Abstract
The diagnosis of liver damage induced by mushroom poisoning is still challenging. This study aims to screen the early biological indexes that could predict acute mushroom poisoning with liver damage. The patients with acute mushroom poisoning and liver damage admitted to The First Affiliated Hospital of Dalian Medical University,China from July 2007 to August 2017 were analyzed retrospectively. A total of 66 patients were enrolled in this study, with 44 and 22 patients in the liver injury group and liver failure group, respectively. Ten patients in the liver failure group died, with a mortality of 45.5% in this group. Multivariable Cox regression showed that the blood ammonia (NH3) and lactic acid (Lac) at the time of admission were independently associated with the in-hospital time to death for patients with liver failure induced by mushroom poisoning. Lactate and blood ammonia at the time of admission could be used to predict the prognosis of patients with acute mushroom poisoning and liver failure.
Keywords: lactate, blood ammonia, biomarkers, mushroom poisoning, liver failure
Introduction
Toadstool poisoning, a foodborne disease, is a leading cause of death every year worldwide [1–5]. Although there are more than 10 000 species of mushrooms worldwide, around 50–100 of them are potentially harmful. Amanita phalloides is one of the most poisonous mushrooms and is responsible for most fatal cases due to mushroom poisoning. Three toxins present in this species include amatoxins, phallotoxins, and virotoxins. Of these three toxins, amatoxins, especially α-amanitin, can have particularly toxic and varied effects. One of the mechanisms of this toxicity has been proposed via inhibition of RNA polymerase II, leading to a deficit of protein and cell death. The liver is often the main organ targeted during this toxicity [6, 7]. The mushroom poisoning includes an initial asymptomatic phase, followed by gastrointestinal symptoms, liver and kidney injuries. Although several mechanisms have been proposed and investigated regarding mushroom poisoning, the treatment strategies are still limited [8]. At present, the treatment of mushroom poisoning includes blocking the enterohepatic circulation of toxins, promoting the excretion of toxins, and protecting organs from damage. For patients with severe liver injury, liver transplantation may be the final treatment plan [9].
However, the criteria for emergency liver transplantation are still unclear. Also, as many hospitals and healthcare workers cannot identify the mushroom species responsible for the toxicity, amatoxin poisoning is often a challenge in the field [10]. Thus, there is a critical requirement to identify biological markers that could predict the prognosis of poisoning and liver failure associated with mushroom consumption.
Background
Ammonia is a metabolic waste generated during the process of amino acid catabolism. This toxic waste metabolite is converted into urea in the liver; thus, liver dysfunction is often associated with hyperammonemia [11]. Patients with chronic liver disease were found to have elevated lactate levels [12]. Studies also show that arterial ammonia levels can predict mortality with 78% sensitivity and 77.5% diagnostic accuracy in acute liver failure [13]. Similarly, lactate levels and lactate clearance have been shown to reflect the severity of disease in critically ill patients with liver cirrhosis [14]. Indeed, both serum lactate kinetics and ammonia have been proposed as prognostic markers for high mortality risk associated with liver cirrhosis and organ failure [15, 16].
Objectives
In this study, we decided to investigate early markers that could assist in the prognosis of patients with mushroom poisoning-associated liver damage. The current lack of methods to predict mushroom poisoning necessitates the need to develop tools for the same. Previous studies indicated that the prognostic role of lactate and ammonia levels could assess the risk of death associated with liver failure. Hence, we attempted to probe if these markers could predict mortality linked to liver damage associated with mushroom poisoning.
Materials and Methods
Patients and study design
Patients admitted to the First Affiliated Hospital of Dalian Medical University from July 2007 to August 2017 and diagnosed with acute mushroom poisoning with liver damage were analyzed retrospectively. The diagnosis criteria were a clear history of eating wild mushrooms and the appearance of digestive tract symptoms after eating them to rule out liver damage caused by other reasons. Those with existing cirrhosis and new diseases (acute heart failure, acute renal failure, and acute hepatitis) within 26 weeks were excluded.
All patients received primary treatment and blood purification. All liver injury groups were treated with hemoperfusion to remove toxins based on liver function and renal function after admission. CRRT was used to reduce organ damage in patients with multiple organ damage. If the liver function was rapidly deteriorating, combined with plasma exchange. The liver failure group chose CRRT combined with plasma exchange as the main treatment and hemoperfusion to remove bilirubin in patients with high bilirubin.
