Abstract
Caffeine consumption is able to interfere in cellular processes related to inflammatory mechanisms by acting through the adenosinergic system. This study aimed to recognize alterations related to adenosinergic system and inflammatory process in the cerebellum of University of Chile Bibulous (UChB) rats after the consumption of ethanol and caffeine. UChB and Wistar rats, males at 5 months old, were divided into the groups (n = 15/group): (i) Control (Wistar rats receiving water); (ii) Ethanol group (UChB rats receiving ethanol solution at 10%) and (iii) Ethanol+caffeine group (UChB rats receiving ethanol solution at 10% added of 3 g/L of caffeine). The cerebellar tissue was collected and processed for immunohistochemistry, Reverse transcription polymerase chain reaction (RT-PCR) and western blotting techniques for the adenosinergic receptors A1 and A2a and inflammatory markers, including Nuclear factor kappa B (NFkB), TLR4, TLR2, MyD88, TNF-α, COX-2, iNOS and microglial marker Iba-1. Results showed ethanol and caffeine consumption differentially altering the immunolocalization of adenosinergic receptors and inflammatory markers in the cerebellar tissue. The A2a receptor was overexpressed in the Ethanol group and was evident in the glial cells. The Ethanol group had increased protein levels for NFκB and TLR4, expressively in Bergmann glia and Purkinje cells. Caffeine reduced the expression of these markers to levels similar to those found in the Control group. The A1 gene was upregulated the Ethanol group, but not its protein levels, suggesting post-transcriptional interference. In conclusion, caffeine seems to attenuate ethanol-induced inflammation in the cerebellum of UChB rats through the A1 and A2a modulation, playing a neuroprotective role in the chronic context of ethanol consumption.
Keywords: caffeine, cerebellum, ethanol, inflammation, adenosinergic receptor, UChB rats
Graphical abstract
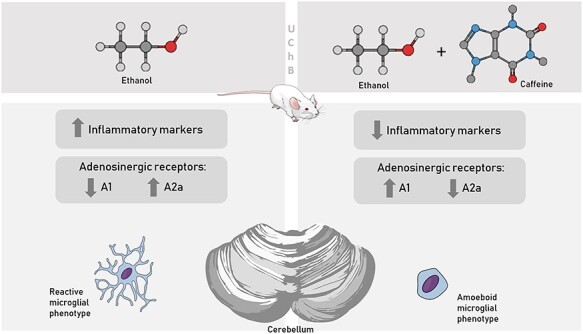
Introduction
Caffeine is one of the most consumed psychostimulant agents around the world due its cognitive enhancement properties [1, 2]. It is a methylxanthine alkaloid, considered a nonselective and competitive antagonist of adenosinergic receptors that counteracts the tonic action of endogenous adenosine on central adenosinergic system, increasing the arousal and alertness [3, 4]. Pharmacological studies indicate that the central nervous system (CNS) effects of caffeine are mediated primarily by its antagonistic actions at the A1 and A2A subtypes of adenosine receptors [5], which belongs to the super-family of G-coupled receptors and are distributed within the brain at variable densities [6, 7].
One of the highest densities of A1 receptors are present within the cerebellar layers [6]. According to studies, brain adenosinergic modulation of ethanol-induced motor incoodination is a receptor mediated response and the adenosine receptor subtype involved in the modulation appeared to be A1 [8, 9]. The A2A subtype is densely distributed in the striatal and olfactory tubercle and sparsely in other brain regions [7]. However, in the literature, the A2A is recognized as the receptor, and one of the pathways, that links the inflammatory process to the adenosinergic system. The A2a receptor can be found in the surface of immune cells, evidencing its role in the immune response [10].
In this way, a broad literature has shown that caffeine consumption is able to interfere in cellular processes inherent to the inflammatory mechanisms by acting through the adenosinergic system, especially when related to the A1 and A2a receptors, attenuating the neuroinflammation [11–13]. Confirming this hypothesis, epidemiological studies suggest that caffeine consumption is inversely related to the onset of neurological dysfunctions whose axis of pathogenesis includes the chronic neuroinflammation such as Alzheimer’s and Parkinson’s diseases [14, 15].
The ethanol consumption is a common habit in society and one of the main risk factors to health, accounting for 5.9% of the world’s total deaths [16]. The alcoholism is a condition that leads to the increase of molecules known to be involved with the immune response, evidencing the installation of neuroinflammatory process [17, 18]. The microglia, responsible cell for immune response within the CNS and the neurons, responds by signaling through the stimulation of toll-like receptors (TLRs), which directly or indirectly activates the NFkB signaling pathway, proinflammatory miRNAs and cytokines [19, 20, 21]. It is also known that the cerebellum is one of the most affected central organs by the ethanol consumption [22, 23]. The susceptibility of the cerebellar neurons to acute and chronic variations of the ethanol blood levels is well documented and differs according to the cellular type [24, 25, 26]. Modifications in the cellular structure were observed after chronic consumption of ethanol by experimental models of alcoholism, such as dilation of the smooth endoplasmic reticulum in Purkinje neurons accompanied by neuronal death [27], granular neurons with apoptotic nuclei, cytoplasm with accumulation of lipid droplets and deterioration of the myelin [28, 29].
In the hall of altered parameters under the alcoholic condition, the adenosinergic tone and the adenosinergic receptors are also modified by the ethanol consumption [30]. There is a belief, particularly among adolescents and young adults, that mixing caffeinated drinks with alcohol may counter the cognitive and motor impairments related to acute alcohol intoxication [31]. However, this antagonism between alcohol and caffeinated drinks shows no consistent pattern in acute conditions [32] and the literature supports the association between alcohol mixed with energy drinks with increased risk for injuries or alcohol self-administration [33].
Nevertheless, the chronic intake of caffeine and its association with alcoholism are still poorly understood. Considering that, the aim of the study herein was to identify if caffeine is able to interfere in the chronic ethanol-induced neuroinflammation and its relation with adenosinergic receptors A1 and A2a in the cerebellum of an experimental ad libitum and chronic ethanol feeding model, the UChB rats. The UChB rat is a well-established experimental model for ethanol voluntary consumption [34], original from the University of Chile (UCh). The UChB rats present high levels of voluntary ethanol consumption (5–7 g of ethanol per kilogram of bodyweight per day) that refers to the human context of alcoholism in a variety of aspects [34, 35]. In this study, we also aimed to evaluate the differential response for the inflammatory markers and adenosinergic receptors on cerebellar subpopulation of neurons and glial cells, considering the potential ethanol and caffeine interference in the cerebellar microenvironment.
Material and Methods
UChB standardization and experimental procedures
The selection of ethanol-drinking rats and standardization of the UChB variety was performed according to the protocol by Mardones and Segovia-Riquelme [36] and the experimental group procedures was also described by Rossetto et al. [12].
The experimentation period started at 80th day of age, when 30 male UChB rats were housed in individual boxes, with solid floor and shavings, under controlled conditions of luminosity (12 h of darkness and 12 h of lightness) and temperature (20–25°C). During this period, the animals received chow (Purina), water ad libitum and ethanol solution (1:10). At 95th day, 15 male rats standardized as UChB were randomly selected to the Ethanol+caffeine group. In the Ethanol+caffeine group, the bottle of ethanol solution was replaced by a bottle containing the ethanol solution (1:10) added by caffeine in a concentration of 3 g/l. For the preparation of the solutions, the ethanol used was ethanol P.A. (CAS-6417-5, Neon) and the caffeine (1,3,7-trimethylxanthine, 98.5–101.0%, Fisher Chemicals, Fair Lawn, USA). The dose of 3 g/l of caffeine was based on previous studies from our group [12, 26, 37]. From the end of the selection period to euthanasia (80–150 days old), the ethanol consumption was measured every week for the UChB rats (7 days).
As the UChB strain are derived from Wistar rats (Rattus norvergicus) and the lineage pass through a selection process when is necessary to consume ethanol for being standardized, one-third group was proposed as control for the UChB model, the Wistar group. The Control group did not intake alcohol during any period of experimentation: it was composed by 15 male Wistar rats consuming water ad libitum, being euthanized at 150th day.
Thus, we constitute the following groups:
Control group (CT): 15 Wistar male rats that consumed water ad libitum until 150th day,
Ethanol group (EtOH): 15 UChB rats that consumed ethanol solution (1:10) and water ad libitum from the 80th to 150th day. Excluding the days for standardization, this group consumed ethanol during 70 consecutive days. During this time, the rats had free choice for water or ethanol, being the bottles alternately positioned inside the box each 7 days.
Ethanol + caffeine group (EtOH+caf): 15 UChB rats that consumed ethanol solution (1:10) and water ad libitum from the 80th to 95th day. At the 95th day, caffeine at a dose of 3 g/l was added to the ethanol solution. This group consumed ethanol solution isolated during 15 days, excluding the days for standardization, and the solution of ethanol with caffeine for 55 consecutive days. During this time, the rats had free choice for water or ethanol+caffeine solution, being the bottles alternately positioned inside the box each 7 days.
The experimental protocol followed the ethical principles according to National Council for the Control of Animal Experimentation (Brazil) and the Committee on Ethics in the Use of Animals (CEUA) from UNICAMP (protocol 4651-1/2017) and UFSCar (protocol 9 895 280 815) previously approved it. After the experimental period, the rats were perfused with saline solution (NaCl 0.09%) by the left ventricle and euthanized by overdose of anesthesia (Xylazine 300 mg/kg and Ketamine 40 mg/kg, via intramuscular injection). Cerebellar tissue samples were collected and processed for the techniques. They were fixed in paraformaldehyde (4%) or stored in a freezer at −80°C.
