Abstract
Purpose
The 2018 Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) showed Nigeria’s progress toward the UNAIDS 90-90-90 targets: 47% of HIV-positive individuals knew their status; of these, 96% were receiving antiretroviral therapy (ART); and of these, 81% were virally suppressed. To improve identification of HIV-positive individuals, Nigeria developed an Enhanced Community Case-Finding Package (ECCP). We describe ECCP implementation in nine states and assess its effect.
Methods
ECCP included four core strategies (small area estimation [SAE] of people living with HIV [PLHIV], map of HIV-positive patients by residence, HIV risk-screening tool [HRST], and index testing [IT]) and four supportive strategies (alternative healthcare outlets, performance-based incentives for field testers, Project Extension for Community Healthcare Outcomes, and interactive dashboards). ECCP was deployed in nine of 10 states prioritized for ART scale-up. Weekly program data (October 2019–March 2020) were tracked and analyzed.
Results
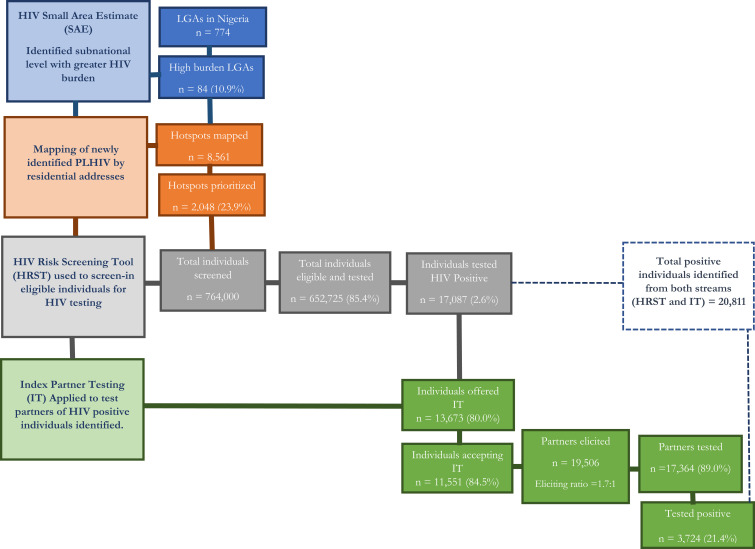
Of the total 774 LGAs in Nigeria, using SAE, 103 (13.3%) high-burden LGAs were identified, in which 2605 (28.0%) out of 9,294 hotspots were prioritized by mapping newly identified PLHIV by residential addresses. Over 22 weeks, among 882,449 individuals screened using HRST, 723,993 (82.0%) were eligible and tested for HIV (state range, 43.7–90.4%), out of which 20,616 were positive. Through IT, an additional 3,724 PLHIV were identified. In total, 24,340 PLHIV were identified and 97.4% were linked to life-saving antiretroviral therapy. The number of newly identified PLHIV increased 17-fold over 22 weeks (week 1: 89; week 22: 1,632). Overall mean HIV positivity rate by state was 3.3% (range, 1.8–6.4%).
Conclusion
Using ECCP in nine states in Nigeria increased the number of PLHIV in the community who knew their status, allowing them to access life-saving care and decreasing the risk of HIV transmission.
Keywords: ART Surge, small area estimation, HIV risk assessment tool, index partner testing
Introduction
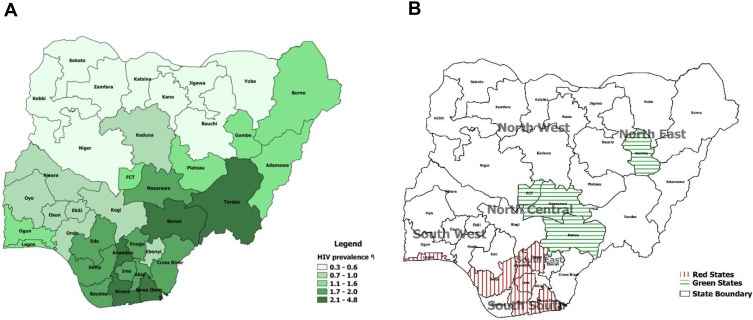
In 2014, the Joint United Nations Programme on AIDS/HIV (UNAIDS) set the 90-90-90 targets for HIV epidemic control by 2020: 90% of people living with HIV (PLHIV) are aware of their HIV status; of these, 90% are receiving sustained antiretroviral therapy (ART); and of these, 90% have viral load suppression.1 To determine progress toward the 90-90-90 targets and to characterize the HIV epidemic fully, Nigeria conducted the 2018 Nigeria AIDS Indicator and Impact Survey (NAIIS), estimating the national HIV prevalence at 1.3% (95% confidence interval: 1.2–1.4%). NAIIS results showed higher prevalence in the South-South, South-East, and part of North Central geopolitical zones (Figure 1A). Nigeria's progress toward the UNAIDS 90-90-90 targets was 47-96-81, with approximately 1 million PLHIV yet to be identified.2 As such, HIV epidemic control is lower than that reported for some African countries, especially for the first 90 target.3
Figure 1.
State-level HIV prevalence in Nigeria and the nine states that implemented the enhanced community case-finding package (ECCP) as part of the Nigeria antiretroviral therapy surge, October 2019–March 2020. (A) Prevalence of HIV by state (NAIIS 2018). (B) Targeted Surge states.
Abbreviations: NAIIS, Nigeria HIV/AIDS Indicator and Impact Survey; FCT, Federal Capital Territory.
