Abstract
Objective
This study was designed to investigate the effects of liquefied petroleum gas (LPG) on hematotoxic, cardiotoxic, and hepatotoxic indices and the modifying influence of selected polyphenols.
Methods
Adult male Wistar rats were exposed to1000 ppm LPG for 10 min at 12-h interval for 30 days with or without cotreatment with 50 mg/kg rutin, quercetin, tannic acid, or gallic acid followed by hematological, biochemical, and histopathological evaluations in animal tissues.
Results
Exposure to LPG induced hematotoxicity, cardiotoxicity, and hepatotoxicity. This is reflected in alterations to levels or activities of blood parameters (hemoglobin, packed cell volume, red blood cells, mean corpuscular volume, mean corpuscular hemoglobin, and platelets), enzymatic and nonenzymatic oxidative stress markers, nitrite, lactate dehydrogenase, creatine kinase-MB, transaminases, γ-glutamyl transpeptidase, bilirubin, and plasma albumin. LPG exposure also caused dyslipidemia and histoarchitectural changes. Treatment with the selected polyphenols effectively attenuated LPG-induced toxicity in rat tissues.
Conclusion
The results indicate that continuous exposure to LPG could lead to blood-, heart-, and liver-related diseases and dietary polyphenols could provide benefits in diseases associated with LPG inhalation toxicity.
Keywords: liquefied petroleum gas, cardiac and hepatic disorders, hematotoxicity, occupational/domestic exposure, polyphenols
Graphical abstract

Introduction
Liquefied petroleum gas (LPG) is an industrial gas that is used in almost every modern manufacturing process and the domestic sphere. There are many benefits of industrial gases. However, several hazards are associated with their use, and they could be harmful if not handled properly. Gas leaks can affect entire neighborhoods or even cities, causing enormous environmental impacts [1]. LPG is a generic term for the flammable mixture of hydrocarbons (usually propane and butane) available for domestic, commercial, and industrial uses [2]. LPG is colorless and odorless and is prepared by refining petroleum or natural gas. For safety purposes, it contains a sulfur-based odorizing agent to allow leaks to be more easily detected.
The use of LPG is rapidly increasing in developing countries like Nigeria [3]. Domestically, LPG is mainly used for household cooking, while industrially, it is used in metallurgical industries, steel plants, and pharmaceutical industries for processes like gas cutting, gas welding, and glass cutting. Toxicity through inhalation of the gas causes different symptoms from a simple headache, respiratory distress, cough, skin irritation, and dizziness to coma and death [4]. Occupational exposure to LPG constitutes a major risk for serious toxicity because of regular, daily exposure to the gas that commonly occurs at the workplace. The inhaled gas is transported by the blood to various organs including the liver and heart where it could eventually cause harmful effects.
Polyphenols are naturally occurring compounds present in plants and have been found to have essential physiological and pharmacological actions. They comprise a wide family of molecules bearing one or more phenolic rings and are present in many food sources like wine, green tea, grapes, vegetables, red fruits, and coffee [5]. Tannic acid (TAN) is a naturally occurring plant polyphenol composed of a central glucose molecule derivatized at its hydroxyl groups with one or more galloyl residues. Gallic acid (GAL), also known as 3,4,5-trihydroxybenzoic acid, is a monomer of TAN. Both TAN and GAL are some of the most important polyphenolic substances in plants [6]. Quercetin and its glycosylated form, rutin, are natural flavonoids found in many fruits and vegetables [7]. Experimental, clinical, and epidemiologic studies have shown that many polyphenols including rutin, quercetin, GAL, and TAN have antioxidant and anti-inflammatory properties that could have preventive and/or therapeutic effects on cardiovascular disease, hepatocellular disease, neurodegenerative disorders, hematological disorder, cancer, and obesity [8–15]. Recent studies have shown that polyphenols have vast potentials for drug development [5]. Thus, the present study has been designed to provide an insight into probable hazards to cardiac and hepatic health, posed by exposure to LPG and to ascertain the modulatory effects of polyphenols.
Materials and methods
Quercetin (3,3′4′,5,6-pentahydroxyflavone hydrate) (C27H10O7.xH2O), rutin (Quercetin-3-rutinoside hydrate) (C27H30O16xH2O), GAL (3.4.5-Trihydroxybenzoic acid) ((HO)3C6H2CO2 H2), TAN (Gallotannin) (C27H30O46), thiobarbituric acid (TBA), trichloroacetic acid (TCA), epinephrine, 5′,5′-dithiobis-(2-nitrobenzoic acid) (DTNB or Ellman’s reagent), sodium citrate, sulfanilamide, phosphoric acid, N-(1-naphthyl)ethylenediamine, and 2,4,6-Tripyridyl-s-triazine (TPTZ) were obtained from Sigma-Aldrich (St Louis, MO). All other chemicals and reagents used were of analytical grade. The assay kits used for the study were products of Randox Laboratory Ltd (UK).
Animal handling and treatment
Forty-two adult male Wistar rats weighing 175 ± 25 g were procured from the Department of Veterinary Anatomy, University of Ibadan, Ibadan, Nigeria and used for the study. Animals were housed in the animal research facility of the Department of Biochemistry, the Federal University of Technology, Akure, Nigeria and maintained on standard animal pellets and water ad libitum. They were handled and used following the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 2011. The animals were divided into six groups with seven rats per group. The groups were normal control group, LPG exposed group, groups exposed to LPG and orally administered with 50 mg/kg body weight of rutin, quercetin, GAL, or TAN [15–17]. Exposure to LPG and administration of polyphenols were carried out 6 days per week for 30 days.