Immunostaining for adenosinergic receptors and inflammatory markers
The cerebellar samples fixed in paraformaldehyde (4%) were embedded Paraplast (Sigma-Aldrich, P3558). Cuts into 5-μm thick were obtained on the microtome (Biocut—Model 1130) and collected on silanized slides. Antigen retrieval was performed by incubating the cuts in citrate buffer (pH 6.0) at 100°C in microwave or treatment with proteinase K, depending on the characteristics of the antibody. Endogenous peroxidase blockade was done with H2O2 (0.3% diluted in methanol) and posterior incubation in BSA solution (3%) diluted in TBS-T buffer during 1 h. The tissue sections were then localized with antibodies for A1 (1:35), A2a (1:35), NFkB (1:100), TLR4 (1:200), TLR2 (1:50) and MyD88 (1:50; references for antibodies described in item 2.6) diluted in BSA 1% and stored overnight at 4°C. The Envision HRP kit (Dako Cytomation Inc., EUA) was used for antigen detection, according to the manufacturer’s instructions. The staining was revealed with diaminobenzidine (DAB), counter-colored with Harris’ Hematoxylin.
Quantification method for immunohistochemistry
The following cell types were considered for counting: Bergmann glia, Purkinje cells, Granular neurons, Golgi cells and White matter cells (in the medullary body of the cerebellum, the cells of the glia were counted indifferently and neurons from the nuclei were not included; Supplemental material).
For Purkinje cells and Bergmann glia counting, 10 random fields, without overlapping, were analyzed by animal, using the 40X objective (Nikon Eclipse E-400 light microscope; Nikon, Tokyo, Japan) and was captured by the NIS-Elements/Image and Image Pro-Plus software. In these photomicrographs, the layer of Purkinje cells and Bergmann glia (surrounding the Purkinje cells) was diagonally photographed in order to capture the greatest possible extension of this layer, as demonstrated in Fig. 3A. The linear extension analyzed per photomicrography for these cell types was ~390 μm per image. For Granule neurons and White Matter cells, an area equivalent to 0.072 mm2, containing only the granular layer or White matter, as shown in Figs 1C, D and2A, B, respectively, was photographed. The Golgi cells were observed in the same photomicrographs analyzed for Granule neurons. The number of reactive cells was measured and the frequency of staining was calculated for each cellular type.
Figure 3.
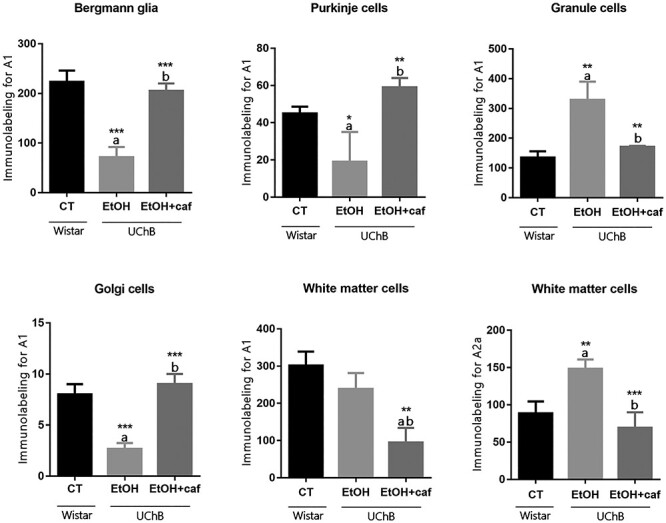
Immunohistochemistry graphs for adenosinergic receptors A1 and A2a for the analyzed cerebellar cell types. Note that the A2a staining was quantitatively measured only for White matter cells. Letters in the top of the column indicate statistical differences: “a” indicates significant statistical difference from the Control group; “b” indicates significant statistical difference from the Ethanol group. *P < 0.05, **P < 0.01 and ***P < 0.001.
Figure 1.
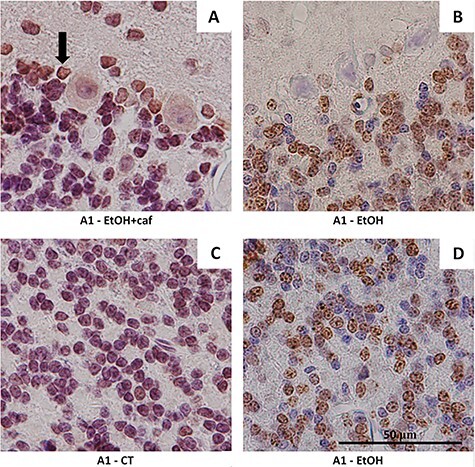
Immunostaining for adenosinergic receptor A1 in cerebellar tissue of the analyzed groups. (A) Evident immunostaining of the antigen in Purkinje cells and Bergmann glia (arrow) in the Ethanol+caffeine group (×100) and low reactivity for the granule neurons (×100). (B) Low reactivity for the Purkinje cells and Bergmann glia in the Ethanol group (×100). (C) Low reactivity for the granule neurons in the Control group (×100). (D) Evident immunostaining for the granule neurons in the Ethanol group (×100).
Figure 2.
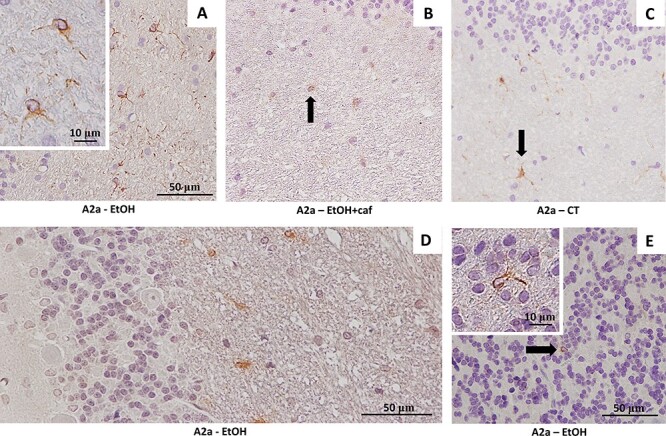
Immunostaining for the A2a adenosinergic receptor in the cerebellar tissue of the analyzed groups. (A) Immunostaining for A2a suggests the presence of immune cells in the cerebellar medullar body of Ethanol group (×40). In detail, the cytoplasmic labeling of the receptor and the form taken by the cell suggest probable microglia in activated state (×100). (B) Cytoplasmic marking in the white matter cell of the Ethanol+caffeine group. Note the less intense positivity (×40). (C) Cytoplasmic marking in the white matter cell of the Control group (×40). (D) Photomicrograph of the molecular, Purkinje and granular cerebellar layers of the Ethanol group, showing immunopositivity restricted to glial cells of the medullary body (×40). (E) Rarely, probable immune cells marked (arrow) were also found in the granular layer (×40). In detail, the A2a-positive immune cell in the cerebellar granular layer of the Ethanol group (×100).
For the NFkB analyses, we also measured the percentage of nuclear staining for this inflammatory marker for the Purkinje cell and Bergmann Glia.
Specifically, TLR2 appeared in a predominant cytoplasmic localization. The immunostaining of this molecule was qualitatively considered, by observing 10 random fields photographed by the 40X objective. The more intense and frequent the DAB staining, the greater the marking was considered.
The immunohistochemistry results were synthetized at the Table 1, which reports the percentages of relative frequency of staining among the groups.
Table 1.
Immunohistochemistry frequency of staining among the groups, according to cerebellar cell types
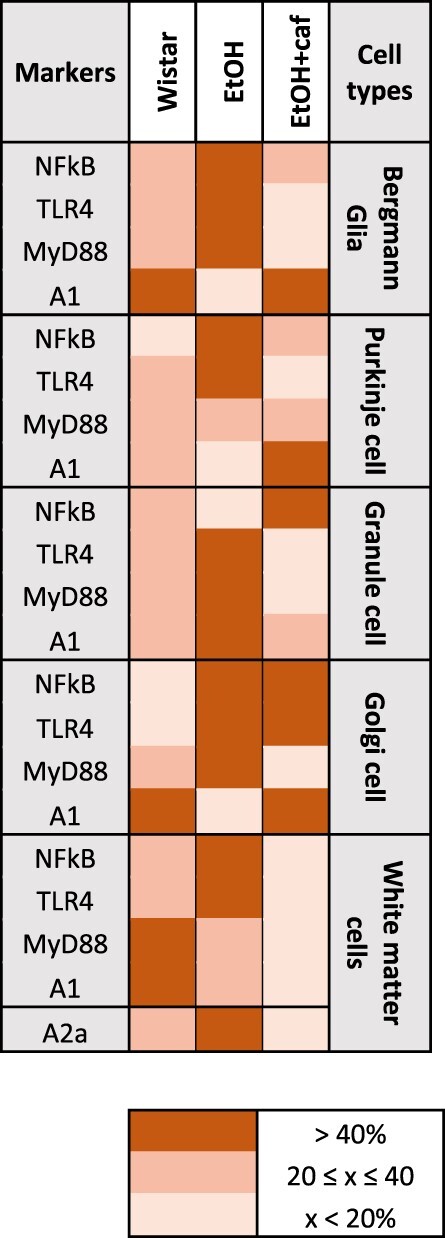
|
In the columns, the analyzed markers are specified for each cerebellar cell type. According to frequency of staining, a color was attributed to the marker and correspondent group, following the legend. In short, the darker the color, the greater the marking.