Progress toward achieving the first 90 target in Nigeria has faced several challenges, especially in identifying PLHIV outside of health facilities. Before NAIIS, case-finding was impeded by inadequate epidemiologic data to guide targeted HIV testing, often leading to misallocation of testing resources and low case-finding. Without epidemiologic data to guide case-finding efforts, HIV program implementing partners used non-targeted testing strategies, such as general house-to-house testing or testing in places of worship, markets, and other locations with high volumes of people but little evidence of HIV burden. Pervasive security concerns and other logistic and social issues in Nigeria also hindered effective community testing. Programs had few rapid test kits, requiring more targeted testing to optimize use of available resources. With epidemiologic data provided by NAIIS, in April 2019, Nigeria reshaped programming to control the epidemic more precisely, scaling up ART programs to provide services to an additional 500,000 PLHIV within 18 months.4,5 At the time, resources for case-finding had been reserved for exclusive use within healthcare facilities and among key populations. Because of low number of positive individuals identified, community testing methods had been discontinued.
The programmatic priority to identify 500,000 PLHIV required testing beyond healthcare facilities and justified the needs for redeployment of targeted community case-finding approach to complement facility-level testing. Testing strategies that improve chances of identifying HIV-positive cases, such as use of HIV risk assessment to prioritize whom to test,6 testing sexual partners of newly-identified HIV-positive cases,7–9 small area estimation and hotspots mapping,10,11 were previously described. However, there were limited studies on the effect of these strategies on case-finding when used together as a package. The US President’s Emergency Plan for AIDS Relief (PEPFAR) Nigeria leveraged these established testing strategies and developed an Enhanced Community Case-Finding Package (ECCP) to accelerate progress toward the first 90 target. In this paper, we categorized these four known strategies as core because each of them was shown to improve chances of identifying HIV-positive individuals. In addition to the four known core strategies above, additional strategies that helped PEPFAR Nigeria to circumvent impediments for successful community HIV testing services (HTS) were added to the ECCP. The four additional strategies are: leveraging community-based organizations (CBOs), incentivizing community testing teams, remote mentorship in security and hard-to-reach locations, and use of data to guide decisions. Here, we describe ECCP implementation and assess community case-finding during implementation in nine of the 10 targeted states.
Methods
Study Design and Ethical Approvals
This was a cross-sectional descriptive study involving PLHIV identified through routine PEPFAR implemented HIV program in Nigeria from October 2019 to March 2020 in nine targeted states. The study was supported by two approved protocols. For results such as small area estimation pertaining to the Nigeria AIDS Indicator and Impact Survey (NAIIS), informed consent was obtained, and the protocol was approved by CDC IRB, University of Maryland Baltimore IRB, and Nigeria Health Research Ethics Committee. For procedures and results pertaining to routine PEPFAR program, de-identified data with no perceived ethical risk to participants were collected, so no informed consent was obtained. PEPFAR routine program implementation and ART Surge were reviewed in accordance with CDC human research protection procedures and were determined to be non-research, public health program activity and received concurrence from the Government of Nigeria Federal Ministry of Health. The study fully complies with the Declaration of Helsinki.
Strategies to Enhance HIV Case-Finding Using ECCP
ECCP consists of a package of strategies designed to ensure improved HIV case-finding in communities while ensuring safety in the face of current security challenges in Nigeria. The various strategies that were implemented are described below.
Core Strategies
We categorized the following four strategies as core strategies because each of them is likely to improve the chances of identifying HIV-positive individuals in a community to varying degrees independent of the others as previously described by several studies.6–11 Additionally, the four core strategies targeted either geographical locations or individuals.
Small area estimation (SAE) was used to identify locations with high treatment coverage gaps. SAE is a model-based estimation that uses other pre-existing survey data, such as NAIIS, and program data.12 The NAIIS SAE model is similar to the model used for an earlier geospatial analysis of HIV prevalence in sub-Saharan Africa.13 NAIIS provided prevalence estimates at the first subnational geographical level (ie, the states in Nigeria), but estimates for the second subnational geographical level (ie, local government area [LGA]) were needed for targeted HIV response. There are 774 LGAs in Nigeria across 36 states and the Federal Capital Territory. SAE helped prioritize LGA-level testing and resource allocation.
After prioritizing LGAs with highest HIV burden and treatment gap through application of SAE, the newly identified PLHIV in the prioritized LGA were mapped by residential address to identify potential hotspots. For the purpose of the Surge, newly identified PLHIV were defined as individuals who reported either not knowing their HIV statuses or reported as known HIV-negative and were tested positive in the community using HIV rapid diagnostic tests. Locations of newly identified PLHIV were mapped using geocodes of landmarks close to residential addresses. Periodically, locations were remapped using newly identified PLHIV to identify additional hotspots for targeted testing.
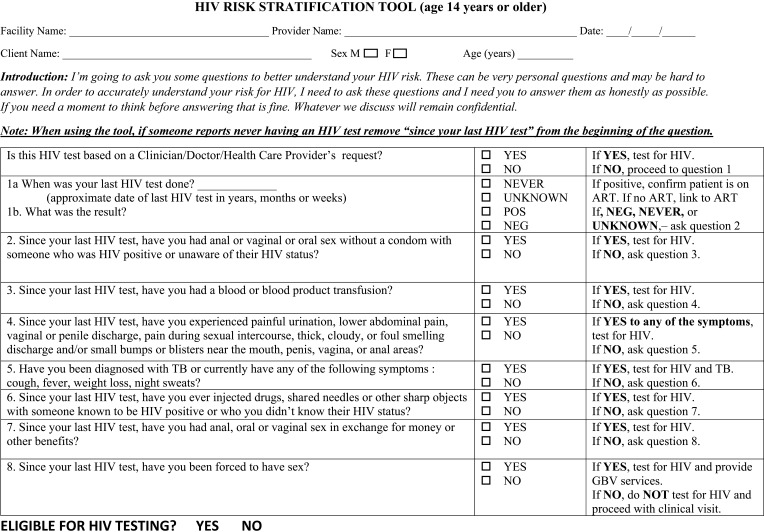
Within identified hotspots, an HIV risk screening tool (HRST) (Figure 2) was used to assess HIV infection risk and to increase the likelihood of identifying PLHIV. Testing eligibility requires individuals to answer “yes” to at least one question assessing HIV risk. Partners of newly identified PLHIV and HIV key populations were exempted from screening using HRST. The HRST was developed and tested by Nigeria HIV Prevention Task Team and included in the package of National HTS data collection tools.
