Abstract
Chronic rhinosinusitis (CRS) affects nearly all individuals with cystic fibrosis (CF) and is thought to serve as a reservoir for microbiota that subsequently colonize the lung. To better understand the microbial ecology of CRS, we generated a 16S rRNA gene sequencing profile of sinus mucus from CF-CRS patients. We show that CF-CRS sinuses harbor bacterial diversity not entirely captured by clinical culture. Culture data consistently identified the dominant organism in most patients, though lower abundance bacteria were not always identified. We also demonstrate that bacterial communities dominated by Staphylococcus spp. were significantly more diverse compared to those dominated by Pseudomonas spp. Diversity was not significantly associated with clinical factors or patient age, however, younger subjects yielded a much wider range of bacterial diversity. These data mirror bacterial community dynamics in the lung and provide additional insight into the role of sinus microbiota in chronic airway disease progression.
Keywords: chronic sinusitis, microbiome, microbial ecology, Staphylococcus aureus, Pseudomonas aeruginosa
1. INTRODUCTION
Chronic rhinosinusitis (CRS) reaches a strikingly high prevalence among CF adults (>99%) [1]. While there are several proposed causes of CF-associated CRS (CF-CRS), including abnormal sinus anatomy and aberrant immune responses [2], bacterial infection remains widely implicated [3, 4]. The “unified airway” model, whereby microbiota adapt to the upper airways prior to migration to the lungs has become a major impetus for studying CF-CRS microbiology [5, 6].
16S rRNA gene sequencing has significantly improved our understanding of CF microbial ecology. Cross-sectional and longitudinal studies of sputum have established that bacterial diversity decreases into adulthood, concomitant with lung function decline [7,8]. Fewer studies have investigated these dynamics in the upper airways, however, CF sinuses harbor significantly lower bacterial diversity compared to non-CF-CRS and non-CRS patients [9]. Clinical culture has shown that Staphylococcus aureus and Pseudomonas aeruginosa are both prevalent and abundant in CF-CRS [10], while their genotypes show within-individual concordance between upper and lower airways [11]. This relationship is particularly striking in lung transplant recipients [12-15], supporting the notion that sinuses serve as a reservoir for CF lung microbiota.
To better understand microbial community ecology in CF-CRS and its role in CF disease progression, we performed 16S rRNA gene sequencing on sinus mucus derived from patients undergoing functional endoscopic sinus surgery (FESS). We explored bacterial diversity with respect to dominance of canonical CF pathogens, patient demographics, and clinical parameters. We discuss these data in the context of interspecies interactions as potential determinants of CF pathogenesis.
2. MATERIALS AND METHODS
The UMN Institutional Review Board approved this study (#1403M49021). Informed consent was obtained from CF adults (n=25) undergoing FESS for treatment of CRS. Sinus secretions were obtained by suction from a single maxillary sinus under endoscopic visualization. Demographic data, clinical history, and co-morbidities were also collected (Table S1). Genomic DNA was extracted from 300 μL of mucus using DNeasy Powersoil kits (Qiagen, Carlsbad, CA) and submitted to the UMN Genomics Center for 16S rRNA gene library preparation. The V4 region was amplified and sequenced using Illumina MiSeq TruSeq 2x300 paired-end technology. Samples and reagent controls were sequenced across four independent runs. Sequence analyses were performed in R (v.4.0.0). Detailed methods are provided in supplementary material. Data are available at https://github.com/hunterlabumn/CF-CRS-Microbiome and NCBI BioProject PRJNA374847.