The study was conducted in accordance with the Basic and Clinical Pharmacology and Toxicology policy for experimental and clinical studies [17]. This study was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University [PJ-KS-JG-QX-2020-77] and written informed consent was waived due to the retrospective nature of this study.
Grouping
Liver injury criteria: gastrointestinal symptoms after poisoning, transaminases more than five times elevated, INR > 2. [18]. Liver failure: extreme weakness, jaundice more than 170 mmol/L or elevation > 17 mmol/L per day, hepatic encephalopathy, INR > 1.5 [19]. Data analysis was carried out between the liver injury group and the liver failure group and between the dead and the survival in the liver failure group.
Data collection and definition
Data for gender, age, poisoning severity score (PSS) [20], days since onset, a series of biochemical indexes at the time of admission, and death during hospitalization were collected from the medical records of the patients. PSS score refers to the recommended scoring for the severity of acute poisoning where the value of the score increases with the severity of poisoning.
Statistical analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for analysis. Continuous variables were represented using the median and interquartile range. Due to the small sample size and the heterogeneous variance between the groups, the nonparametric test of two independent samples was used to analyze and show the difference between the two groups. Categorical variables were shown as frequencies and percentages, and the chi-square (χ2) test was used for their comparison. Univariable and multivariable Cox regression models were used to explore the factors related to time from hospital admission to in-hospital death. Factors with statistical significance in univariable analysis were included in multivariable regression analysis. P-values <0.05 were considered statistically significant.
Results
General characteristics
There were 105 patients eligible for mushroom poisoning. A total of 66 patients met the criteria of liver injury, excluding 39 patients with nonhepatic injury and whose transaminase is always less than five times. Among 66 patients, 44 comprised the liver injury group and 22 were in the liver failure group. We analyzed the liver injury group and liver failure group indexes at the time of admission (Table 1).
Table 1.
Characteristics between liver injury and liver failure groups
| Characteristics | Liver injury group (n = 44) | Liver failure group (n = 22) | P |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 20 (45.5) | 12 (54.5) | <0.01** |
| Female | 24 (54.5) | 10 (45.5) | |
| PSS, median (range) | 8.00 (3.00–13.00) | 1.00 (6.00–7.60) | <0.01** |
| Age (years), median (range) | 54.50 (27.00–79.00) | 51.50 (36.00–72.00) | 0.28 |
| Days since onset (days), median (range) | 2.50 (1.00–9.00) | 2.75 (2.00–8.00) | 0.71 |
| Length of in-hospital stay (days), median (range) | 9.00 (4.00–15.00) | 6.50 (2.00–17.00) | 0.15 |
| AST (U/L), median (range) | 1389.00 (134.00–5263.00) | 4644.00 (97.00–8369.00) | <0.01** |
| ALT (U/L), median (range) | 2152.50 (244.00–6351.00) | 5213.00 (508.00–10388.00) | <0.01** |
| TBIL (μmol/L), median (range) | 82.30 (13.20–99.40) | 8.40 (16.80–214.90) | <0.01** |
| PT (s), median (range) | 16.10 1.90–106.00) | 26.45 (11.40–81.50) | 0.02* |
| INR, median (range) | 1.43 (.99–6.00) | 2.45 (1.13–6.84) | 0.01* |
| Lac(mmol/L),median (range) | 1.10 (.60–2.80) | 3.05 (.80–15.00) | <0.01** |
| ALB (g/L), median (range) | 4.05 (29.10–56.00) | 35.50 (31.00–56.00) | 0.13 |
| TP (g/L), median (range) | 68.20 (43.60–85.00) | 6.90 (48.20–75.80) | 0.02* |
| NH3 (μmol/L), median (range) | 31.00 (8.00–8.00) | 73.50 (16.00–235.00) | <0.01** |
| Hb (g/L), median (range) | 141.00 (81.00–19.00) | 145.00 (11.000–2.00) | 0.25 |
| PLT (109/L), median (range) | 16.50 (6.00–308.00) | 126.50 (22.40–352.00) | 0.05 |
| Amy (U/L), median (range) | 76.50 (31.00–191.00) | 114.00 (42.00–284.00) | 0.01* |
| Lipase (U/L), median (range) | 139.50 (39.00–765.00) | 289.00 (122.00–1713.00) | 0.01* |
| Cre (μmol/L), median (range) | 57.50 (32.00–259.00) | 6.50 (38.75–281.00) | 0.69 |
*P < 0.05.
