Abstract
Renal transporters involved in tubular excretion pathway are considered to be the key concern in drug evaluations in nephrotoxicity. However, the relationship between the alternation of renal transporters and the kinetic process of vancomycin (VCM)-induced nephrotoxicity has not been fully elucidated. The present study investigated the alteration of renal transporters expression in the kinetic process of VCM-induced nephrotoxicity in mice. C57BL/6 mice were administrated with normal saline or VCM for 7 days. Biochemical and pathological analyses were conducted to investigate the nephrotoxicity induced by VCM administration. Renal oxidative status, plasma, and kidney content of VCM were monitored. Quantitative real-time polymerase chain reaction and immunohistochemistry analyses were performed to analyze the expression of renal transporters. Finally, our data showed that the exposure of VCM (400 mg/kg) caused a slight nephrotoxicity in mice, whereas exposure of VCM (600 mg/kg) resulted in the severe nephrotoxicity in mice as evidenced by biochemical parameters and renal morphological changes. In addition, the accumulation of VCM in kidney is higher than plasma. Interestingly, VCM (600 mg/kg, body weight) resulted in the induction of Oct2–Mate1 and Oat1/3–Mrp2/Mrp4/Bcrp pathways. However, VCM (400 mg/kg, body weight) caused the induction of Oct2–Mate1/Mate2 and Oat1/3–Mrp4/Bcrp pathways. The changes of renal transporters in association with the kinetic process of VCM-induced nephrotoxicity may exert important practical implications for its optimal use in clinic.
Keywords: vancomycin, nephrotoxicity, oxidative status, renal transporters
Introduction
Vancomycin (VCM) is one of the most commonly used glycopeptides antibiotics in both adult and pediatric populations against many types of bacterial infections, particularly severe infections. Unfortunately, a major limitation of VCM in clinical utility is nephrotoxicity [1]. Impressively, VCM is mainly excreted via the kidney, where its damaging side effects occurred [2]. It has been reported that VCM-induced nephrotoxicity was occurred in 5–25% of recipient patients, which has emerged as the major clinical cause of renal failure [3]. This underscores the urgent need for understanding the kidney-specific accumulation of VCM and development of novel strategies to prevent this serious side effect.
Renal tubular cell damage, oxidative stress, and VCM cast formation have been implicated in the pathogenesis of VCM-induced nephrotoxicity [4–6]. However, the precise mechanisms underlying VCM-associated nephrotoxicity are largely unknown. It is well-known that kidney proximal tubules are considered as the primary target sites of drug-induced nephrotoxicity, which explain why tubular excretion is an important route for the secretion of drug or toxins from the body [7]. Renal tubular secretion of the majority of drugs or toxins was performed via a variety of renal transporters, such as organic anion transporters (Oats), organic cation transporters (Octs), multidrug resistance-related proteins (Mrps), multidrug and toxin extrusions proteins (Mates), and so on. Differential expression of transporters involved in the secretion of drug and toxins could be a way to limit intracellular accumulation of drug and uremic toxins upon re-exposure [8]. For this reason, renal transporters exert an important role in regulating pharmacodynamics and pharmacokinetics of drugs [9, 10]. Some researchers demonstrated that nephrotoxicity caused by VCM is associated with a selective intracellular accumulation of the drug and endogenous toxins in the renal proximal tubular cells, which results in cell injury, and leads to the renal dysfunction [11]. Additionally, the systemic and renal clearance of VCM is changed in patients with nephrotoxicity, and dose adjustments are required for VCM due to altered pharmacokinetics and/or pharmacodynamics in patients with nephrotoxicity caused by VCM [12]. Thus, understanding the changes of renal transporters involved in renal excretion pathway is beneficial to predicting better the drug disposition and optimizing dosage.
Reports have shown that drug-induced nephrotoxicity is accompanied with changed expressions of renal transporters and renal insufficiency. And the renal tubular cells are particularly vulnerable to drug-induced nephrotoxicity [13]. Because of this, the changes of renal transporters involved in tubular excretion pathway are considered to be the key concern in drug evaluations in renal injury. Additionally, some reporters have shown that the alternation of organic anion transporters 1 (Oat1), organic anion transporters 2 (Oat2), organic anion transporters 3 (Oat3), organic cation transporter 2 (Oct2), multidrug resistance-associated protein 2 (Mrp2), multidrug resistance-associated protein 4 (Mrp4), multidrug and toxin extrusion proteins 1 (Mate1), and P-glycoprotein (P-gp) expression were closely linked to drug-induced nephrotoxicity [14–17]. However, a discrepancy was found in their results. For instance, one study reported that the Mate1 level was significantly increased by gentamicin at doses of 50 mg/kg-induced renal injury but decreased by gentamicin at doses of 100 mg/kg-induced renal injury in rats [18]. This drives us to hypothesize that the changes of these transporter expression are closely associated with the severity of renal injury. Simultaneously, there are limited data about the relationship between the alternation in expression of renal transporters and the kinetic process of VCM-induced nephrotoxicity. In addition, the renal excretion pathway of VCM had not been fully elucidated during the advancement of VCM-induced nephrotoxicity. Therefore, we sought to explore the significance of the renal transporter changes involved in the kinetic process of VCM-induced nephrotoxicity and its implication for VCM excretion at the different degrees of nephrotoxicity in mice in the present study. These information may have important practical implications for optimal use of VCM in clinic.
