Abstract
Glycyrrhiza glabra root (licorice) is a widely used herb for its beneficial effects on health. This study explored the protective effects of licorice extract against oxidative stress and testicular dysfunction caused by methotrexate (MTX). Mice were allocated into (i) negative control group that received saline; (ii) licorice extract group, orally administered with 200 mg/kg body weight (bw) licorice extract for 12 days; (iii) positive MTX-intoxicated group, injected with a single intraperitoneal dose of MTX (20 mg/kg bw) on day 7; and (iv) a protective group that received licorice extract for 12 days and then MTX on day 7 as in groups 2 and 3. Total proteins, albumin, globulins, malondialdehyde, glutathione peroxidase, reduced glutathione, IL-1, and IL-6 were measured in blood and testis samples collected from all groups. Testicular oxidative stress, serum reproductive hormones, and spermogram were examined. The expression of steroidogenesis-associated genes (translocator protein; and P450scc) was examined by quantitative real-time PCR. Bcl-2-associated X protein and cyclogenase-2 genes were examined by immunohistochemical analysis. The bioactive contents of licorice extract were confirmed by gas chromatography–mass spectrometry analysis. Pretreatment with licorice extract ameliorated the toxic effects of MTX on total proteins, albumin, and globulins and oxidative stress biomarkers and reversed the effect of MTX on examined serum and tissue antioxidants. Besides, MTX down-regulated mRNA expression of translocator protein and P450scc genes. Licorice extract averted the decrease in serum testosterone and the increase in IL-1β and IL-6 levels induced by MTX. Moreover, MTX increased sperm abnormalities and percentage of dead sperms and reduced sperm motility. These changes were absent in the licorice preadministered group. Licorice prevented the increase in immunoreactivity of testis for Bcl-2-associated X protein and cyclogenase-2 that were overexpressed in MTX-injected mice. Licorice extracts positively regulated the expression of steroidogenesis genes suppressed by MTX, increased antioxidant enzymes (glutathione peroxidase, reduced glutathione, and catalase) and reduced biomarker of oxidative stress (testicular malondialdehyde) and inflammatory cytokines (IL-1 and -6). Moreover, reduction in testicular tissue immunoreactivity to Bcl-2-associated X protein and cyclogenase-2. In conclusion, licorice extract mitigated the toxic effects of MTX-induced testicular dysfunction at biochemical, molecular, and cellular levels.
Keywords: licorice extract, methotrexate, testis dysfunction, gene expression, oxidative stress
Introduction
Co-administration therapies with safe herbal medication are more efficient and safer in managing various ailments than do monotherapy [1]. Methotrexate (MTX) is widely used for the treatment of rheumatoid arthritis, chronic inflammation, and tumors [2]. However, MTX is cytotoxic and can induce organ toxicity and damage [3]. The effects of MTX are due to its impact on the state of cellular or systemic oxidative stress, inflammation, and apoptosis [3].
Earlier reports suggest that the toxic effect of MTX on testicular activity and reproductive efficiency can be mitigated by the co-administration of protocatechuic acid, vitamin B17, or chrysin alongside MTX [4–6]. Oxidative stress induced by MTX plays a critical role in MTX toxicity in all examined organs [7]. Moreover, the structural damage induced by oxidative stress has been reported in germ cells [8]. MTX leads to problems in testicular tissue by increasing the formation of reactive oxygen species (ROS) and by inducing toxic effects [9]. In parallel, oxidative stress can result in damage of cellular macromolecule by increases in ROS [10, 11]. Therefore, body organs and tissues react by up-regulating their redox homeostasis to alleviate MTX effects that cause ROS and oxidative stress [11]. Till now, MTX has been considered the most effective chemotherapeutic agent used for cancer treatment but has some side effects [12]. Therefore, a combination therapy may be useful alongside MTX to alleviate its toxicity and increase its effectiveness in cancer treatment. It has been confirmed that compounds of herbal origin with antioxidant properties could reduce testicular damage induced by MTX.
Herbal medicines contain natural substances that promote health and alleviate illness. Licorice is one such oriental herbal medicine that can be used for the treatment of many diseases [13]. In traditional medicine, licorice is used to treat several diseases, extending from colds and coughs to hepatic and intestinal disturbances and even cancer [14].
The pharaohs of ancient Egypt used licorice root over 4000 years ago as a flavoring agent in drinks and candies; since then, licorice has been used by herbal medicinal practitioners in most other countries [15] for the management of various ailments. Licorice is used for the treatment of injuries, swelling, and detoxification [16], and its extract can relieve allergic inflammation [17]. Licorice has antimicrobial, antiatherosclerotic, antihepatitis, antinephritic, as well as cardiac and vascular protective functions [18, 19]. Licorice contains numerous active phytochemicals having both anti-inflammatory and antioxidant properties.
The constituents of licorice are majorly saponins, consisting of one molecule of glycyrrhetic acid and two molecules each of polyphenols, polysaccharides, glucuronic acid, and glycyrrhetinic acid [20]. The active portions of licorice are rich in phenolic compounds and bioflavonoids that represent active antioxidants [20]. A few studies have discussed the importance of licorice in male fertility and sperm characteristics. While some concluded that licorice does not impair male reproductive function [21], others stated that it is a potential drug for male infertility [22] and ameliorates male infertility that is associated with obesity [23]. In this study, we tried to confirm whether licorice is beneficial or harmful for testicular dysfunction associated with MTX intoxication.
Herbal medications may have broad safety margins and might have the potential to reduce the cytotoxic effects of synthetic drugs [24, 25]. The beneficial component of herbal medicine can enhance the restorative capacity of damaged cells and tissues induced by toxic materials [25, 26]. Therefore, the use of herbal medications has become commensal with chemical drugs to alleviate the side effects on various organs. The current study assessed the impact of licorice extract in mitigating the oxidative stress, inflammation, and testicular dysfunction associated with MTX intoxication at the biochemical, molecular, and cellular levels.
Materials and Methods
Kits and chemicals
Glutathione peroxidase (GPx), reduced glutathione (GSH), malondialdehyde (MDA), total proteins, albumin, and globulin were obtained from Biodiagnostic Company, Giza, Egypt. Methotrexate and the RNA extraction chemicals of high molecular grade were purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzyme for reverse transcription and various types of DNA molecular weight markers were from MBI (Fermentas, Thermo Fisher Scientific, California, USA). Qiazol for RNA extraction and Oligo dT primers were from QIAGEN (Valencia, CA, USA). Kits for testosterone (catalog no: E-EL-0155) and follicle-stimulating hormone (FSH) hormone (catalog no: E-EL-M0511) were from Elabscience Biotechnology Inc. (Texas, USA).
