Abstract
Background:
Tobacco smoke exposure is a major risk factor for the health of children and adolescents with CF. In this study, we assess whether cessation of smoke exposure is associated with improved outcomes in this population.
Methods:
We used annualized and encounter-based data from the U.S. CF Foundation Patient Registry (2006-2018) on all individuals born 1998-2010. The analytical sample included those who ever reported second-hand smoke exposure (daily or weekly), ever lived with a smoker, or ever reported smoking themselves. We used non-linear mixed models for pulmonary exacerbations and linear mixed models for ppFEV1 and BMI as a function of ceased exposure.
Results:
The sample included 3,633 individuals contributing 19,629 person-years. Cessation of smoke exposure reduced the odds of a pulmonary exacerbation in 12 months by 17% (OR 0.83, p<0.001) in the first year of cessation, with an additional 6% decrease (OR 0.94, p=0.003) for each additional year of cessation. Cessation was associated with improvements in ppFEV1 and BMI: 0.7% ppFEV1 increase (p<0.001) in the first year of cessation and 0.4% increase (p=0.001) for each additional year of cessation; 1% increase in BMI percentile (p<0.001) in the first year of cessation plus 0.4% increase (p=0.009) for each additional year. Three years of cessation reduce the predicted probability of a pulmonary exacerbation in 12 months by 8% and improve ppFEV1 and BMI by 2%.
Conclusion:
Eliminating smoke exposure may reduce pulmonary exacerbations and improve respiratory and nutritional outcomes in children and adolescents with CF. Both smoking cessation and exposure prevention should be prioritized in pediatric CF care.
Keywords: Tobacco, Secondhand smoke, Smoke exposure, Smoking cessation
BACKGROUND
Cystic fibrosis (CF), an autosomal recessive disorder caused by dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR), affects approximately 1 in 3,500 live births in the United States.1 Morbidity from nutritional and respiratory failure has improved considerably with the development of interventions such as pancreatic enzyme replacement, dietary supplementation, airway clearance techniques, mucolytic and anti-inflammatory medications, antibiotics, and CFTR modulators. However, improvements from these treatments may be diminished due to socioeconomic factors and environmental exposures.2,3
Tobacco smoke exposure (TSE) is a major cause of disease, with no safe levels of exposure.4 Children are exposed to higher levels of second-hand smoke than adults, putting them at major health risk.4 TSE is particularly deleterious for the lung health of young people with CF.5-10 By age 6, smoke-exposed children in the U.S. CF Foundation Patient Registry (CFFPR) have approximately 5% lower ppFEV1 than unexposed children, and this deficit persists through age 18.3 Additionally, we recently reported that TSE blunts the clinical efficacy of CFTR modulators: among adolescents age 12-20 in the CFFPR, smoke exposure nullified the therapeutic benefit of the modulator combination tezacaftor-ivacaftor,11 which indicates that cessation may be an important pathway to maintaining benefit from CFTR modulators.
While there is a preponderance of evidence demonstrating that TSE is harmful to patients with CF, less is known about the potential benefits of TSE cessation on CF outcomes. One small study demonstrated a potential for recovery of TSE-induced systemic CFTR dysfunction in non-CF adults.12 Other non-CF studies suggest that some lung damage from smoking or exposure to tobacco smoke may be reversible in animal models13 and non-CF adults.14 The aim of this study is to determine whether cessation of TSE can improve the respiratory and nutritional outcomes of pediatric patients with CF previously exposed to smoke.
METHODS
We used anonymized annualized and encounter-based data from the CF Foundation Patient Registry (CFFPR) for the period 2006-2018; 2006 is the first year when CFFPR began collecting smoke exposure data, and 2018 is the last year of available CFFPR data at the time of analysis. The data included all individuals in the CFFPR born between 1/1/1998 and 12/31/2010. Our analytical sample comprised those who ever reported second-hand smoke exposure (daily or weekly), ever lived with a household member who smoked, or ever reported smoking themselves. Individuals entered the analysis in the year they reported smoke exposure, or at 6 years of age if smoke exposure was reported prior to age 6. The analysis begins at age 6 because it is the earliest age of reproducible spirometry reported to the CFFPR. Individuals were followed until 2018 or until they aged out (>20 years old) of the dataset. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham (protocol 300002076).