Materials and methods
Experimental drugs and reagents
VCM was purchased from VIANEX S.A (Greece). The Milli-Q system was used to purify ultrapure water (Millipore, Bedfordshire, MA). Quantitative real-time polymerase chain reaction (RT-qPCR) reagents were purchased from TaKaRa Biotechnology (Dalian) Co. Ltd (Dalian, China).
Ethical statement
The guide for the care and use of laboratory animals of the National Institutes of Health was strictly implemented. The animal experiment protocol was approved by the Animal Ethics Committee of the Shanghai Children’s Hospital (Shanghai, China, Permit number: 2015003) in this study.
Animals and administration of VCM
Male C57BL/6 mice (22 ± 2 g, 7 weeks of age) were purchased from Shanghai Model Organisms Center, Inc. (Shanghai, China). All animals were placed in the controlled environment with temperature (22 ± 1°C) and humidity (60 ± 5%) on a 12-h-light–dark cycle; all mice had free access to food and water. VCM was dissolved in saline and intraperitoneally injected into each mouse at a dose of 400 mg/kg (n = 5) or 600 mg/kg (n = 5) per body weight (BW) at 24-h intervals for 7 day, respectively. At the end of the experiment, all mice were fasted overnight.
Serum biochemistry
The plasma levels of blood urea nitrogen (BUN) and creatinine were determined with commercially available kits in an automatic biochemical analyzer (HITACHI 7080, Japan).
Histological analysis
Kidney samples from all groups were fixed in 10% formaldehyde (neutral-buffered formalin), dehydrated, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin–eosin (H&E) according to standard protocols. Shanghai Showbio Biotech Inc. performed histological assessments.
Plasma and renal concentrations of VCM
The measure of plasma and renal concentrations of VCM was conducted by Hunan Demeter Instrument Co. Ltd (Changsha, China). Plasma and renal concentrations of VCM quantification method were used and improved on the basis of previous study [19]. The method of plasma concentration of VCM quantification was as followed: briefly, a volume of 200 μl of plasma was pipetted into a 2-ml tube, then 600 μl of 10% trichloroacetic acid–acetonitrile mixture (7:1, v:v) was added to the tube. Subsequently, the mixture was vortexed for 30 s and centrifuged at 20000 × g for 5 min. A 200-μl aliquot of the supernatant was injected into the 2D-LC system for analysis. The methods for the determination of renal VCM concentration was as followed: the sliced kidney samples were weighed for 30 mg, mixed with 300 μl of normal saline (NS) and homogenized in an ice-bath. Renal samples (50 μl) collected were added to 50 μl of deionized water and 400 μl of methyl alcohol, then vortex mixed for 30 s, followed by centrifugation at 12000 rpm for 10 min for deproteinization. Then 400 μl of the upper organic layer was transferred into a new polythene tube and dried with nitrogen at 40°C. The dried residue was dissolved in 400 μl using the mobile phase, and a 200-μl aliquot was injected into the 2D-LC system for analysis.
RNA extraction and RT-qPCR
RNAiso Plus® Reagent Kit (Takara Biotechnology, Dalian, China) was employed to extract total kidney RNA. RNA was reverse transcribed into cDNA (1 μg/μl) using a PrimeScript® RT Reagent Kit with gDNA Eraser (Takara Biotechnology, Dalian, China). Gene-specific primers were designed online with Primers 3. Supplementary table 1 presents the primers for the detected genes. Quantification of gene expression was performed with real-time PCR (Applied Biosystems, USA) with SYBR® Premix Ex Taq™ Kit (Takara Biotechnology, Dalian, China). All reactions were performed in triplicate with the following thermal cycling conditions: initial denaturation at 95°C for 30 s followed by 50 cycles of 95°C for 5 s and 60°C for 30 s. Gapdh was used as an internal reference to calculate the relative expression of target genes.
ELISA of KIM-1
The enzyme-linked immunosorbent assay (ELISA) kits were purchased from Abcam (Cambridge, UK) to assess renal KIM-1 of mice. All procedures were conducted in accordance with the manufacturer’s instructions.