Preparation of licorice ethanolic extract
Fresh licorice roots were purchased from a local market in Taif (Hyper Panda Market, Saudi Arabia) and were identified by a botanist from the College of Science, Taif University, Saudi Arabia. A voucher number (2020/0424) was assigned after identification of the licorice root from the Department of Botany, Taif University. These freshly dried roots were powdered, and 100 g of licorice powder was added to 500 ml ethanol (1:5 w/v). The mixture was gently stirred in a shaker (horizontal shaker, Bio-Rad) at room temperature for 24 h [27]. The mixture was then filtered through Whatman filter papers #1 [28], concentrated using a “Buchi” rotary evaporator (Perkins Elmer, New York, USA) after evaporation at 40°C with moderate rotation. The end yield was 9.8 g from the initial 100 g licorice powder used.
Gas chromatography–mass spectrometry analysis
The gas chromatography–mass spectrometry (GC/MS) analysis was carried out to analyze licorice extract using Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS (Thermo Fisher Scientific, California, USA). The TG-5MS fused silica capillary column had 30-m, 0.251- and 0.1-mm film thickness. An electron ionization system with ionization energy of 70 eV was used for GC/MS detection. Carrier gas used was helium and was used at a constant flow rate of 1 ml/min. The temperature of injector and MS transfer line was set at 280°C. The temperature of oven was programmed at temperature of 50°C for 2 min (initial hold) and then at 150°C with an increasing rate of 7°C/min. Later, it is increased to 270 with an increasing rate of 5°C/min (hold: 2min) and then to 310 as a final temperature at an increasing rate of 3.5°C/min (hold: 10 min). All the identified components were quantified using a percent relative peak area. To identify the detected compounds, a tentative identification was performed after comparing the relative retention time of identified compounds and mass spectra with those of the NIST-WILEY library data of the GC/MS system.
Animals and experimental design
Forty male mice (8 weeks old), weighing 30 ± 2 g, were used for this study. The Ethical Committee of Turabah University College, Taif University, approved all the procedures and the number of animals used for this study (Approval number: TURSP-2020-09). Animals had free access to food and water; they were maintained at conditioned room temperature (22 ± 4°C) in a laboratory at Turabah University College and were handled manually for seven days to overcome treatment stress. Four groups (10 mice per group) were used for the following treatments: (i) the negative control (CNT) group received saline and free food and water access; (ii) the licorice extract group, orally administered with 200 mg/kg body weight licorice extract for 12 days consecutively [29, 30]; (iii) a positive MTX-intoxicated group, injected with a single dose of MTX (20 mg/kg bw intraperitoneally) on day 7 [31, 32]. The dose for MTX is optimal to induce organ toxicities [32]; and (iv) the protective group received licorice extract for 12 days and then MTX on day 7, same as explained in groups 2 and 3, and licorice was continued orally for five days following MTX injection. The experimental design for this study is shown in Fig. 1.
Figure 1 .
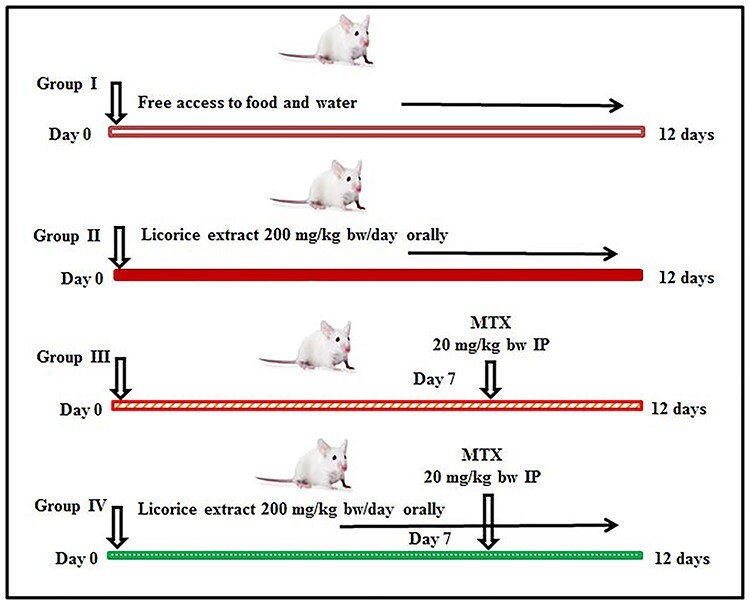
a schematic diagram of the experimental design.
After 12 days of experimental treatments, mice were decapitated under light anesthesia and an intramuscular injection of Ketanest (10 mg/kg bw). Samples of blood and testis tissue were collected. Serum was extracted and preserved at –20°C for measurement of MDA, antioxidants, cytokines, and reproductive hormones. Qiazol was used to preserve some parts from right testicular tissue samples for RNA extraction and subsequent quantitative real-time PCR (qRT-PCR). Phosphate buffer (ice cold) was used to preserve and homogenize some parts from left testes tissue to measure antioxidant activity (MDA and catalase), and 10 neutral formalin buffer (10% NFB) was used for histological and immunohistochemical analyses.
Biochemical and cytokine measurements
Mouse ELISA kits for IL-1 beta and IL-6 (catalog numbers E-EL-M0037 and E-EL-M0044, respectively) were purchased from Elabscience, Texas, USA. Serum cytokine levels were measured using ELISA kits and BIO-RAD instrument in accordance with the user’s manuals and reference tables supplied with each kit. In short, these kits were based on sandwich enzyme-linked immune-sorbent assay technology. Anti-IL-1b or IL-6 antibodies were precoated onto 96-well plates. Then, the biotin conjugated anti-IL-1b or IL-6 antibody was used as detection antibodies. The standards, test samples, and biotin-conjugated detection antibody were added to the wells subsequently and then washed with wash buffer. HRP-Streptavidin was added and unbound conjugates were washed away with wash buffer. TMB substrates were used to visualize HRP enzymatic reaction.