Gene expressions for inflammatory markers and adenosinergic receptors in the cerebellum
Five cerebellar samples per group were homogenized using Politron®, after added to PBS and Tryzol®. After the RNA extraction, the material was aliquoted and stored at –80°C. The samples were submitted to electrophoresis in agarose gel at 1% for the RNA integrity verification.
For the cDNA synthesis, the reverse transcription was performed using the commercial kit, High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), according to the manufacturer’s instructions. For each 1 μg of RNA was added 2.5 μl of Reverse Trasncriptase Buffer, followed of 1 μl of dNTP’s, 2.5 μl of Random Primers and 1.25 μl of the MultiScribeTM enzyme, completing the volume with DEPC water to 20 μl. The differential expression of genes was quantified NFKB (Assay ID Rn00595794_m1), TLR4 (Assay ID Rn00569848_m1), A1 (Assay ID Rn00567668_m1), A2a (Assay ID Rn00583935_m1), MYD88 (Assay ID Rn01640049_m1) e TLR2 (Assay ID Rn02133647_s1). The β-actin gene was used as endogenous control (Assay ID Rn00667869_m1).
With the cDNA obtained from the samples, an amplification was performed through quantitative polymerase chain reaction (PCR-RT) in real time using TaqMan Master Mix (Applied Biosystems). For the quantitative analysis of the gene expressions were used the commercially available systems TaqMan Assay-on-demand, composed of oligonucleotides and probes (Applied Biosystems). A gadget of PCR detection in real time 7500 Real Time PCR System (Applied Biosystems) was used with the software Sequence Detection System for obtaining the CT values. The reactions of Real-Time PCR were performed in duplicates using TaqMan Master Mix (Applied Biosystems, EUA). The amplification was performed in a final volume of 10 μl, using 5 μl of the specific reagent TaqMan Master Mix, 0.5 μl of each specific probe and 4.5 μl of cDNA diluted in 1:10. The data were exported to Excel spreadsheets to calculate ΔCT. The standard conditions for the amplification were 50°C during 2 min, 95°C during 10 min, followed by 40 cycles of 95°C during 15 s and 60°C during 1 min (simultaneous annealing and extension).
The variation in expression among samples was calculated by the 2-∆∆ Ct method with the mean ∆Ct value for a group of five samples from Control rats being used as a calibrator.
Extraction of proteins and western blotting
Five cerebellar samples of each group were homogenized through Polytron ® during 1 min in 50 μl/mg of RIPA extraction buffer containing 10% (v/v) Triton X-100 and 10 μl/ml of cocktail protease inhibitor (Sigma-Aldrich, St. Louis, Mo., USA). Tissue extracts were obtained by centrifugation for 20 min at 14 000 rpm at 4°C. Aliquot of each sample was used to determine the protein concentration using Bradford’s reagent (Bio-Rad Laboratories, Hercules, CA, USA). Samples were mixed (1:1) with 3X sample buffer (100 mM Tris–HCl pH 6.8, 10% β-mercaptoetanol, 4% SDS and 20% glycerol), incubated in dry bath at 95°C during 5 min. Fifty micrograms of proteins were loaded onto the SDS-polyacrylamide gel. After electrophoresis, the material was electrically transferred (Hoefer System) to nitrocellulose membranes (Amersham) at 120 V during 90 min. Membranes was blocked in BSA 3% diluted in TBS-T during 1 h and then incubated with NFkB (sc-8008, monoclonal anti-mouse, Santa Cruz Biotechnology, 1:350), TLR4 (sc-53 462, monoclonal anti-mouse, Santa Cruz Biotechnology, 1:250), TLR2 (b2-1019R, polyclonal anti-rabbit, Bioss, 1:500), MyD88 (bs-1047R, polyclonal anti-rabbit, Bioss, 1:350), A1 (bs-4235R, polyclonal anti-rabbit, Bioss, 1:500), A2a (sc-32 261, monoclonal anti-mouse, Santa Cruz Biotechnology, 1:200), TNF-α (ab8348, monoclonal anti-mouse, 1:250), COX-2 (sc376861, monoclonal anti-mouse, 1:500), iNOS (ab178945, monoclonal anti-rabbit, 1:500) and Iba-1 (Wako 019–19 741, anti-rabbit, 1:500) antibodies. After washing with TBS-T buffer, the membranes were incubated for 2 h with the conjugated HRP anti-rabbit or anti-mouse secondary antibodies at 1: 2000 dilution in 1% BSA. After a further series of TBS-T washes, 10 ml of chemiluminescent solution (Super Signal West Pico Chemiluminescent/Thermo Scientific/34080) was added and the peroxidase activity was revealed by a luminescent signal from the western blotting bands, which were captured by the G-BOX and chemicamera equipment (Syngene, Cambridge, UK). Antibody for β-actin was used as an endogenous control (sc-47 778, monoclonal anti-mouse, 1:500).
The software UN-SCAN-IT 5.1 Graph Digitizer Software (Silk Scientific, Orem, UT, USA) was used to quantify the protein levels. The total pixel value of each band was calculated by the software, converting graph images to (x, y) data. The obtained values from the chosen markers were divided by the correspondent total pixel value from β-actin bands of the membrane. Data were exported to Excel and processed at GraphPad Prism 7.0 (GraphPad Prism, Inc., San Diego, CA, USA), as described in item 2.7.
Statistical analyses
All data were exported to Excel and transferred to GraphPad Prism 7.0 (GraphPad Prism, Inc., San Diego, CA, USA). All values obtained for each technique were considered parametric within the group analyzed using the Shapiro–Wilk normal distribution test. Statistical significance was calculated by One-way ANOVA, with Tukey post-test (P < 0.05).
Results
Ethanol and caffeine differentially altered the immunolocalization of adenosinergic receptors in the cerebellar tissue
For the A1 receptor, the labeling in the cerebellar layers was more evident than for A2a (Figs 1 and 2). For Bergmann glia, Purkinje cells and Golgi neurons A1 immunostaining decreased in the Ethanol group when compared with Ethanol+caffeine or Control group (Fig. 1A and B). For Granule neurons, tissue response was totally different to that observed in Bergmann glia and Purkinje cells, showing frequent A1 staining in the Ethanol group rather than less reactivity in the Ethanol+caffeine and Control groups (Fig. 1C and D). For White matter cells, diminished A1 staining was identified after the caffeine consumption in the Ethanol+caffeine group. The immunopositivity for this antigen was the same in the Ethanol and Control groups.
For the A2a adenosine receptor, low immunoreactivity was noted in all layers of the cerebellar cortex, in exception to cerebellar White matter cells. Thus, the higher number of positive cells was observed in the Ethanol group and lower immunolabeling was observed for the animals of the Ethanol+caffeine group. Eventually, A2a positive cells were observed in the granular layer (Fig. 2). The graphs of the immunohistochemistry analysis for A1 and A2a are represented at Fig. 3. The percentage of staining among the groups is demonstrated at Table 1.
Ethanol and caffeine differentially altered the immunolocalization of inflammatory mediators in the cerebellar tissue
The NFkB marker showed high frequency of labeled Bergmann glia cells in the Ethanol group. For Purkinje cells, the ethanol consumption led to higher immunostaining for the Ethanol and Ethanol+caffeine groups when compared with the Control group. Increase of nuclear NFkB staining was found in the Ethanol group for Bergmann glia and Purkinje cells (Fig. 4). The granule neurons presented high immunostaining in the Control group and low immunostaining in the Ethanol group. For Golgi neurons, it was observed that ethanol consumption increased immunostaining in the Ethanol and Ethanol+caffeine groups. For the White matter cells, significant decrease in labeling was observed for the Ethanol+caffeine group (Fig. 4).
Figure 4.
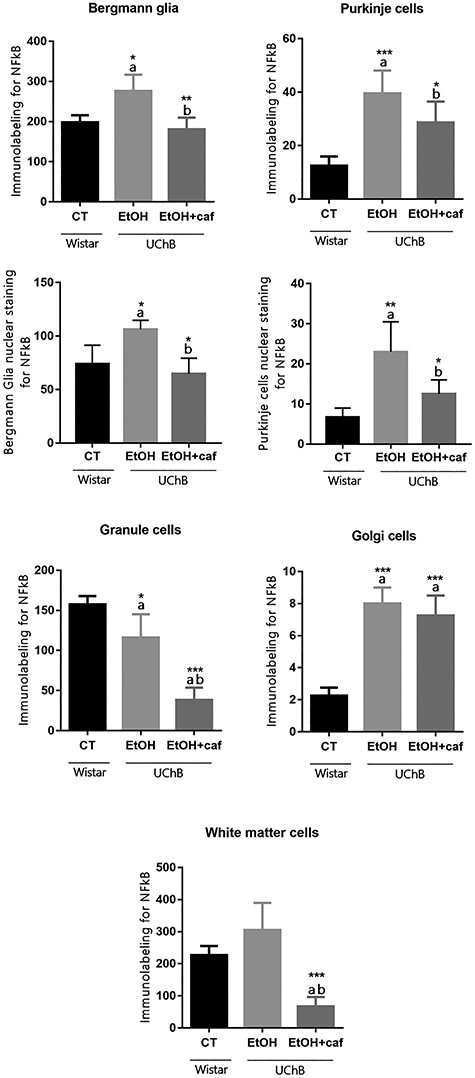
Immunohistochemistry graphs for NFkB for the analyzed cerebellar cell types. Note that specific NFkB nuclear staining considered for Bergmann Glia and Purkinje cells. Letters in the top of the column indicate statistical differences: “a” indicates significant statistical difference from the Control group; “b” indicates significant statistical difference from the Ethanol group. *P < 0.05, **P < 0.01 and ***P < 0.001.