The outcome variables were pulmonary exacerbations, lung function, and body mass index (BMI). The number of pulmonary exacerbations in 12 months was obtained from annualized CFFPR data. Although that number ranged from 0 to 13, in any given year only 30-45% experienced a pulmonary exacerbation. Therefore, the variable was transformed into a dichotomous variable (0/≥1) to account for the skewed nature of the data. Lung function, measured as forced expiratory volume in 1 second, percent predicted (ppFEV1), calculated with the Global Lung Function Initiative reference equations, was obtained from encounter-based data. Annualized measures of lung function for each individual were created by calculating the median ppFEV1 across encounters for that calendar year. BMI was calculated as weight (kg)/height (m)2 from height and weight recorded at each clinical encounter and transformed into percentiles using the U.S. Centers for Disease Control and Prevention reference for age and sex.15 Annualized measures of BMI percentile for each individual were created by calculating the median BMI percentile across all encounters for that year.
The exposure variable, TSE cessation, measured whether an individual previously exposed to tobacco smoke is no longer exposed. Cessation was assumed to occur halfway between the year reporting TSE and the year reporting no TSE. TSE was defined as (1) reporting daily or weekly second-hand exposure, (2) living with a smoker, or (3) smoking themselves. We examined whether cessation impacts the intercept and the slope of each dependent variable. Change in the intercept due to cessation is a dichotomous time-varying variable, meaning that individuals can experience cessation and then be re-exposed again. Change in the slope is measured with a spline that indicates the time since cessation. If an individual becomes re-exposed, the spline no longer increases with increasing time. The measure of cessation as a change in the slope can be thought of as a measure of cumulative years of cessation at that point in time, whereas the dichotomous time-varying measure of cessation accounts for whether an individual is still exposed. This coding scheme ensures consistency in meaning both within individuals and across individuals. An example of the coding scheme is provided in Supplementary Table 1.
Analyses controlled for non-time varying variables, including age when first entered the sample, sex, race/ethnicity (White non-Hispanic, non-White non-Hispanic, Hispanic), paternal education (less than high school, high school, some college, college degree), genotype (F508del heterozygous, homozygous, other), and newborn screening. Time-varying covariates included household size, income (from <$10,000 to >$90,000, in $10,000 increments), health insurance (private, public, both, none), CFTR modulator use, P. aeruginosa, and B. cepacia.
Statistical analyses
We used nested non-linear mixed models with a binomial distribution to examine pulmonary exacerbations in 12 months (yes/no) as a function of TSE cessation, and linear mixed models to examine BMI percentile and ppFEV1 as a function of TSE cessation. For pulmonary exacerbations, we present both odds ratios and predicted probabilities because we are interested in both the significance and magnitude of TSE cessation. The linear mixed models correct for the interdependency created by using multiple observations from the same person. We included an individual-specific error term for the random effects of intercept for all three outcomes, and a random slope for BMI percentile and ppFEV1. The slope is measured as years since entering the sample. Because individuals enter the sample at the age they are when smoke exposure is first reported, we used years since entering the sample, rather than age, as a measure of time. Thus, time is measured as years since the first recorded smoke exposure. We also included a quadratic of time to account for the expected non-linear change in slope for both lung function and BMI percentile as children age. A change in the intercept for cessation indicates the immediate and sustained benefit from cessation. A change in the slope for cessation indicates the average annual change due to continued cessation. For example, if cessation increased the intercept of ppFEV1 by 1% and the slope by 0.5%, lung function would improve by 1% within the first year of cessation and by an additional 0.5% for every year the cessation continues. In that example, after ten years, an individual who experienced cessation may have 5.5% higher ppFEV1 than a counterpart who never experienced cessation (1% improvement from cessation plus 0.5% improvement for 9 years of cessation). For ease of interpretation, all coefficients are graphed to demonstrate the predicted difference between the two groups three years after cessation. All models adjusted for the same sociodemographic and clinical covariates: age at entering the sample, sex, race/ethnicity, paternal education, household size, household income, health insurance, genotype, newborn screening, CFTR modulator use, P. aeruginosa, and B. cepacia. Age at entering the sample, sex, race/ethnicity, paternal education, genotype, and newborn screening are non-time varying covariates. Household size, household income, health insurance, CFTR modulator use, P. aeruginosa, and B. cepacia are time-varying covariates.
Missing data.