The total proteins, albumin, and globulin were measured colorimetrically using spectrophotometer (Smart-tech, Bio-Rad, Philadelphia, USA) based on kits purchased from Biodiagnostic Company (Dokki, Giza, Egypt), and the instruction manual was supplied for each kits. Total proteins were measured based on the hypothesis that proteins react with copper ions (II) to produce a blue violet color compound in alkaline medium. The color intensity is proportional to the concentration of total proteins present in the sample [33]. For albumin measurements, albumin is bound by the BCG dye to produce an increase in the blue green color in a pH 3.8 acid medium. The color increase is proportional to the concentration of albumin present in the sample [34].
Spermogram
The spermatozoa from epididymis were collected from its tail within 5 min of animal anesthesia and decapitation. Collected spermatozoa were concentrated by centrifuging at 1200 rpm for 5 min at 30°C. The spermatozoa cells were dispersed in Tris-fructose-citrate (TFC) medium. Individual sperm motilities, the percentages of sperm motility, live and dead sperms, and the percentage of abnormal spermatozoa were estimated [35].
Preparation of testicular homogenates for measurement of oxidative stress
For measuring catalase and MDA activities, 500 mg of testes were homogenized in ice-cold buffer (5 ml; 50 mM potassium phosphate buffer, pH 7.4; 1 mM EDTA; 1 ml/l Triton X-100). The samples to be examined were centrifuged at 4000 xg for 10 min at 4°C. The clear layer of supernatant was stored at –20°C for measurement of MDA (nmol/g testicular tissue) and catalase (U/g testicular tissue) using ELISA plate reader (Bio-Rad Co, NY, USA) [36, 37]. The kit of catalase was based on the fact that catalase can react with a known quantity of H2O2. The reaction is stopped after exactly 1 min with catalase inhibitor. In the presence of peroxidase (HRP), remaining H2O2 reacts with 3,5-Dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4-aminophenazone (AAP) to form a chromophore with a color intensity inversely proportional to the amount of catalase in the original sample [37]. For MDA, the idea of the kit was depend on the fact that Thiobarbituric acid (TBA) can reacts with malondialdehyde (MDA) in acidic medium at temperature of 95°C for 30 min to form thiobarbituric acid reactive product the absorbance of the resultant pink product can be measured at 534 nm [36].
Serum antioxidants assays
MDA levels as a tissue degradation marker was measured in serum spectrophotometrically. The principle of the assay was based on the fact that thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) in acidic medium at temperature of 95°C for 30 min to form thiobarbituric acid reactive product the absorbance of the resultant pink product can be measured at 534 nm [36]. GPx and GSH as antioxidant markers were measured using previously published methods [38, 39]. As, GSH catalyzes the reduction of oxidized glutathione (GSSG) to reduced glutathione (GSH), therfore, glutathione reductase catalyzes the reduction of glutathione (GSSG) in the presence of NADPH, which is oxidized to NADPH+. The decrease in absorbance at 340 nm is measured. The assay of GPx is an indirect measure of the activity of c-GPx. Oxidized glutathione (GSSG), produced upon reduction of an organic peroxide by c-GPx, is recycled to its reduced state by the enzyme glutathione reductase [38, 39].
qRT-PCR analysis
Total RNA from right testis was extracted using Qiazol reagent and quantified spectrophotometrically at 260 nm. RNA integrity was checked and confirmed on a denatured agarose gel. Complementary DNA (cDNA) synthesis was performed using 2 μg of total RNA using the kit of MyTaq Red Mix (Bioline, Meridian Bioscience, USA). cDNA was amplified using SYBR Green master mix (Thermo Scientific, USA). Sequences of translocator protein (TSPO), cholesterol side-chain cleavage enzyme (P450scc), and β-actin primers used for gene amplification are mentioned in Table 1. Data were analyzed using the 2−ΔΔCt method of Bio-Rad CFX96 Touch™ Real-Time PCR machine (Bio-Rad Co, USA). Comparative CT (cycle threshold) values expressed and reflected the changes in gene intensity after normalization with the house-keeping β-actin gene.
Table 1.
primer sequence used for quantitative real-time PCR in mice in kidney.
| Gene | Accession number | Product size (bp) | Direction | Primer sequence |
|---|---|---|---|---|
| TSPO | NM_009775.4 | 105 | Sense | TCCCAGAGTGAAGGCACCCA |
| Antisense | AGGTAGACCAGCAGGCCCAA | |||
| P450scc | AF195119.1 | 128 | Sense | ATGGGTCGAGATCCGGGCTT |
| Antisense | CCCAGACACTGCCGAACACC | |||
| β-Actin | NM_007393.5 | 140 | Sense | CCAGCCTTCCTTCTTGGGTA |
| Antisense | CAATGCCTGGGTACATGGTG |
Histology and immunohistochemistry of testis
To study testis histology, testicular samples were sliced, dehydrated, soaked, and embedded in paraffin. Slices of testis were cut into segments of 3 μm for hematoxylin and eosin (H&E) staining. Slides were examined using the Nikon Eclipse 80i microscope and photographed using the Canon digital camera, SX620 H, Osaka, Japan.
For testicular immunohistochemistry, testes slices were embedded in paraffin, then deparaffinized, and rehydrated. Testicular slices were then soaked in H2O2 (2%) for 15 min and washed in PBS three times to inhibit peroxidase activity. Bovine serum albumin (5%) was used to block nonspecific binding sites. BAX Antibody (Catalog No. MS711B0) and cyclooxygenase (COX-2) antibody (Catalog No. RM9121R7) were procured from Lab Vision (Fremont, CA), and polyclonal antibodies (Santa Cruz Biotechnology, USA) were diluted to 1:200 and incubated overnight at 4°C. The slides were then washed carefully thrice with PBS and incubated with biotin-conjugated secondary antibody (1:1500 dilution, cat# sc-2040, Santa Cruz Biotechnology, USA). Antibody binding and reactivity were visualized using diaminobenzidine and counterstaining with hematoxylin at room temperature for 10 s.
Data analysis
Data from ten different mice for each treatment are expressed as the mean ±standard error of mean (SEM). Data were analyzed using SPSS data analysis software (v 20.0, Chicago, USA) using one-way ANOVA and Duncan’s post hoc descriptive test. Values with P < 0.05 were statistically significant.
Results
GC-MS analysis of ethanolic licorice extract
The GC-MS analysis of licorice ethanolic extract (Table 2, Fig. 2) showed the detailed presence of 20 different phytochemicals. The most abundant constituents are phenols, cyclohexenes, Ocimene, Pentadecane, Hexadecane, and other known compounds seen in Table 2. These constituents are the active ingredients that may induce the medical potential of licorice extract in oxidative stress relief.