Renal immunohistochemistry
Renal sections (4 μm) were deparaffinized and rehydrated in a graded ethanol series. After antigen retrieval and blocking by normal goat serum, sections were incubated with Oct2 monoclonal antibody (Abcam, 1:200 dilution) and Mrp2 monoclonal antibody (Abcam, 1:100 dilution) at developed with 4°C overnight. The localization of peroxidase conjugates was determined using a DAB kit (Servicebio, Wuhan, China). Slides were examined under a microscope, and the signals were analyzed using Image software analysis tools.
Statistical analysis
All data were presented as mean ± standard deviation (SD). Data were statistically analyzed by one-way analysis of variance and Student’s t-test (SPSS 24.0 software; SPSS, Inc., Chicago, USA). P < 0.05 was considered as statistically significant.
Results
The change of the physiological and renal function parameters induced by VCM treatment in mice
A mice model of nephrotoxicity was established by intraperitoneal injection of VCM. All mice started with similar mean BW. As shown in Figure 1A, the markedly changes of BW are observed in VCM-treated mice. In addition, renal hypertrophy was shown in VCM-treated mice as evidenced by the significant increase of kidney weight and the mages of kidney size when compared with the vehicle group (Fig. 1B and C). As compared with the vehicle group, plasma creatinine, plasma BUN, and the kidney injury molecule 1 (KIM-1) mRNA in kidney were dramatically increased in the VCM (600 mg/kg) group, whereas the slight increase of plasma level of creatinine and BUN, renal KIM-1 mRNA were observed in mice with treatment of VCM at the dosage of 400 mg/kg per BW (Fig. 1D and E). ELISA showed that the increased renal KIM-1protein level in mice with VCM (600 mg/kg) and VCM (400 mg/kg) treatment (Fig. 1F). Moreover, VCM treatment increased the number of terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling-positive cells in dose-dependent manner (Supplementary Fig. 1). These above data demonstrated that the exposure of VCM at dosage of 400 and 600 mg/kg developed the different degrees of nephrotoxicity in mice.
Figure 1.
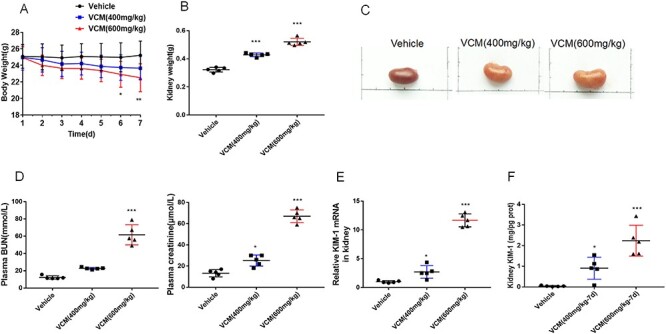
the physiological and renal function parameters in VCM-induced nephrotoxicity. BW curve over time (A). The kidney weight (B). The image of kidney size (C). Plasma creatinine and BUN level (D). Relative mRNA expression of KIM-1 in kidney (E). Relative protein expression of KIM-1 in kidney (F). Data are presented as mean ± SD. n = 5, *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle group, respectively.
The alternation of renal morphology induced by VCM treatment in mice
H&E staining revealed that the section of renal tissue from vehicle group showed normal histological structure of the renal corpuscles and renal tubules. However, the extensive dilatation, vacuolar degeneration, epithelial desquamation, necrosis, infiltration with inflammatory cells, and edema occurred predominantly in the proximal tubules were undergoing in VCM (400 mg/kg, BW)-treated mice, and the damage gradually became more severe at the dosage of 600 mg/kg per BW (Fig. 2). These finding support the different changes of renal function including plasma creatinine as well as plasma BUN induced by different dosage of VCM.
Figure 2.
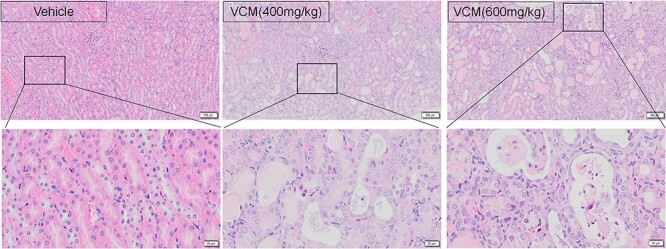
the morphology and pathology condition of the kidneys in VCM-induced nephrotoxicity. Kidney histological examination was conducted by H&E staining in mice (×100 magnification), n = 3.
The change of oxidative status induced by VCM treatment in mice
Oxidative status induced by VCM treatment was indicated by a marked increase in renal malondialdehyde (MDA) accompanied with the decrease in renal glutathione (GSH). In our study, a markedly increase of renal MDA was observed in VCM (600 mg/kg) group when compared with the vehicle group. However, there was no significant difference in MDA level between the vehicle group and the VCM (400 mg/kg) group. In addition, the renal GSH level was significantly increased by VCM treatments at dosage of 400 mg/kg but decreased by VCM treatments at dosage of 600 mg/kg per BW (Fig. 3). These findings indicated that renal oxidative status is difference at different stages of renal injury induced by VCM administration, and the decrease in renal antioxidant enzymatic protection could aggravate the damage of oxidative stress induced by VCM.
