >~10min) [14]. Most patients presenting in cardiac arrest after injury will be beyond the electrical phase secondary to prehospital transport time. In the circulatory phase of cardiac arrest, the most important therapy is to initiate a technique to provide oxygen delivery to ischemic tissue. After ~10 min without perfusion, cardiac arrest patients may progress into the metabolic phase, beyond which point survival is poor even if circulation returns secondary to global ischemia-reperfusion. Because there is only a limited amount of time until patients progress from the circulatory phase into the metabolic phase, techniques to restore perfusion that are not completed rapidly are likely to be futile. Based on this framework, speed of EDT may represent a modifiable risk factor for mortality in trauma patients arriving in cardiac arrest.
A critical barrier to the study of technique and speed as a modifiable risk factor for mortality in post-injury cardiac arrest patients undergoing EDT is data collection. Prospective data collection by research personnel is challenging because patients requiring emergency department thoracotomies arrive unexpectedly and infrequently. Prospectively collecting data on EDTs requires research personnel that are continuously present and available, a resource that is beyond most centres. Prospective real-time data collection is additionally challenged by the rapid and chaotic nature of these emergent events, which may lead to imprecise or incomplete capture of data elements [15]. Important aspects such as timing and technique of EDT are not recorded or incompletely captured in the electronic medical record, which may limit the utility of retrospective chart review as an option for reliable data collection [16].
Use of trauma video review (TVR) with continuous high-definition audiovisual recording systems may present a solution to the near absence of granular data on timing and technique of emergency department thoracotomy. As a first step towards generating procedural benchmarks for EDT, we hypothesized that we could rigorously define the distribution of times to achieve critical procedural milestones using TVR as a data collection tool. We further hypothesized that compared to TVR, data collection using retrospective chart review would have higher rates of missing data and be associated with substantial bias with respect to timing of procedural milestones.
Methods
The John Paul Pryor Shock Trauma and Resuscitation Center at the University of Pennsylvania evaluates ~2700 contacts annually, of which ~21% are victims of penetrating trauma. The state of Pennsylvania is located in the northeastern region of the United States and is the fifth most populous state in the country. The Trauma Quality Improvement Audiovisual Program has been an integral part of quality improvement and education efforts at our centre since the early 1990 s. At our institution, each of the 5 resuscitation bays is equipped with a continuously recording, high-definition audiovisual camera at the foot of the bed and another camera at the head of the bed (Fig. 1). These cameras continuously record all activity within the field of view 24-hours a day, seven days a week. Recordings are stored on a secure server for 28 days, after which time they are automatically deleted. Resuscitations with opportunities for improvement (including multiple patient scenarios, combative patients, and patients in extremis) are selected and reviewed in a monthly multidisciplinary conference with a focus on education and continuous quality improvement. As part of these efforts, we noted that there appeared to be significant variability in the times to completion of EDT which led to a quality improvement effort focused on this procedure.
Fig. 1.
Representative views from the foot-of-the-bed (A) and overhead (B) high-definition video recording cameras located in each resuscitation bay.
For this project, we reviewed the resuscitations of every patient who underwent an emergency department thoracotomy between April 2016 and September 2017 at our institution. All resuscitations were reviewed by one of three reviewers (DH, RD, KC). 10% of videos were co-abstracted independently and reviewed together to ensure time points were similarly defined. We used TVR to carefully review every resuscitation in which a patient underwent a EDT, from the moment the patient arrived until the patient moved on to the next phase of care. Reviewers were able to play, pause, rewind and fast-forward recordings. The ability to pause and resume playback allows for very accurate data collection in a prospective fashion as-if it the resuscitation is occurring in real-time.
The first aim of this study was to describe the central tendencies and variability in times to completion of procedural milestones. Based on the cardinal steps of emergency department thoracotomy and available literature [17], we defined four procedural milestones for EDT: 1. Decompression of the right chest (tube thoracostomy, finger thoracostomy, or clamshell thoracotomy with transverse sternotomy performed in conjunction with left anterolateral thoracotomy) 2. Retractor deployment 3. Pericardiotomy 4. Aortic Cross-clamp (Table 1). Using TVR, we rigorously measured the time to completion of these procedures from a.) patient arrival in the trauma bay and b.) from the time of emergency department thoracotomy skin incision. Because right chest decompression ideally occurs by a second provider and in conjunction with left chest entry, it is possible for the right chest to be decompressed prior to completion of the left anterolateral thoracotomy. We defined times to procedural milestones as high outliers if they met or exceeded the 75th percentile of time to completion of at least one milestone or those cases which were deficient in one or more milestones (e.g. no right chest decompression). The second aim of this study was to compare the data collection using TVR compared to review of the electronic medical record (EMR). We compared the rates of missingness for TVR and EMR data abstraction. Because TVR recordings contain a running time-date clock, we also calculated the percentage bias between time measurements derived from TVR and EMR for each of the four procedural milestones defined above.