Table 2.
chemical composition of licorice extract as identified by GC-MS.
| Number | Retention time (min) | Area, % | Group or compound name (from Central Library Search Unit) |
|---|---|---|---|
| 1 | 9.36 | 0.75 | Undecane |
| 2 | 10.21 | 0.76 | Beta-ocimene |
| 3 | 11.41 | 0.58 | Cyclohexen |
| 4 | 13.58 | 11.55 | Heptadiene |
| 5 | 14.00 | 4.99 | 3-Cyclohexene-1-methanol |
| 6 | 15.54 | 1.18 | trans,trans-nona-2, 4 dienol |
| 7 | 16.96 | 54.79 | Phenol; 5-methyl-2(1-methylethyl) |
| 8 | 17.19 | 16.15 | Phenol; 2-methyl-5(1-methylethyl) |
| 9 | 19.25 | 0.64 | Docosane |
| 10 | 19.99 | 2.06 | Transcaryophyllene |
| 11 | 22.44 | 0.72 | Phenol, 2,4-bis (1,1-dimethylethyl) |
| 12 | 23.95 | 0.49 | Caryophyllene oxide |
| 13 | 24.00 | 0.72 | 1-Pentadecene |
| 14 | 26.34 | 1.10 | Pentadecane |
| 15 | 28.30 | 1.02 | 1-Heptadecanol |
| 16 | 28.45 | 0.32 | 2-Myristynoyl pantetheine and dotriacontane |
| 17 | 30.29 | 0.71 | Naphthalene |
| 18 | 30.66 | 0.80 | Cyclohexene |
| 19 | 32.21 | 0.33 | 17-Pentatriacontene and tridecanol |
| 20 | 54.87 | 0.35 | 2,4,6,8,10 Tetradecapentaenoic acid |
Figure 2 .
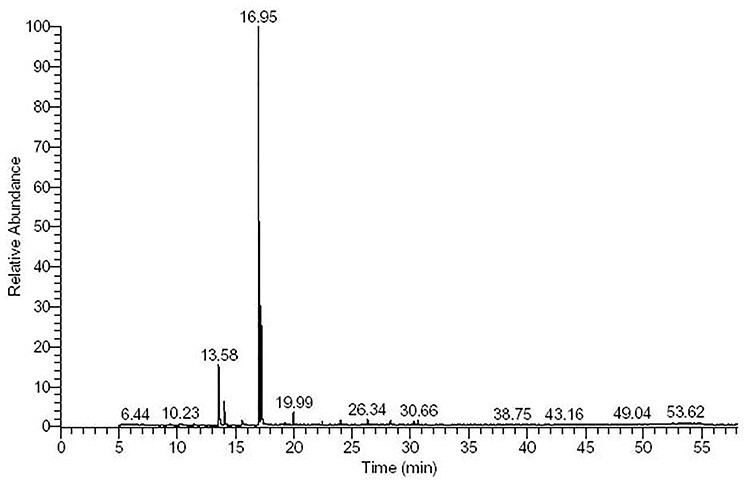
GC-MS analysis of ethanolic licorice extract.
Impact of licorice extract on serum proteins and changes in cytokine levels due to methotrexate
MTX induced a decrease in serum levels of total proteins, albumin, and globulins. MTX intoxication showed an increase in the serum levels of inflammatory cytokines, as there are increased levels of IL-1β and IL-6 in MTX-injected mice. Licorice preadministration to MTX-intoxicated mice showed improvement and protections for the changes in total proteins, albumin, globulins, IL-1, and IL-6 (Table 3). MTX injection decreases serum levels of total proteins, albumins, and globulins (Table 3). Preadministration of licorice extract recovered these changes compared to MTX-intoxicated mice.
Table 3.
protective effects of licorice on MTX-induced changes on serum proteins.
| Total proteins (g/dl) | Albumin (g/dl) | Globulins (g/dl) | IL-1β (pg/ml) | Il-6 (pg/ml) | |
|---|---|---|---|---|---|
| Control | 7.8± 0.56 | 4.1 ± 0.38 | 4.65 ± 0.39 | 134 ± 9.1 | 71 ± 7 |
| Licorice | 7.4 ± 0.56 | 3.5 ± 0.7 | 4.1± 0.61 | 156.3 ± 11.5 | 77 ± 9.5 |
| Methotrexate | 3.8 ± 0.3* | 1.99 ± 0.4* | 1.85 ± 0.05* | 340 ± 35* | 210 ± 24* |
| Licorice + methotrexate | 5.7 ± 0.3** | 3.02± 0.26** | 4.5 ± 0.05** | 168.2 ± 16** | 110± 9.9** |
Values are means ± standard error (SEM) for seven different mice per each treatment. Values are statistically significant at *P < 0.05 versus control and licorice groups. **P < 0.05 versus methotrexate.
Effect of licorice extract on testicular oxidative stress induced by methotrexate
MTX injection induced tissue degradation as MDA levels in testis were increased (Table 4). In contrast, catalase activity was decreased in testis of MTX-intoxicated mice (Table 4). Mice that had been pretreated with licorice extract and then intoxicated by MTX showed significant protection against toxic effects of MTX on MDA and catalase (Table 4).
Table 4.
protective effect of licorice extract on MTX-induced testicular oxidative stress.
| MDA (nmol/g testicular tissue) | Catalase (U/g testicular tissue) | |
|---|---|---|
| Control | 8.1± 0.3 | 17.1 ± 1.3 |
| Licorice | 7.8 ± 0.21 | 15 ± 1.3 |
| Methotrexate | 28.1 ± 1.9* | 6.3 ± 1.5* |
| Licorice + methotrexate | 14.2 ± 1.7** | 13.5 ± 1.4** |
Values are means ± standard error (SEM) of seven different mice per each treatment. Values are statistically significant at *P < 0.05 versus control licorice groups, and **P < 0.05 versus methotrexate group.
Effect of licorice extract on oxidative stress index in methotrexate-intoxicated mice
MTX injection led to an increase in lipid peroxidation marker, MDA, in the serum of MTX-intoxicated mice (Table 5). Unlike MTX findings, mice that received licorice extract showed an increase in GPx and GSH levels. Interestingly, mice that received licorice extract for 12 days and a single dose of MTX on day 7 showed less pronounced changes and protections in MDA, GPx, and GSH.