Figure 3.
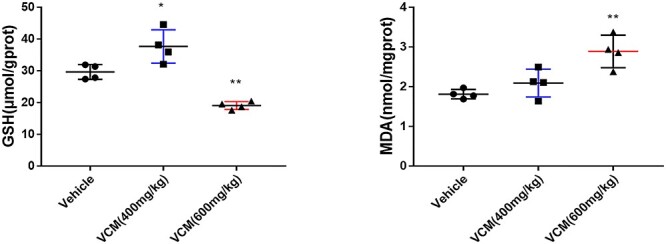
renal oxidative status in VCM-induced nephrotoxicity. The concentrations of MDA and GSH in kidney were measured. Data are presented as mean ± SD. n = 4, *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle group, respectively.
The alternation of renal transporters induced by VCM treatment in mice
The renal excretion of the most drugs is generally affected due to the change of tubular transport in nephrotoxicity. In addition, many endogenous toxins were the substrates of the transporter Oat1, Oat3, Mrp2, and so on. Therefore, we investigated the mRNA expression of renal transporter in mice with VCM treatment. As shown in Figure 4A, the exposure of VCM at dosage of 400 or 600 mg/kg significantly decreased the expression of uptake transporters, including Oat1, Oat3, and Oct2. Synchronously change of Oct2 protein level was observed in the group of VCM (600 mg/kg) and VCM (400 mg/kg) treatment by immunohistochemical staining (Fig. 5). However, there is no obvious change in the expression of organic anion transporter 4c1 (Oatp4c1). In addition, the mRNA expression levels of efflux transporter Mrp2 in the VCM (600 mg/kg) group, but not VCM (400 mg/kg) group, were markedly lower than in the vehicle group. Conversely, the mRNA expression levels of efflux transporter multidrug and toxin extrusion proteins 2 (Mate2) in the VCM (400 mg/kg) group, but not VCM (600 mg/kg) group, were markedly higher than in the vehicle group. Interestingly, the exposure of VCM at dosage of 400 or 600 mg/kg significantly decreased the expression of efflux transporters Mate1, breast cancer resistance protein (Bcrp) and Mrp4, whereas the change of efflux transporters P-gp expression in kidney was not observed in the groups with VCM treatment compared with the group with vehicle treatment (Fig. 4B). Consistently, the result of immunohistochemical staining of Mrp2 (scale bars, 20 μm; black arrows indicate the positive cells) in the kidneys also demonstrated that the protein level of efflux transporter Mrp2 in the VCM (600 mg/kg) group, but not VCM (400 mg/kg) group, were markedly lower than in the vehicle group (Fig. 5).
Figure 4.
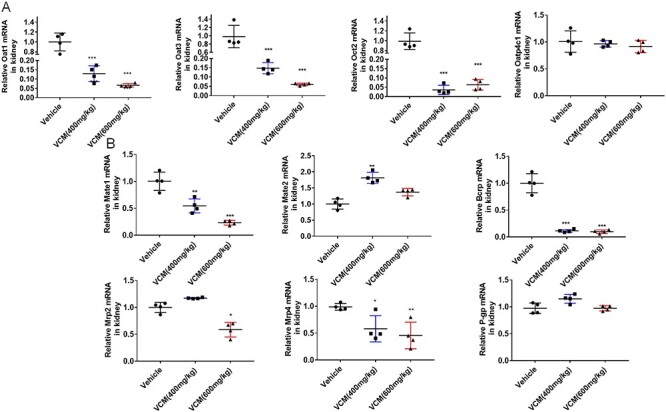
the mRNA expression profile of renal transporters in VCM-induced nephrotoxicity. RT-qPCR analysis was performed to measure the uptake transporters associated gene expression of Oat1, Oat3, Oct2, Oatp4c1 (A) and the efflux transporters associated gene expression of Mrp2, Mrp4, Mate1, Mate2, Bcrp, and P-gp (B) in the kidney. All data are shown as the mean ± SD. n = 4, *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle group, respectively.
Figure 5.