3. RESULTS
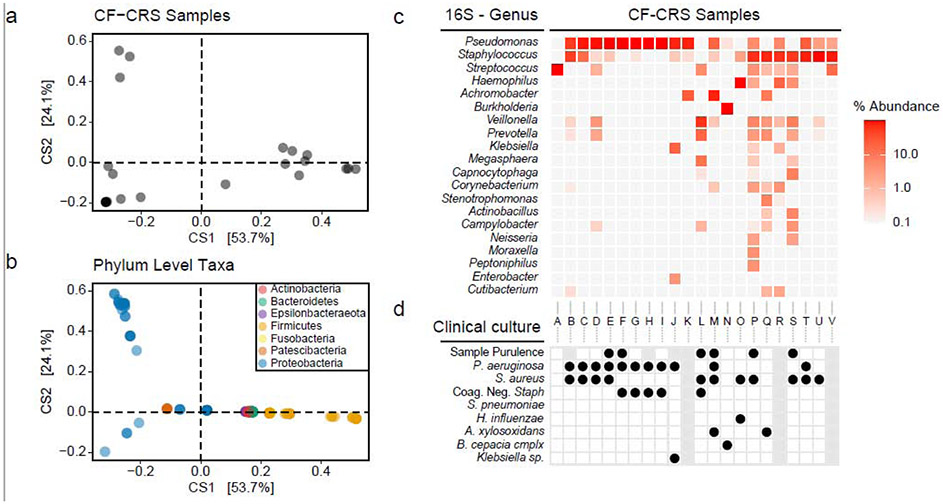
Using 16S rRNA gene sequencing, we profiled bacterial community membership of CF-CRS mucus. Double principal coordinate analysis (DPCoA) was used to assess sample similarity with respect to taxonomic identity, phylogenetic relationships, and relative abundance (Figure 1a,b). Samples clustered into three groups defined by Firmicutes and Proteobacteria, with 53.7% of variation described on the first axis. Multivariate analysis showed no association between clinical parameters or comorbidities (polyps, GERD, asthma, allergies, prior sinus surgery, CFTR genotype) and the DPCoA (Figure S1).
Figure 1. CF-CRS sinus communities differentiate by Proteobacteria or Firmicutes.
(a,b) Double principal coordinates analysis (DPCoA) of CF-CRS sinus bacterial communities. In (a) each point represents a single sample/community (n=22), while (b) shows corresponding ASVs, colored by Phylum. (c) Hierarchical clustered heatmap of CF-CRS samples with the top 20 genera by mean relative abundance. (d) Sinus culture results corresponding to each sample. Grey boxes indicate that culture data were not available.
Hierarchical clustering and heatmapping were used to visualize relative abundances of bacterial genera (Figure 1c). Indeed, samples were differentiated by Firmicutes (Streptococcus, Staphylococcus) or Proteobacteria (Pseudomonas). Other Proteobacteria (Haemophilus, Achromobacter, and Burkholderia) were abundant, but at lower prevalence, and accounted for second axis variation. Several amplicon sequence variants (ASVs) were assigned specific epithets (i.e. 100% sequence identity to no more than one species in a database) (Table S2), allowing for comparisons to culture-based data (Figure 1d). With exceptions, the dominant genus identified by sequencing was also identified by culture, though the second most abundant genus was often not. Notably, Streptococcus was third most abundant and comprised primarily of ASVs matching to the S. milleri group (SMG; S. anginosus, S. constellatus, S. intermedius). However, the SMG are not generally recovered clinically, underscoring the need for expanded use of molecular methods for bacterial identification and broadening the range of organisms targeted by clinical lab culture.
Many other taxa identified by sequencing, particularly those with fastidious growth requirements (e.g. anaerobiosis) are not reported by clinical culture. This was particularly apparent in Staphylococcus-dominated samples, which tended to have greater bacterial diversity overall. Notwithstanding their lower abundance, we note that fastidious organisms (e.g. Prevotella, Veillonella) may also play a role in CRS, either through their own pathogenic potential or by affecting pathogen biology via interspecies interactions.