Table 5.
effects of licorice extract on serum antioxidant levels.
| MDA (nmol/ml) | GPx (U/l) | GSH (nmol/l) | |
|---|---|---|---|
| Control | 12 ± 1.5 | 170 ± 27.5 | 3.9 ± 0.1 |
| Licorice | 13.6 ± 0.5 | 221 ± 23* | 4.9 ±0.4* |
| Methotrexate | 41 ± 2.3** | 102 ± 8.5** | 2.37 ± 0.11** |
| Licorice + methotrexate | 20.1 ± 1.7*** | 150 ± 8.9*** | 3.1± 0.2*** |
Values are means ± standard error (SEM) for seven different mice per each treatment. Values are statistically significant at **P < 0.05 versus control; **P < 0.05 versus control and licorice groups. ***P < 0.05 versus methotrexate.
Impacts of licorice on sperms
MTX-injected mice showed a decrease in sperm count, motility, and concentration and an increase in sperm abnormalities (Table 6). Preadministration of licorice extract significantly improved (P < 0.05) the changes in the spermogram induced by MTX. Moreover, licorice preadministration improved and decreased sperm defects and abnormalities reported in the MTX-intoxicated group (Table 6).
Table 6.
sperm analysis.
| Motility | Viability | Concentration (×1000) | Abnormal morphology | |
|---|---|---|---|---|
| Control | 45 ± 1.2 | 66.0 ± 3.3 | 71 ± 4.7 | 16.1 ± 1.04 |
| Licorice | 52.1 ± 3.1 | 75.2 ± 4.2 | 80.2 ± 6.56 | 14.2 ± 1.1 |
| Methotrexate | 21.2 ± 1.3* | 37.2 ± 2.9* | 31.1 ± 3.1* | 43.2 ± 4.5* |
| Licorice + methotrexate | 35.2 ± 2.2** | 54.1 ± 3.2** | 58.0 ± 4.3** | 23.1 ± 1.45** |
Values are means ± standard error (SEM) for seven different rats per each treatment. Values are statistically significant at *P < 0.05 versus control and **P < 0.05 versus methotrexate.
Impact of licorice on serum testosterone and FSH levels
MTX-injected mice showed a decrease in serum testosterone and FSH levels (Table 7). Preadministration of licorice extract significantly improved (P < 0.05) these alterations that are shown in Table 7. Collectively, licorice effects showed in Tables 6 and 7 confirm its role in sperm quality and male reproductive hormones.
Table 7.
effects of licorice extract on serum testosterone and FSH levels.
| Testosterone (ng/ml) | FSH (pg/ml) | |
|---|---|---|
| Control | 1.27 ± 0.1 | 11.1 ± 1.01 |
| Licorice | 1.6 ± 0.3 | 12.5 ± 0.9 |
| Methotrexate | 0.5 ± 0.1* | 5.3 ± 0.4* |
| Licorice + methotrexate | 1.21 ± 0.18** | 12.9± 1.1** |
Values are means ± standard error (SEM) for seven different mice per each treatment. Values are statistically significant at *P < 0.05 versus control and licorice groups. **P < 0.05 versus methotrexate.
Impact of licorice extract on the quantitative expression of spermatogenesis-associated genes
Injection of MTX induced down-regulation of TSPO and P450scc expression in testis (Figs 3 and 4) compared to control mice. Of note, the administration of licorice extract alone showed slight up-regulation in TSPO and P450scc expression. The down-regulation of examined testicular genes was restored and returned to a normal expression pattern when licorice was preadministered to MTX-injected groups (Figs 3 and 4).
Figure 3 .
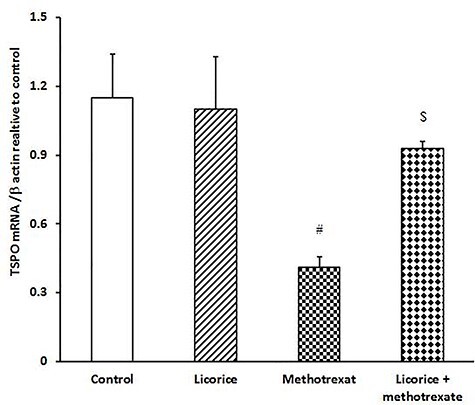
ameliorative impact of licorice extract on TSPO mRNA expression in MTX-injected mice; a graphic presentation of testicular TSPO mRNA expression by qRT-PCR in different groups of mice compared with that of β-actin; #P < 0.05 versus control and licorice extract groups, and $P < 0.05 versus MTX group.
Figure 4 .
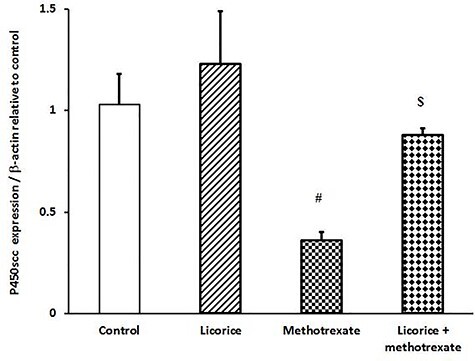
ameliorative impact of licorice extract on P450scc mRNA expression in MTX-injected mice; graphic presentation of testicular mRNA expression by qRT-PCR for P450scc in different groups of mice compared with that of β-actin; #P < 0.05 versus control and licorice extract groups and $P<0.05 versus MTX group.
Histopathological examination
Testis of the control group showed the normal histology of seminiferous tubules and interstitial Leydig cells (Fig. 5A), while the testis of the licorice group showed the same histological status (Fig. 5B). MTX-intoxicated group showed degeneration of spermatogonial cells with edema of interstitial tissue (Fig. 5C). Finally, testis of the MTX group pretreated with licorice extract showed restoration of normal architecture with mild thickening of interstitial tissue (Fig. 5D).
Figure 5 .
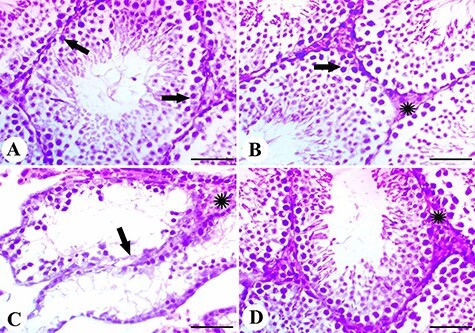
results of histopathological examination; (A) the control group showing normal testicular histology with normal spermatogonial cells (arrows); (B) testis of the licorice group showing normal cells (arrow) and interstitial Leydig cells (*); (C) testis of the MTX group showing degeneration of spermatogonial cells (arrow) with edema of interstitial tissue (*); (D) testis of the licorice and MTX group showed restoration of normal architecture with mild thickening of interstitial tissue; scale bar = 50 μm.