The results showed reduced immunostaining for TLR4 marker in the Ethanol+caffeine group in all cell groups analyzed (Fig. 5). In Bergmann glia, the immunostaining between the Ethanol and the Control group was similar, but in the Ethanol+caffeine group, this molecule had significantly reduced in relation to the other groups. When the Purkinje cells were labeled, the most frequently positivity was identified for the antigen in the Ethanol group when compared with the Control and Ethanol+caffeine groups. The Golgi neurons presented a tissue response similar to Bergmann glia The White matter cells presented high TLR4 immunostaining for the Control group in relation to the other groups (Fig. 5).
Figure 5.
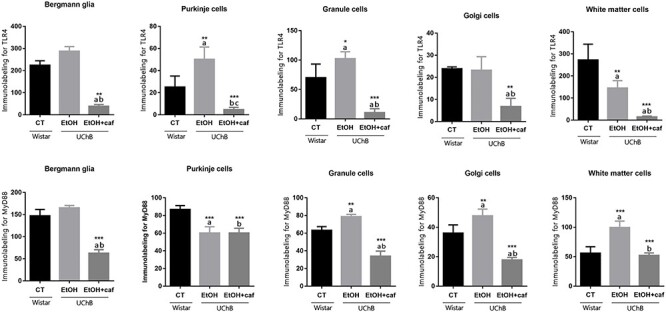
Immunohistochemistry graphs for TLR4 and MyD88 for the analyzed cerebellar cell types. Letters in the top of the column indicate statistical differences: “a” indicates significant statistical difference from the Control group; “b” indicates significant statistical difference from the Ethanol group. *P < 0.05, **P < 0.01 and ***P < 0.001.
The MyD88 marker presented a trend of high expression in the Ethanol and Control groups. For the Bergmann glia, the immunolabeling between the Ethanol and Control groups were statistically equivalent and the Ethanol+caffeine group presented lower frequency for this marker. The tissue response was similar for Granule and Golgi neurons, occurring elevated frequency for MyD88 for the Ethanol group when compared with Control and Ethanol+caffeine group. For the White matter cells, increased staining was identified for the Ethanol group when compared with Ethanol+caffeine and Control group (Fig. 5).
The Fig. 6 shows representative images of immunohistochemistry analysis for NFkB, TLR4 and MyD88. The percentage of staining among the groups is demonstrated at Table 1.
Figure 6.
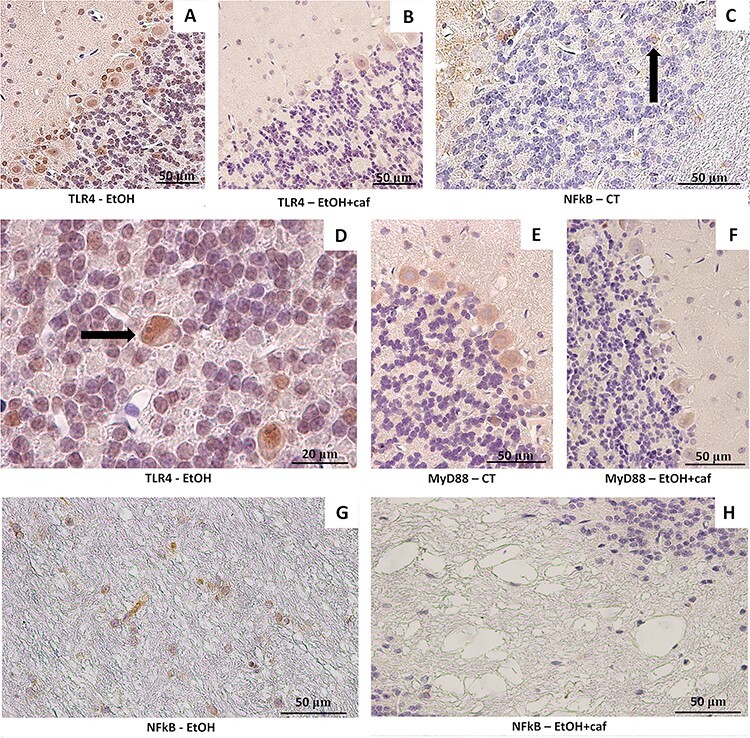
Immunohistochemistry plate for inflammatory markers TLR4, NFkB and MyD88 in the cerebellum of Ethanol, Ethanol+caffeine and Control group. (A) Immunostaining for TLR4 in the Ethanol group. Frequent labeling was noted for Bergmann’s glia and Purkinje cells (×40). (B) Immunostaining for TLR4 in the Ethanol+caffeine. Less frequent labeling was noted for Bergmann glia and Purkinje cells (×40). (C). Immunolabeling for NFkB in the granule neurons in the Control group (×40). (D) Immunostaining for TLR4 in the granule neurons in the Ethanol group. The positive Golgi neuron for this antigen is pointed (arrow). (E) Immunostaining for MyD88 in the cerebellum of Control group. Frequent positivity was observed for Purkinje cells (×40). (F) Immunostaining for MyD88 in the cerebellum of Ethanol+caffeine group (×40). (G) Immunostaining for NFkB in the White matter cells of the Ethanol group. Great immunoreactivity was noted for this antigen (×40). (H) Immunostaining for NFkB in the White matter cells of the Ethanol+caffeine group. Low reactivity was noted for the NFkB antingen (×40).
The TLR2 presented high qualitative immunoreactivity for the Ethanol and Control groups. The Ethanol+caffeine group presented decreased immunoreactivity for this antigen (Fig. 7).
Figure 7.
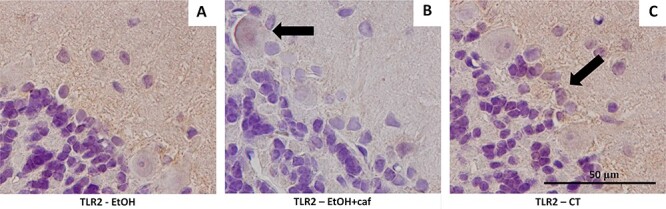
Immunostaining for TLR2 in cerebellar tissue. (A) Immunostaining for molecular, granular and Purkinje layers of the Ethanol group. Moderate and dispersed labeling was noted around Bergmann’s glia and the molecular layer (×100). (B) Immunostaining for Purkinje cell in the Ethanol+caffeine group (arrow). Less marked labeling was noticed when compared with the Ethanol group (×100). T. Immunostaining for Control group was similar to that found in the Ethanol group. It was dispersed around the Bergmann glia (arrow).
Caffeine consumption attenuated adenosinergic and inflammatory signaling by transcriptional and post-transcriptional mechanisms
Ethanol exposure resulted in a significant increase of A1 and A2 gene expression, whereas caffeine was responsible by reduction of both receptors, being similar to the control group (Fig. 8A and C). Unlike gene expression results, caffeine induced a significant increase of A1 protein levels, suggesting a possible participation of this substance in transcriptional and posttranscriptional mechanisms related more specifically to A1 receptor (Fig. 8B). Regarding A2a, the protein levels confirmed the downregulation of this receptor, being similar to the Control group (Fig. 8D).
Figure 8.
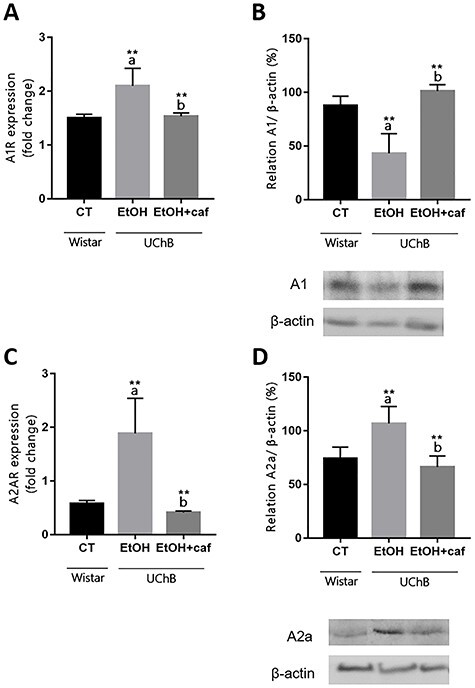
Gene expression and protein level analyses for A1 and A2a markers in the cerebellum. Data are expressed as the mean ± standard deviation. Letters in the top of the column indicate statistical differences: “a” indicates significant statistical difference from the Control group; “b” indicates significant statistical difference from the Ethanol group. *P < 0.05, **P < 0.01 and ***P < 0.001.