Exposure to LPG
LPG was obtained under pressure in a gas cylinder from a gas station in Akure, Nigeria. A 36 l glass inhalation chamber (30 cm × 41 cm × 30 cm) with two outlets on the opposite sides, with the outlet higher than the inlet, was used for animal exposure to LPG. Animals were subjected to inhalation of 1000 ppm LPG (the concentration was calculated using the ideal gas law PV = nRT) for 10 min at 12-h intervals, 6 days a week for 30 days, through a delivery tube connected to a pressure regulator and passed into the exposure chamber housing the animal. Normal control animals were placed in a similar exposure chamber for the same period but without exposure to LPG. After each exposure session, the animals were returned to their cages. Twenty-four hours after the last treatment, animals were sacrificed by cervical dislocation. Blood was collected by cardiac puncture into ethylenediamine tetra-acetic acid (EDTA) tubes for hematological analysis and preparation of plasma, whereas livers and hearts were excised and processed for biochemical estimations and histopathological evaluation.
Evaluation of hematological parameters
Hematotoxicity was assessed by measuring hemoglobin (Hb), packed cell volume (PCV), red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cells (WBC), and platelets (PLT) using established methods [18].
Biochemical analyses
Liver and heart were excised, washed in ice-cold 1.15% potassium chloride solution, blotted with filter paper, and homogenized in phosphate-buffered saline (PBS; pH 7.4, 1:10 w/v) using a Teflon homogenizer. The homogenate obtained was centrifuged at 10000 × g at 4°C for 30 min and the resulting supernatant was used for biochemical analyses.
Evaluation of oxidative stress, inflammation, and tissue damage indices
Assessment of lipid peroxidation
The formation of TBA reactive substances was measured to assess the extent of lipid peroxidation [19]. Brain supernatant (0.4 ml) was mixed with 1.6 ml of Tris-KCl buffer and 0.5 ml of 30% TCA. Then, 0.5 ml of 0.75% TBA was added and the mixture was placed in a water bath for 45 min at 80°C. The mixture was cooled and centrifuged at 3000 × g. The absorbance of the supernatant was measured against a reference blank at 532 nm. Malondialdehyde level was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1.
Determination of reduced glutathione level
Reduced glutathione (GSH) concentration in homogenates was measured as described in a previous report [20]. The absorbance of the yellow-colored thionitrobenzoic acid produced after the reaction of the homogenate with DTNB was measured at 412 nm.
Determination of ferric-reducing antioxidant power
Ferric-reducing antioxidant power (FRAP) was determined as previously described [21]. The homogenate and FRAP reagent (consisting of sodium acetate buffer (300 mM, pH 3.6), 10 mM TPTZ in 0.1 M HCl, and 20 mM FeCl3 in a ratio of 10:1:1 v/v/v) were mixed, vortexed, and then incubated at 37°C for 30 min in the dark. Absorbance was read at 593 nm. FRAP was deduced using a standard curve prepared with FeSO4·7H2O (0–2 mM). Results were expressed as μmol/l sample.
Evaluation of superoxide dismutase activity
Superoxide dismutase (SOD) activity of homogenates was determined as described in a previous report [22]. An aliquot of a dilution of homogenate in distilled water was added to 2.5 ml of 0.05 M carbonate buffer (pH 10.2). Then, 0.3 ml of freshly prepared 0.3 mM adrenaline was added. The reference cuvette contained 2.5 ml of buffer, 0.3 ml of adrenaline, and 0.2 ml of water. The increase in absorbance at 480 nm was monitored every 30 s for 150 s. One unit of SOD activity was given as the amount of protein necessary to cause 50% inhibition of the oxidation of adrenaline to adrenochrome under assay conditions.
Evaluation of nitrite level
The nitrite level of the samples was measured by the Griess reaction as described previously [23]. Sodium nitroprusside (2.7 ml, 10 mM) in PBS was added to 0.3 ml of the tissue homogenate and incubated at 25°C for 150 min. Then, 0.5 ml of the incubated aliquot was added to 0.5 ml of Griess reagent: (1% (w/v) sulfanilamide, 2% (v/v) orthophosphoric acid, and 0.1% (w/v) naphthylethylene diamine hydrochloride). The absorbance was measured at 546 nm with a spectrophotometer.
Determination of lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity was determined based on a previously reported method [24]. Blood was collected by cardiac puncture into EDTA tubes, properly mixed, and centrifuged at 3000 × g for 15 min. The clear supernatant obtained was used to estimate LDH activity using kits obtained from Randox Laboratories Ltd (UK).
Evaluation of markers of cardiovascular injury
Blood was collected by cardiac puncture into EDTA tubes, properly mixed, and centrifuged at 3000 × g for 15 min. The clear supernatant obtained was used to estimate creatine kinase-MB (CKMB) activity and levels of triglycerides (TRIG), total cholesterol (CHOL), and high-density lipoprotein cholesterol (HDL-C) using kits obtained from Randox Laboratories Ltd (UK). Low-density lipoprotein cholesterol (LDL-C) and very-low-density lipoprotein cholesterol (VLDL-C) were calculated using Friedewald’s formula, whereas the atherogenic and coronary risk indices were computed as previously reported [25].
Evaluation of markers of hepatocellular injury
Amounts of direct bilirubin, total bilirubin, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT) were estimated in plasma using assay kits obtained from Randox Laboratories Ltd, (Antrim, UK) following the instructions of the manufacturer.
Histopathological assessment
Formalin-fixed, paraffin-embedded tissues were routinely processed and stained with hematoxylin and eosin [26]. Histopathological assessment was carried out using an Acuscope® (China) microscope with TSView® Software (China) for imaging at ×400 magnification.
Statistical analysis
Results were analyzed using appropriate analysis of variance followed by Tukey’s multiple comparison tests. In all the tests, P < 0.05 was taken as the criterion for statistical significance. The statistical software used to analyze the data was GraphPad Prism 6.01 (GraphPad Software Inc., CA).