Sexual partners of index cases (newly identified PLHIV through HRST) were tested to increase likelihood of identifying additional PLHIV. Each index client was asked to provide contact information of sexual partners in the previous year; measures were taken to ensure confidentiality and to decrease stigma. To improve confidentiality, counseling was conducted individually and in a secure location. The state Ministry of Health sent messages via short message service to identified sexual partners to invite them to take advantage of free community health services. Partners of index patients were tested without HRST screening.
Figure 2.
Nigeria HIV risk stratification (screening) tool (HRST): one of the four core strategies of enhanced community case-finding package (ECCP) deployed as part of the Nigeria antiretroviral therapy surge, October 2019–March 2020.
Supportive Strategies
We categorized the following four strategies as supportive because they helped in circumventing obstacles hindering accesses to a community or might have provided indirect support for optimal implementation of the four core strategies. The supportive strategies may be unlikely to contribute substantially in identifying new PLHIV in the community without at least one of the four established core strategies. The first three supportive strategies targeted HTS providers, while the fourth strategy served as crosscutting intervention that guided decision making across all other seven strategies.
Partnering community-based organizations (CBOs) and alternative healthcare outlets were leveraged to ensure that testing services are locally adapted and appropriate. Alternative healthcare outlets, such as traditional and spiritual healers, traditional birth attendants, pharmacists, and patent medical vendors, serve many Nigerians.14,15 These alternative healthcare outlets and CBOs were mapped in targeted communities, and staff were trained and provided with HIV rapid test kits. PLHIV identified through CBOs were actively linked to healthcare facilities for treatment.
Performance-based incentives (PBIs) helped engage field testers. Testing targets were allocated per field staff or team, and appraisals were conducted every 2 months. A performance-incentive scale was developed such that achieving 50–74%, 75–89%, and ≥90% of assigned monthly testing targets was rewarded with 30%, 50%, and 100% of monthly bonus compensation, respectively. Alternative healthcare outlets and CBOs were incentivized with free rapid HIV test kits (at least three test kits) or cash (between 2–3 US dollars) per identified HIV-positive client.
Project Extension for Community Healthcare Outcomes (ECHO) and peer-to-peer learning ensured continuous quality improvement of testing services. Project ECHO is a collaborative model of medical education and care management that is operated via remote, low-bandwidth technology to share and disseminate best practices among colleagues.16 We leveraged Project ECHO for weekly virtual mentorship of field testers. Each state conducted weekly meetings to review performance and challenges and to replicate best practices among state teams. Learning exchange visits also were organized between states.
A performance dashboard was used to monitor trends and provide timely feedback. We deployed a user-friendly interactive Microsoft Excel dashboard to support program and commodity (test kit and antiretroviral drugs) tracking. Performance trends were monitored weekly to develop appropriate interventions to improve performance. We considered performance to be good and appropriate when both the number of newly identified PLHIV increased (equals or above assigned weekly targets) and the positivity rate was above state prevalence.
Selection of Implementation States
Using NAIIS data, Nigeria selected six states for priority interventions given high treatment coverage gaps (categorized as red states) and four states that were closer to epidemic control (categorized as green states). Those states were Rivers, Akwa-Ibom, and Delta (South-South zone); Enugu and Imo (South-East zone); Lagos (South-West zone); Federal Capital Territory (FCT), Benue, and Nasarawa (North-Central Zone); and Gombe (North-East Zone; Figure 1B). Akwa-Ibom was excluded from this assessment due to lack of access to state data. Despite high HIV prevalence and treatment gap, Taraba State was not included because the state was not supported by PEPFAR. However, following successful implementation of ECCP in the ten targeted states, Taraba State is now supported by PEPFAR and added to the ten targeted states. The estimated HIV treatment gap in nine of the ten targeted states was 490,908, about 50% of total HIV treatment gap in Nigeria.17 ECCP and ART coverage targets constituted the Nigeria ART Surge, and prioritized states were referred to as Surge states.
Field Implementation Approach
Community testing teams were trained on ethics, the use of HRST, and index testing of sexual partners. Teams comprised an HRST administrator, counselor-tester, and linkage/treatment supporter. Field testers were recruited from the states where they were deployed, ensuring familiarity with the local language and culture. A total of 362 teams were deployed across the nine targeted states, with Delta and Rivers states having the highest number of teams (125 and 115 teams, respectively). Testing targets and terrains determined the number of teams assigned to each state. Both Rivers and Delta states have higher testing targets with complex geographical terrains and higher security threats. Teams operated in the community as mobile units and prioritized locations where more HIV-positive individuals were identified as guided by mapping of newly identified PLHIV by residential addresses. To destigmatize HIV testing, the Surge integrated general health services (eg, weight check, blood pressure check, blood sugar, and rapid malaria tests) with community HIV testing. HIV risk screening using HRST and potential HIV testing were provided after most other general health services were received. Participants were general population in the communities who accepted free general health services from October 2019 to March 2020. Individuals who reported not knowing their HIV status or reported an HIV-negative status were eligible for HRST screening. Individuals who reported an HIV-positive status were ineligible. Individuals screened-in by HRST underwent HIV pre-test counseling before rapid HIV testing based on the national HIV testing algorithm (serial testing with Determine as the screening test, Unigold as the confirmatory test, and Stat-Pak as the tiebreaker). Individuals with HIV-negative test results were counseled on how to minimize risk and practice safe sex; those with HIV-positive test results received post-test counseling and were actively referred for ART services at the treatment center of their choice. Additionally, individuals who tested HIV-positive were provided with a telephone number to call if they need further counseling, psychosocial support, and support for partner violence for individuals who consented to partner notification services (index partner testing) even though all provided services were strictly confidential.