Table 1.
Descriptions, definitions, purposes, and aetiologies of cardiac arrest addressed for procedural milestones of EDT.
| EDT Procedural Milestone | Description | Timing Definition | Critical purpose | Traumatic shock aetiology addressed |
|---|---|---|---|---|
| Right Chest Decompression | Insertion of decompressing tube into right pleural space or incision through right chest wall | Patient arrival to decompression of the right pleural space | Diagnosis and treatment of right pneumothorax or hemothorax Direct control of bleeding structures in the right chest | Right Tension Pneumothorax Right Tension Hemothorax Right Chest Bleeding |
| Retractor Deployment | Anterolateral incision of the left chest wall from skin to pleural space | Patient arrival to placement of chest wall retractor | Surgical exposure for subsequent milestones Diagnosis and treatment of left pneumothorax or hemothorax Direct control of bleeding structures in the left chest | Left Tension Pneumothorax Left Tension Hemothorax Left Chest Bleeding |
| Pericardiotomy | Opening of the pericardium, direct inspection of the heart | Patient arrival to delivery of the heart from the pericardial sac | Diagnosis and treatment of cardiac tamponade Diagnosis and treatment of cardiac injury Exposure for internal cardiac compressions | Cardiac Tamponade Cardiac Injury |
| Aortic Occlusion | Clamping of the descending thoracic aorta just above the diaphragm | Patient arrival to clamping of the descending aorta | Maximize perfusion of cerebral and coronary circulation by shunting available cardiac output Control of infradiaphragmatic hemorrhage | Abdominopelvic bleeding Lower extremity bleeding |
| Open Cardiac Massage | Open compression of the heart | Patient arrival to initiation of internal compressions | Provide cardiac output through manual compression of the non-beating heart | Cardiac failure |
Patient variables collected for this project included patient demographic information (age, sex, race), mechanism of injury and injury severity (as measured by Injury Severity Score (ISS) and New Injury Severity Score (NISS)). Outcome data collected included return of spontaneous circulation (RSOC) and mortality. We collected training level (resident, fellow, or attending) for physician providers. Descriptive statistics were used where appropriate and the distribution of continuous data was visually inspected and tested for normality using Shapiro-Wilk test. To compare rates of missingness between data collected from TVR and EMR abstraction, we used McNemar’s test for paired dichotomous data. We defined two-tailed statistical significance as α < 0.05. Stata v14.0 was used for all statistical analyses (StataCorp, College Station. TX).
Results
During the study period, 44 EDTs were reviewed and abstracted and included in the final analysis. Patients had a median age of 30 [IQR 25–44] and were predominantly African-American 42/44 (95%) males 41/44 (93%) with penetrating trauma 42/44 (95%). The demographics of the cohort are displayed in Table 2. The population was severely injured with a median ISS of 25 [IQR 16–25] and a NISS of 36 [IQR 25–66]. Two-thirds of the patients arrived by police transport. 11/44 (25%) of patients had signs of life upon arrival to the emergency department. From patient arrival, median time in minutes to achieve procedural milestones were as follows: right chest decompression: 5.33 [IQR 4.0–6.80], retractor deployment 5.02 [IQR3.67–6.68], pericardiotomy 5.83 [IQR 4.85–8.68], aortic cross-clamp 8.03 [IQR 5.81–10.11] (Fig. 2). From skin incision, median times in minutes to milestones were as follows: right chest decompression: 2.11 [IQR 0.68–2.83], retractor deployment 1.35 [IQR 0.96–1.85], pericardiotomy 2.35 [IQR 1.85–3.75], aortic cross-clamp 3.71 [IQR 2.83–5.77] (Fig. 3). 20/44 (45%) of the procedures were performed by senior-level residents (PGY4–5), while 19/44 (43%) of thoracotomies were performed by trauma fellows with attendings performing the remaining 4/44 (9%) of procedures. We identified that 28/44 (64%) of procedures were high outliers (as defined by >75th percentile of time or omission of a milestone) (Table 3). Procedural milestones consisting of pericardiotomy, right chest decompression, and or aortic cross-clamp were not performed in 9/44 (20%) of cases.