Results
Hematological indices
As shown in Table 1, Hb, PCV, RBC, MCV, MCH, MCHC, and PLT were decreased, whereas the WBC was increased in LPG-exposed rats compared with the control group. These alterations were attenuated in the groups treated with polyphenols.
Table 1.
effects of rutin (RUT), quercetin (QUE), tannic acid (TAN), and gallic acid (GAL) on hematological profile of rats exposed to liquefied petroleum gas (LPG)
| GROUP | PCV (%) | HB (g/dl) | RBC (×109/l) | WBC (×109/l) | MCV (fl) | MCH (pg) | MCHC (g/l) | PLT (×106/l) |
|---|---|---|---|---|---|---|---|---|
| Control | 39.3 ± 0.01 | 12.9 ± 0.01 | 4.07 ± 0.00 | 5.90 ± 0.01 | 9.77 ± 0.00 | 3.53 ± 0.01 | 33.83 ± 0.01 | 134.3 ± 0.04 |
| LPG | 33.3 ± 0.06a | 12.0 ± 0.01a | 3.70 ± 0.00a | 11.9 ± 0.02a | 9.63 ± 0.00a | 3.43 ± 0.00a | 29.05 ± 0.01a | 124.5 ± 0.05a |
| LPG + Rut(50 mg/kg) | 38.3 ± 0.01b | 13.5 ± 0.00b | 3.90 ± 0.00b | 7.03 ± 0.02b | 9.87 ± 0.00b | 3.47 ± 0.00b | 33.37 ± 0.00b | 135.3 ± 0.08b |
| LPG + Que(50 mg/kg) | 41.3 ± 0.04b | 13.8 ± 0.01b | 3.97 ± 0.00b | 6.17 ± 0.01b | 10.4 ± 0.00b | 3.50 ± 0.01b | 35.57 ± 0.01b | 137.6 ± 0.11b |
| LPG + Gal (50 mg/kg) | 41.0 ± 0.01b | 13.8 ± 0.00b | 4.13 ± 0.00b | 7.37 ± 0.00b | 9.87 ± 0.01b | 3.50 ± 0.00b | 33.07 ± 0.01b | 133.3 ± 0.08b |
| LPG + Tan (50 mg/kg) | 45.3 ± 0.01b | 14.9 ± 0.00b | 4.60 ± 0.00b | 6.30 ± 0.01b | 9.97 ± 0.01b | 3.59 ± 0.00b | 35.37 ± 0.01b | 136.3 ± 0.07b |
Results are expressed as mean ± SD (n = 7).
aP < 0.0001 vs. control
bP < 0.0001 vs. LPG
Evaluation of oxidative stress, inflammation, and tissue damage indices
Results of hepatic and cardiac antioxidant parameters are presented in Figures 1–4. LPG induced hepatic and cardiac oxidative stress in rats. This is observed in the diminished GSH level (Fig. 1), FRAP score (Fig. 2), and SOD activity (Fig. 3) along with the exacerbated lipid peroxidation (Fig. 4) in LPG–toxified rats compared with the control group (P < 0.0001). The redox stress was suppressed in gas-toxified groups administered with the polyphenols (P < 0.0001). As shown in Figure 5, nitrite level was markedly increased in the LPG-exposed group but decreased in the polyphenol-treated groups. Also, in Figure 6a, LDH activity was increased in the rats exposed to LPG compared with the control group (P < 0.0001), whereas treatment of exposed animals with the polyphenols reversed this effect of LPG. Quercetin and TAN were more effective in reducing the activity of LDH than rutin and GAL, respectively.
Figure 1 .

effect of rutin, quercetin, gallic acid, and tannic acid on GSH level of rats exposed to LPG. Results are expressed as mean ± SD (n = 7). aP < 0.0001 vs. CTRL, bP < 0.0001 vs. LPG. CTRL: control, RUT: rutin, QUE: quercetin, GAL: gallic acid, TAN: tannic acid, LPG: liquefied petroleum gas.
Figure 4 .

effect of rutin, quercetin, gallic acid, and tannic acid on lipid peroxidation level (LPO) of rats exposed to LPG. Results are expressed as mean ± SD (n = 7). aP < 0.0001 vs. CTRL, bP < 0.0001 vs. LPG. CTRL: control, RUT: rutin, QUE: quercetin, GAL: gallic acid, TAN: tannic acid, LPG: liquefied petroleum gas.
Figure 2 .

effect of rutin, quercetin, gallic acid, and tannic acid on FRAP of rats exposed to LPG. Results are expressed as mean ± SD (n = 7). aP < 0.0001 vs. CTRL, bP < 0.0001 vs. LPG. CTRL: control, RUT: rutin, QUE: quercetin, GAL: gallic acid, TAN: tannic acid, LPG: liquefied petroleum gas.
Figure 3 .

effect of rutin, quercetin, gallic acid, and tannic acid on SOD activity of rats exposed to LPG . Results are expressed as mean ± SD (n = 7). aP < 0.0001 vs. CTRL, bP < 0.0001 vs. LPG. CTRL: control, RUT: rutin, QUE: quercetin, GAL: gallic acid, TAN: tannic acid, LPG: liquefied petroleum gas SD: standard deviation.
Figure 5 .

effect of rutin, quercetin, gallic acid, and tannic acid on nitrite level (NO) of rats exposed to LPG. Results are expressed as mean ± SD (n = 7). aP < 0.0001 vs. CTRL, bP < 0.0001 vs. LPG. CTRL: control, RUT: rutin, QUE: quercetin, GAL: gallic acid, TAN: tannic acid, LPG: liquefied petroleum gas.
Figure 6 .