Data Collection and Analysis
For mapping of potential hotspots (mapping of HIV-positive individuals by residential addresses), coordinates of each testing point in the community were captured using a smartphone Global Positioning System receiver, and geocodes of major landmarks close to residential addresses of newly identified PLHIV were documented. To assess ECCP for each state, daily summaries were compiled with the following indicators and analyzed weekly for a period of 22 weeks (October 2019–March 2020): total number of individuals screened-in using HRST, number of individuals screened-out using HRST, total number of individuals tested, total number of individuals with HIV-positive results, total number of HIV-positive clients who accepted index partner testing, total number of partners of index cases elicited, total number of partners tested, total number of partners tested positive, HIV positivity rate, and total number of individuals with HIV-positive results who were linked to treatment.
Results
Over 22 weeks, of the 882,449 individuals screened using HRST (range per state, 11,105 [Gombe]–557,467 [Rivers]), 723,993 (82%) were eligible and tested for HIV (range per state, 43.7% [Enugu]–90.4% [Rivers]). Overall, 158,456 (18%) people were screened-out (not eligible for testing; screened-out range, 9.6% [Rivers]–56.3% [Enugu]). Among the 723,993 individuals tested, 69.6% (n=504,149) were from Rivers State (Table 1).
Table 1.
Results of Enhanced Community Case-Finding (ECCP) Package by Individual Strategy Among the Nine Surge States in Nigeria, March 2019–October 2020
| Rivers | Benue § | Nasarawa § | Delta | Gombe | FCT | Imo | Enugu | Lagos | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| State HIV prevalence (%) | 3.6 | 4.8 | 1.8 | 1.7 | 1.2 | 1.4 | 1.7 | 1.8 | 1.3 | 1.3 |
| Small Area Estimation (SEA): | ||||||||||
| Estimated state PLHIV (n) | 183,029 | 192,116 | 66,549 | 57,895 | 25,795 | 57,965 | 54,021 | 55,918 | 126,783 | 820,071 |
| PLHIV on treatment (n) | 26,041 | 110,878 | 36,372 | 15,208 | 14,377 | 38,185 | 12,057 | 18,110 | 57,935 | 329,163 |
| Treatment gap (n) | 156,988 | 81,238 | 30,177 | 42,687 | 11,418 | 19,780 | 41,964 | 37,808 | 68,848 | 490,908 |
| Treatment gap (%) | 85.8 | 42.3 | 45.3 | 73.7 | 44.3 | 34.1 | 77.7 | 67.6 | 54.3 | 59.9 |
| Total LGAs in the state (n) | 23 | 23 | 13 | 25 | 11 | 6 | 27 | 17 | 20 | 165 |
| Targeted LGAs (n) | 9 | 8 | 11 | 11 | 4 | 4 | 27 | 9 | 20 | 103 |
| Estimated PLHIV in targeted LGAs (n) | 67,003 | 65,920 | 26,255 | 26,081 | 8970 | 14,493 | 54,021 | 27,272 | 114,160 | 404,175 |
| Geocode Mapping of New PLHIV | ||||||||||
| Total hotspots mapped in targeted LGAs (n) | 7396 | 261 | 472 | 293 | 493 | 75 | 131 | 63 | 110 | 9294 |
| Hotspots that produced at least 90% of the positives (n) | 1734 | 205 | 352 | 121 | 50 | 34 | 46 | 41 | 22 | 2605 |
| Hotspots that produced at least 90% of the positives (%) | 23.4 | 78.5 | 74.6 | 41.3 | 10.1 | 45.3 | 35.1 | 65.1 | 20.0 | 28.0 |
| HIV Risk Screening Tool (HRST) | ||||||||||
| Individuals screened (n) | 557,467 | 63,212 | 55,237 | 106,650 | 11,105 | 31,778 | 18,483 | 22,158 | 16,359 | 882,449 |
| Individuals screened-out (test kits saved) (n) | 53,318 | 28,694 | 18,487 | 22,521 | 4100 | 12,814 | 3059 | 12,473 | 2990 | 158,456 |
| Individuals tested (n) | 504,149 | 34,518 | 36,750 | 84,129 | 7005 | 18,964 | 15,424 | 9685 | 13,369 | 723,993 |
| % screened-out | 9.6 | 45.4 | 33.5 | 21.1 | 36.9 | 40.3 | 16.6 | 56.3 | 18.3 | 18.0 |
| % tested | 90.4 | 54.6 | 66.5 | 78.9 | 63.1 | 59.7 | 83.4 | 43.7 | 81.7 | 82.0 |
| Positive individuals identified (n) | 13,578 | 1719 | 1810 | 1359 | 344 | 763 | 267 | 321 | 455 | 20,616 |
| % positive (positivity rate) | 2.7 | 5.0 | 4.9 | 1.6 | 4.9 | 4.0 | 1.7 | 3.3 | 3.4 | 2.8 |
| Index Partner Testing (IT) | ||||||||||
| Individuals offered IT (n) | 9878 | - | - | 1359 | 543 | 763 | 267 | 408 | 455 | 13,673 |
| Individuals who accepted IT (n) | 8890 | - | - | 816 | 343 | 654 | 224 | 321 | 303 | 11,551 |
| % accepting IT | 90.0 | - | - | 60.0 | 63.2 | 85.7 | 83.9 | 78.7 | 66.6 | 84.5 |
| Partners elicited (n) | 15,380 | - | - | 1476 | 469 | 860 | 291 | 420 | 610 | 19,506 |
| Partners tested (n) | 14,872 | - | - | 417 | 376 | 602 | 130 | 388 | 579 | 17,364 |
| Positive partners identified (n) | 2885 | - | - | 132 | 127 | 190 | 20 | 68 | 302 | 3724 |
| Elicitation ratio | 1.7 | - | - | 1.8 | 1.4 | 1.3 | 1.3 | 1.3 | 2.0 | 1.7 |
| Testing acceptance among elicited partners (%) | 96.7 | - | - | 28.3 | 80.2 | 70.0 | 44.7 | 92.4 | 94.9 | 89.0 |
| IT positivity rate | 19.4 | - | - | 31.7 | 33.8 | 31.6 | 15.4 | 17.5 | 52.2 | 21.4 |
| Total tested (RST and IT) (n) | 519,021 | 34,518 | 36,750 | 84,546 | 7381 | 19,566 | 15,554 | 10,073 | 13,948 | 741,357 |
| Total positive (RST and IT) (n) | 16,463 | 1719 | 1810 | 1491 | 471 | 953 | 287 | 389 | 757 | 24,340 |
| Positivity rate (RST and IT) (%) | 3.2 | 5.0 | 4.9 | 1.8 | 6.4 | 4.9 | 1.8 | 3.9 | 5.4 | 3.3 |
| Community HTS teams (n) | 115 | 20 | 37 | 125 | 13 | 14 | 12 | 20 | 6 | 362 |
| Performance appraisal review cycles conducted for field teams | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 18 |
| Virtual mentorship sesssions held (n) | 44 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 220 |
| Dashboard review meetings conducted (n) | 44 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 220 |
Note:§States did not implement IT during the study period.