Table 2.
Characteristics of patients in the study.
| Patient characteristic | Patients (n = 44) |
|---|---|
| Age in years | 30 (IQR 25–44) |
| Male sex | 41 (93%) |
| Race | |
| African American | 42 (95%) |
| Caucasian | 1 (2%) |
| Missing from record | 1 (2%) |
| Injury Mechanism | |
| Gunshot wound | 37 (84%) |
| Stab wound | 5 (11%) |
| Motor Vehicle | 1 (2%) |
| Motorcycle | 1 (2%) |
| Injury Severity Score | 25 (IQR 16–25) |
| New Injury Severity Score | 36 (IQR 25–66) |
| Prehospital Transport | |
| Police | 29 (66%) |
| Ambulance | 13 (30%) |
| Other | 2 (4%) |
| SOL in Emergency Department | 11 (25%) |
| Outcomes | |
| ROSC | 13 (30%) |
| Mortality | 43(98%) |
| Survival | 1 (2%) |
| Neurologically Intact Survival | 1 (2%) |
Abbreviations: IQR = Interquartile range. SOL = Signs of Life.
Fig. 2.
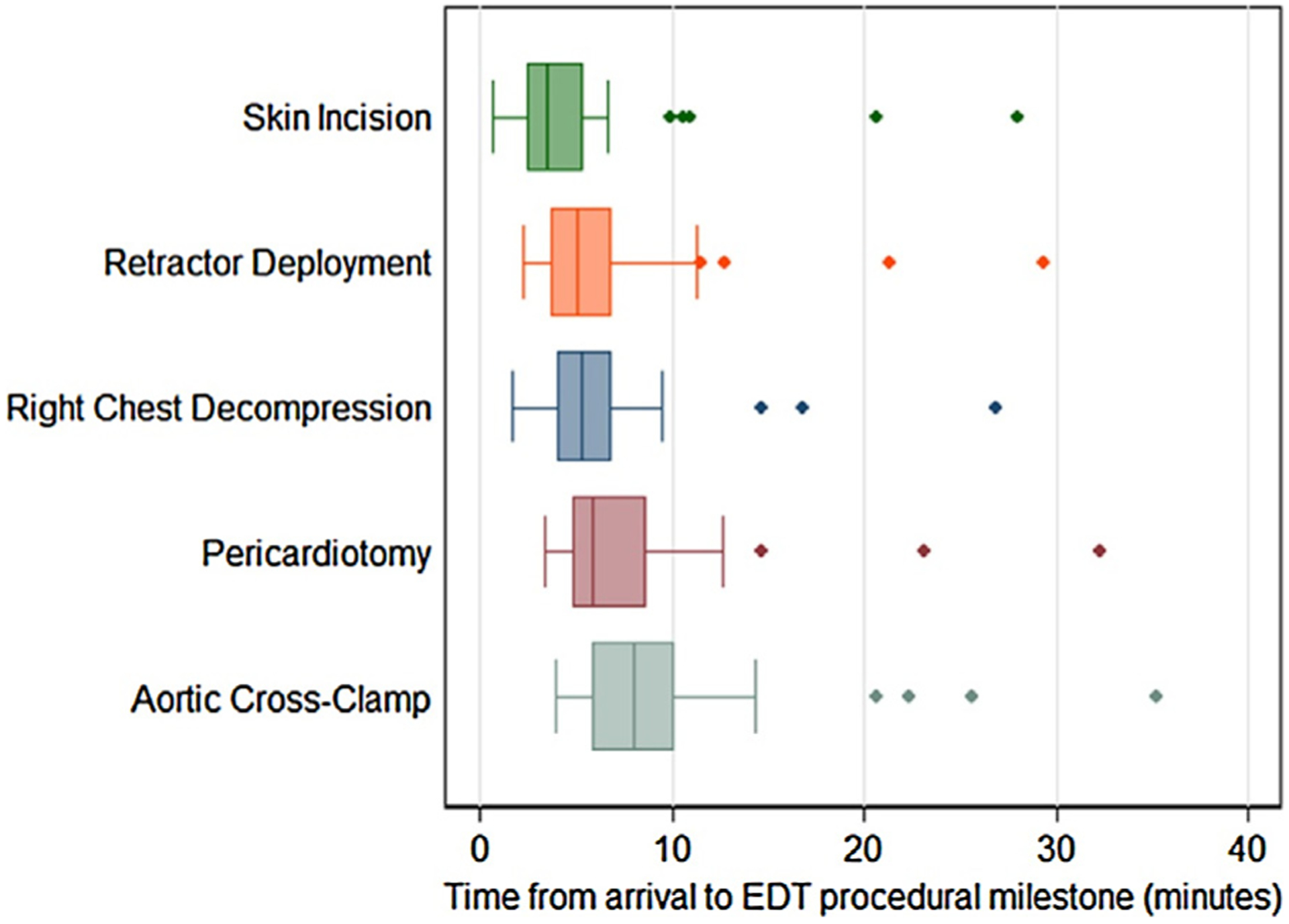
Box plots of times from patient arrival to completion of EDT procedural milestones in minutes. Whiskers represent highest and lowest non-outlier values (outliers defined as 1.5(IQR) beyond 1st or 3rd quartile of time).