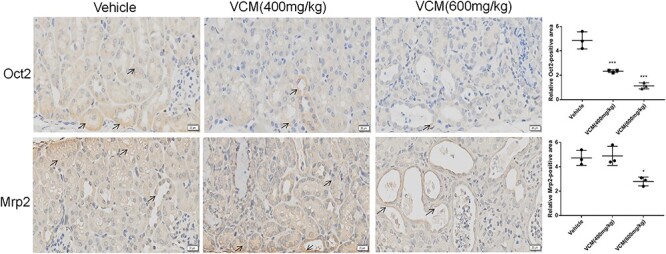
representative images and quantification for immunohistochemical staining of Oct2 and Mrp2 (scale bars, 20 μm; black arrows indicate the positive cells) in the kidneys. Six random fields were taken from each kidney. Data are expressed as mean ± SD; n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle group, respectively
Dose-dependent changes in levels of systemic and renal exposure to VCM in mice
Dose-adjusted concentration was calculated by dividing the concentration by the corresponding 24-h dose on mg/kg basis. Interestingly, the accumulation of VCM in kidney tissues is about two times higher than the plasma concentration, which indicating highly contributes to VCM-induced nephrotoxicity. As shown in Figure 6A, the dose-adjusted plasma VCM concentration in the VCM (600 mg/kg) group was increased by 1.7-fold compared with the VCM (400 mg/kg) group. Simultaneously, the dose-adjusted kidney VCM concentration in the VCM (600 mg/kg) group was increased by 3.5 fold compared with the VCM (400 mg/kg) group (Fig. 6B).
Figure 6.
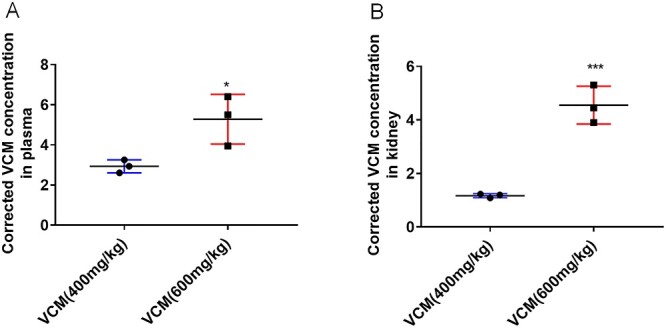
plasma and renal concentrations of VCM after intraperitoneal administration at the doses of 400 or 600 mg/kg per BW. Plasma concentrations (A). Renal concentrations (B). All data are shown as the mean ± SD. n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle group, respectively
Discussions
It is well known that nephrotoxicity is the most frequent complication of VCM, despite its clinical application in the treatment of life-threatening gram-positive bacterial infections. Apart from underlying patient’s susceptibility to kidney injury, the inherent nephrotoxicity of drugs and the renal handling (transport and metabolism) of medications are the major cause of nephrotoxicity [20]. Several studies have reported that the changes of renal transporter expression are coming with renal injury, a discrepancy was found in their results [14–17]. The current study demonstrated that the changes of renal transporters expression in the kinetic process of VCM-induced nephrotoxicity were closely associated with the severity of nephrotoxicity.
In order to mimic the different degrees of nephrotoxicity, mice were intraperitoneally injected with VCM at the dosage of 400 and 600 mg/kg per BW at 24-h intervals for consecutive 7 days. Our results showed that the exposure of VCM (400 mg/kg) caused a slight nephrotoxicity in mice, whereas exposure of VCM (600 mg/kg) resulted in the severe nephrotoxicity in mice as evidenced by biochemical parameters and renal morphological changes. Subsequently, to clarify the relationships between the changes in expression of renal transporters and the kinetic process of VCM-induced nephrotoxicity in vivo, we investigated the effect of the different degrees of nephrotoxicity on renal transporter system. In the present study, VCM exposure (400 or 600 mg/kg) led to the downregulation of kidney Oct2, Oat1, Oat3, Mate1, Bcrp, and Mrp4 expression but did not affect the expression of Oatp4c1 and P-gp in kidney. Interestingly, the mRNA expression levels of Mrp2 in the VCM (600 mg/kg) group, but not VCM (400 mg/kg) group, were markedly lower than the vehicle group. The increased expression of Mate2 was observed in mice treated with VCM (400 mg/kg, BW) but not observed in mice treated with VCM (600 mg/kg, BW). Thus, the renal transporters changes in kidney support the difference in the changes of renal function including the elevations of plasma creatinine and plasma BUN as well as the increase of levels of systemic and renal exposure to VCM in mice.