**P < 0.01.
PSS: poisoning severity score; AST: aspartate transaminase; ALT: alanine transaminase; TBIL: total bilirubin; PT: prothrombin time; INR: international normalized ratio; Lac: lactic acid; ALB: albumin; TP: total protein; NH3: blood ammonia; Hb: hemoglobin; PLT: platelet; Amy: amylase; Cre: creatinine.
Significant differences were noted in gender, PSS, AST, ALT, TBIL, PT, INR, TP, NH3, Amy, and lipase at the time of admission between the liver injury and the liver failure group (all P < 0.05). Patients in the liver failure group had more females (54.5% vs. 45.5%, P < 0.01), higher PSS score (1.0 [6.0–7.6] vs. 8.0 [3.0–13.0], P < 0.01), severe liver damage, and pancreatic damage (Table 1).
Blood purification treatment
All patients were divided into liver injury group and liver failure group according to their condition at the time of admission. The liver injury group and liver failure group underwent different frequencies of hemoperfusion, hemofiltration and plasma exchange according to the damage of the organs. The specific treatment conditions are shown in (Table 2).
Table 2.
The treatment situation is shown in the figure below
| Liver injury group (n = 44) | Liver failure group (n = 22) | |||
|---|---|---|---|---|
| Treatment times | Person times | Treatment times | Person times | |
| HP | 0 | 1 | 0 | 5 |
| 1 | 17 | 1 | 6 | |
| 2 | 6 | 2 | 9 | |
| 3 | 10 | 5 | 1 | |
| 4 | 5 | 6 | 1 | |
| 5 | 5 | |||
| CRRT | 0 | 22 | 0 | 10 |
| 1 | 5 | 1 | 6 | |
| 2 | 5 | 2 | 4 | |
| 3 | 9 | 3 | 1 | |
| 4 | 1 | 14 | ||
| 6 | 1 | |||
| 7 | 1 | |||
| PE | 0 | 19 | 0 | 2 |
| 1 | 7 | 1 | 7 | |
| 2 | 8 | 2 | 3 | |
| 3 | 7 | 3 | 4 | |
| 4 | 2 | 4 | 3 | |
| 5 | 1 | 5 | 3 | |
Survival and death subgroups
All patients in the liver injury group survived after treatment. The patients in the liver failure group were further divided into survival and death subgroups, and the indexes at the time of admission for different subgroups in the liver failure group were analyzed (Table 3).
Table 3.
Characteristics between survival and death subgroups in the liver failure group
| Characteristics | Survival subgroup (n = 12) | Death subgroup (n = 10) | P |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 9 (75.0) | 3 (3.0) | 0.03* |
| Female | 3 (25.0) | 7 (7.0) | |
| PSS, median (range) | 1.00 (6.00–12.00) | 12.00 (7.00–17.00) | 0.05* |
| Age (years), median (range) | 47.50 (36.00–6.00) | 57.50 (42.00–72.00) | 0.04* |
| Days since onset (days), median (range) | 2.00 (2.00–8.00) | 3.00 (2.00–4.00) | 0.96 |
| Length of in-hospital stay (days), median (range) | 9.00 (6.00–17.00) | 3.00 (2.00–5.00) | <0.01** |
| AST (U/L), median (range) | 5365.50 (97.00–8268.00) | 402.50 (716.00–8369.00) | 0.49 |
| ALT (U/L), median (range) | 5213.00 (1005.00–7049.00) | 503.50 (508.00–10388.00) | 0.87 |
| TBIL (μmol/L), median (range) | 59.30 (44.30–214.90) | 87.35 (16.80–155.70) | 0.59 |
| PT (s), median (range) | 2.45 (11.40–32.90) | 33.25 (16.30–81.50) | 0.01** |
| INR, median (range) | 2.14 (1.13–2.90) | 2.90 (1.48–6.84) | <0.01** |
| Lac (mmol/L), median (range) | 2.25 (.80–3.50) | 7.55 (2.08–15.00) | <0.01** |
| ALB (g/L), median (range) | 40.30 (34.30–56.00) | 34.10 (31.00–36.10) | <0.01** |
| TP (g/L), median (range) | 64.65 (49.70–75.80) | 53.05 (48.20–65.40) | 0.02** |
| NH3 (μmol/L), median (range) | 50.50 (16.00–121.00) | 94.00 (28.00–235.00) | 0.02** |
| Hb (g/L), median (range) | 159.00 (12.00–2.00) | 139.50 (11.00–168.00) | 0.06 |
| PLT (109/L), median (range) | 150.50 (65.00–213.00) | 73.50 (22.40–352.00) | 0.74 |
| Amy (U/L), median (range) | 107.00 (42.00–158.00) | 116.00 (9.00–284.00) | 0.15 |
| Lipase (U/L), median (range) | 233.50 (122.00–984.00) | 557.00 (261.00–1713.00) | 0.02 |
| Cre (μmol/L), median (range) | 64.00 (39.00–88.00) | 57.00 (38.75–281.00) | 0.41 |
*P < 0.05.