Regarding inflammatory markers, both experimental conditions did not change NFkB and TLR4 gene expression; however, western blotting analysis showed a significant increase of both markers resulting from ethanol consumption alone and a reduction when associated with caffeine (Fig. 9A–D). TLR2 gene expression decreased after consumption of ethanol alone or with caffeine, but the protein levels increased because ethanol exposure (Fig. 9E). Interestingly, the increase of TLR2 protein was attenuated in the presence of caffeine (Fig. 9F). Similarly, the presence of caffeine induced a downregulation of MyD88 gene when compared with ethanol alone (Fig. 9G). The level of MyD88 protein also confirmed the downregulation by caffeine presence, whereas ethanol alone did not cause any change (Fig. 9H). Together, these results indicated that caffeine attenuated the activation of TLRs/NFkB pathway caused by ethanol.
Figure 9.
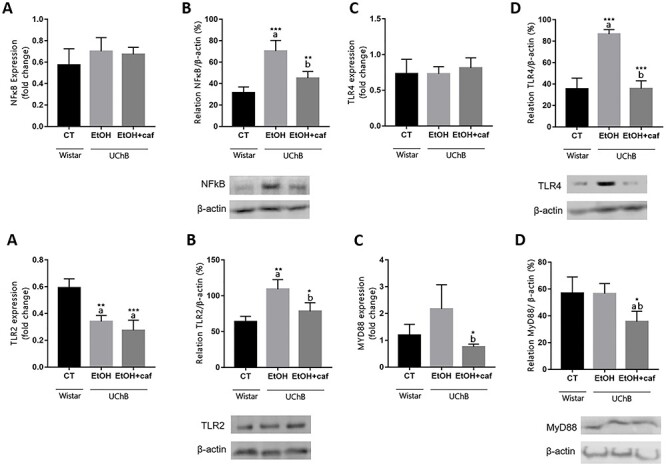
Gene expression and protein level analyses for NFkB, TLR4, TLR2 and MyD88 markers in the cerebellum. Data are expressed as the mean ± standard deviation. Letters in the top of the column indicate statistical differences: “a” indicates significant statistical difference from the Control group; “b” indicates significant statistical difference from the Ethanol group. *P < 0.05, **P < 0.01 and ***P < 0.001.
Caffeine consumption decreased proinflammatory markers and downregulates microglial activation
We observed an increased protein level of TNF-α and iNOS associated with increased levels of Iba-1 in the Ethanol group. Caffeine consumption was able to reduce Iba-1 protein level, a marker directly associated with downregulation of microglial activation with concomitant reduction of TNF-α and iNOS levels, relevant proinflammatory markers in the Ethanol+caffeine group. However, Ethanol or caffeine did not alter COX-2 protein levels (Fig. 10).
Figure 10.
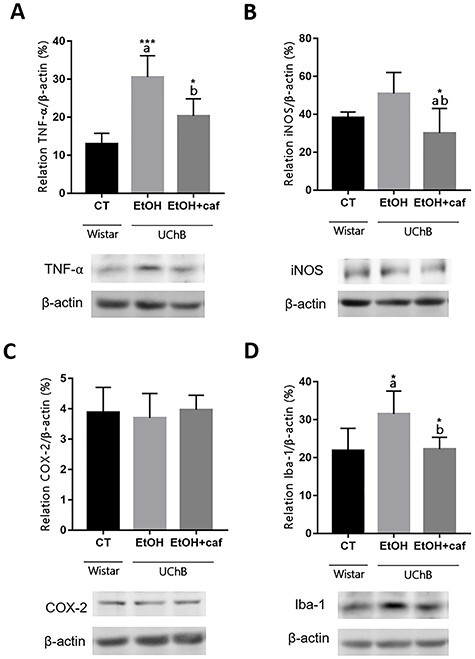
Protein level analyses for TNF-α, iNOS, COX-2 and Iba-1 markers in the cerebellum. Data are expressed as the mean ± standard deviation. Letters in the top of the column indicate statistical differences: “a” indicates significant statistical difference from the Control group; “b” indicates significant statistical difference from the Ethanol group. *P < 0.05, **P < 0.01 and ***P < 0.001.
Discussion
The present study identify that the components of the cerebellar circuitry had differential response in the cerebellar tissue when stimulated by ethanol or ethanol and caffeine in front of the inflammatory markers and the adenosinergic receptors. The NFkB immunolabeling was more often localized in the Bergmann Glia, Purkinje cells and Granule neurons in the Ethanol group. The same tissue response was found for the TLR4, suggesting the hypotheses of an inflammatory process mediated by TLR4-MyD88-NFkB pathway in these cellular types.
Functionally, the Bergmann glia is an integral part of the adult cerebellar circuit, where they play an important role in extracellular ion homeostasis [38], synapse stability [39, 40], plasticity [41], metabolic function and neuroprotection [42]. The cell bodies of Bergmann glia are contained within the Purkinje layer, but their vertically oriented processes extend through the depth of the molecular layer to the pial surface, unsheathing Purkinje cells dendrites and dendritic spines along their paths [43, 44]. In the alcoholism context, it was already reported the impairment in the Bergmann glia front the ethanol exposure, mainly during the cerebellum development [44]. Yang et al. [45] demonstrated a reduction of Bergmann glia ramification after chronic ethanol consumption. The NFkB and TLR4 increase in this cellular type can indicate degeneration process, once the neuroinflammation is one axis of neurodegeneration [46]. Studies indicated that the maintenance of these astrocytes’ morphology is essential for the Purkinje cells surveillance in adult cerebellum [47, 48]. Considering the recurrent presence of NFkB and TLR4 immunolabeling in the Purkinje cells in the Ethanol group, we can suggest a possible relation between the inflammation process established in the Bergmann glia and the Purkinje cells. In this way, the ethanol exposure possibly affects the Purkinje cells directly and indirectly, through the Bergmann Glia impairment.
According to Martinez et al. [26] the inflammatory response leads to neuronal deaths through apoptosis, increasing the Caspase-3 expression after the ethanol consumption in UChB rats. The same authors evaluated the concomitant intake of ethanol and caffeine and verified a diminished Caspase-3 expression, with consequently attenuation of neuronal deaths in the group that ingested caffeine. In the present study, NFkB and TLR4 protein levels were found decreased after simultaneously expose to ethanol and caffeine, when compared with ethanol alone or control group, pointing out the action of caffeine on the neuroinflammation signaling.
In relation to cerebellar granule cells, caffeine administration associated with the ethanol consumption attenuated the NFkB and TLR4 immunolocalization reaching levels lower than the Ethanol and Control groups. Oliveira et al. [28] reported increased Caspase-3 and XIAP expression for granule neurons of UChA rats submitted to ethanol consumption. The action of ethanol around granule neurons is admittedly harmful leading to apoptosis of this cellular type through the activation of pathways as the NFkB [49].
Concerning to the adenosinergic receptors, particularly the immunopositivity for the A1 antigen was observed in the Bergmann glia, Purkinje and Golgi neurons for the Ethanol+caffeine group, when compared with the Ethanol group. The same biological response was found for the Control group, characterizing the tendency to return to normality found in the Ethanol+caffeine group. The granular neuron was the only cell type that had an increase in A1 receptor for the Ethanol group.
The present study demonstrated significant increase in the genic expression and protein levels of A2a for the cerebellum of Ethanol group. Thus, it is inferred that there was induction of greater microglial reactivity for this group due to ethanol consumption, consistent with the apparent increase in neuroinflammation. Specific immunostaining of this antigen was found in glial cells of cerebellar medullary body suggesting its localization in immune cells such as the microglia. The Ethanol+caffeine group showed reduced levels of this receptor, comparable with the Control group. It suggests that caffeine may attenuate the microglial reactivity, suppressing inflammation and promoting neuroprotection [50]. Besides this, microglial morphology in the cerebellum of Ethanol group resembles to reactive microglia phenotypes, being branched or activated. In the Control and Ethanol+caffeine groups, its aspect is apparently amoeboid [51].
It is known that A1 receptor plays a neuroprotective role, once its activation leads to lower glutamate release and consequent neuron hyperpolarization [52]. In fact, under acute ethanol ingestion conditions, A1 activation attenuates brain damage and its blocking seems to exacerbate damage in adult rats [53]. Under chronic toxic condition, such as chronic alcoholism, which leads to increased adenosine release, the desensitization of this receptor occurs due to a long-term potentiation process [54]. Through this process, decreased expression of A1 receptors is found in CNS [54]. In the present study, we observed a significant decrease in the A1 receptor for the Ethanol group probably due to the chronic situation of alcoholism to which these animals were submitted. Concomitantly, a considerable increase in protein expression of inflammatory markers was evidenced for TLR4, NFkB and MyD88. Caffeine administration was able to restore A1 expression in the Ethanol+caffeine group cerebellum.
In contrast, A2a receptors increased in the brain under toxic chronic conditions and their blockade provides effective neuroprotection in adult rats [52]. In the present study, the increase in A2a is also correlated with the increase in inflammatory markers, once observed the immune role of this receptor. Caffeine was able to decrease A2a expression, contributing to neuroprotection. Thus, it is inferred that adenosinergic receptors are associated with the activation of the inflammatory process in the cerebellum of UChB animals and that caffeine administration was able to articulate the attenuation of the cerebellum inflammation process through modulation of A1 and A2a in the Ethanol+caffeine group.