Abbreviations: LGAs, Local Government Areas; ECCP, Enhanced Community Case-Finding Package; HTS, HIV testing services.
Seven of the nine states implemented community index testing within the study period; the two remaining states (Benue and Nasarawa) implemented community index testing after the study period. Of the total 20,811 PLHIV identified in the seven states, 3724 (17.9%) were identified through index testing. Index testing was offered to 13,673 (80.0%) of the 17,087 PLHIV identified through use of HRST in the 7 states and 11,551 (84.5%) accepted, among whom 19,506 partners were elicited, for an overall elicitation ratio of 1.7:1 (range, 1.3:1 [FCT, Imo, and Enugu]–2.0:1 [Lagos]). The positivity rate of index testing stream was 21.4% (range, 15.5 [Imo]–52.1% [Lagos]; Table 1). Figure 3 summarizes the cascade of results from 7 states that deployed community index testing during the study period and hence benefited from full complement of ECCP core strategies.
Figure 3.
Cascade of results from implementation of full complement of enhanced community case-finding package (ECCP) in seven of the nine Surge states as part of the Nigeria antiretroviral therapy surge, October 2019–March 2020.
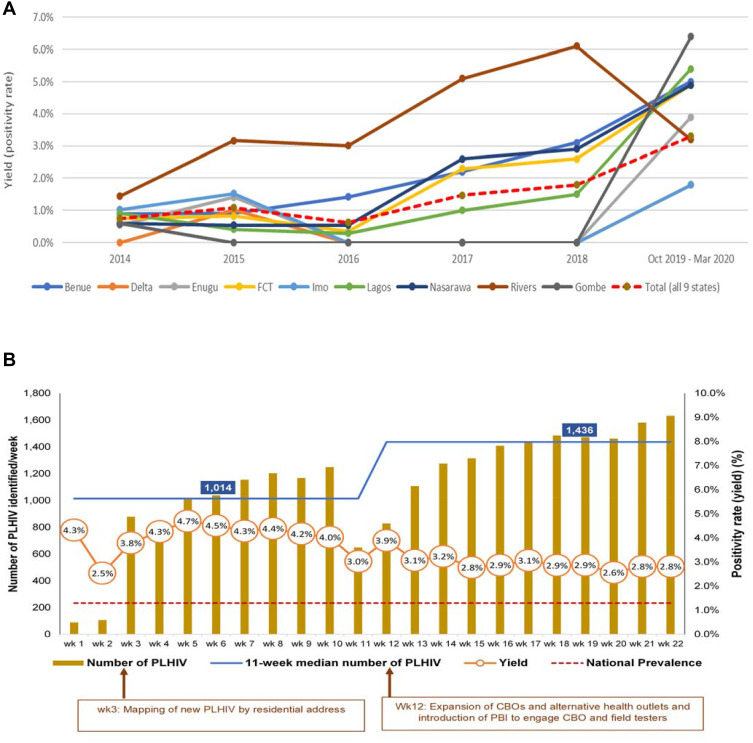
Average positivity rate during the Surge period was above the positivity rate obtained in the previous five years among eight of the nine states. There was an observed decline in positivity rate in Rivers State from 6.1% in 2018 to 3.2% in 2019 (Figure 4A). Similarly, across all nine states, the volume of newly identified PLHIV in the community increased 17-fold over 22 weeks (week 1: 89; week 22: 1632). Median increase in newly identified and initiated PLHIV between the first 11 weeks and second 11 weeks was 41.6% (median: first 11 weeks, 1014; second 11 weeks, 1436). The number of PLHIV identified increased steadily after week 3, when mapping of PLHIV by residence was introduced (Figure 4). The number of CBOs and alternative healthcare outlets supported and PBIs were increased in week 12, which also increased the number of PLHIV identified (Figure 4). Overall, a total of 24,340 PLHIV were identified in the communities using ECCP, of which 23,703 (97.4%) were linked to life-saving ART.
Figure 4.
Community HIV positivity rate trend in nine states that implemented the enhanced community case-finding package (ECCP) as part of the Nigeria antiretroviral therapy surge. (A) 5-year (2014–2018) positivity rate trend by state versus Surge period (October 2019–March 2020). (B) Weekly positivity rate and number of newly identified PLHIV by state during the Surge (October 2019–March 2020).
Abbreviations: CBO, community-based organization; PBI, performance-based incentives; wk., week; PLHIV, People Living with HIV.