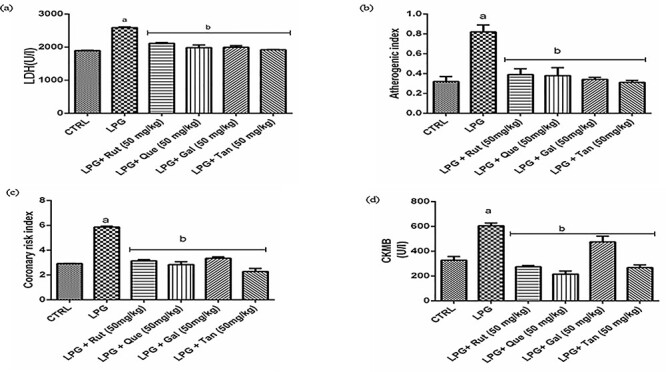
effect of rutin, quercetin, gallic acid, and tannic acid on (a) LDH activity (b) atherogenic index (c) coronary risk index (CRI) (d) CKMB of rats exposed LPG. Results are expressed as mean ± SD (n = 7). aP < 0.0001 vs. CTRL, bP < 0.0001 vs. LPG. CTRL: control, RUT: rutin, QUE: quercetin, GAL: gallic acid, TAN: tannic acid, LPG: liquefied petroleum gas.
Cardiovascular injury markers
Exposure to LPG resulted in ~25%, 100%, and 39% increase in CHOL, TRIG, and LDL-C, respectively, but a 50% decrease in HDL-C level (P < 0.0001) (Table 2). Polyphenol administration ameliorated LPG-induced dyslipidemia. Also, LPG exposure increased the atherogenic and coronary risk indices, whereas polyphenol administration reversed the increase (Fig. 6b and c). The activity of the myocardial injury marker, CK-MB, was significantly increased by exposure to LPG, but treatment with the polyphenols blunted this increase (Fig. 6d). Again, quercetin was more effective than rutin, whereas TAN was more effective than GAL.
Table 2.
effect of rutin (RUT), quercetin (QUE), tannic acid (TAN), and gallic acid (GAL) on lipid profile of rats exposed to liquefied petroleum gas (LPG)
| GROUP | CHOL (mg/dl) | TRIG (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) | HDL (mg/dl) |
|---|---|---|---|---|---|
| Control | 103.32 ± 3.02 | 66.67 ± 5.93 | 66.18 ± 0.21 | 13.27 ± 3.61 | 43.68 ± 1.19 |
| LPG | 129.36 ± 4.16a | 155.78 ± 4.89a | 92.46 ± 0.43a | 31.02 ± 3.48a | 23.52 ± 0.05a |
| LPG + Rut (50 mg/kg) | 106.00 ± 4.26b | 119.80 ± 4.14b | 79.67 ± 0.78b | 23.96 ± 1.63b | 30.80 ± 0.12b |
| LPG + Que (50 mg/kg) | 105.96 ± 2.90b | 111.39 ± 2.90b | 64.41 ± 0.56b | 22.27 ± 3.56b | 36.12 ± 0.21b |
| LPG + Gal (50 mg/kg) | 119.56 ± 1.56b | 63.22 ± 3.45b | 72.58 ± 4.88b | 12.65 ± 1.63b | 40.11 ± 3.56b |
| LPG + Tan (50 mg/kg) | 111.51 ± 3.56b | 59.38 ± 5.93b | 64.41 ± 1.92b | 11.88 ± 4.56b | 48.72 ± 0.21b |
Results are expressed as mean ± SD (n = 7).
aP < 0.0001 vs. control
bP < 0.0001 vs. LPG
Hepatocellular injury markers
The hepatotoxicity of LPG in exposed animals was revealed by increased levels of total and direct bilirubin, increased activities of GGT, ALT, and AST, and decreased plasma albumin level (P < 0.0001). All the polyphenols ameliorated these LPG-induced hepatocellular changes (Table 3).
Table 3.
effect of rutin (RUT), quercetin (QUE), tannic acid (TAN) and gallic acid (GAL) on liver function biomarkers of rats exposed to liquefied petroleum gas (LPG)
| GROUP | AST (U/I) | ALT (U/I) | GGT (U/I) | ALB (G/DL) | DB (μmol/l) | TB (μmol/l) |
|---|---|---|---|---|---|---|
| Control | 115.13 ± 2.52 | 46.65 ± 6.73 | 393.90 ± 2.37 | 4.33 ± 0.04 | 2.24 ± 0.51 | 1.84 ± 0.15 |
| LPG | 169.51 ± 3.60a | 82.98 ± 9.30a | 509.10 ± 0.10a | 4.02 ± 0.08a | 3.79 ± 0.57a | 2.55 ± 0.20a |
| LPG + Rut (50 mg/kg) | 125.84 ± 3.96b | 67.89 ± 7.87b | 484.10 ± 3.65b | 4.22 ± 0.05b | 2.54 ± 0.56c | 2.17 ± 0.13b |
| LPG + Que (50 mg/kg) | 113.43 ± 3.24b | 66.48 ± 2.39b | 482.90 ± 1.64b | 4.26 ± 0.05b | 2.53 ± 0.49c | 1.67 ± 0.12b |
| LPG + Gal (50 mg/kg) | 124.65 ± 6.48b | 51.85 ± 5.53b | 398.40 ± 7.74b | 4.16 ± 0.09b | 2.18 ± 0.49b | 1.71 ± 0.12b |
| LPG + Tan (50 mg/kg) | 108.33 ± 3.24b | 51.39 ± 1.06b | 351.30 ± 0.90b | 4.20 ± 0.01b | 1.68 ± 0.30b | 1.68 ± 0.18b |
Results are expressed as mean ± SD (n = 7). TB: total bilirubin; DB: direct bilirubin; ALB: albumin.
aP < 0.0001 vs. control
bP < 0.0001
cP < 0.001 vs. LPG
Histopathological observation
Livers of the control rats showed no visible lesions. On the contrary, vacuolar degeneration of hepatocytes was detected (black arrow) in LPG-exposed rats. The liver of rats treated with rutin, quercetin, GAL, and TAN showed no visible lesions (Fig. 7).