Renal transports exert an important role in the pharmacokinetics of drugs and renal toxicity [21]. Differential expression of transports involved in the uptake and efflux of drug participate in limiting intracellular accumulation of drug upon re-exposure [8]. It has been reported that Oct2, Mate1, and Mate2 work in concert in the secretion of cationic drugs from the kidney [22]. Interestingly, VCM is a canonic drug. Therefore, we investigated the effect of VCM on the renal Oct2, Mate1, and Mate2 expression in the present study. Previous study has demonstrated that Oct2 are critical to the nephrotoxicity of cationic drugs such as cisplatin [23]. In present study, Oct2 expression in kidney was downregulated in VCM-treated mice, which may be an adaptive and protective response against kidney injury by preventing high concentrations of VCM from reaching the renal tubular cells and subsequently inhibit the development of nephrotoxicity. This finding is consistent with previously reported results with the downregulation of Oct2 in renal injury [16]. In contrast to the basal uptake transporter Oct2, Mate1 and Mate2 expressed in the apical membrane of proximal tubule epithelial cells contribute to the urinary efflux of cationic drugs. Previous study has demonstrated that Mates are essential for the active secretion of canonic drug into renal proximal tubular cells [24]. Ondansetron, a Mate inhibitor, significantly increases the levels of creatinine and BUN in mice receiving cisplatin treatment due to increasing cisplatin concentration in the proximal tubules induced by the inhibition of Mate1 and Mate2 [25]. In the present study, we found that Mate1 expression was markedly decreased by VCM (600 mg/kg) and VCM (400 mg/kg), which is lined with the higher accumulation of VCM in kidney compared with the accumulation of VCM in plasma. However, the increased expression of Mate2 was observed in mice treated with VCM (400 mg/kg, BW) but not observed in mice treated with VCM (600 mg/kg, BW), which may partly contribute to the aggravated renal injury as evidenced by the higher level of creatinine and BUN in VCM (600 mg/kg, BW) group as compared with the VCM (400 mg/kg, BW) group. Taken together, the alternation of Oct2, Mate1, and Mate2 level may be confounding factors contributing to the changes in the disposition of VCM and subsequently nephrotoxicity. However, the present study is still not validated the potent inhibition of VCM on Oct2, Mate1, and Mate2 function. It is unknown whether VCM treatment has an effect on the disposition of the cationic drugs transported by Oct2, Mate1, and Mate2 in vivo and in vitro. Future studies are urgent to validate the interaction of VCM with Oct2, Mate1, and Mate2 using metformin as the probe substrate toward these transporters.
Previous study reported that drug-induced nephrotoxicity led to the change of the expressions of renal transporters, which resulting in the decreased excretion of endogenous toxins. Afterward, because of the elimination of uremic toxins is inhibited, the accumulated endogenous toxins in kidney could accelerated the progression of nephrotoxicity [14, 15]. Thus, promoting the secretion of endogenous toxins is an effective route for mitigating the nephrotoxicity induced by drugs. Our results showed that the exposure of VCM at the dosage of 600 mg/kg per BW markedly increased the concentration of plasma creatinine and BUN. Several emerging evidences have demonstrated that renal transport participated in the renal elimination of creatinine, such as Oct2, Oat1, and Oat3 [26, 27]. The present results demonstrated that the reduced membrane expression of renal Oat1 and Oat3 were observed in mice with VCM administration at the dosage of 400 and 600 mg/kg per BW, which might be a compensatory protective response against nephrotoxicity induced by the accumulation of VCM. Apart from Oats transporters, previous studies revealed that the transporters Oct2 play an important role in mediating the renal tubular excretion of creatinine [28]. Thus, an increase of serum creatinine induced by the exposure of VCM could be at least partly explained by the attenuation in Oct2, Oat1, and Oat3 expression in our study. Although the expression of Oct2, Oat1, and Oat3 were also decreased by VCM (400 mg/kg, BW) exposure, the plasma creatinine level did not synchronously change. This could be related to the complex interactions between excretion and reabsorption in kidney. Besides, Mrps expressed in the apical membrane of proximal tubule epithelial cells contribute to the urinary efflux of endogenous toxins. Interestingly, previous study demonstrated that inhibition of Mrps could cause accumulation of endogenous toxins in proximal tubule cells and consequently lead to nephrotoxicity [29]. Notably, Mrp2 is an efflux transporter that mediates the secretion of oxidized GSH and lipid peroxides generated during renal injury, in turn, alleviates the oxidative stress [30]. MDA is participated in lipid peroxidation as well as its content reflects the level of lipid peroxidation, and it is regarded as a suitable index of oxidative damage in vivo. It has been previously reported that Mrp2 was involved in the tubular secretion of MDA [31]. The present results showed that the renal MDA was significantly increased by VCM (600 mg/kg, BW) treatment, but not changed by VCM (400 mg/kg, BW) treatment, which might be closely related to the downregulated Mrp2 mRNA level in mice with the treatment of VCM (600 mg/kg, BW). As previously reported, GSH is another substrate of Mrp2 [32]. In our study, a significant reduce of the kidney GSH level was observed in VCM treatments at dosage of 600 mg/kg, but an increase in VCM treatments at dosage of 400 mg/kg per BW. Interestingly, the renal Mrp2 mRNA level in mice with the treatment of VCM (400 mg/kg, BW) is comparable to the mice with NS treatment, which was consistent with the change of kidney GSH level. Consistently, previous study also investigated the changes of several renal transporters in rats with VCM treatment. The research similarly showed that Mrp2, Oat1, Oat3, and Oct2 decreased in rats with VCM-induced nephrotoxicity [33]. Interestingly, P-gp was also reported [33] to be downregulated, which was different from unchanged P-gp levels in the study. However, due to lack of experiments results about different dose of VCM in rats, the differential outcomes of P-gp were difficult to determine whether it is due to the extent of the renal injury, differences in species, or some other cause. Besides, Bcrp and Mrp4 are also the efflux transporters of GSH. Overexpression of Bcrp and Mrp4 would decrease the level of GSH [34, 35]. In the present study, the mRNA expression of Bcrp and Mrp4 were downregulated by VCM, but no obvious difference was observed between VCM (400 mg/kg, BW) and VCM (600 mg/kg, BW) groups. Therefore, the most plausible explanation for the observed differences in excretion of MDA and GSH in VCM (600 mg/kg, BW)-treated mice compared with VCM (400 mg/kg, BW) mice is the impaired efflux of MDA and GSH from renal tubular secretion into urine because of the downregulated Mrp2 expression. Meanwhile, the changes of renal transporters in different VCM group support the difference in the changes of renal function including the elevations of plasma creatinine and BUN as well as the increase of levels of systemic and renal exposure to VCM in mice. Taken together, the accumulation of endogenous toxins induced by VCM-induced nephrotoxicity could be at least partly explained by the attenuation in Oct2, Oat1, Oat3, Mrp2, and Mrp4 expression in our study.