Approximately 22% of the sample was missing annual smoke exposure data. Missing TSE data was more common in the earlier years and decreased at later years. In 2006, the first year of collecting TSE data, about 44% had missing data on smoke exposure; by 2011, the missing TSE data drops down to 30%; in 2012, to 15%; and in 2018, to 11%. Nearly all respondents (98.5%) had valid TSE data reported in at least one year. Only complete TSE data were used to determine cessation (change in the intercept) and time since cessation (change in the slope). See Supplementary Table 1 for additional information on how ‘time since cessation’ was coded for those who did not have TSE data across all years. In additional analyses, we imputed TSE cessation and time since TSE cessation and found substantively similar results. Among covariates, there were high levels of missingness (28%) on income; all other covariates had less than 10% missing data. Due to the high level of missingness on the income variable, in the imputation phase we included an auxiliary measure of income that is a good approximation of the value of the missing income. Specifically, for each year of the survey data, we calculated zip-code income based on residential zip-codes in the CFFPR and publicly available zip-code income data from the Internal Revenue Service. Additionally, measures of paternal education and health insurance were included in both the imputation and analysis models. Zip-code income, paternal education, and health insurance accounted for 45% of the variation in participant income in 2006, confirming that these variables perform well as imputation variables. We also conducted sensitivity analyses comparing non-imputed and imputed estimates, and found that the results are substantively the same. Missing covariate data were addressed with multiple imputations (n=10 data sets) using Markov Chain Monte Carlo,16 as discussed in our previous work using this CFFPR data set.3 Analyses were performed with Stata 16 (College Station, TX: StataCorp LLC).
RESULTS
The analytic sample included 3,633 individuals, who contributed 19,629 person-years (mean=5.4, range 1-13). A STROBE diagram of the patient population is included in Supplementary Figure 1.
Sociodemographic and clinical characteristics of the sample, overall and by smoking cessation status, are summarized in Table 1. Data are presented for individuals’ last survey year (rather than for 2018) because approximately 20% of the sample aged out prior to 2018. In their last survey year, about 47% of the sample were still exposed to tobacco smoke. Specifically, 22% were always exposed, 39% experienced intermittent cessation but were re-exposed, and 40% experienced permanent cessation. Those who experienced permanent cessation had more favorable sociodemographic characteristics compared to those who remained exposed: higher annual household income ($54,000 vs $45,000; p<0.001), higher paternal education (36% vs 23% college degree; p<0.001), and private health insurance (33% vs 22%; p<0.001). Hispanics were more likely to experience permanent cessation than non-Hispanic Whites (p=0.010). Those who experienced intermittent cessation had sociodemographic characteristics that were in between the ‘permanent cessation’ and ‘never cessation’ groups.
Table 1.
Characteristics of the sample in their final survey year, by cessation of smoke exposure (N=3,633)
| Total N=3,633 |
Cessation of Exposure | ||||
|---|---|---|---|---|---|
| Never N=784 (21.6%) |
Intermittent N=1,412 (38.9%) |
Permanent N=1,437 (39.6%) |
p-value1 | ||
| SOCIODEMOGRAPHIC | |||||
| Age at entry 2 | 7.7 (4.8) | 10.1 (5.0) | 5.9 (3.9) | 8.15 (4.7) | <0.001 |
| Female sex, % | 48.2 | 44.5 | 48.7 | 49.6 | 0.062 |