Figure 7 .

effect of rutin (RUT), quercetin (QUE), gallic acid (GAL), and tannic acid (TAN) on the histopathology of liver of rat exposed to liquefied petroleum gas (LPG) and administered rutin, quercetin, tannic acid, and gallic acid: ×400. Control: no visible lesion; LPG-exposed: vacuolar degeneration of hepatocytes was detected (black arrow); treatment with polyphenol reduced pathological changes showing no visible lesion.
Figure 8 shows representative photomicrographs of hearts from rats in all groups. The hearts of the control rats and polyphenol-treated groups showed no visible lesions. In the LPG-exposed rats, the disintegration of myofibres (black arrow) and adipose tissues within the interstitium (white arrow) could be observed.
Figure 8 .
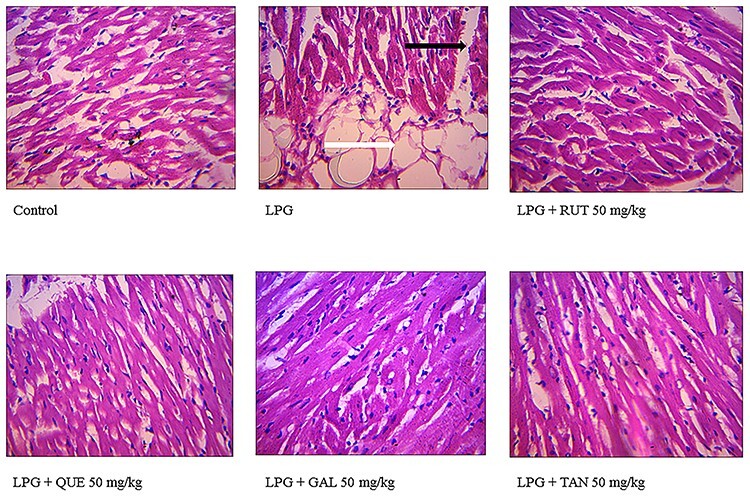
effect of rutin (RUT), quercetin (QUE), gallic acid (GAL), and tannic acid (TAN) on the histopathology of heart of rat exposed to LPG and administered rutin, quercetin, tannic acid, and gallic acid: ×400. Control: no visible lesion; LPG-exposed: disintegration of myofibres (black arrow) and the white arrow indicates adipose tissues within the interstitium; treatment with polyphenol reduced pathological changes showing no visible lesion.
Discussion
Occupational exposure to LPG is a major risk for serious toxicity. This is because of the regular exposure to this industrial gas at the workplace. Lack of adequate data on the toxicity of LPG among users can make problems associated with the toxicity go undetected and hinder the search for possible remedies. This informed the simulation of a scenario of regular exposure to the gas in this study to assess the toxicological effects on blood, liver, and heart. The remedial effects of administering two common flavonoids namely, quercetin and rutin (its glycoside derivative), and two well-known phenolic acids, GAL and TAN, were also investigated.
Blood can act as a pathological and physiological indicator of animal health [27]. Following inhalation, gas is transported by the blood to various organs including the liver and heart, where they may cause harmful effects. Free radicals and reactive oxygen species generated by toxic gases can disrupt the hematological system of organisms and compromise the ability of blood to maintain homeostasis. Deviation of hematological parameters from normal levels represents the presence of toxicity or disease [28]. In this study, the alterations in blood parameters resulting from LPG exposure may be suggestive of hemolysis, anemia, inflammatory processes, and weakened immunity. The observed decrease in Hb, PCV, RBC, MCV, MCH, MCHC, and PLT of toxified rats in our study agrees with previous findings [29, 30]. WBCs function primarily in defense against foreign bodies, usually through leukocytosis and antibody production. In the present study, the increased WBC count in toxified rats also agrees with previous findings [31]. Leukocyte increase may indicate a response to inflammatory processes or increased antibody activity [32]. The selected polyphenols effectively alleviated hematotoxicity in toxified rats and this supports previous reports of the antihemolytic activity of phenolics [33–35].
The effects of toxic substances on tissues often manifest in oxidative stress [36]. However, there are active defense mechanisms in the organism to counter redox stress. For example, endogenous antioxidant enzymes act jointly to mop reactive oxygen species, which promote oxidative stress. In the current study, exposure to LPG impaired the antioxidant defense system and elicited oxidative stress. Effects of LPG on the hepatic and cardiac FRAP, SOD activity, GSH level, and lipid peroxidation confirm the induction of oxidative stress [30, 37]. The polyphenols repaired the impaired antioxidant system by increasing hepatic and cardiac GSH levels and SOD activity while attenuating lipid peroxidation. This culminated in the amelioration of the LPG-imposed oxidative stress. The observed redox-modifying effect of the polyphenols agrees with their well-known antioxidant properties [25]. Nitric oxide is regarded as a proinflammatory mediator when excessively produced in abnormal conditions [38]. The observed increase in cardiac and hepatic nitrite level of LPG-exposed animals implies the occurrence of damaging inflammatory events in these organs, whereas the decrease in the concentration of nitrite in polyphenol-treated groups can be attributed to the anti-inflammatory effects of the polyphenols [39–41]. LDH, which is present in high concentrations in cardiac muscle and liver, is an important diagnostic enzyme. It is released into the bloodstream upon the destruction of cells by lipid peroxidation. Therefore, its increased activity in the LPG-exposed group indicates the tissue-damaging effect of the gas. Quercetin, rutin, GAL, and TAN ameliorated the damaging effect of LPG, probably through a reduction of lipid peroxidation in the heart.