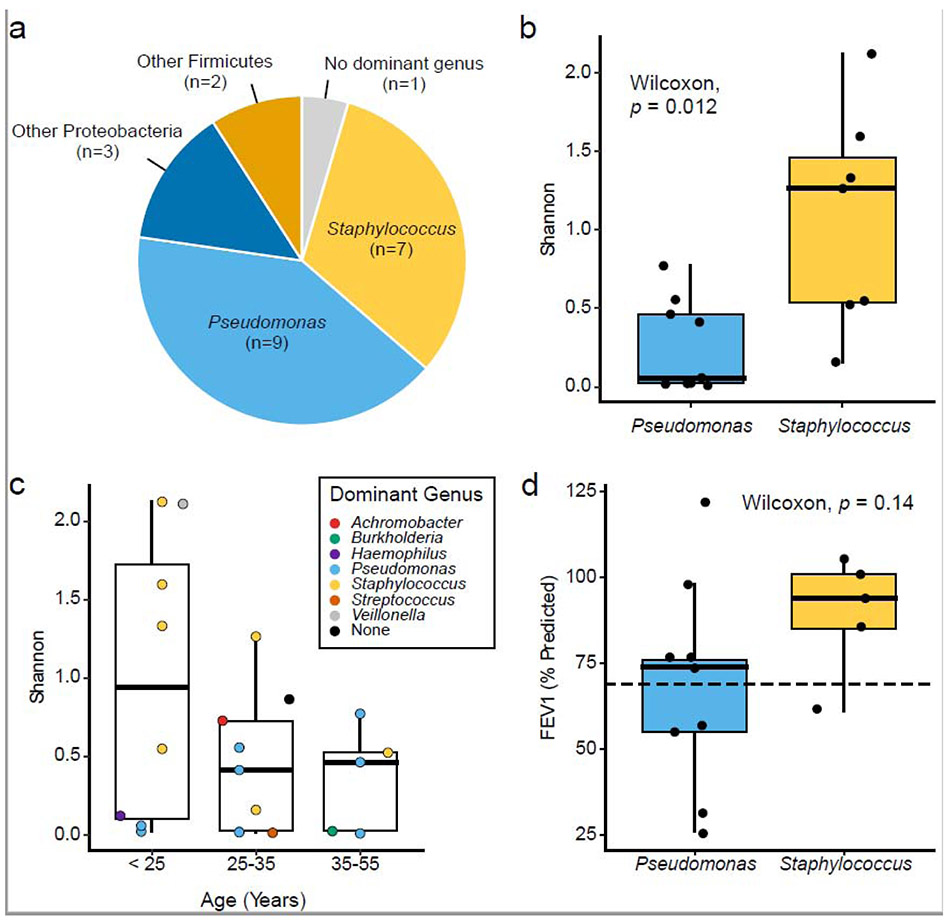
In most cases (21 of 22 samples), communities were structured by a taxon that comprised at least 50% of community membership and was more than twice as abundant as the next highest-ranking taxon (Figure 2a). The top two dominant genera were Pseudomonas (9 samples, 41%) and Staphylococcus (7 samples, 32%). Achromobacter, Burkholderia, Haemophilus, Streptococcus, and Veillonella were dominant in one sample each. Veillonella was noteworthy as it is not commonly attributed to pathogenesis (we also note, however, that presence does not necessarily equate to causality). We then compared alpha diversity (richness and evenness) between samples.
Figure 2. Shannon diversity and bacterial dominance.
(a) Number and proportion of samples with a dominant genus present (n = 22). (b) Shannon diversity compared between Pseudomonas or Staphylococcus dominance (Wilcoxon test, p=0.012). (c) Shannon diversity relative to CF patient age (Kruskal-Wallis, p=0.3). Points are colored by dominant genus. (d) Lung function measured by FEV1% (% predicted) compared between dominant genera (Wilcoxon test, p=0.2). Dashed line denotes level below which CF disease is considered to be moderate to severe.
Staphylococcus-dominated communities were significantly more diverse than those dominated by Pseudomonas (Wilcoxon, p=0.012)(Figure 2b). S. aureus is a known early colonizer of the CF lung, but as patients age, colonization with P. aeruginosa becomes more prevalent and coincides with a reduction in bacterial diversity [16]. Here, a significant relationship between age and Shannon diversity was not observed (Figure 2c), though diversity in samples from younger patients (16-25 years old) varied greatly compared to older age groups. Additionally, samples dominated by Staphylococcus in this group (50%) were greater than any other age. These data are consistent with the temporal acquisition of S. aureus and P. aeruginosa in the CF lung. Indeed, we identified a strong agreement between CRS sequence data and pathogen detection in the sinus and lung by clinical culture (Figure S2).
Finally, colonization of the lung by either S. aureus or P. aeruginosa has been associated with pulmonary function decline [17]. Given the “unified airway” hypothesis, we asked whether pathogen dominance in the sinuses fits this association (Figure 2d). While we found no statistically significant relationship, only patients with moderate to severe lung disease (FEV1%<70) yielded sinus mucus dominated by Pseudomonas. These data suggest that sinus microbiology may reflect lung function among a larger patient cohort.