Fig. 3.
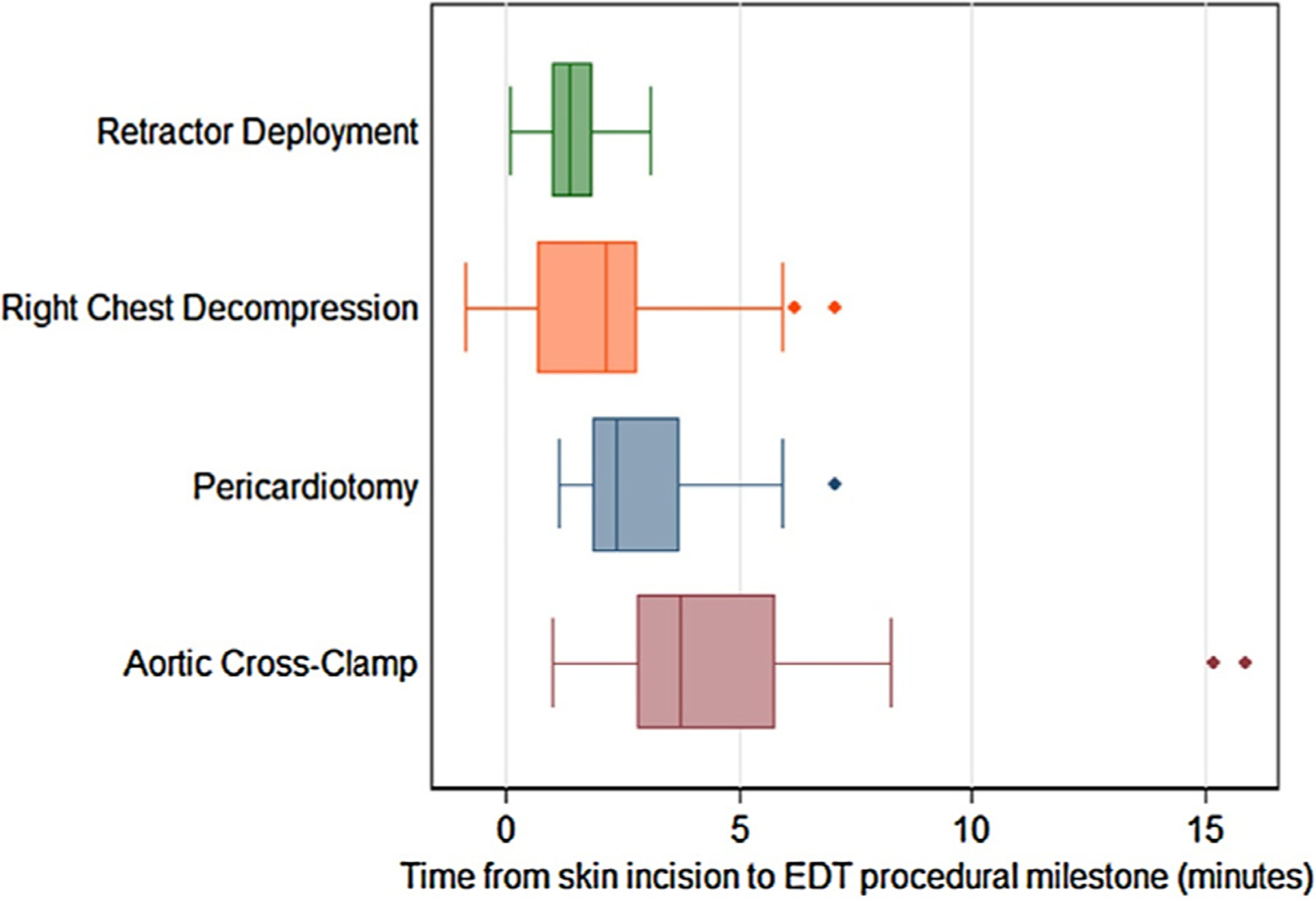
Box plots of times from skin incision to completion of EDT procedural milestones in minutes. Minimum times for right chest tubes may be less than zero referent to skin incision start times for EDT because in some cases right chest decompression occurred prior to initiation of EDT. Whiskers represent highest and lowest non-outlier values (outliers defined as 1.5(IQR) beyond 1st or 3rd quartile of time).