**P < 0.01.
Among the 22 patients in the liver failure group, 10 patients were deceased, including 3 males and 7 females, with a mortality of 45.5% in the liver failure group. There were no significant differences in the days since onset, ALT, AST, Hb, TBIL, PLT, and Amy at the time of admission between the survival and death subgroups (all P > 0.05; Table 3). However, there were significant differences in age, PSS score, PT, INR, Lac, ALB, TP, NH3 at the time of admission, and length of in-hospital stay between the survival and death subgroups (all P < 0.05; Table 3).
Predictive factors of survival and death
The Cox regression model was established to determine the length of in-hospital stay with the time variable; the death as the survival outcome variable; and PSS, AST, ALT, TBIL, PT, INR, Lac, ALB, TP, NH3, Hb, PLT, Amy, Lipase, Cre covariates as independent variables. Results revealed that the NH3 [hazard ratio (HR): 1.011, 95% confidence interval (CI): 1.000–1.021, P = 0.042] and Lac (HR: 1.187, 95% CI: 1.026–1.372, P = 0.021) at the time of admission were independently associated with in-hospital time to death for patients with liver failure induced by mushroom poisoning (Fig. 1, Table 4)).
Figure 1.
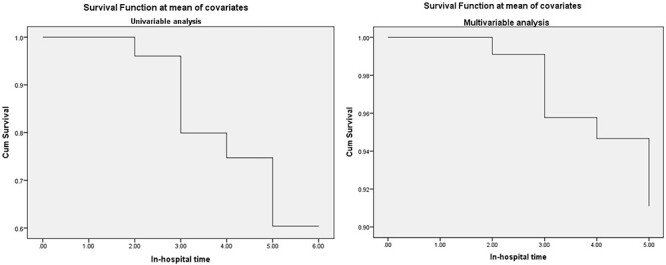
Survival function at mean of covariates.
Table 4.
Predictive factors of the time to death in the liver failure group
| Index | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| PSS | 1.266 | 1.008–1.589 | 0.043* | - | - | - |
| AST (U/L) | - | - | 0.449 | - | - | - |
| ALT (U/L) | - | - | 0.801 | - | - | - |
| TBIL (μmol/L) | - | - | 0.645 | - | - | - |
| PT (s) | 1.031 | 1.007–1.055 | 0.011* | - | - | - |
| INR | 1.426 | 1.056–1.925 | 0.021* | - | - | - |
| Lac (mmol/L) | 1.090 | 1.047–1.353 | 0.008** | 1.187 | 1.026–1.372 | 0.021** |
| ALB (g/L) | 0.749 | 0.588–.955 | 0.020* | - | - | - |
| TP (g/L) | 0.926 | 0.853–1.004 | 0.026 | - | - | - |
| NH3 (μmol/L) | 1.012 | 1.002–1.022 | 0.014* | 1.011 | 1.000–1.021 | 0.042** |
| Hb (g/L) | - | - | 0.144 | - | - | - |
| PLT (109/L) | - | - | 0.668 | - | - | - |
| Amy (U/L) | - | - | 0.231 | - | - | - |
| Lipase (U/L) | 1.001 | 1.000–1.003 | 0.055 | - | - | - |
| Cre (μmol/L) | - | - | 0.638 | - | - | - |
*P < 0.05.