4. DISCUSSION
CRS studies commonly use CF as an exclusionary criterion, limiting both culture-based and molecular surveys of CF-CRS microbiota. However, the importance of sinus microbial ecology is underscored by the concordance of bacterial genotypes between upper and lower CF airways [11]. Building on our prior work on the sinus-lung axis in CF airway disease [18], we (i) use 16S rRNA gene sequencing together with DADA2 for detailed analyses of CRS microbiota, and (ii) establish their phylogenetic relationships and associations with clinical metrics and co-morbidities. Altogether, data presented here support the notion that the sinuses serve as a site for bacterial adaptation prior to chronic lung infection.
Bacterial diversity in CF sputum negatively correlates with age and pulmonary function decline [7, 19]. Similarly, lung infections by the two most prevalent CF pathogens, S. aureus and P. aeruginosa, correlate with age; S. aureus is generally isolated from young CF patients and P. aeruginosa becomes the primary pathogen in adulthood [20]. Co-colonization also occurs and is associated with poor clinical outcomes [21]. Interestingly, many of these trends were observed in our CF-CRS cohort; (i) nearly all sinus mucus was dominated by either Pseudomonas or Staphylococcus (ii) 13 of 22 (59%) were cocolonized, (iii) community diversity and Staphylococcus-dominated samples were highest among younger subjects and declined with increasing age, and (iv) only sinus samples from patients exhibiting moderate to severe lung function were dominated by Pseudomonas. The small size of our cohort limited the ability to further test hypotheses related to organism dominance, diversity, and other clinical parameters, though collectively, these data suggest that CRS microbial dynamics may reflect, or even prognosticate, trajectories of CF lung infections.
By using amplicon sequence variants (ASVs), we improved upon the resolution by which microbiota associated with CF-CRS are surveyed (Table S2). We showed an increased diversity of bacterial taxa in Staphylococcus-dominated communities, including those from Veillonella (V. dispar, V. parvula), Megasphaera (M. micronuciformis), Prevotella (P. melaninogenica, P. salivae), Corynebacterium (C. tuberculostearicum), several Streptococcus spp., and others. While it is likely that this increased diversity reflects an early disease state and fewer environmental perturbations (e.g. antibiotics), the presence of these less prevalent and less abundant taxa raises the question of whether interbacterial interactions are also determinants of canonical pathogen dominance and/or virulence potential. Expanding in vitro investigations of interbacterial interactions will be critical to identify the contributions of community ecology to CF-CRS.
Sinusitis and its contributions to CF pathogenesis are poorly understood. Once thought to be sterile, sinuses are now known to harbor diverse polymicrobial communities that influence chronic bacterial colonization of the lung. While further comparative genomic analyses are necessary to establish metastatic relationships the directionality of exchange between upper and lower airway microbiota [22], this work adds to our emerging understand of the role of sinus microbiota in CF morbidity.
Supplementary Material
Highlights.
16S rRNA gene sequencing and amplicon sequence variant (ASV) analysis reveals an unrealized diversity of CRS microbiota not captured by clinical culture
CF-CRS microbiota dominated by Staphylococcus aureus harbor significantly more bacterial diversity relative to those dominated by Pseudomonas aeruginosa.
Sinus bacterial diversity does not correlate with CRS co-morbidities
CF-CRS microbiology mirrors bacterial community dynamics in the CF lung
ACKNOWLEDGEMENTS.
This work was supported by an Investigator-Sponsored Research Grant from Gilead Sciences to RCH and a National Institutes of Health T32 Fellowship (#T90 DE 0227232) awarded through the National Institute of Dental and Craniofacial Research to SKL. We thank Abayo Itabiyi and Ali Stockness of the Department of Otolaryngology, members of UMN BioNet for sample acquisition, and the UMN Genomics Center for sequencing assistance.
Abbreviations:
- ASV
amplicon sequence variant
- CRS
chronic rhinosinusitis
- CF-CRS
cystic fibrosis-associated chronic rhinosinusitis
- CFTR
cystic fibrosis transmembrane conductance regulator
- DPCoA
double principal coordinate analysis
- FESS
endoscopic sinus surgery
- GERD
gastroesophageal reflux disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT. The authors declare no conflicts.