| Race/ethnicity, % | |||||
| White non-Hispanic | 83.5 | 84.1 | 85.8 | 80.9 | |
| Non-White non-Hispanic | 8.7 | 8.9 | 7.9 | 9.3 | 0.004 |
| Hispanic | 7.8 | 7.0 | 6.3 | 9.7 | |
| Household size | 4.3 (1.6) | 4.4 (1.6) | 4.3 (1.6) | 4.2 (1.5) | 0.195 |
| Household income, in $10K | 4.9 (3.2) | 4.5 (3.3) | 4.6 (3.3) | 5.4 (3.5) | <0.001 |
| Father’s education, % | |||||
| Less than high school | 6.7 | 8.0 | 6.3 | 6.1 | |
| High school | 40.6 | 47.1 | 42.8 | 34.8 | |
| Some college | 23.7 | 21.7 | 25.4 | 23.3 | <0.001 |
| College | 29.0 | 23.2 | 25.5 | 35.8 | |
| Health insurance type, % | |||||
| Private | 26.6 | 22.1 | 22.6 | 32.9 | |
| Public | 63.5 | 67.8 | 67.6 | 57.2 | |
| Private and Public | 8.7 | 8.4 | 8.7 | 8.9 | <0.001 |
| None/other | 1.2 | 1.7 | 1.1 | 1.0 | |
| CLINICAL | |||||
| Pulmonary exacerbations in 12 mos. | 0.8 (1.4) | 0.8 (1.4) | 0.9 (1.5) | 0.7 (1.4) | 0.004 |
| One or more, % | 39.1 | 40.0 | 41.6 | 36.5 | 0.019 |
| Two or more, % | 19.5 | 18.9 | 21.9 | 17.4 | 0.009 |
| BMI percentile | 50.7 (28.8) | 49.3 (29.3) | 50.3 (28.7) | 51.8 (28.6) | 0.137 |
| ppFEV1 | 84.6 (21.5) | 83.2 (21.3) | 84.2 (21.2) | 85.7 (21.6) | 0.026 |
| Genotype, % | |||||
| Heterozygous | 49.7 | 46.8 | 51.2 | 49.9 | |
| Homozygous | 37.1 | 37.9 | 37.2 | 36.7 | 0.106 |
| Other | 13.1 | 15.3 | 11.6 | 13.4 | |
| Newborn screening, % | 29.1 | 27.9 | 29.7 | 29.1 | 0.690 |
| CFTR modulator use, % | |||||
| Ivacaftor | 8.3 | 8.3 | 8.1 | 8.4 | 0.944 |
| Lumacaftor-ivacaftor | 9.3 | 7.5 | 11.0 | 8.6 | 0.014 |
| P. aeruginosa, % | |||||
| None | 67.1 | 67.6 | 66.9 | 67.5 | |
| Non-mucoid | 14.8 | 14.7 | 14.8 | 14.9 | 0.991 |
| Mucoid | 17.9 | 17.7 | 18.3 | 17.7 | |
| B. cepacia, % | 2.3 | 2.2 | 3.1 | 1.5 | 0.015 |
Significance level for the difference between those who experienced cessation and those who never experienced cessation.
Continuous measures are shown as mean (standard deviation).
Table 2 shows results from non-linear mixed models of pulmonary exacerbations and linear mixed models of lung function and BMI. After adjusting for covariates, TSE cessation was associated with lower odds of a pulmonary exacerbation in 12 months: a 17% decrease in the intercept (OR 0.83, 95% CI 0.75–0.91, p<0.001) and 6% decrease in the slope (OR 0.94, 95% CI 0.91–0.98, p=0.003). Conversely, after adjusting for covariates, TSE cessation was associated with increase in ppFEV1 and BMI: for ppFEV1, 0.7% increase in the intercept (β=0.69, 95% CI 0.34–1.04, p<0.001) and 0.4% increase in the slope (β=0.39, 95% CI 0.16–0.61, p=0.001); for BMI percentile, 1% increase in the intercept (β=1.03, 95% CI 0.57–1.50, p<0.001) and 0.4% increase in the slope (β=0.42, 95% CI 0.10–0.74, p=0.009). Changes in the intercept and slope correspond to changes in the first year of cessation and each additional year of cessation, respectively.
Table 2.
Unadjusted and adjusted models of pulmonary exacerbations, ppFEV1, and BMI as a function of smoke exposure cessation (N=3,633)
| Unadjusted1 | Adjusted2 | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Pulmonary exacerbations | ||||||||
| Cessation: Intercept | 0.79 | 0.71 | 0.87 | <0.001 | 0.83 | 0.75 | 0.91 | <0.001 |
| Cessation: Slope | 0.94 | 0.90 | 0.97 | 0.001 | 0.94 | 0.91 | 0.98 | 0.003 |
| β | β | |||||||
| ppFEV1 | ||||||||
| Cessation: Intercept | 0.73 | 0.37 | 1.08 | <0.001 | 0.69 | 0.34 | 1.04 | <0.001 |
| Cessation: Slope | 0.38 | 0.15 | 0.62 | 0.001 | 0.39 | 0.16 | 0.61 | 0.001 |
| BMI percentile | ||||||||
| Cessation: Intercept | 1.00 | 0.53 | 1.46 | <0.001 | 1.03 | 0.57 | 1.50 | <0.001 |
| Cessation: Slope | 0.41 | 0.09 | 0.73 | 0.013 | 0.42 | 0.10 | 0.74 | 0.009 |
Includes time for all three outcomes and a quadratic of time for ppFEV1 and BMI percentile.