**P < 0.01.
Discussion
Most of the acute mushroom poisoning patients admitted to our hospital suffered from acute gastroenteritis and liver damage. We experienced a total mortality rate of 9.5% and a mortality rate of 45.4% in patients with acute liver failure. We found that blood ammonia levels and lactic acid were independently associated with time to death for patients with mushroom poisoning associated with liver failure. Thus, we suggested the NH3 and Lac as possible predictive factors of the in-hospital mortality rate for patients with liver failure caused by mushroom poisoning.
Although the mortality observed in our study is consistent with previous studies [21], it is also lower than that reported in some studies [22]. This indicated that some patients with liver failure could survive through comprehensive treatment and without liver transplantation.
There is no specific antidote for acute mushroom poisoning. It has been previously reported that blood purification treatment can significantly eliminate toxins and improve prognosis [23–25]. In this study, we observed that patients in the liver injury group survived post active blood purification treatment.
Cox regression model was established for patients in the liver failure group with the length of in-hospital stay as time variable and death as the survival outcome variable, and the results showed that lactate and ammonia at the time of admission were the risk factors of death. It was consistent with previous studies that show that the prognosis of patients with mushroom poisoning can be predicted 3 days post-onset of disease [18].
More than 95% of mushroom poisoning-related deaths worldwide are related to mushrooms with amatoxin [26]. The main species of toadstools in northern China are Amanita. Most patients were admitted to our hospital after 3 days of the onset of the disease when typical liver damage had already appeared clinically. Moreover, patients with liver failure often died 3 days after admission, consistent with previous studies where most of the patients with severe mushroom poisoning died within 1 week [21, 27]. Previous studies have also detected elevated lactate levels in patients with mushroom-induced acute liver failure [21]; however, the possibility of using these levels as prognostic markers was not suggested.
Limitations
First, all data in our study were from one medical center, and the results are not necessarily suitable for other regions or countries. Secondly, as a retrospective analysis, there was selective bias, which could have impacted the conclusion.
Conclusion
Our study found that blood purification treatment could improve the rescue success rate of mushroom poisoning patients with liver injury. NH3 and Lac were shown to be the predictive factors of the in-hospital time to death for patients with liver failure induced by mushroom poisoning, providing a clinical reference for physicians confronting this issue.
Ethics approval and consent to participate
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies. This study was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University (No. PJ-KS-JG-QX-2020-77) and written informed consent was waived due to the retrospective nature of this study.
Patient consent for publication
Not applicable.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest statement
None declared.
Acknowledgments
None. No funding was received for this study.
Contributor Information
Yanguo Gao, Department of Neurology, Xinhua Hospital Affiliated to Dalian University, No. 156 Wansui Street, Shahekou District, Dalian, China.
Hongqiao Zhang, Department of Emergency, the First Affiliated Hospital of Dalian Medical University, No.222 Zhongshan Road, Xigang District Dalian, China.
Hua Zhong, Department of Medical Record, the First Affiliated Hospital of Dalian Medical University, No.222 Zhongshan Road, Xigang District Dalian, China.
Suosuo Yang, Department of Emergency, the First Affiliated Hospital of Dalian Medical University, No.222 Zhongshan Road, Xigang District Dalian, China.
Qiuyan Wang, Department of Emergency, the First Affiliated Hospital of Dalian Medical University, No.222 Zhongshan Road, Xigang District Dalian, China.