REFERENCES
- [1].Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27(5):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–32. [DOI] [PubMed] [Google Scholar]
- [3].Vickery TW, Ramakrishnan VR, Suh JD. The Role of Staphylococcus aureus in Patients with Chronic Sinusitis and Nasal Polyposis. Curr Allergy Asthma Rep. 2019;19(4):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hoggard M, Wagner Mackenzie B, Jain R, Taylor MW, Biswas K, Douglas RG. Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease. Clin Microbiol Rev. 2017;30(1):321–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Godoy JM, Godoy AN, Ribalta G, Largo I. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg. 2011;145(4):673–6. [DOI] [PubMed] [Google Scholar]
- [6].Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med. 2014;20(6):623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5(6):e11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Nat Acad Sci USA. 2012; 109:58095814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cope EK, Goldberg AN, Pletcher SD, Lynch SV. Compositionally and functionally distinct sinus microbiota in chronic rhinosinusitis patients have immunological and clinically divergent consequences. Microbiome. 2017;5(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sobin L, Kawai K, Irace AL, Gergin O, Cunningham M, Sawicki GS, et al. Microbiology of the Upper and Lower Airways in Pediatric Cystic Fibrosis Patients. Otolaryngol Head Neck Surg. 2017;157(2):302–8. [DOI] [PubMed] [Google Scholar]
- [11].Johansen HK, Aanaes K, Pressler T, Nielsen KG, Fisker J, Skov M, et al. Colonisation and infection of the paranasal sinuses in cystic fibrosis patients is accompanied by a reduced PMN response. J Cyst Fibros. 2012;11(6):525–31. [DOI] [PubMed] [Google Scholar]
- [12].Ciofu O, Johansen HK, Aanaes K, Wassermann T, Alhede M, von Buchwald C, et al. Pseudomonas aeruginosa in the paranasal sinuses and transplanted lungs have similar adaptive mutations as isolates from chronically infected CF lungs. J Cyst Fibros. 2013;12(6):729–36. [DOI] [PubMed] [Google Scholar]
- [13].Mainz JG, Naehrlich L, Schien M, Käding M, Schiller I, Mayr S, et al. Concordant genotype of upper and lower airways Pseudomonas aeruginosa and Staphylococcus aureus isolates in cystic fibrosis. Thorax. 2009;64(6):535–40. [DOI] [PubMed] [Google Scholar]
- [14].Syed SA, Whelan FJ, Waddell B, Rabin HR, Parkins MD, Surette MG. Reemergence of Lower-Airway Microbiota in Lung Transplant Patients with Cystic Fibrosis. Ann Am Thorac Soc. 2016;13(12):2132–42. [DOI] [PubMed] [Google Scholar]
- [15].Choi KJ, Cheng TZ, Honeybrook AL, Gray AL, Snyder LD, Palmer SM, et al. Correlation between sinus and lung cultures in lung transplant patients with cystic fibrosis. Int Forum Allergy Rhinol. 2018;8(3):389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep. 2015;5:10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahlgren HG, Benedetti A, Landry JS, Bernier J, Matouk E, Radzioch D, et al. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm Med. 2015;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lucas S, Yang R, Dunitz JM, Boyer HC, Hunter RC. 16S rRNA gene sequencing reveals sitespecific signatures of the upper and lower airways of cystic fibrosis patients. J Cyst Fib. 2018;17:204212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Klepac-Ceraj V, Lemon KP, Martin TR, Allgaier M, Kembel SW, Knapp AA, et al. Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ Microbiol. 2010;12(5):1293–303. [DOI] [PubMed] [Google Scholar]
- [20].Salsgiver EL, Fink AK, Knapp EA, LiPuma JJ, Olivier KN, Marshall BC, et al. Changing Epidemiology of the Respiratory Bacteriology of Patients With Cystic Fibrosis. Chest. 2016;149(2):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Limoli DH, Yang J, Khansaheb MK, Helfman B, Peng L, Stecenko AA, et al. Staphylococcus 5 aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur J Clin Microbiol Infect Dis. 2016;35(6):947–53. [DOI] [PubMed] [Google Scholar]
- [22].Hansen SK, Rau MH, Johansen HK, Ciofu O, Jelsbak L, Yang L et al. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for lung infection. J Cyst Fib. 2012;6:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.