BAX and COX 2 immunohistochemistry
Testis samples in control and licorice groups showed no BAX reactivity (Fig. 6A and B), while those of the MTX group showed strong expression for BAX in spermatogonial cells (Fig. 6C). Mice that received licorice plus MTX showed normal and reduced BAX expression with normal testicular histological structure (Fig. 6D).
Figure 6 .
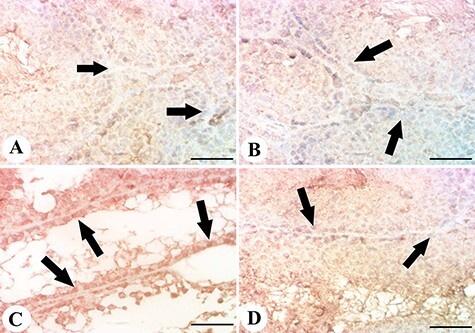
results of the immunohistochemical examination of BAX. (A, B) testis of control and licorice groups showing mild expression of BAX in the testicular cells; (C) testis of MTX group showing enhanced BAX expression and reactivity in spermatogonial cells; (D) testis of licorice + MTX group showing mild BAX expression; scale bar = 50 μm.
Figure 7A–D shows changes in COX-2 expression in testicular tissues. Control and licorice-administered mice exhibited mild changes in COX-2 expression (Fig. 7A and B). Mice from the MTX group exhibited a significant level of COX-2 expression and immunoreaction (Fig. 7C). When licorice was administered before MTX, the improved changes were obvious. It is therefore highly likely that MTX-induced BAX and COX-2 up-regulation is inhibited by licorice extract (Figs 6D and 7D).
Figure 7 .
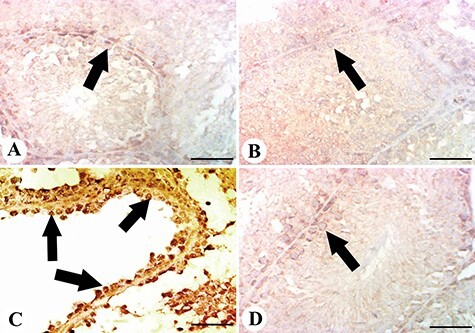
results of the immunohistochemical examination of COX-2. (A, B) testis of control and licorice groups showing faint and little expression of COX-2 among testicular cells; (C) testis of the MTX group showing increased Cox-2 expression in spermatogonial cells (arrows); (D) testis of licorice + MTX group showing mild COX-2 expression; scale bar = 50 μm.
Discussion
MTX is the most commonly used anticancer drug because of its significant benefits. However, it also exerts side effects on all organs of the body. Gonads are the main component of the reproductive system. Therefore, protection of the germinal cells and normal testicular function is important for patients under MTX therapy.
The current findings showed that MTX significantly increases serum and testicular MDA levels and activity, confirming testicular oxidative stress and damage. Moreover, MTX caused a decrease in catalase, GPx, and GSH activities. These effects were restored by the antioxidative impacts of licorice extract. MTX alters the male fertility index, which was restored by the preadministration of licorice extract. Testicular histology and immunohistochemical findings showed the extent of MTX-induced testicular damage and its recovery by licorice extract.
MTX induced a reduction in both seminiferous tubule length and epithelium thickness and reduction in the sperm number and mobility [40]. These findings are in accordance with ours and those reported earlier [40]. We reported here that MTX decreased both FSH and testosterone levels, which were restored by the preadministration of licorice extract. This confirms the importance of licorice in improving male fertility [9]. MTX induces its effect through testicular oxidative stress and ROS.
MTX causes oxidative damage, which is associated with increased ROS production [41]. Here, testicular oxidative stress was confirmed by the increase in levels of MDA and its activity in both serum and testicular tissues, reduced antioxidant activity, and increased oxidative stress index. The reduced expression of GSH is the main cause of disruption in the antioxidant defense system [42]. As known, MTX induced impairment in tissue redox [43] and a decrease in antioxidant enzymes and tissue damage due to release of ROS [44]. Our findings, as well as those reported in previous studies [22, 23, 29], show that MTX causes damage to the testis and other organs through lipid peroxidation and redox impairment. Preadministration of licorice extract increased GPx, GSH, and catalase levels and their activities in testis and serum of MTX-injected group.
Antioxidant enzymes react with O2 and H2O2. GPx captures alkyl radicals formed from oxidized membrane components that use GSH (substrate) [45]. Detoxification of free radicals in oxidative stress is induced by GSH and GPx [45]. For the efficient functioning of the testes, the antioxidant defense mechanisms must be protected against ROS. Antioxidant enzymes, such as GPx and CAT, play important roles in the protection of testicular and male reproductive organs against ROS [46].
TSPO is a cholesterol-binding protein found at particularly high levels in steroid synthesizing cells of the testis in the outer mitochondrial membrane. TSPO interacts with steroidogenic acute regulatory protein to transport cholesterol into mitochondria [47]. Reduced levels of these steroids have been linked to reduced sexual function. Although testosterone-replacement therapy is available, still undesirable side effects present. To regulate steroid levels in the testis and brain, TSPO ligands are proposed as therapeutic agents [48–50]. P450scc is responsible for the conversion of insoluble cholesterol to soluble pregnenolone [50]. It is the first rate-limiting step in steroidogenesis [51]. Overexpression of this enzyme indicates the activity of testis and steroidogenesis. We examined the effect of licorice on TSPO and P450scc expression and confirmed for the first time that licorice ameliorates MTX-induced disruption in steroidogenesis and, consequently, male fertility. Some studies about the effects of licorice extract on the reproductive system indicate that it could maintain sperm mobility and improve the fertilizing ability of BALB/cA mouse sperm in vitro [52]. However, others failed to conclude any significant correlation between licorice, sperms, and reproductive functions [21]. In this study, MTX was found to induce a decrease in serum testosterone levels, while MTX preadministered with licorice extract showed significant recovery in the levels. When testosterone levels were measured on day 14, there was a decrease in testosterone levels in the MTX group, compared to that in the control group [9].