Discussion
Deploying ECCP during the Nigeria ART Surge increased identification of PLHIV in the communities. Integrating ECCP core and supportive strategies into normal activities helped target services to identify PLHIV who did not know their HIV-positive status. HRST helped to target testing and to screen-out many individuals without risk or with low risk of HIV infection, which helped conserve limited testing resources. Increased positivity rates, which were above estimated state prevalence in almost all states, also indicate testing was more targeted. We assumed testing as targeted and acceptable when the positivity rate is higher than state prevalence because prevalence rates estimated through population-based household surveys such as NAIIS were without any testing strategy and were random results, hence the targeted testing positivity rate should be above random results if the strategy is effective. Finally, the absolute number of newly identified PLHIV increased substantially, and almost all these individuals were linked to life-saving ART.
Small area estimation was used to target testing in LGAs with high estimated HIV prevalence. Before NAIIS, surveys were less precise and provided misleading estimates. Identifying hotspots for community testing relied on number of patients receiving treatment in hospitals, so LGAs in which these hospitals are located were targeted for community testing. This approach was ineffective because most of the time patients tend to access care in hospitals far from their residences due to stigma.18 Mapping newly identified PLHIV by residence narrowed the geographical area to identify potential hotspots, leading to progressive increases in both positivity rates and number of newly identified PLHIV, as observed from week 3. The LGA mapping of hotspots, using newly identified PLHIV, is reported to be an effective way of identifying hotspots in high-burden areas for targeted testing.19 By deploying this strategy, only about one-quarter of identified hotspots that produced at least 90% of newly identified PLHIVs were targeted, thereby reducing the potential number of field testers that would have been spread to over 8000 hotspots, the majority of which are less likely to produce a substantial number of PLHIV.
Within the hotspots, HRST was used to prioritize testing of individuals who were likely to have an HIV-positive result based on their HIV risk exposures. Because sexual partners of newly identified PLHIV are likely to be HIV-positive,20 index testing was offered without HRST. In Malawi, HIV risk screening before rapid test using a checklist like HRST was found to be very effective.21 Similarly, in Zimbabwe, an HIV-screening tool decreased the tests needed to identify an HIV-positive individual by 55%.22 In our analysis in Nigeria, HRST resulted in a saving of about 158,000 rapid diagnostic test kits and likely contributed to the 3.3% total positivity rate observed, which is 2.5 times higher than the national prevalence of 1.3%. Lower positivity rates in some states were likely due to sub-optimal application of HRST (eg, low proportion of screening-out), indicating that low-risk individuals were inappropriately screened-in for testing. The huge treatment gaps in Rivers, Imo, and Delta states likely pushed testers not to use HRST judiciously in desperation to get more positives, and this was proven by the observed low proportion of individuals screened-out, 9.6%, 16.6%, and 21.1%, respectively, resulting in low positivity rates either below or equal to state prevalence. Lagos also reported a low number of individuals screened-out but with a very high positivity rate of 3.4% compared to state prevalence of 1.3%. This is because the individuals screened in Lagos were predominantly key populations with higher HIV prevalence,23 and key populations were also exempted from HRST. Similarly, eight of the nine Surge states demonstrated improvement in positivity rate when compared with the yield obtained in the previous five years when Surge was not implemented. Rivers State demonstrated a decline in positivity rate from 6.1% in 2018 to 3.2% during the Surge period. This decline was due to the reason highlighted earlier. Index testing is shown to improve positivity rates and testing efficiency,7,8 which in this study also improved our chances of identifying more positive individuals, even though index clients sometimes were reluctant to provide information about their sexual partners due to stigma and fear of rejection24 as observed by low overall elicitation ratio of 1.7:1. The overall positivity rate from the index testing stream in this study was 21.4%, which implied that every 1 out of 5 partners tested was positive. This is also about 17 times higher than national prevalence of 1.3%. About one-sixth of the new PLHIV identified were identified through the index testing stream. All the seven states that implemented index testing during the study period demonstrated improved overall positivity rate when compared with HRST positivity rate. Integrated healthcare approaches, similar to those reported in Uganda,25 were adapted to destigmatize HIV testing during HRST and during partner elicitation for index testing, which possibly improved testing acceptance in the communities. Despite a modest index testing acceptance rate of 84.5% among newly identified PLHIV, unfortunately the elicitation ratio was low. With elicitation ratio of 1:3, we could have possibly doubled the number of new PLHIV we identified through the index testing stream, hence this finding highlighted the need for deploying initiatives to reduce stigma and other factors contributing to low partner elicitation among newly identified PLHIV going forward. A similar study in Kenya reported index testing acceptance rate of 89% and elicitation ratio of 1:1.3.26
We observed that the four supportive strategies were particularly important to overcome key programmatic difficulties we faced during community HTS implementation, such as pervasive security challenges, distrust and suspicion whenever community testers were seen in communities, insufficient mentorship, low morale among field testers, and a culture of low data use to guide decisions among program implementers. Leveraging alternative healthcare outlets, such as traditional and spiritual healers, traditional birth attendants, pharmacists, and patent medical vendors, as well as involvement of community leaders and influencers,27 helped to provide services safely in security-challenged areas. These alternative healthcare outlets, including CBOs, are used by many individuals in Nigeria and other developing countries due to religious beliefs, trust in local alternative healthcare, and lack of adequate affordable healthcare.14,15,25,28 This approach is buttressed by previous experience recruiting traditional birth attendants to support prevention of mother-to-child HIV transmission as reported in Nigeria and Malawi.29,30 The number of identified PLHIV increased substantially in week 12, when more CBOs and alternative healthcare outlets joined the study (Figure 4).