Conclusions
In summary, our results showed that VCM (600 mg/kg, BW)-induced nephrotoxicity led to the downregulated expression of the Oct2–Mate1 and Oat1/3–Mrp2//Mrp4/Bcrp pathways. However, VCM (400 mg/kg, BW) caused the decreased expression of the Oct2–Mate1/Mate2 and Oat1/3–Mrp4/Bcrp pathways but did not decrease the expression of Mrp2. In addition, the increased expression of Mate2 was observed in mice with VCM (400 mg/kg, BW) treatment but not in mice with VCM (600 mg/kg, BW) treatment. The current work on the mechanistic basis of VCM-induced nephrotoxicity opens up new avenues for further evaluation of pharmacologic approaches for renoprotection through targeting the renal transporters. Moreover, the changes in expression of renal transporters in association with the kinetic process of VCM-induced nephrotoxicity in vivo may have important practical implications for its optimal use.
Author contributions
Z.L., H.S., and Q.Y. conceived and designed the research. Q.Y., H.L., and M.G. conducted the experiments and analysis. Q.Y., H.L., M.G., and L.D. analyzed data. Q.Y. and H.L. drafted the manuscript. Q.Y., Z.L., H.L., and H.S. revised the manuscript. L.D., L.Y., and H.S. contributed to discussion of the work. All authors reviewed and approved the manuscript.
Supplementary Material
Acknowledgments
We would like to acknowledge the members of Hunan Demeter Instrument Co. Ltd (Changsha, China) for performing the measure of plasma and kidney concentration of vancomycin.
Contributor Information
Hongjing Li, Department of Pharmacy, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200062, China.
Qiaoling Yang, Department of Pharmacy, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200062, China; Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Mingzhu Gui, Department of Pharmacy, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200062, China; Department of Pediatrics, Shanghai Baoshan Luodian Hospital, Shanghai 201908, China.
Lili Ding, Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Li Yang, Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Huajun Sun, Department of Pharmacy, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200062, China.
Zhiling Li, Department of Pharmacy, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200062, China.
Funding
National Natural Science Foundation of China (Grant No. 81603199), Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (Grant No. CXYJY2019MS003) to Z.L.; Interdisciplinary Program of Shanghai Jiao Tong University (Grant No. YG2019QNA03) to Q.Y.
Conflict of Interest
The authors declared that they have no conflicts of interest to this work.