As stated before, the inflammatory factors produced due to COX-2 activation is involved partially in the regulation of Leydig cell dysfunction [53]. In this study, MTX not only increased proinflammatory cytokine levels but also induced testicular oxidative stress. COX-2 activates the synthesis of prostaglandins from arachidonic acid. Prostaglandins play a critical role in the development of the inflammatory response [54]. The increase in levels of proinflammatory cytokines due to COX-2 activation results in the activation of specific proapoptotic signals such as caspase-3 and BAX [55]. These findings supported our histopathological findings that MTX significantly up-regulates the immunoreactivity of the inflammatory marker, COX-2 and BAX (apoptosis-associated gene), compared to those in the control. MTX-induced testicular oxidative stress can be attributed to the stimulation of the TNF-α/COX-2 pathway and, subsequently, of apoptosis. Interestingly, licorice has the potential to restore and control both cytokines and factors associated with inflammation and apoptosis. Overall, we speculate that the antioxidants, as well as inflammatory and cytokine signaling pathways, are the target for licorice against MTX-induced testicular dysfunction.
Conclusion
This study confirmed the role of MTX in ROS generation and induction of oxidative stress in testis. MTX up-regulated apoptotic gene expression and redox imbalance. Preadministration of licorice extract ameliorated these effects and restored the levels of relevant markers in testis and sera. In addition, licorice down-regulated the expression of genes related to inflammation and regulated BAX and COX-2 cellular immunoreactivity. These findings established the suitability of licorice in reducing the side effects associated with MTX therapy.
Contributor Information
Adil Aldhahrani, Clinical Laboratory Sciences Department, Turabah University College, Taif University, Taif, 21995, Saudi Arabia.
Mohamed Mohamed Soliman, Clinical Laboratory Sciences Department, Turabah University College, Taif University, Taif, 21995, Saudi Arabia; Biochemistry Department, Faculty of Veterinary Medicine, Benha University, Benha 13736, Egypt.
Fayez Althobaiti, Biotechnology Department, College of Science, Taif University, Taif, 21995, Saudi Arabia.
Adel Alkhedaide, Clinical Laboratory Sciences Department, Turabah University College, Taif University, Taif, 21995, Saudi Arabia.
Mohamed Abdo Nassan, Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt.
Wafaa Abdou Mohamed, Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt.
Gehan B A Youssef, Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Benha University, Benha 13736, Egypt.
Alshaimaa Mohammed Said, Biochemistry Department, Faculty of Veterinary Medicine, Benha University, Benha 13736, Egypt.
Authors’ contributions
All authors approved the final version of this study and contributed equally to this finished work.
Acknowledgements
We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020/197), Taif University, Taif, Saudi Arabia.
Funding
This study was supported by the Taif University Researchers Supporting Project (TURSP-2020/197), Taif University, Taif, Saudi Arabia.
Conflict of Interest
None declared.
References
- 1.Mokhtari RB, Homayouni TS, Baluch N et al. Combination therapy in combating cancer . Oncotarget 2017;8:38022–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers . Nat Rev Rheumatol 2016;12:731–42. [DOI] [PubMed] [Google Scholar]
- 3.El-Sheikh AA, Morsy MA, Abdalla AM et al. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediators Inflamm 2015;2015:859383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owumi SE, Ochaoga SE, Odunola OA et al. Protocatechuic acid inhibits testicular and epididymal toxicity associated with methotrexate in rats . Andrologia 2019;51:e13350. [DOI] [PubMed] [Google Scholar]
- 5.Felemban SG, Aldubayan MA, Alhowail AH et al. Vitamin B17 ameliorates methotrexate-induced reproductive toxicity, oxidative stress, and testicular injury in male rats. Oxid Med Cell Longev 2020;2020:4372719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belhan S, Çomaklı S, Küçükler S et al. Effect of chrysin on methotrexate-induced testicular damage in rats . Andrologia 2019;51:e13145. [DOI] [PubMed] [Google Scholar]
- 7.Daggulli M, Dede O, Utangac MM et al. Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats . Int J Clin Exp Med 2014;7:5511–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Ateşşahin A, Karahan İ, Türk G et al. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats . Reprod Toxicol 2006;21:42–7. [DOI] [PubMed] [Google Scholar]
- 9.Nouri HS, Azarmi Y, Movahedin M. Effect of growth hormone on testicular dysfunction induced by methotrexate in rats . Andrologia 2009;41:105–10. [DOI] [PubMed] [Google Scholar]
- 10.Finkel T. Signal transduction by reactive oxygen species . J Cell Biol 2011;194:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease . Crit Rev Food Sci Nutr 2004;44:275–95. [DOI] [PubMed] [Google Scholar]
- 12.Paroha S, Verma J, Dubey RD et al. Recent advances and prospects in gemcitabine drug delivery systems. Int J Pharm 2021;592:120043. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZY, Nixon DW. Licorice and cancer . Nutr Cancer 2001;39:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Parlar A, Arslan SO, Çam SA. Glabridin alleviates inflammation and nociception in rodents by activating BKCa channels and reducing NO levels . Biol Pharm Bull, 2020;43:884–97. [DOI] [PubMed] [Google Scholar]
- 15.Aboelsoud NH. Herbal medicine in ancient Egypt . J Med Plant Res 2010;4:082–6. [Google Scholar]
- 16.Fukai T, Marumo A, Kaitou K et al. Anti-Helicobacter pylori flavonoids from licorice extract . Life Sci 2002;71:1449–63. [DOI] [PubMed] [Google Scholar]
- 17.Belinky PA, Aviram M, Fuhrman B et al. The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation . Atherosclerosis 1998;137:49–61. [DOI] [PubMed] [Google Scholar]
- 18.Fukai T, Satoh K, Nomura T et al. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra . Fitoterapia 2003;74:624–9. [DOI] [PubMed] [Google Scholar]
- 19.Kim SC, Byun SH, Yang CH et al. Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage) . Toxicology 2004;197:239–51. [DOI] [PubMed] [Google Scholar]
- 20.Shirwaikar A, Malini S, Kumari SC. Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin induced nephrotoxicity in rats . Indian J Exp Biol 2003;41:58–62. [PubMed] [Google Scholar]