Table 3.
Median times to defined EDT procedural milestones from skin incision presented as median (IQR). Outliers defined as milestones achieved at ≥75th percentile of time or milestones not performed.
| Procedural Milestone | Time to completion in minutes Median (IQr) | Outliers (≥75th percentile OR milestone not performed) N (%) |
Milestone not performed N (%) |
|---|---|---|---|
| Retractor Deployment | 1.35 (0.96–1.85) | 12 (27%) | 0 (0%) |
| Right Chest Decompression | 2.11 (0.68–2.83) | 18 (41%) | 7(16%) |
| Pericardiotomy | 2.35 (1.85–3.75) | 16 (36%) | 2 (5%) |
| Aortic Cross Clamp | 3.71 (2.83–5.77) | 14 (32%) | 4 (9%) |
Abbreviations: IQR = Interquartile range.
With respect to the quality of data abstracted by TVR compared to EMR, only 25/44 (57%) of the patients had any procedural time data recorded in the EMR at all. No patients had all time points for the four milestones documented in the EMR. Timing data for EDT procedural milestones was frequently absent and ranged in missingness from 21/44 (48%) (time of aortic cross-clamp) to 1/44 (98%) (time of pericardiotomy). In contrast, data abstracted from TVR was missing in 0% to 2.7% of cases and was less likely to be missing for each procedural milestone than data collected from the EMR (p < 0.001) (Table 4). When data was actually present in the EMR, bias associated with time measurements was substantial, ranging from 10.7% to 28%. With respect to clinical outcomes in the overall cohort, 13/44 (30%) patients had return of spontaneous circulation (ROSC). Overall survival in the cohort was 2%, neurologically intact survival was also 2%.
Table 4.
Missingness and bias associated with EDT time data abstracted from the EMR compared to data abstracted from TVR. P values are for McNemar’s test for paired dichotomous data.
| Procedural milestone | % Bias vs. TVR Median (IQR) | Timing data absent in EMR N (%) |
Timing data absent in TVR N (%) |
p |
|---|---|---|---|---|
| Skin incision (n = 44) | 14.5 (5.8–18.9) | 30 (68%) | 0 (0%) | <0.001 |
| Retractor deployment (n = 44) | 28.0 (14.5–110.5) | 40 (91%) | 0 (0%) | <0.001 |
| Right chest decompression (n = 37) | 17.1 (10.5–72.5) | 32 (86%) | 1 (3%) | <0.001 |
| Pericardiotomy (n = 42) | 10.8 (10.8–10.8) | 41 (98%) | 0 (0%) | <0.001 |
| Aortic cross clamp (n = 40) | 10.7 (6.3–31.9) | 19 (48%) | 1 (3%) | <0.001 |
Discussion
Using data abstraction from a continuously running audiovisual recording system, we were able to describe the variability in time to completion of the procedural milestones of emergency department thoracotomy and use this data to define benchmarks of quality. To our knowledge, this study is the first of its kind. Surgeons emphasize the need for speed during resuscitative procedures such as EDT and tube thoracostomy, but this the first investigation to quantify the time a procedure takes to complete and underscores the variability in speed with which specific procedural milestones take place.
This investigation was only possible through the use of our continuously recording audiovisual review system, which has been a part of our performance improvement and education efforts since the 1990 s. Although our program is now well established, there are some common barriers to implementation that have been identified in the literature. In their survey of American trauma centre videotaping practices, Ellis et al found that a common barrier was the time and personnel needed to run a trauma video review program [18]. In a similar survey conducted by Campbell et al. [19], the investigators found that the most common barrier was medicolegal concerns. This was supported by a third survey distributed to paediatric centres that identified similar concern [20].