The chronic ethanol exposure leads to the neuroinflammation establishment process [55, 56] and it commonly involves microglia activation through the TLR4-MyD88-NFkB pathway [57, 58]. In the cerebellum of UChB rats, the exposure to ethanol alone induced an inflammatory microenvironment, which was confirmed by TLR4, NFkB and MyD88 protein levels increase. According to Flores-Bastías and Karahanian [59] this process seems to be initiated at the molecular level, with the overexpression of inflammatory genes. However, molecular alterations were not identified for TLR4 and NFKB in any of the other groups. In fact, there is a strong hypothesis of ethanol-induced tolerance in some molecular aspects when the UChB is used as experimental model [60] and it was indicated by levels maintenance of the gene expression between the alcoholic groups and the control group, composed by Wistar rats. Similarly, caffeine also did not alter the gene expression of these inflammatory markers.
Considering that, previous studies of our research group have already reported the capability of the ethanol and caffeine in altering miRNAs levels in the cerebellum and blood of the UChB model, suggesting an explanation to the observed changes in the immunohistochemical and protein levels results for the NFkB and TLR4 markers among the groups. Rossetto et al. [12] showed an elevated expression of the miR-155-5p in the cerebellum after the ethanol intake, one of the proinflammatory miRNAs linked to microglial activation [61]. The same authors reported that caffeine ingestion mitigated the level of this miRNA, suggesting a recovery of the inflammatory process previously established in the group that only consumed ethanol [12]. The miRNA-155 expression is stimulated by binders of receptors as the TLRs and by cytokines as the interferon-γ (IFN-γ) [62, 63].
Taken together, our results highlighted an inflammatory microenvironment in the Ethanol group with the augment of NFkB, TLR4, TLR2 and MyD88 in molecular or protein levels. According to Baoning et al. [64] the ethanol consumption elevates the levels of NFkB through the TLR2/MyD88 activation. The TLR2 receptor has an extracellular domain, a trans-membrane region and an intracellular domain. In brief, the extracellular domain recognizes a variety of antigens. The intracellular domain, with the help of other intracellular proteins and adaptors, such as MyD88, signals the activation of transcription factor NF-κB and ultimately produces inflammatory cytokines that are responsible for mounting a more effective innate response and initiating adaptive immunity [65, 66]. The intracellular domain is usually referred as toll/IL-1R (TIR) domains [67]. As an essential adaptor for the TLR4 and TLR2 intracellular domains and activation [68], the increase of MyD88 in the Ethanol group suggests major recruitment of this signaling due to more activation of the TLR4-MyD88-NFkB pathway.
Interestingly, the Ethanol+caffeine group had the most part of the inflammatory markers reduced to levels comparable to the Control group. Concomitantly, the ethanol isolated or added of caffeine altered the gene expression of A1 and A2a in the analyzed groups.
Caffeine immunomodulator action is not well understood [69] but it is established that caffeine acts through the ligation to these purine analogues, the adenosinergic receptors [70, 71]. The A1 receptor is the most abundant in the encephalon and, after binding the A1 receptor, the adenosine induces the adenililciclase decrease leading to consequent AMPc decrease and cellular hyperpolarization through K+ efflux and inhibition of circulating calcium. Thus, the actions arising from A1 activation are summarily inhibitory [72].
According to Fang et al. [30], the ethanol consumption presents effects around the A1 receptor, once it increases the adenosine endogenous levels [73]. After acute ethanol intake, an increased expression of A1 receptor is observed and a consequently diminution in the cAMP occurs [74]. However, in the chronic consumption, the decrease of adenylyl cyclase and cAMP occurs by desensitization of A1 receptors [75]. In the present study, the gene expression of A1 increased in the Ethanol group. In contrast, the A1 protein level decreased for the same group, indicating a decrease of the adenosinergic tone for the A1 receptor after the ethanol consumption. According to Kovacs and Toldy [75], the A1 diminution is expected after de chronic ethanol consumption. Based on that, it could suggest the increase in A1 gene expression, found in the Ethanol group, as an attempt to answer the low protein levels. In this way, the ethanol can possibly be exerting a post-transcriptional action towards the A1 gene transcription. Interestingly, the presence of caffeine led to recovery of A1 receptor levels in the cerebellum, despite the ethanol consumption, inhibiting this observed action of the ethanol. It is reported that the chronic consumption of caffeine increases the levels of A1 in the CNS [76].
Beyond the A1 receptor, caffeine also presents high affinity to bind the A2a receptors which expression is mainly circumscribed to striatum, olfactory tubercle and accumbens nuclei [10]. However, the A2a is recognized as the receptor that links the inflammatory process to the adenosinergic system because it can be found in the surface of immune cells evidencing its role in the immune response [10]. The A2a receptor activation plays an excitatory role and increases the adenylylcyclase and cAMP concentration. Once caffeine binds the A2a receptor, it activates the adenylylcyclase and converts the ATP in cAMP. This condition induces to intracellular cascade mediated by increased cAMP via phosphodiesterase inhibition. Thus, the extracellular caffeine binding amplifies cAMP actions in intracellular compartment, inhibiting the phosphodiesterase. High concentrations of cAMP activates the PKA (protein kinase A), which in change, blocks the release of proinflammatory citokynes [77]. In the CNS, the A2a activation exacerbates the inflammatory response, mostly under chronic conditions [78]. The mechanisms by which blockade of A2a confers neuroprotection remain unclear; however, one of the central hypotheses is based in the control of microglia-mediated neuroinflammation [58, 79]. Studies have suggested that A2a receptor activation results in reactive microglia phenotypes, leading to the increase of neurotoxic factors and non-cellular elements culminating in the establishment of neuroinflammation [79, 80].
The present study showed caffeine consumption attenuating Iba-1 protein levels in the Ethanol+caffeine group. In activated microglia, the Ionized binding protein 1 (Iba-1), participates in actin-bundling, membrane ruffling, cell migration and phagocytosis [81, 82, 83]. The upregulation of Iba-1 is associated with microglia activated phenotype [80]. When presented with a teratogen such as alcohol, microglia release cytokines that can promote inflammation and ultimately lead to neurodegeneration [84]. In association with increased Iba-1 for the Ethanol group, upregulation of TNF-α and iNOS protein levels was found for the same group. High TNF-α is implicated in the induction of other pro-inflammatory cytokines that play an important role in neuroinflammation and neurodegenerative disorders [85].
Conclusion
The present study concluded that the ethanol consumption interferes in the inflammatory process and adenosinergic signaling of the cerebellum, increasing inflammatory mediators NFkB, TLR4, MyD88, TLR2 and proinflammatory markers such as TNF-α and iNOS. Caffeine added to the ethanol solution evidently contributed to the reestablishment of cerebellar tissue, considering the response of the markers presented by the Control group as standard parameter for normality, interfering in the inflammatory markers through the modulation of the adenosinergic receptors A1 and A2a, also reducing microglial activation state.
Important differential sensitivity of the cellular components in the cerebellum was detected, referring to the reactivity of the cells facing the organ impairment when it is submitted to the chronic consumption of ethanol and, subsequently, treated with caffeine.
Future studies focusing on microglial modifications related to the ethanol and caffeine consumption and its relation with the adenosinergic receptors A1 and A2a can perform a valuable perspective for the understanding of the cerebellar microenvironment under the chronic use of these substances and its toxicological conditions.
Supplementary Material
Acknowledgments
We would like to thank the São Paulo Research Foundation, FAPESP (2016/07410-8), and National Council for Scientific and Technological Development (CNPq) for supporting this research.
Contributor Information
Isabela Maria Urra Rossetto, Department of Structural and Functional Biology, University of Campinas (UNICAMP), 255 Monteiro Lobato St, Campinas, SP 13083-862, Brazil.
Valéria Helena Alves Cagnon, Department of Structural and Functional Biology, University of Campinas (UNICAMP), 255 Monteiro Lobato St, Campinas, SP 13083-862, Brazil.
Larissa Akemi Kido, Department of Food and Nutrition, University of Campinas (UNICAMP), 80 Monteiro Lobato St, Campinas, SP 13083-862, Brazil.
Fermino Sanches Lizarte Neto, Department of Surgery and Anatomy, University of São Paulo (USP), 3900 Bandeirantes Ave, Ribeirão Preto, SP 14049-900, Brazil.
Luís Fernando Tirapelli, Department of Surgery and Anatomy, University of São Paulo (USP), 3900 Bandeirantes Ave, Ribeirão Preto, SP 14049-900, Brazil.
Daniela Pretti da Cunha Tirapelli, Department of Surgery and Anatomy, University of São Paulo (USP), 3900 Bandeirantes Ave, Ribeirão Preto, SP 14049-900, Brazil.
Luiz Gustavo de Almeida Chuffa, Department of Structural and Functional Biology, State University of São Paulo (UNESP), 250 Prof. Dr. Antônio Celso Wagner Zanin St, Botucatu, SP 18618-689, Brazil.
Francisco Eduardo Martinez, Department of Structural and Functional Biology, State University of São Paulo (UNESP), 250 Prof. Dr. Antônio Celso Wagner Zanin St, Botucatu, SP 18618-689, Brazil.
Marcelo Martinez, Department of Morphology and Pathology, Federal University of São Carlos (UFSCar), 13571 Biblioteca Comunitária Ave, São Carlos, SP 13565-905, Brazil.
Funding
The São Paulo Research Foundation, FAPESP (process 2016/07410-8) and National Council for Scientific and Technological Development (CNPq) supported the research presented herein.