Project ECHO enabled remote mentorship and support, especially in locations with major security threats. For example, in the Rivers State in the Gulf of Guinea, the world’s worst piracy hotspot with high rates of kidnapping,31,32 CBOs and community field testers were mentored remotely, and performance was monitored continuously using Project ECHO. PBIs is shown to engage field testers and motivate staff, thereby improving program success and public health outcomes, and improves commitment and fosters healthy competition among public health workers.33,34 The PBI strategy might have influenced HTS field testers, which likely resulted in increased numbers of newly identified PLHIV and treatment referrals. To ensure individuals were not coerced as a result of PBIs, we emphasized ethics and volunteerism during training of community fieldworkers before fieldwork. Additionally, we ensured strict compliance with national testing guidelines. Finally, weekly data review, using an interactive Microsoft Excel dashboard, provided implementers access to data to drive discussions and rapidly adapt and improve programs. Without close data monitoring using the dashboard, key programmatic decisions, such as mapping of newly identified PLHIV by residential addresses, increasing the number of CBOs, and providing PBIs, would have been missed, decreasing our ability to adapt strategies after week 12 to identify more PLHIV.
There are at least two limitations to our findings. First, as a programmatic evaluation, we could not quantify the impact of each strategy independently especially of the four supportive strategies. Second, we were unable to determine whether all identified HIV-positive cases are truly new cases since we relied on self-report awareness; some PLHIV may have already known their status and decided to conceal and got re-tested. To minimize this limitation, we are currently implementing patient biometric system among all patients who consented to have their fingerprints captured and conduct deduplication at national data repository level.
Conclusion
Overall, implementing ECCP helped identify 24,340 PLHIV from the nine Surge states within 22 weeks and saved 158,456 test kits. Using our overall positivity rate of 3.3%, the saved 158,456 test kits may identify an additional 5229 PLHIV in the future. Using the combination of community case-finding strategies in ECCP, including HIV risk assessment, index testing, small area estimates, and HIV hotspot mapping, we identified more PLHIV in the community and linked them to care. Adapting the strategies presented in this paper and implementing them as a package may improve HIV case-finding in similar settings. ECCP can be used by countries with generalized HIV epidemics and relatively low HIV prevalence to accelerate HIV case-finding and linkage to care.
Acknowledgments
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of CDC Cooperative Agreement No. CDC-RFAGH17-1753.
CDC Nigeria ART Surge Team: Chidozie Meribe, Obinna Ogbanufe, Chibuzor Onyenuobi, Timothy A. Efuntoye, Uzoma Ene, Omodele Johnson Fagbamigbe, Ayodele Fagbemi, Nguhemen Tingir, Orji Bassey, Matthias Alagi, Ibrahim Dalhatu, Jerry Gwamna, Shane Diekman, Michelle Williams-Sherlock, Akpan Sebong, Greg Ashefor, Prosper Okonkwo, Patrick Dakum, Charles Mensah, Ahmad Aliyu, Bolanle Oyeledun, John Oko, Ifunanya Mgbakor.
The authors acknowledge the enormous contributions from the nine state ministries of health (Benue, Delta, Enugu, FCT, Gombe, Imo, Lagos, Nasarawa, Rivers) and the four PEPFAR implementing partners (APIN Public Health Initiative [APIN], Center for Integrated Health Program in Nigeria [CIHP], Catholic Caritas Foundation Nigeria [CCFN], Institute of Human Virology Nigeria [IHVN]).
Disclosure
Dr Aliyu Gambo reports grants from The Global Funds, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.UNAIDS. Accelerating towards 90-90-90; 2018. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2018/july/90-90-90-targets-workshop. Accessed July30, 2021.
- 2.Federal Ministry of Health, Nigeria. Nigeria HIV/AIDS Indicator And Impact Survey (NAIIS) 2018: technical report. Abuja, Nigeria. 2019. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Five African countries approaching control of their HIV epidemics. Available from: https://www.cdc.gov/globalhivtb/who-we-are/features/phia_lesotho_uganda.html. Accessed July30, 2021.
- 4.Boyd AT, Ogbanufe O, Onyenuobi C, et al. Scale-up of antiretroviral treatment access among people living with HIV in Rivers State, Nigeria, 2019–2020. AIDS. 2021;35:1127–1134. doi: 10.1097/QAD.0000000000002858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirlikov E, Jahun I, Odafe SF, et al. Rapid scale-up of an antiretroviral therapy program before and during the COVID-19 pandemic — nine States, Nigeria, March 31, 2019–September 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:421–426. doi: 10.15585/mmwr.mm7012a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FHI360. Reaching the first 90: improving HIV testing yield in Zimbabwe. Available from: https://www.fhi360.org/sites/default/files/media/documents/zhct-hiv-testing-yield-may18.pdf. Accessed July30, 2021.
- 7.Katbi M, Adegboye A, Adedoyin A, et al. Effect of clients strategic index case testing on community-based detection of HIV infections (STRICT study). Int J Infect Dis. 2018;74:54–60. doi: 10.1016/j.ijid.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 8.Jubilee M, Park FJ, Chipango K, et al. HIV index testing to improve HIV positivity rate and linkage to care and treatment of sexual partners, adolescents and children of PLHIV in Lesotho. PLoS One. 2019;14(3):e0212762. doi: 10.1371/journal.pone.0212762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph Davey D, Wall KM, Serrao C, et al. HIV positivity and referral to treatment following testing of partners and children of PLHIV index patients in public sector facilities in South Africa. J Acquired Immune Deficiency Syndromes. 2019;81(4):365–370. doi: 10.1097/qai.0000000000002048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau P. HIV hotspot mapping: an evidence-informed HIV response. Available from:https://www.ee.co.za/article/hiv-hotspot-mapping-evidence-informed-hiv-response.html. Accessed July30, 2021.
- 11.Pebody R. Mapping local HIV epidemics can help target resources to areas with the greatest need. Available from: https://www.aidsmap.com/news/jul-2016/mapping-local-hiv-epidemics-can-help-target-resources-areas-greatest-need. Accessed July30, 2021.