Even at busy trauma centres, annual individual provider volume for such high-impact low frequency procedures such as EDT may be low, making continuous quality improvement efforts focused on these procedures particularly warranted. Accurate measurement of processes is a key aspect of quality improvement, and centres that do not measure process and outcome variables related to resuscitation run the risk operating without insight into their objective performance. Despite the fact that ours is a centre with relatively large volume of penetrating trauma and the second-highest annual volume of EDT in the state of Pennsylvania, we were somewhat discouraged to learn that standard procedural steps for EDT such as pericardiotomy, right chest decompression, and aortic cross-clamping were not performed up to 1 in 5 cases. While some of these omissions may have been secondary to futility discovered upon inspection of wounding patterns to the thoracic viscera [8], others appeared to be omissions of oversight. We also found significant levels of variability in the time to completion of procedural milestones, with high outliers identified for every step. For example, the shortest times from patient arrival to aortic cross-clamp observed were approximately 4–5 minutes; the longest times were over 20 min. This finding underscores the wide variability in the speed with which this procedure is performed and emphasizes the need to identify opportunities to minimize procedural variability. For patients in cardiac arrest, warm ischemic time attributable to slower procedural times may contribute to some of the high rates of mortality observed in patients undergoing EDT over the past 5 decades. Although our study is underpowered to show that speed makes a difference in outcomes, there is strong face validity to the concept that in patients with survivable injuries who are rapidly transported to trauma centres, EDT procedural speed may be an important determinant of ROSC and patient survival. As “thoracotomy trainers” become more widely available, we hope to use the results of our investigation to implement simulation training at all provider levels. Such training has been shown to improve provider speed during resuscitative procedures [21].
Our study also incorporates and quantifies both the time elapsed from patient arrival to completion of each procedural milestones as well as the time from skin incision to completion of the milestones. We chose to present the data in two different ways because measuring time elapsed from patient arrival to completion of procedural milestones includes the time it takes to make the clinical decision to proceed with EDT, while measuring the times from skin incision to completion of procedural milestones reflects the speed of the actual procedure. The procedure itself may be done quickly, but if the decision to proceed is delayed or the initiation of the procedure is delayed then technical speed is of little consequence. Conversely, protracted procedural times may negate any potential benefit of early EDT initiation. Cognitive and procedural aspects of EDT therefore represent overlapping but distinct avenues for quality improvement, and only by measuring both can targets for interventions be identified.
Risk factors for mortality in patients undergoing EDT have been described [22], but unfortunately none of them are subject to modification by treating centres or providers. The mortality in our series is significantly higher compared to European studies, a recent systematic review of European resuscitative thoracotomies found that the overall survival for thoracotomies performed in the emergency department for penetrating trauma was over 40% [23]. This difference is largely driven by the absence of signs of life in 3 out of 4 patients in our series and high proportions of gunshot wounds. Although the concept that perfusion should be restored as rapidly as possible has strong face validity based on literature from other cardiac arrest patient populations [24–27], there is essentially no literature to support this contention in trauma cohorts. Study of the relationship between EDT procedural factors such as speed and survival after traumatic cardiac arrest thus represents one area where modifiable risk factors for mortality may yet be discovered. Until recently, such investigations would have been limited by the absence of granular, unbiased, non-missing data necessary for rigorous study. In comparing times abstracted from the TVR to times abstracted from the EMR, we found high rates of missingness and bias for EDT timing data in the EMR. This finding underscores the problems and ineffectiveness of chart review for quality improvement and process improvement initiatives. This finding was also highlighted by Oakley et al, who only found a 20% error detection rate using traditional EMR review compared to video review [16]. Based on our findings in this study, we believe that using TVR as a data collection modality for quality improvement and research efforts focused on resuscitation is a vast improvement over abstraction from the medical record and should be considered the new gold standard. TVR can be used to study everything from ATLS protocol adherence, universal precaution utilization, leadership as well as system errors [16,28–32]. Hoyt et al described the first use of TVR as a quality improvement and educational tool in 1988. They recorded over 2500 resuscitations with a simple portable TV stand on which a VCR, camera and TV was mounted. Using TVR they were able to demonstrate a decrease in time to definitive care for matched patient-groups cared for by the same resuscitation team over a three-month period [33]. The authors concluded that one of the principle benefits of TVR as an educational tool is the ability to start, stop, pause and rewind footage in a relaxed constructive setting in which immediate feedback is possible.