Conflict of Interest Statement
The authors declare that there was no conflict of interest.
Ethics Approval
The experimental protocol followed the ethical principles according to National Council for the Control of Animal Experimentation (Brazil) and Committee on Ethics in the Use of Animals (CEUA) from UNICAMP (protocol 4651-1/2017) and UFSCar (protocol 9 895 280 815) approved it.
Availability of Data and Material
All the authors assure that data reported here are accurate and comes from original research.
Authors’ Contributions
M. Martinez and I.M.U. Rossetto contributed to the study conception and design; L.A. Kido, F.S.N. Lizarte, L.F. Tirapelli, D.P.C. Tirapelli, L.G.A. Chuffa, F.E. Martinez and I.M.U. Rossetto performed material preparation, data collection and analysis; I.M.U. Rossetto wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. M. Martinez and V.H.A. Cagnon provided funding acquisition and resources. All the authors had relevant contributions in the research and agreed in sending this paper to Toxicology Research review.
References
- [1].Brice C, Smith A. The effects of caffeine on simulated driving, subjective alertness and sustained attention. Hum Psychopharmacol 2002;16:523–31. 10.1002/hup.327. [DOI] [PubMed] [Google Scholar]
- [2]. Fiani B, Zhu L, Musch B L et al. The neurophysiology of caffeine as a central nervous system stimulant and the resultant effects on cognitive function. Cureus 2021;13:e15032. doi: 10.7759/cureus.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peleli M, Fredholm BB, Sobrevia L et al. Pharmacological targeting of adenosine receptor signaling. Mol Aspects Med 2017;55:4–8. 10.1016/j.mam.2016.12.002. May 26, 2020, doi 10.1101/2020.05.26.114769 [DOI] [PubMed] [Google Scholar]
- [4].Franceschini S, Lulli M, Bertoni S et al. Caffeine improves text reading and global perception. J Psychopharmacol 2020;34:315–25. 10.1177/0269881119878178. [DOI] [PubMed] [Google Scholar]
- [5].Fredholm BB. Adenosine--a physiological or pathophysiological agent? J Mol Med (Berl) 2014;92:201–6. 10.1007/s00109-013-1101-6. [DOI] [PubMed] [Google Scholar]
- [6].Goodman RR, Snyder SH. Autoradiographic localization of adenosine receptors in rat brain using [3H] cyclohexyladenosine. J Neurosci 1982;2:1230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jarvis MF, Jackson RH, Williams M. Autoradiographic localization of high affinity adenosine A2 receptor in rat brain. Brain Res 1989;484:111–1. [DOI] [PubMed] [Google Scholar]
- [8].Saeed Dar M. Mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination: possible involvement of cAMP. Brain Res 1997;749:263–74. 10.1016/s0006-8993(96)01263-2. [DOI] [PubMed] [Google Scholar]
- [9].Saeed Dar M. Modulation of ethanol-induced motor incoordination by mouse striatal A(1) adenosinergic receptor. Brain Res Bull 2001;55:513–20. 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- [10].Moreau JL, Huber G. Central adenosine A2A receptors: an overview. Brain Res Rev 1999;31:65–82. [DOI] [PubMed] [Google Scholar]
- [11].Iris M, Tsou P-S, Sawalha AH. Caffeine inhibits STAT1 signaling and downregulates inflammatory pathways involved in autoimmunity. Clin Immunol 2018;192:68–77. 10.1016/j.clim.2018.04.008. [DOI] [PubMed] [Google Scholar]
- [12].Rossetto IMU, Cagnon VHA, Lizarte FSN et al. Ethanol and caffeine consumption modulates the expression of miRNAs in the cerebellum and plasma of UChB rats. Life Sci 2019;229:180–6. 10.1016/j.lfs.2019.05.016. [DOI] [PubMed] [Google Scholar]
- [13].Di Martino E, Bocchetta E, Tsuji S et al. Defining a time window for neuroprotection and glia modulation by caffeine after neonatal hypoxia-ischaemia. Mol Neurobiol 2020;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brothers HM, Marchalant Y, Wenk GL. Caffeine attenuates lipopolysaccharide-induced neuroinflammation. Neurosci Lett 2010;480:97–100. 10.1016/j.neulet.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].A Oñatibia-Astibia, R. Franco, E. Martínez-Pinilla. Health benefits of methylxanthines in neurodegenerative diseases. Mol Nutr Food Res 2017, 61, 6. doi: 10.1002/mnfr.201600670. [DOI] [PubMed] [Google Scholar]
- [16].World health Organization (WHO) . Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- [17].Crews FT, Lawrimore CJ, Walter TJ et al. The role of neuroimmune signaling in alcoholism. Neuropharmacology 2017;122:56–73. 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang D, Xiong W, Jackson MF et al. Ethanol tolerance affects endogenous adenosine signaling in mouse hippocampus. J Pharmacol Exp Ther 2016;358:31–8. 10.1124/jpet.116.232231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vetreno RP, Lawrimore CJ, Rowsey PJ et al. Persistent adult neuroimmune activation and loss of hippocampal neurogenesis following adolescent ethanol exposure: blockade by exercise and the anti-inflammatory drug indomethacin. Front Neurosci 2018;12:200. 10.3389/fnins.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nkpaa KW, Owoeye O, Amadi BA et al. Ethanol exacerbates manganese-induced oxidative/nitrosative stress, pro-inflammatory cytokines, nuclear factor-κB activation, and apoptosis induction in rat cerebellar cortex. J Biochem Mol Toxicol 2020;35:e22681. 10.1002/jbt.22681. [DOI] [PubMed] [Google Scholar]
- [21].Ahearn OC, Watson MN, Rawls SM. Chemokines, cytokines and substance use disorders. Drug Alcohol Depend 2021;220:108511. 10.1016/j.drugalcdep.2021.108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luo J. Effects of ethanol on the cerebellum: advances and prospects. Cerebellum 2015;14:383–5. 10.1007/s12311-015-0674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stowell RD, Majewska AK. Acute ethanol exposure rapidly alters cerebellar and cortical microglial physiology. Eur J Neurosci 2020;00:1–10. 10.1111/ejn.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ghimire SR, Saxena AK, Rai D et al. Effect of maternal alcohol consumption on cerebellum of rat pups: a histological study. Nepal Med Coll J 2009;4:268–71. [PubMed] [Google Scholar]
- [25].Bagheri F, Goudarzi I, Lashkarbolouki T et al. Melatonin prevents oxidative damage induced by maternal ethanol administration and reduces homocysteine in the cerebellum of rat pups. Behav Brain Res 2015;287:215–25. 10.1016/j.bbr.2015.03.022. [DOI] [PubMed] [Google Scholar]
- [26].Martinez M, Rossetto IMU, Neto FSL et al. Interactions of ethanol and caffeine on apoptosis in the rat cerebellum (voluntary ethanol consumers). Cell Biol Int 2018;42:1575–83. 10.1002/cbin.11054. [DOI] [PubMed] [Google Scholar]
- [27].Dlugos CA. Ethanol-induced alterations in Purkinje neuron dendrites in adult and aging rats: a review. Cerebellum 2015;14:466–73. 10.1007/s12311-014-0636-6. [DOI] [PubMed] [Google Scholar]
- [28].Oliveira SA, Chuffa LGA, Fioruci-Fontanelli BA et al. Apoptosis of Purkinje and Granular cells of the cerebellum following chronic ethanol intake. Cerebellum 2014;13:728–38. 10.1007/s12311-014-0591-2. [DOI] [PubMed] [Google Scholar]
- [29].Martinez M, Sauce R, Oliveira SA et al. Ethanol intake-induced apoptosis in glial cells and axonal disorders in the cerebellar white matter of UChA rats (voluntary ethanol consumers). Tissue Cell 2015;47:389–94. 10.1016/j.tice.2015.05.006. [DOI] [PubMed] [Google Scholar]
- [30].Fang T, Dong H, Xu X-H et al. Adenosine A2A receptor mediates hypnotic effects of ethanol in mice. Sci Rep 2017;7:12678. 10.1038/s41598-017-12689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scalese M, Denoth F, Siciliano V et al. Energy Drink and Alcohol mixed Energy Drink use among high school adolescents: association with risk taking behavior, social characteristics. Addict Behav 2017;72:93–9. 10.1016/j.addbeh.2017.03.016. [DOI] [PubMed] [Google Scholar]
- [32].Benson S, Tiplady B, Scholey A. Attentional and working memory performance following alcohol and energy drink: a randomised, double-blind, placebo-controlled, factorial design laboratory study. PLoS One 2019;14:e0209239. 10.1371/journal.pone.0209239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holstein SE, Barkell GA, Young MR. Caffeine increases alcohol self-administration, an effect that is independent of dopamine D2 receptor function. Alcohol 2021;8:S0741-8329(20)30316-5. 10.1016/j.alcohol.2020.12.004. [DOI] [PubMed] [Google Scholar]
- [34].Mardones J, Segovia N, Hederra A. Heredity of experimental alcohol preference in rats. II. Coefficient of heredity. Q J Stud Alcohol 1953;1:14,1–2. [PubMed] [Google Scholar]
- [35].Bell RL, Hauser SR, Liang T et al. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 2017;122, 201–243. 10.1016/j.neuropharm.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol 1983;5:171–8. [PubMed] [Google Scholar]
- [37].Marcelo Martinez, Isabela M. U. Rossetto, Renata M. S. Arantes, et al. Serum miRNAs are differentially altered by ethanol and caffeine consumption in rats. Toxicol Res 2019;8(6):842–849. doi: 10.1039/c9tx00069k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang F, Xu Q, Wang W et al. Bergmann glia modulate Purkinje cell bistability via Ca2+−dependent K+ uptake. Proc Natl Acad Sci U S A 2012;109:7911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Iino M, Goto K, Kakegawa W et al. Glia-synapse interaction through Ca2+−permeable AMPA receptors in Bergmann glia. Science 2001;292:926–9. [DOI] [PubMed] [Google Scholar]