- 21.Shin S, Jang JY, Choi BI et al. Licorice extract does not impair the male reproductive function of rats . Exp Anim 2008;57:11–7. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Jin Y, Jin Y. Promoting effect of licorice extract on spermatogonial proliferation and spermatocytes differentiation of neonatal mice in vitro . In Vitro Cell Dev Biol Anim 2016;52:149–55. [DOI] [PubMed] [Google Scholar]
- 23.Ghorbanlou M, Rostamkhani S, Shokri S et al. Possible ameliorating effects of Glycyrrhiza Glabra (Licorice) on the sperm parameters in rats under high fat diet . Endocr Regul 2020;54:22–30. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Qi F, Cui Y et al. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics . Biosci Trends 2018;12:220–39. [DOI] [PubMed] [Google Scholar]
- 25.Bedi O, Bijjem KRV, Kumar P et al. Herbal induced hepatoprotection and hepatotoxicity: a critical review . Indian J Physiol Pharmacol 2016;60:6–21. [PubMed] [Google Scholar]
- 26.Gupta R, Kannan GM, Sharma M et al. Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats . Environ Toxicol Pharmacol 2005;20:456–64. [DOI] [PubMed] [Google Scholar]
- 27.Mae T, Kishida H, Nishiyama T et al. A licorice ethanolic extract with peroxisome proliferator-activated receptor-gamma ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats . J Nutr 2003;133:3369–77. [DOI] [PubMed] [Google Scholar]
- 28.Ouédraogo M, Lamien-Sanou A, Ramdé N et al. Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits . Exp Toxicol Pathol 2013;65:335–9. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoud AM, Hussein OE, Hozayen WG et al. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: protective effect of 18β-glycyrrhetinic acid. Chem Biol Interact 2017;270:59–72. [DOI] [PubMed] [Google Scholar]
- 30.Aksoy N, Dogan Y, Iriadam M et al. Protective and therapeutic effects of licorice in rats with acute tubular necrosis . J Ren Nutr 2012;22:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuksel Y, Yuksel R, Yagmurca M et al. Effects of quercetin on methotrexate-induced nephrotoxicity in rats . Hum Exp Toxicol 2017;36:51–61. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Daim MM, Khalifa HA, Abushouk AI et al. Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice. Oxid Med Cell Longev 2017;2017:3281670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassault A, Grafmeyer D, de Graeve J et al. Quality specifications and allowable standards for validation of methods used in clinical biochemistry . Ann Biol Clin (Paris) 1999;57:685–95. [PubMed] [Google Scholar]
- 34.Burnett RW. Accurate estimation of standard deviations for quantitative methods used in clinical chemistry . Clin Chem 1975;21:1935–8. [PubMed] [Google Scholar]
- 35.Saad DY, Soliman MM, Mohamed AA et al. Protective effects of sea cucumber (Holothuria atra) extract on testicular dysfunction induced by immune suppressant drugs in Wistar rats . Andrologia 2018;50:e13017. [DOI] [PubMed] [Google Scholar]
- 36.Ohkawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction . Anal Biochem 1979;95:351–8. [DOI] [PubMed] [Google Scholar]
- 37.Selvaratnam J, Robaire B. Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production. Exp Gerontol 2016;84:12–20. [DOI] [PubMed] [Google Scholar]
- 38.Nishikimi M, Appaji N, Yagi K, The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen . Biochem Biophys Res Commun 1972;46:849–54. [DOI] [PubMed] [Google Scholar]
- 39.Bahrami N, Goudarzi M, Hosseinzadeh A et al. Evaluating the protective effects of melatonin on di(2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed Pharmacother 2018;108:515–23. [DOI] [PubMed] [Google Scholar]
- 40.Pınar N, Çakırca G, Özgür T et al. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed Pharmacother 2018;97:1486–92. [DOI] [PubMed] [Google Scholar]
- 41.Eki Nci-Akdemi RF, Yildirim S, Mehmet Kandemi̇r F et al. The effects of casticin and myricetin on liver damage induced by methotrexate in rats . Iran J Basic Med Sci 2018;21:1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khafaga AF, El-Sayed YS. Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation. Life Sci 2018;196:9–17. [DOI] [PubMed] [Google Scholar]
- 43.Uzar E, Koyuncuoglu HR, Uz E et al. The activities of antioxidant enzymes and the level of malondialdehyde in cerebellum of rats subjected to methotrexate: protective effect of caffeic acid phenethyl ester . Mol Cell Biochem 2006;291:63–8. [DOI] [PubMed] [Google Scholar]
- 44.Sherif IO, Uroprotective mechanism of quercetin against cyclophosphamide-induced urotoxicity: effect on oxidative stress and inflammatory markers . J Cell Biochem 2018;119:7441–8. [DOI] [PubMed] [Google Scholar]
- 45.Yuluğ E, Türedi S, Alver A et al. Effects of resveratrol on methotrexate-induced testicular damage in rats. Sci World J 2013;2013:489659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Türedi S, Yuluğ E, Alver A et al. Effects of resveratrol on doxorubicin induced testicular damage in rats . Exp Toxicol Pathol 2015;67:229–35. [DOI] [PubMed] [Google Scholar]
- 47.Midzak A, Papadopoulos V. Binding domain-driven intracellular trafficking of sterols for synthesis of steroid hormones, bile acids and oxysterols . Traffic 2014;15:895–914. [DOI] [PubMed] [Google Scholar]
- 48.Papadopoulos V, Aghazadeh Y, Fan J et al. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol 2015;408:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller WL. Steroid hormone synthesis in mitochondria . Mol Cell Endocrinol 2013;379:62–73. [DOI] [PubMed] [Google Scholar]
- 50.Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis . Best Pract Res Clin Endocrinol Metab 2012;26:771–90. [DOI] [PubMed] [Google Scholar]
- 51.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders . Endocr Rev 2011;32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tung NH, Shoyama Y, Wada M et al. Two activators of in vitro fertilization in mice from licorice . Biochem Biophys Res Commun 2015;467:447–50. [DOI] [PubMed] [Google Scholar]
- 53.Matzkin ME, Mayerhofer A, Rossi SP et al. Cyclooxygenase-2 in testes of infertile men: evidence for the induction of prostaglandin synthesis by interleukin-1β . Fertil Steril 2010;94:1933–6. [DOI] [PubMed] [Google Scholar]
- 54.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation . Arterioscler Thromb Vasc Biol 2011;31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neamatallah T, el-Shitany NA, Abbas AT et al. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway . Food Funct 2018;9:3743–54. [DOI] [PubMed] [Google Scholar]