Adjusted for age at entry, sex, race/ethnicity, household size, income, father's education, health insurance, genotype, newborn screening, CFTR modulator use, P.aeruginosa, and B. cepacia; includes the quadratic for time.
Note: Changes in the intercept and slope correspond to changes in the first year of cessation and each additional year of cessation, respectively.
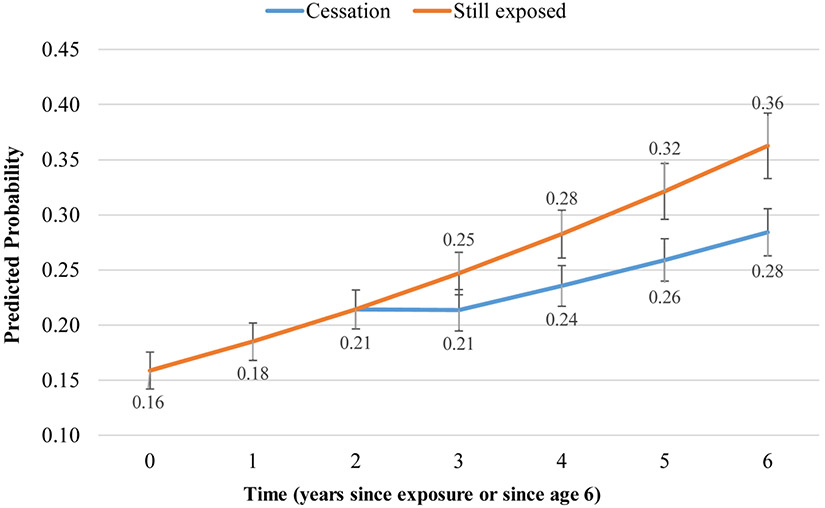
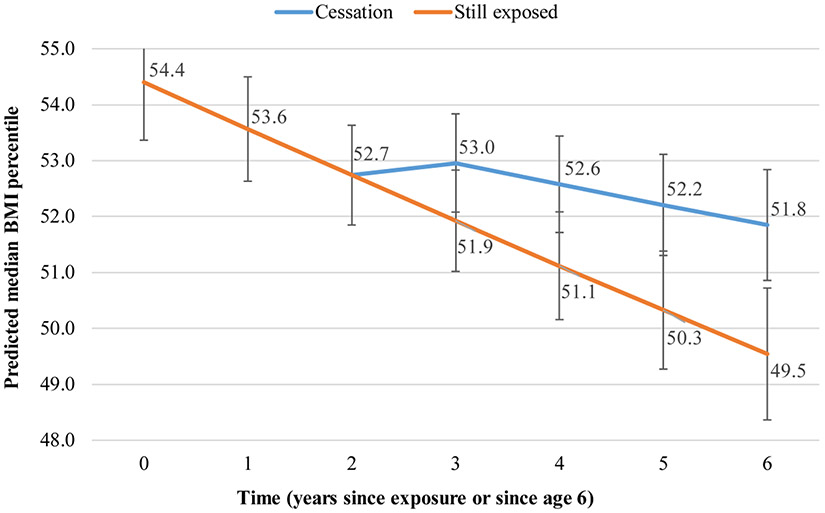
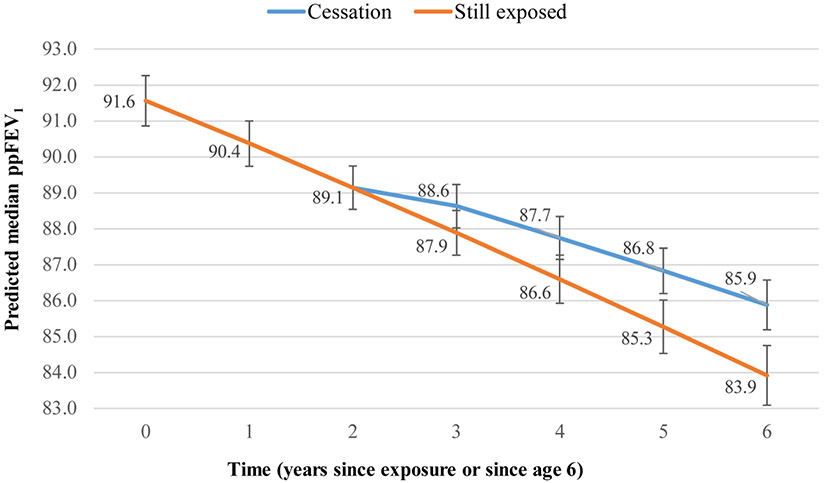
Figures 1-3 display graphically what changes in outcomes can be expected to occur within three years after cessation based on this dataset. Three years after cessation, those no longer exposed to smoke would have a 8% lower probability of experiencing a pulmonary exacerbation in the past 12 months than their still-exposed counterparts: 0.28 (95% CI 0.26–0.31) vs 0.36 (95% CI 0.33–0.39) (Figure 1). Three years after cessation, those no longer exposed to smoke would have a 2% higher ppFEV1 than their still-exposed counterparts: 85.9 (95% CI 85.2–86.6) vs 83.9 (95% CI 83.1–84.7) (Figure 2). Similarly, three years after cessation, those no longer exposed to smoke would have 2.3% higher BMI than their still-exposed counterparts: 51.8 (95% CI 50.9–52.8) vs 49.5 (95% CI 48.4–50.7) (Figure 3).
Figure 1. Predicted probability of a pulmonary exacerbation over time, by cessation status*.
*Adjusted for age at entry, sex, race/ethnicity, household size, income, father’s education, health insurance, genotype, newborn screening, CFTR modulator use, P.aeruginosa, and B. cepacia
Figure 3. Predicted median BMI percentile over time, by cessation status*.
*Adjusted for age at entry, sex, race/ethnicity, household size, income, father’s education, health insurance, genotype, newborn screening, CFTR modulator use, P.aeruginosa, and B. cepacia
Figure 2. Predicted median ppFEV1 over time, by cessation status*.
*Adjusted for age at entry, sex, race/ethnicity, household size, income, father’s education, health insurance, genotype, newborn screening, CFTR modulator use, P.aeruginosa, and B. cepacia
DISCUSSION