Our study has several limitations which must be discussed. Although our centre has relatively high volume of EDT, we are still limited by a small sample size. Since the outcome of survival is rare, we chose to focus on a process metrics (time to completion of specific procedural milestones for EDT) rather than outcome metrics. We made this decision based on what we feel is understood about the relationship between warm ischemic time and survival after cardiac arrest, but literature supporting this relationship in traumatic cardiac arrest in human subjects is not strong. It is also important to emphasize that although this work focuses on defining benchmarks for EDT procedural milestones, not every patient requiring an EDT meets indications for all milestones. For instance, a patient with an isolated stab wound the right ventricle may not require a right chest tube or aortic cross-clamping. However, since the majority of patients in this study were victims of gun violence, in our patient population such cases are the exception rather than the rule. By focusing on procedural milestones defined here as key components of EDT, it is our hope that when specific milestones are omitted this will represent a carefully considered decision rather than an oversight. We also used the 75th percentile of time to define outliers in our procedural benchmarks. Arguments could be made for more or less stringent thresholds, but data from other institutions to support or refute a specific threshold is lacking. Further multicentre study with larger sample sizes may will help identify empirically defined cut points that clinicians can use as goals. Additionally, we do not have autopsy data on these patients, therefore, with the exception of the patients that went to the operating room, the true burden of injury is underestimated as we only captured chest ISS and NISS for those patients that expired in the emergency department.
In a high-impact, high-mortality procedure like emergency department thoracotomy, surgeons should continuously seek to improve patient outcomes. Defining the time it takes for a procedure to be completed and the amount of variability is the first step in identifying a problem and targeting interventions. Our investigation successfully used trauma video review to define ‘normative EDT’ times and identified high outliers while highlighting the shortcomings of the Electronic Medical Record. Future interventions should focus on minimizing procedural variability and targeting outliers.
Conflicts of interest and source of funding
No authors have conflicts to declare. DNH is currently supported by a training grant through the National Heart, Lung, and Blood Institute. (K08HL131995)
Footnotes
Meetings at which this was presented:
The Eastern Association for the Surgery of Trauma, January 10th, 2018.
References
- [1].Seamon MJ, Haut ER, Van Arendonk K, Barbosa RR, Chiu WC, Dente CJ, et al. An evidence-based approach to patient selection for emergency department thoracotomy: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015;79:159–73. [DOI] [PubMed] [Google Scholar]
- [2].Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years22. J Am Coll Surg 2000;190:288–98. [DOI] [PubMed] [Google Scholar]
- [3].Brown TB, Romanello M, Kilgore M. Cost-utility analysis of emergency department thoracotomy for trauma victims. J Trauma 2007;62:1180–5. [DOI] [PubMed] [Google Scholar]
- [4].Moriwaki Y, Sugiyama M, Yamamoto T, Tahara Y, Toyoda H, Kosuge T, et al. Outcomes from prehospital cardiac arrest in blunt trauma patients. World J Surg 2011;35:34–42. [DOI] [PubMed] [Google Scholar]
- [5].Moore EE, Knudson MM, Burlew CC, Inaba K, Dicker RA, Biffl WL, et al. Defining the limits of resuscitative emergency department thoracotomy: a contemporary western trauma association perspective. J Trauma Inj Infect Crit Care 2011;70:334–9. [DOI] [PubMed] [Google Scholar]
- [6].Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000;190:288–98. [DOI] [PubMed] [Google Scholar]
- [7].Kavolius J, Golocovsky M, Champion HR. Predictors of outcome in patients who have sustained trauma and who undergo emergency thoracotomy. Arch Surg 1993;128:1158–62. [DOI] [PubMed] [Google Scholar]
- [8].Seamon MJ, Goldberg AJ, Schwab CW. Emergency department thoracotomy for gunshot wounds of the heart and great vessels. J Trauma Inj Inf Crit Care 2010;68:1514–5. [DOI] [PubMed] [Google Scholar]
- [9].Seamon MJ, Pathak AS, Bradley KM, Fisher CA, Gaughan JA, Kulp H, et al. Emergency department thoracotomy: still useful after abdominal exsanguination? J Trauma Inj Inf Crit Care. 2008;64:1–7. [DOI] [PubMed] [Google Scholar]