References
- 1.Li Y, Huang Y, Yang J et al. Bacteria and poisonous plants were the primary causative hazards of foodborne disease outbreak: a seven-year survey from Guangxi, South China. BMC Public Health 2018;18:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu D, Cheng H, Bussmann RW et al. An ethnobotanical survey of edible fungi in Chuxiong City, Yunnan, China. J Ethnobiol Ethnomed 2018;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavassoli M, Afshari A, Arsene AL et al. Toxicological profile of Amanita virosa—a narrative review. Toxicol Rep 2019;6:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G, Yuan Q, Wang L et al. Epidemiology of foodborne disease outbreaks from 2011 to 2016 in Shandong Province, China. Medicine 2018;97:e13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perisetti A, Raghavapuram S, Sheikh AB et al. Mushroom poisoning mimicking painless progressive jaundice: a case report with review of the literature. Cureus 2018;10:e2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santi L, Maggioli C, Mastroroberto M et al. Acute liver failure caused by Amanita phalloides poisoning. Int J Hepatol 2012;2012:487480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sai Latha S, Naveen S, Pradeep CK et al. Toxicity assessment of wild mushrooms from the Western Ghats, India: an in vitro and sub-acute in vivo study. Front Pharmacol 2018;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadpour B, Tajoddini S, Rajabi M et al. Mushroom poisoning in the Northeast of Iran; a retrospective 6-year epidemiologic study. Emergency (Tehran, Iran) 2017;5:e23. [PMC free article] [PubMed] [Google Scholar]
- 9.Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol 2005;42:202–9. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Mu M, Yuan L et al. Challenges in the early diagnosis of patients with acute liver failure induced by amatoxin poisoning: two case reports. Medicine 2018;97:e11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Lkhagva E, Chung HJ et al. The pharmabiotic approach to treat hyperammonemia. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheiner B, Lindner G, Reiberger T et al. Acid-base disorders in liver disease. J Hepatol 2017;67:1062–73. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut 2006;55:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drolz A, Horvatits T, Rutter K et al. Lactate improves prediction of short-term mortality in critically Ill patients with cirrhosis: a multinational study. Hepatology (Baltimore, Md) 2019;69:258–69. [DOI] [PubMed] [Google Scholar]
- 15.Gao F, Huang XL, Cai MX et al. Prognostic value of serum lactate kinetics in critically ill patients with cirrhosis and acute-on-chronic liver failure: a multicenter study. Aging 2019;11:4446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalimar SMF, Mookerjee RP et al. Prognostic role of ammonia in patients with cirrhosis. Hepatology (Baltimore, Md) 2019;70:982–94. [DOI] [PubMed] [Google Scholar]
- 17.Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol 2018;123:233–5. [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Lee D, Lee JH et al. Predictors of poor outcomes in patients with wild mushroom-induced acute liver injury. World J Gastroenterol 2017;23:1262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibáñez-Samaniego L, Bañares R. Acute liver failure caused by mushroom poisoning: still a fork in the road. Liver Int 2016;36:952–3. [DOI] [PubMed] [Google Scholar]
- 20.Persson HE, Sjöberg GK, Haines JA et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol 1998;36:205–13. [DOI] [PubMed] [Google Scholar]
- 21.Karvellas CJ, Tillman H, Leung AA et al. Acute liver injury and acute liver failure from mushroom poisoning in North America. Liver Int 2016;36:1043–50. [DOI] [PubMed] [Google Scholar]
- 22.Pajoumand A, Shadnia S, Efricheh H et al. A retrospective study of mushroom poisoning in Iran. Hum Exp Toxicol 2005;24:609–13. [DOI] [PubMed] [Google Scholar]
- 23.Bergis D, Friedrich-Rust M, Zeuzem S et al. Treatment of Amanita phalloides intoxication by fractionated plasma separation and adsorption (Prometheus®). J Gastrointest Liver Dis 2012;21:171–6. [PubMed] [Google Scholar]
- 24.Chaudhary S, Chaurasia RK, Patel S et al. Clinical profile and outcome of patients presenting with mushroom poisoning in a tertiary care center of eastern Nepal. JNMA J Nepal Med Assoc 2013;52:543–8. [PubMed] [Google Scholar]
- 25.Larsen FS, Schmidt LE, Bernsmeier C et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol 2016;64:69–78. [DOI] [PubMed] [Google Scholar]
- 26.Okumu MO, Patel MN, Bhogayata FR et al. Acute poisonings at a regional referral hospital in Western Kenya. Trop Med Infect Dis 2018;3:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erden A, Esmeray K, Karagöz H et al. Acute liver failure caused by mushroom poisoning: a case report and review of the literature. Int Med Case Rep J 2013;6:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