Dyslipidemia is associated with abnormal levels and proportions of lipids in the blood and disturbed lipoprotein metabolism [42]. It is a major risk factor for atherosclerotic (coronary) heart disease and increases the susceptibility of the heart to chemical toxicity [43–45]. Dyslipidemia was observed in this study and shows the potential of LPG to cause cardiovascular problems. Treatment with the selected polyphenols reversed dyslipidemia in agreement with a previous report [46]. The dyslipidemic reversing ability of polyphenols has been linked to several mechanisms including prevention of LDL oxidation and decreased intestinal cholesterol absorption [47]. Atherogenic indices were derived to optimize the predictive capacity of the lipid profile [48]. Individuals with a high total-cholesterol/HDL cholesterol ratio have greater cardiovascular risk owing to the imbalance between the cholesterol carried by atherogenic and protective lipoproteins. The LPG-exposed group showed high atherogenic and coronary risk indices, which is an additional indication of the cardiotoxicity of the gas, whereas the lowered indices observed in the polyphenol-treated groups suggest the beneficial effects of the selected polyphenols on LPG cardiotoxicity.
A further indication of cardiac injury was revealed in the activity of the cardiac tissue marker enzyme, CKMB. CKMB activity is a sensitive indicator of an early stage of acute myocardial damage. In this study, the activity of CKMB increased significantly in the LPG-exposed group indicating acute myocardial damage that was ameliorated by treatment with the polyphenols. This may be linked to the prevention of membrane impairment by polyphenols [49].
Hepatotoxicity was evident in LPG-exposed rats as adjudged by the increase in activities of ALT, AST, γ-GGT, and plasma albumin level, which are consistent marks of hepatic injury. ALT and AST enter the bloodstream after hepatocellular structural integrity damage [13]. The effects of the gas on the various hepatotoxicity indices indicate that the synthetic and secretory functions of the liver were affected [50]. This corroborates the results from a previous study [51]. The extent of the hepatocellular injury was limited by treatment with the phenolic compounds supporting the well-reported hepatoprotective property of polyphenols [42, 52].
The histopathological findings support the biochemical improvements of the toxicological effects of LPG by quercetin, rutin, GAL, and TAN reported. There were increased incidence and severity of histopathological hepatic and cardiac lesions, but regenerative changes were noticed in groups treated with polyphenols, thus confirming their potent hepatoprotective and cardioprotective properties.
Of the two phenolic acids evaluated in this study, the activity of TAN was superior to that of GAL. This may be because of the polymeric nature of TA and the presence of more free hydroxyl groups [10]. For the flavonoids, quercetin showed better bioactivity compared with rutin. The reduced bioactivity of rutin may be attributed to its glycosylation [53]. Overall, TA showed the best activity at the employed dose. This agrees with the reported antioxidant, anti-inflammatory [54], cardioprotective [55], and hepatoprotective [13] properties of TA.
Conclusively, these findings show that workers continuously exposed to LPG are at a higher risk of developing clinical abnormalities especially blood, heart, and liver-related disorders. This study further shows that quercetin, rutin, GAL, and TAN could have beneficial health effects in people exposed to LPG due to their antioxidant, hematoprotective, hepatoprotective, and cardioprotective activities.
Contributor Information
Olayinka Oluwaseun Aladesanmi, Phytomedicine, Biochemical Pharmacology and Toxicology Laboratories, Department of Biochemistry, School of Sciences, The Federal University of Technology, Akure 340001, Nigeria.
Femi Emmanuel Ojo, Phytomedicine, Biochemical Pharmacology and Toxicology Laboratories, Department of Biochemistry, School of Sciences, The Federal University of Technology, Akure 340001, Nigeria.
Morenikejimi Bello, Phytomedicine, Biochemical Pharmacology and Toxicology Laboratories, Department of Biochemistry, School of Sciences, The Federal University of Technology, Akure 340001, Nigeria.
Bobola Jeremiah Taiwo, Phytomedicine, Biochemical Pharmacology and Toxicology Laboratories, Department of Biochemistry, School of Sciences, The Federal University of Technology, Akure 340001, Nigeria.
Afolabi Akintunde Akindahunsi, Phytomedicine, Biochemical Pharmacology and Toxicology Laboratories, Department of Biochemistry, School of Sciences, The Federal University of Technology, Akure 340001, Nigeria.
Funding
There was no special funding or grant for this research.
Conflict of interest statement
None declared.