- [10].Clevenger FW, Yarbrough DR, Reines HD. Resuscitative thoracotomy: the effect of field time on outcome. J Trauma 1988;28:441–5. [DOI] [PubMed] [Google Scholar]
- [11].Seamon MJ, Fisher CA, Gaughan J, Lloyd M, Bradley KM, Santora TA, et al. Prehospital procedures before emergency department thoracotomy: “scoop and run” saves lives. J Trauma Inj Infect Crit Care 2007;63:113–20. [DOI] [PubMed] [Google Scholar]
- [12].Powell DW, Moore EE, Cothren CC, Ciesla DJ, Burch JM, Moore JB, et al. Is emergency department resuscitative thoracotomy futile care for the critically injured patient requiring prehospital cardiopulmonary resuscitation? J Am Coll Surg 2004;199:211–5. [DOI] [PubMed] [Google Scholar]
- [13].Champion HR, Danne PD, Finelli F. Emergency thoracotomy. Arch Emerg Med 1986;3:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA 2002;288:3035–8. [DOI] [PubMed] [Google Scholar]
- [15].Mackenzie CF, Xiao Y. Video techniques and data compared with observation in emergency trauma care. Qual Saf Health Care 2003;12:ii51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oakley E, Stocker S, Staubli G, Young S. Using video recording to identify management errors in pediatric trauma resuscitation. Pediatrics 2006;117:658–64. [DOI] [PubMed] [Google Scholar]
- [17].Hunt PA, Greaves I, Owens WA. Emergency thoracotomy in thoracic trauma-a review. Injury 2006;37:1–19. [DOI] [PubMed] [Google Scholar]
- [18].Ellis DG, Lerner EB, Jehle DV, Romano K, Siffring C. A multi-state survey of videotaping practices for major trauma resuscitations. J Emerg Med 1999;17:597–604. [DOI] [PubMed] [Google Scholar]
- [19].Campbell S, Sosa JA, Rabinovici R, Frankel H. Do not roll the videotape: effects of the health insurance portability and accountability act and the law on trauma videotaping practices. Am J Surg 2006;191:183–90. [DOI] [PubMed] [Google Scholar]
- [20].Rogers SC, Dudley NC, McDonnell W, Scaife E, Morris S, Nelson D. Lights, camera, action … spotlight on trauma video review: an underutilized means of quality improvement and education. Pediatr Emerg Care 2010;26:803–7. [DOI] [PubMed] [Google Scholar]
- [21].Custalow CB, Kline JA, Marx JA, Baylor MR. Emergency department resuscitative procedures: animal laboratory training improves procedural competency and speed. Acad Emerg Med 2002;9:575–86. [DOI] [PubMed] [Google Scholar]
- [22].Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000;190:288–98. [DOI] [PubMed] [Google Scholar]
- [23].Narvestad JK, Meskinfamfard M, Søreide K. Emergency resuscitative thoracotomy performed in European civilian trauma patients with blunt or penetrating injuries: a systematic review. Eur J Trauma Emerg Surg 2016;42:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European resuscitation council guidelines for resuscitation 2010 section 4. Adult advanced life support. Resuscitation 2010;81:1305–52. [DOI] [PubMed] [Google Scholar]
- [25].Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA: J Am Med Assoc 1990;263:1106–13. [PubMed] [Google Scholar]
- [26].Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, et al. Part 9: Post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122:S768–86. [DOI] [PubMed] [Google Scholar]
- [27].Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. [DOI] [PubMed] [Google Scholar]
- [28].DiGiacomo JC, Hoff WS, Rotondo MF, Martin K, Kauder DR, [106_TD $DIFF][86_TD$DIFF]Anderson HL 3rd, et al. Barrier precautions in trauma resuscitation: real-time analysis utilizing videotape review. AmJ Emerg Med 1997;15:34–9. [DOI] [PubMed] [Google Scholar]
- [29].Hoff WS, Reilly PM, Rotondo MF, DiGiacomo JC, Schwab CW. The importance of the command-physician in trauma resuscitation. J Trauma 1997;43:772–7. [DOI] [PubMed] [Google Scholar]
- [30].Ritchie PD, Cameron PA. An evaluation of trauma team leader performance by video recording. Aust N Z J Surg 1999;69:183–6. [DOI] [PubMed] [Google Scholar]
- [31].Santora TA, Trooskin SZ, Blank CA, Clarke JR, Schinco MA. Video assessment of trauma response: adherence toATLS protocols. AmJ EmergMed 1996;14:564–9. [DOI] [PubMed] [Google Scholar]
- [32].Townsend RN, Clark R, Ramenofsky ML, Diamond DL. ATLS-based videotape trauma resuscitation review: education and outcome. J Trauma 1993;34:133–8. [DOI] [PubMed] [Google Scholar]
- [33].Hoyt DB, Shackford SR, Fridland PH, Mackersie RC, Hansbrough JF, Wachtel TL, et al. Video recording trauma resuscitations: an effective teaching technique. J Trauma 1988;28:435–40. [DOI] [PubMed] [Google Scholar]