- [40].Saab AS, Neumeyer A, Jahn HM et al. Bergmann glial AMPA receptors are required for fine motor coordination. Science 2012;337:749–53. [DOI] [PubMed] [Google Scholar]
- [41].Balakrishnan S, Jackson C, Russell N et al. Ectopic release sites lack fast vesicle recycling mechanisms, causing long-term depression of neuron-glial transmission in rat cerebellum. Glia 2011;59:82–93. [DOI] [PubMed] [Google Scholar]
- [42].Poblete-Naredo I, Guillem AM, Juárez C et al. Brain-derived neurotrophic factor and its receptors in Bergmann glia cells. Neurochem Int 2011;8:1133–44. 10.1016/j.neuint.2011.10.002. [DOI] [PubMed] [Google Scholar]
- [43].Palay S, Chan-Palay V. Cerebellar Cortex. Berlin, Heidelberg, New York: Springer-Verlag, 1974. [Google Scholar]
- [44].Dlugos CA, Pentney RJ. Quantitative immunocytochemistry of glia in the cerebellar cortex of old ethanol-fed rats. Alcohol 2001;63:63–9. 10.1016/s0741-8329(00)00143-9. [DOI] [PubMed] [Google Scholar]
- [45].Yang Y, Tang Y, Xing Y et al. Activation of Liver X receptor is protective against ethanol-induced developmental impairment of Bergmann Glia and Purkinje neurons in the mouse cerebellum. Mol Neurobiol 2014;49:176–86. [DOI] [PubMed] [Google Scholar]
- [46].Heimfarth L, Loureiro SO, Dutra MF et al. Disrupted cytoskeletal homeostasis, astrogliosis and apoptotic cell death in the cerebellum of preweaning rats injected with diphenyl ditelluride. Neurotoxicology 2013;34:175–88. 10.1016/j.neuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- [47].Cui W, Allen ND, Skynner M et al. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia 2001;34:272–82. [DOI] [PubMed] [Google Scholar]
- [48].Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int 2002;77:94–108. [DOI] [PubMed] [Google Scholar]
- [49].Luo J. Mechanisms of ethanol-induced death of cerebellar granule cells. Cerebellum 2012;1:145–54. 10.1007/s12311-010-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Madeira MH, Boia R, Ambrósio AF et al. Having a Coffee Break: the impact of caffeine consumption on microglia-mediated inflammation in neurodegenerative diseases. Mediators Inflamm 2017;2017:4761081. 10.1155/2017/4761081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cai Z, Hussain MD, Yan L-J. Microglia, neuroinflammation, and Aβ in Alzheimer's disease. Int J Neurosci 2014;124:307–24. 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- [52].Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signal 2005;1:111–34. 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ade M, Sebastião AM, Ribeir JA. Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev 2000;2-3:258–74. [DOI] [PubMed] [Google Scholar]
- [54].Wetherington JP, Lambert NA. Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. J Neurosci 2002;22:1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Crews FT. Cytokines and alcohol. Alcohol Clin Exp Res 2006;30:720–30. 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- [56].Melbourne JK, Thompson KR, Peng H et al. Its complicated: the relationship between alcohol and microglia in the search for novel pharmacotherapeutic targets for alcohol use disorders. Prog Mol Biol Transl Sci 2019;167:179–221. 10.1016/bs.pmbts.2019.06.011. [DOI] [PubMed] [Google Scholar]
- [57].Li Q, Liu D, Pan F et al. Ethanol exposure induces microglia activation and neuroinflammation through TLR4 activation and SENP6 modulation in the adolescent rat hippocampus. Neural Plast 2019;12:1648736. 10.1155/2019/1648736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ibáñez F, Montesinos J, Ureña-Peralta JR et al. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation 2019;16:136. 10.1186/s12974-019-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Flores-Bastías O, Karahanian E. Neuroinflammation produced by heavy alcohol intake is due to loops of interactions between Toll-like 4 and TNF receptors, peroxisome proliferator-activated receptors and the central melanocortin system: a novel hypothesis and new therapeutic avenues. Neuropharmacology 2018;128:401–7. 10.1016/j.neuropharm.2017.11.003. [DOI] [PubMed] [Google Scholar]
- [60].Quintanilla ME, Israel Y, Sapag A et al. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol 2006;11:310–23. [DOI] [PubMed] [Google Scholar]
- [61].Sun X-H, Song M-F, Song H-D et al. miR-155 mediates inflammatory injury of hippocampal neuronal cells via the activation of microglia. Mol Med Rep 2019;4:2627–35. 10.3892/mmr.2019.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gaudet AD, Fonken LK, Watkins LR et al. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 2018;24:221–45. 10.1177/1073858417721150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lippai D, Bala S, Csak T et al. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS One 2013;9:e70945. 10.1371/journal.pone.0070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Qi B, Shi C, Meng J et al. Resveratrol alleviates ethanol-induced neuroinflammation in vivo and in vitro: involvement of TLR2-MyD88-NF-κB pathway. Int J Biochem Cell Biol 2018;103:56–64. 10.1016/j.biocel.2018.07.007. [DOI] [PubMed] [Google Scholar]
- [65].Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Rev Biochem Biophys Res Commun 2009;388:621–5. 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- [66].Chen E, Bakr MM, Firth N et al. Inflammatory cell expression of Toll-like receptor-2 (TLR2) within refractory periapical granuloma. F1000Res 2018;7:1819. 10.12688/f1000research.16678.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003;21:335–76. 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [68].O'Neill LAJ, Dunne A, Edjeback M et al. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J Endotoxin Res 2003;1:55–9. 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- [69].Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology 2018;12:818–25. 10.1016/j.imbio.2018.08.011. [DOI] [PubMed] [Google Scholar]
- [70].Judelson DA, Preston AG, Debra L. Miller, et al. Lieberman. Effects of theobromine and caffeine on mood and vigilance. J Clin Psychopharmacol 2013;4:499–506. 10.1097/JCP.0b013e3182905d24. [DOI] [PubMed] [Google Scholar]
- [71].Iglesias I, Albasanz JL, Martín M. Effect of caffeine chronically consumed during pregnancy on Adenosine A1 and A2A receptors signaling in both maternal and fetal heart from Wistar rats. J Caffeine Res 2014;4:115–26. 10.1089/jcr.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kanyshkova T, Pawlowski M, Meuth P et al. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci 2009;27:8847–57. 10.1523/JNEUROSCI.0689-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hack SP, Christie MJ. Adaptations in adenosine signaling in drug dependence: therapeutic implications. Crit Rev Neurobiol 2003;15:235–74. doi: 10.1615/critrevneurobiol.v15.i34.30. [DOI] [PubMed] [Google Scholar]
- [74].Elmenhorst E-M, Elmenhorst D, Benderoth S et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A 2018;115:8009–14. 10.1073/pnas.1803770115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kovács GL, Toldy E. Basal and isoproterenol-stimulated cyclic-adenosine monophosphate levels in mouse hippocampus and lymphocytes during alcohol tolerance and withdrawal. Alcohol Alcohol 2003;38:11–7. 10.1093/alcalc/agg018. [DOI] [PubMed] [Google Scholar]
- [76].Jacobson KA, von Lubitz DK, Daly JW et al. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci 1996;17:108–13. 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001;414:20–7. 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- [78].Rodrigo A. Cunha, Iang-Fan Chen, Michail V. Sitkovsky. Opposite modulation of peripheral inflammation and neuroinflammation by Adenosine A2A Receptors. In: Malva JO, Rego AC, Cunha RA, et al. (eds) Interaction Between Neurons and Glia in Aging and Disease. Springer, Boston, MA. 2007. doi: 10.1007/978-0-387-70830-0_3. [DOI] [Google Scholar]
- [79].Santiago AR, Baptista FI, Santos Paulo F.et al. Role of microglia adenosine A2A receptors in retinal and brain neurodegenerative diseases. Mediators Inflamm 2014. 10.1155/2014/465694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lowe PP, Morel C, Ambade A et al. Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. J Neuroinflammation 2020;17:296. 10.1186/s12974-020-01972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ito M. The cerebellum: from structure to control. Trends Cogn Sci 1998;2:371. 10.1016/s1364-6613(98)01217-0. [DOI] [PubMed] [Google Scholar]
- [82].Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol 2006;78:272–303. 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- [83].Ohsawa K, Imai Y, Sasaki Y et al. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem 2004;88:844–56. 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- [84].Topper LA, Baculis BC, Valenzuela CF. Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation 2015;12:160. 10.1186/s12974-015-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Frankola KA, Greig NH, Luo W et al. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets 2011;10:391–403. 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the authors assure that data reported here are accurate and comes from original research.