References
- 1.Gomes JBA, Rodrigues JJPC, Rabêlo RAL et al. IoT-enabled gas sensors: technologies, applications, and opportunities. J Sens Actuator Netw 2019;8. doi: 10.3390/jsan8040057. [DOI] [Google Scholar]
- 2.Ihemtuge TU, Aimikhe VJ. Optimization of liquefied petroleum gas (LPG) distribution in Nigeria. Int J Eng Tech Res 2020;10. doi: 10.31873/ijetr.10.5.83. [DOI] [Google Scholar]
- 3.Puzzolo E, Pope D, Stanistreet D et al. Clean fuels for resource-poor settings: a systematic review of barriers and enablers to adoption and sustained use. Environ Res 2016;146:218–34. doi: 10.1016/j.envres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Jafar N, Simin H, Mortaza S. A case report: convulsion and reduced level of consciousness in two children following liquefied petroleum gas inhalation. J Environ Anal Toxicol 2017;07:10–1. doi: 10.4172/2161-0525.1000444. [DOI] [Google Scholar]
- 5.Silva RFM, Pogačnik L. Polyphenols from food and natural products: neuroprotection and safety. Antioxidants 2020;9:1–13. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinyemi AJ, Olabiyi AA, Ogunmodede OT et al. Inhibitory effect of tannic acid and its derivative (gallic acid) against cisplatin – induced thiobarbituric acid reactive substances (TBARS) production in rat kidney – in vitro. Int J Adv Res 2015;3:116–26. [Google Scholar]
- 7.Lee S, Lee J, Lee H et al. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J Food Biochem 2019;43:1–9. doi: 10.1111/jfbc.13002. [DOI] [PubMed] [Google Scholar]
- 8.Akinmoladun AC, Olaniyan OO, Famusiwa CD et al. Ameliorative effect of quercetin, catechin, and taxifolin on rotenone-induced testicular and splenic weight gain and oxidative stress in rats. J Basic Clin Physiol Pharmacol 2020;31:1–9. doi: 10.1515/jbcpp-2018-0230. [DOI] [PubMed] [Google Scholar]
- 9.Cory H, Passarelli S, Szeto J et al. The role of polyphenols in human health and food systems: a mini-review. Front Nutr 2018;5:1–9. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akomolafe SF, Akinyemi AJ, Anadozie SO. Phenolic acids (gallic and tannic acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int Sch Res Not 2014;2014:1–8. doi: 10.1155/2014/984709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barszcz M, Taciak M, Tuśnio A et al. Effects of dietary level of tannic acid and protein on internal organ weights and biochemical blood parameters of rats. PLoS One 2018;13. doi: 10.1371/journal.pone.0190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ademosun AO, Oboh G, Bello F et al. Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J Evid Based Complementary Altern Med 2016;21:NP11–7. doi: 10.1177/2156587215610032. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Song Q, Han X et al. Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int Immunopharmacol 2017;47:95–105. doi: 10.1016/j.intimp.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Akbari G. Molecular mechanisms underlying gallic acid effects against cardiovascular diseases: an update review. Avicenna J Phytomed 2020;10:11–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Olaleye MT, Crown OO, Akinmoladun AC et al. Rutin and quercetin show greater efficacy than nifedipin in ameliorating hemodynamic, redox, and metabolite imbalances in sodium chloride-induced hypertensive rats. Hum Exp Toxicol 2014;33:602–8. doi: 10.1177/0960327113504790. [DOI] [PubMed] [Google Scholar]
- 16.Manjunatha S, Shaik AH, MP E et al. Combined cardio-protective ability of syringic acid and resveratrol against isoproterenol induced cardio-toxicity in rats via attenuating NF-kB and TNF-α pathways. Sci Rep 2020;10:1–13. doi: 10.1038/s41598-020-59925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turgut Coşan D, Saydam F, Özbayer C et al. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology 2015;67:97–105. doi: 10.1007/s10616-013-9661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheesbrough M (ed). In: District Laboratory Practice in Tropical Countries, Part 2. South Africa: Cambridge University Press, 2006. [Google Scholar]
- 19.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 1990;58:733–43. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–8. [PubMed] [Google Scholar]
- 21.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P, Das B. Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984;21:130–2. [PubMed] [Google Scholar]
- 23.Sangameswaran B, Balakrishnan BR, Deshraj C et al. In vitro antioxidant activity of roots of Thespesia lampas Dalz and Gibs. Pak J Pharm Sci 2009;22:368–72. [PubMed] [Google Scholar]
- 24.Tienhaara R, Meany JE. The lactate dehydrogenase catalyzed reduction of pyruvate. active substrate and substrate inhibition. Biochemistry 1973;12:2067–70. doi: 10.1021/bi00735a007. [DOI] [PubMed] [Google Scholar]
- 25.Akinmoladun AC, Adegbamigbe AD, Okafor NR et al. Toxicological and pharmacological assessment of a multiherbal phytopharmaceutical on triton X-1339-induced hyperlipidemia and allied biochemical dysfunctions. J Food Biochem 2020;1–13. doi: 10.1111/jfbc.13238. [DOI] [PubMed] [Google Scholar]
- 26.Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- 27.H Jorum O, M Piero N. Haematological effects of dichloromethane-methanolic leaf extracts of Carissa edulis (Forssk.) Vahl in normal rat models. J Hematol Thromboembolic Dis 2016;04. doi: 10.4172/2329-8790.1000232. [DOI] [Google Scholar]
- 28.Owoade AO, Adetutu A, Olorunnisola OS. Hematological and biochemical changes in blood, liver and kidney tissues under the effect of tramadol treatment. J Alcohol Drug Depend 2019;07:1–7. doi: 10.35248/2329-6488.19.7.326. [DOI] [Google Scholar]
- 29.Fauzie AK, Venkataramana GV. Exposure to organic and inorganic traffic-related air pollutants alters haematological and biochemical indices in albino mice Mus musculus. Int J Environ Health Res 2020;30:117–33. doi: 10.1080/09603123.2019.1577367. [DOI] [PubMed] [Google Scholar]
- 30.Sirdah MM, Al LNA, El MRA. Possible health effects of liquefied petroleum gas on workers at filling and distribution stations of Gaza governorates. 289–94. [PubMed]