- 12.Gutreuter S, Ehimario I, Njeri W, et al. Improving estimates of district HIV prevalence and burden in South Africa using small area estimation techniques. PLoS One. 2019;14(2):e0212445. doi: 10.1371/journal.pone.0212445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer-Lindgren L, Cork MA, Sligar A, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570:189–193. doi: 10.1038/s41586-019-1200-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isola OI. The ‘relevance’ of African traditional medicine (alternative medicine) to healthcare delivery system in Nigeria. J Developing Areas. 2013;47(1):319–338. doi: 10.1353/jda.2013.0004 [DOI] [Google Scholar]
- 15.Okoronkwo I, Onyia-Pat JL, Okpala P, Agbo MA, Ndu A. Patterns of complementary and alternative medicine use, perceived benefits, and adverse effects among adult users in Enugu Urban, Southeast Nigeria. Evid Based Complement Alternat Med. 2014;2014:239372. PMID: 24803945; PMCID: PMC3996953. doi: 10.1155/2014/239372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Project ECHO. Project ECHO's proven success. Available from: https://echo.unm.edu/about-echo/. Accessed July30, 2021.
- 17.The United States President's Emergency Plan for AIDS Relief (PEPFAR). Nigeria Country Operational Plan (COP) 2019 strategic direction summary. Available from: https://www.state.gov/wp-content/uploads/2019/09/Nigeria_COP19-Strategic-Directional-Summary_public.pdf. Accessed July30, 2021.
- 18.Adam NA, Aggrey M, Gillian AL, et al. People living with HIV travel farther to access healthcare: a population-based geographic analysis from rural Uganda. Int AIDS Soc. 2016;19(1):20171. doi: 10.7448/IAS.19.1.20171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuadros DF, Sartorius B, Hall C, et al. Capturing the spatial variability of HIV epidemics in South Africa and Tanzania using routine healthcare facility data. Int J Health Georg. 2018;17:27. doi: 10.1186/s12942-018-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gbadamosi SO, Itanyi IU, Menson WNA, et al. Targeted HIV testing for male partners of HIV-positive pregnant women in a high prevalence setting in Nigeria. PLoS One. 2019;14(1):e0211022. doi: 10.1371/journal.pone.0211022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moucheraud C, Chasweka D, Nyirenda M, et al. Simple screening tool to help identify high-risk children for targeted HIV testing in Malawian inpatient wards. J Acquir Immune Defic Syndr. 2018;79(3):352–357. doi: 10.1097/QAI.0000000000001804 [DOI] [PubMed] [Google Scholar]
- 22.Bandason T, Dauya E, Dakshina S, et al. Screening tool to identify adolescents living with HIV in a community setting in Zimbabwe: a validation study. PLoS One. 2018;13(10):e0204891. doi: 10.1371/journal.pone.0204891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Agency for the Control of AIDS (NACA). Nigeria integrated biobehavioral surveillance survey (IBBSS 2014). Available from: https://naca.gov.ng/final-nigeria-ibbss-2014-report/. Accessed July30, 2021.
- 24.Bilardi JE, Hulme-Chambers A, Chen MY, et al. The role of stigma in the acceptance and disclosure of HIV among recently diagnosed men who have sex with men in Australia: a qualitative study. PLoS One. 2019;14(11):e0224616. doi: 10.1371/journal.pone.0224616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aurelie B, Patricia WM, Angela A, et al. Expanding HIV testing and counselling into communities: feasibility, acceptability, and effects of an integrated family planning/HTC service delivery model by Village Health Teams in Uganda. Health Policy Plan. 2016;31(8):1050–1105. doi: 10.1093/heapol/czw035 [DOI] [PubMed] [Google Scholar]
- 26.Kariuki RM, Rithaa GK, Oyugi E, et al. What is the level of uptake of partner notification services in HIV testing in selected health facilities in Gatanga Sub County, Muranga County – kenya, a retrospective study. BMC Infect Dis. 2020;20:432. doi: 10.1186/s12879-020-05146-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The World Health Organization (WHO). How community engagement prevents and protects health care from attacks in north-east Nigeria. Available from: https://www.who.int/news-room/feature-stories/detail/how-community-engagement-prevents-and-protects-health-care-from-attacks-in-north-east-nigeria. Accessed July30, 2021.
- 28.James PB, Wardle J, Steel A, et al. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Global Health. 2018;3:e000895. doi: 10.1136/bmjgh-2018-000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nsirim RO, Iyongo JA, Adekugbe O, et al. Integration of traditional birth attendants into prevention of mother-to-child transmission at primary health facilities in Kaduna, North-West Nigeria. J Public Health Africa. 2015;6(1):455. doi: 10.4081/jphia.2015.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamela G, Kabondo C, Tembo T, et al. Evaluating the benefits of incorporating traditional birth attendants in HIV prevention of mother to child transmission service delivery in Lilongwe, Malawi. Afr J Reprod Health. 2014;18(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- 31.The Economist. Crime waves, the Gulf of Guinea is now the world’s worst piracy hotspot. International, June 29th, 2019 edition. Available from: https://www.economist.com/international/2019/06/29/the-gulf-of-guinea-is-now-the-worlds-worst-piracy-hotspot. Accessed July30, 2021.
- 32.US Consolate General Lagos. OSAC: Nigeria 2019 crime & safety report: Lagos. Available from: https://www.osac.gov/Content/Report/4a5eaf52-3655-43e6-b540-1684bcb6f3de. Accessed July30, 2021.
- 33.Schuster RC, de Sousa O, Rivera J, et al. Performance-based incentives may be appropriate to address challenges to delivery of prevention of vertical transmission of HIV services in rural Mozambique: a qualitative investigation. Hum Resour Health. 2016;14:60. doi: 10.1186/s12960-016-0157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain H, Mori AT, Khan AJ, et al. The cost-effectiveness of incentive-based active case finding for tuberculosis (TB) control in the private sector Karachi, Pakistan. BMC Health Serv Res. 2019;19:690. doi: 10.1186/s12913-019-4444-z [DOI] [PMC free article] [PubMed] [Google Scholar]