References
- 1.Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab 2016;7:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies SW, Efird JT, Guidry CA et al. Vancomycin-associated nephrotoxicity: the obesity factor. Surg Infect (Larchmt) 2015;16:684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwamoto T, Kagawa Y, Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull 2003;26:876–9. [DOI] [PubMed] [Google Scholar]
- 4.Faruk O, Koyuncu AM, Fehmi O et al. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 2005;215:227–33. [DOI] [PubMed] [Google Scholar]
- 5.Christine D, Angela P, Sylvia L et al. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 2009;107:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luque Y, Louis K, Jouanneau C et al. Vancomycin-Associated Cast Nephropathy. J Am Soc Nephrol 2017;28:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diekjürgen D, Grainger DW. Drug transporter expression profiling in a three-dimensional kidney proximal tubule in vitro nephrotoxicity model. Pflugers Arch 2018;470:1311–23. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Han L. Roles of renal drug transporter in drug disposition and renal toxicity. Adv Exp Med Biol 2019;1141:341–60. [DOI] [PubMed] [Google Scholar]
- 9.Shibata M, Toyoshima J, Kaneko Y et al. A drug-drug interaction study to evaluate the impact of peficitinib on OCT1- and MATE1-mediated transport of metformin in healthy volunteers. Eur J Clin Pharmacol 2020;76:1135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boof ML, Halabi A, Ufer M et al. Impact of the organic cation transporter 2 inhibitor cimetidine on the single-dose pharmacokinetics of the glucosylceramide synthase inhibitor lucerastat in healthy subjects. Eur J Clin Pharmacol 2020;76:431–7. [DOI] [PubMed] [Google Scholar]
- 11.Humanes B, Carlos Jado J, Camano S et al. Protective effects of cilastatin against vancomycin-induced nephrotoxicity. Biomed Res Int 2015;10:704382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloprogge F, Hill LF, Booth J et al. Revising pediatric vancomycin dosing accounting for nephrotoxicity in a pharmacokinetic-pharmacodynamic model. Antimicrob Agents Chemother 2019;63:e00067–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naud J, Michaud J, Beauchemin S et al. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos 2011;39:1363–9. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Meng Q, Liu Q et al. JBP485 improves gentamicin-induced acute renal failure by regulating the expression and function of Oat1 and Oat3 in rats. Toxicol Appl Pharmacol 2013;271:285–95. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Meng Q, Wang C et al. Changes in expression of renal Oat1, Oat3 and Mrp2 in cisplatin-induced acute renal failure after treatment of JBP485 in rats. Toxicol Appl Pharmacol 2012;264:423–30. [DOI] [PubMed] [Google Scholar]
- 16.Ji L, Masuda S, Saito H, Inui K. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kidney Int 2002;62:514–24. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Shibata T, Oba T et al. Multidrug-resistance-associated protein plays a protective role in menadione-induced oxidative stress in endothelial cells. Life Sci 2009;84:211–7. [DOI] [PubMed] [Google Scholar]
- 18.Ma YR, Luo X, Wu YF et al. Alteration of renal excretion pathways in gentamicin-induced renal injury in rats. J Appl Toxicol 2018;38:968–77. [DOI] [PubMed] [Google Scholar]
- 19.Sheng Y, Zhou B. High-throughput determination of vancomycin in human plasma by a cost-effective system of two-dimensional liquid chromatography. J Chromatogr 2017;1499:48–56. [DOI] [PubMed] [Google Scholar]
- 20.Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 2009;4:1275–83. [DOI] [PubMed] [Google Scholar]
- 21.Tett SE, Kirkpatrick CM, Gross AS et al. Principles and clinical application of assessing alterations in renal elimination pathways. Clin Pharmacokinet 2003;42:1193–211. [DOI] [PubMed] [Google Scholar]
- 22.Li LP, Song FF, Weng YY et al. Role of OCT2 and MATE1 in renal disposition and toxicity of nitidine chloride. Br J Pharmacol 2016;173:2543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipski KK, Mathijssen RH, Mikkelsen TS et al. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 2009;86:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Yonezawa A, Hashimoto S et al. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol 2010;80:1762–7. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Guo D, Dong Z et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol 2013;273:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Warren MS, Zhang X et al. Impact on creatinine renal clearance by the interplay of multiple renal transporters: a case study with INCB039110. Drug Metab Dispos 2015;43:485–9. [DOI] [PubMed] [Google Scholar]
- 27.Lepist EI, Zhang X, Hao J et al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int 2014;86:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urakami Y, Kimura N, Okuda M et al. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res 2004;21:976–81. [DOI] [PubMed] [Google Scholar]
- 29.El-Sheikh AA, Greupink R, Wortelboer HM et al. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Transl Res 2013;162:398–409. [DOI] [PubMed] [Google Scholar]
- 30.Liu DM, Yang D, Zhou CY et al. Aloe-emodin induces hepatotoxicity by the inhibition of multidrug resistance protein 2. Phytomedicine 2020;68:153148. [DOI] [PubMed] [Google Scholar]
- 31.Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther 2006;109:1–11. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich CG, Ottenhoff R, de Waart DR et al. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology 2001;167:73–81. [DOI] [PubMed] [Google Scholar]
- 33.Wen SJ, Wang CY, Huo XK et al. JBP485 attenuates vancomycin-induced nephrotoxicity by regulating the expressions of organic anion transporter (Oat) 1, Oat3, organic cation transporter 2 (Oct2), multidrug resistance-associated protein 2 (Mrp2) and P-glycoprotein (P-gp) in rats. Toxicol Lett 2018;295:195–204. [DOI] [PubMed] [Google Scholar]
- 34.Krzyżanowski D, Bartosz G, Grzelak A. Collateral sensitivity: ABCG2-overexpressing cells are more vulnerable to oxidative stress. Free Radical Biol Med 2014;76:47–52. [DOI] [PubMed] [Google Scholar]
- 35.Bai J, Lai L, Yeo HC et al. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int J Biochem Cell Biol 2004;36:247–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