- 31.Qasim F, Ahmed A. Effects of welding fume particles on heamatological parameters in male albino rats. Zanco J Med Sci 2013;17:422–8. doi: 10.15218/zjms.2013.0027. [DOI] [Google Scholar]
- 32.Tête N, Afonso E, Bouguerra G et al. Blood parameters as biomarkers of cadmium and lead exposure and effects in wild wood mice (Apodemus sylvaticus) living along a pollution gradient. Chemosphere 2015;138:940–6. doi: 10.1016/j.chemosphere.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Audomkasok S, Singpha W, Chachiyo S et al. Antihemolytic activities of green tea, safflower, and mulberry extracts during Plasmodium berghei infection in mice. J Pathog 2014;2014:1–4. doi: 10.1155/2014/203154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh T, Biswas MK, Chatterjee S et al. In-vitro study on the hemolytic activity of different extracts of indian medicinal plant Croton bonplandianum with phytochemical estimation: a new era in drug development. J Drug Deliv Ther 2018;8:155–60. doi: 10.22270/jddt.v8i4.1747. [DOI] [Google Scholar]
- 35.Guemmaz T, Zerargui F, Boumerfeg S et al. Anti-hemolytic, anti-lipid peroxidation, antioxidant properties and acute toxicity of Xanthium strumarium leaves extracts. Annu Res Rev Biol 2018;24:1–12. doi: 10.9734/arrb/2018/40024. [DOI] [Google Scholar]
- 36.Adaramoye OA, Akintayo O, Achem J et al. Lipid-lowering effects of methanolic extract of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vasc Health Risk Manag 2008;4:235–41. doi: 10.2147/vhrm.2008.04.01.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odunola OA, Uka E, Akinwumi KA et al. Exposure of laboratory mice to domestic cooking gas: - implications for toxicity. Int J Environ Res Public Health 2008;5:172–6. doi: 10.3390/ijerph5030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangal CSK, Anitha R, Lakshmi T. Inhibition of nitric oxide production and nitric oxide synthase gene expression in LPS activated RAW 264.7 macrophages by thyme oleoresin from thymus vulgaris. J Young Pharm 2018;10:481–3. doi: 10.5530/jyp.2018.10.104. [DOI] [Google Scholar]
- 39.Nair V, Bang WY, Schreckinger E et al. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (lps)-induced inflammation in macrophage cells. J Agric Food Chem 2015;63:6355–65. doi: 10.1021/acs.jafc.5b00928. [DOI] [PubMed] [Google Scholar]
- 40.Ou Q, Zheng Z, Zhao Y et al. Impact of quercetin on systemic levels of inflammation: a meta-analysis of randomised controlled human trials. Int J Food Sci Nutr 2020;71:152–63. doi: 10.1080/09637486.2019.1627515. [DOI] [PubMed] [Google Scholar]
- 41.Owumi SE, Nwozo SO, Effiong ME et al. Gallic acid and omega-3 fatty acids decrease inflammatory and oxidative stress in manganese-treated rats. Exp Biol Med 2020;245:835–44. doi: 10.1177/1535370220917643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aderibigbe MA, Obafemi TO, Olaleye MT et al. Effects of gender, age and treatment duration on lipid profile and renal function indices in diabetic patients attending a teaching hospital in South-Western Nigeria. Afr Health Sci 2018;18:900. doi: 10.4314/ahs.v18i4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang C, Ning B, Waqar AB et al. Bisphenol A exposure enhances atherosclerosis in WHHL rabbits. PLoS One 2014;9:e110977. doi: 10.1371/journal.pone.0110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miltonprabu S, Sumedha NC. Diallyl trisulfide ameliorates arsenic induced dyslipidemia in rats. Food Sci Biotechnol 2015;24:725–33. doi: 10.1007/s10068-015-0094-z. [DOI] [Google Scholar]
- 45.Muthumani M, Milton PS. Silibinin attenuates arsenic induced alterations in serum and hepatic lipid profiles in rats. J Appl Pharm Sci 2013;3:132–8. doi: 10.7324/JAPS.2013.30223. [DOI] [Google Scholar]
- 46.Musolino V, Gliozzi M, Nucera S et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis 2019;18:115. doi: 10.1186/s12944-019-1061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imran A, Arshad MU, Mehmood S et al. Oxidative stress diminishing perspectives of green and black tea polyphenols: a mechanistic approach. Polyphenols. Published Online First 2018. doi: 10.5772/intechopen.75933. [DOI] [Google Scholar]
- 48.Obafemi TO, Akinmoladun AC, Olaleye MT et al. Antidiabetic potential of methanolic and flavonoid-rich leaf extracts of Synsepalum dulcificum in type 2 diabetic rats. J Ayurveda Integr Med 2017;8. doi: 10.1016/j.jaim.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Famurewa AC, Ugwu-Ejezie CS, Iyare EE et al. Hepatoprotective effect of polyphenols isolated from virgin coconut oil against sub-chronic cadmium hepatotoxicity in rats is associated with improvement in antioxidant defense system. Drug Chem Toxicol. Published Online First 2019. doi: 10.1080/01480545.2019.1598428. [DOI] [PubMed] [Google Scholar]
- 50.Vicente-Sánchez C, Egido J, Sánchez-González PD et al. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol 2008;46:2279–87. doi: 10.1016/j.fct.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Obodo BN, Iyevhobu KO, Idara IU et al. Effects of duration of exposure on biochemical and haematological profile in liquefied petroleum gas (LPG) plant workers. Int J Res Publ 2020;58. doi: 10.47119/ijrp100581820201352. [DOI] [Google Scholar]
- 52.xin DJ, J ying O, Dai H et al. Antioxidant and antiapoptotic polyphenols from green tea extract ameliorate CCl4-induced acute liver injury in mice. Chin J Integr Med 2020;26:736–44. doi: 10.1007/s11655-019-3043-5. [DOI] [PubMed] [Google Scholar]
- 53.Xu D, Hu MJ, Wang YQ et al. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019;24. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashafaq M, Tabassum H, Parvez S. Modulation of behavioral deficits and neurodegeneration by tannic acid in experimental stroke challenged wistar rats. Mol Neurobiol 2017;54:5941–51. doi: 10.1007/s12035-016-0096-8. [DOI] [PubMed] [Google Scholar]
- 55.Hu X, Wang H, Lv X et al. Cardioprotective effects of tannic acid on isoproterenol-induced myocardial injury in rats: further insight into ‘French paradox’. Phyther Res 2015;29:1295–303. doi: 10.1002/ptr.5376. [DOI] [PubMed] [Google Scholar]