We conducted a longitudinal analysis of CF Foundation Patient Registry data from 2006 to 2018 to determine if cessation of smoke exposure can reverse the harmful effects of tobacco smoke in children and adolescents with CF. This is the first study to report that ceased smoke exposure is associated with statistically and clinically significant improvement of respiratory and nutritional outcomes in children and adolescents with CF previously exposed to tobacco smoke. After adjusting for covariates, three years of cessation reduced the predicted probability of a pulmonary exacerbation within the last year by 8% and improved both ppFEV1 and BMI percentile by approximately 2%. These estimates represent a dose-dependent effect, as they also include the partial benefit from intermittent cessation experienced by individuals who were later re-exposed. The effect sizes offset the average annual loss of lung function in pediatric CF17-19 and are comparable to the effect of some established cystic fibrosis therapies.20 These findings may be used by clinical care providers as a conversation starter with CF families about smoking cessation, and as a motivator for individuals to quit smoking for the benefit of the child with CF.
Our data also offer clarity about the prevalence of smoke exposure and the extent of cessation efforts in the U.S. CF population. Despite the adverse effects of tobacco smoke on CF lung health,2,3,5,7,21 exposure remains pervasive, with approximately 30% of U.S. children with CF being exposed to tobacco smoke (compared to 40% of children in the general population22). Of those exposed between 2006 and 2018, 40% experienced the benefits of permanent cessation, 39% experienced intermittent cessation but were re-exposed, and 22% remained always exposed. These statistics compare somewhat favorably to the cessation rates in the general U.S. population in 2018, where 55% reported making a quit attempt in the past year, 8% reported successfully quitting in the past year, and 62% of all who ever smoked are now former smokers.23
Children and adolescents with CF in disadvantaged families were less likely to experience cessation of smoke exposure. These results corroborate previous reports that unfavorable socioeconomic circumstances reduce the likelihood of a successful cessation attempt23-25 and mirror the increased risk of smoke exposure in low-income and non-college-educated CF families.4 A notable exception were children of Hispanic ethnicity, which were more likely to experience TSE cessation than their non-Hispanic counterparts. This finding is in line with research in non-CF populations which finds that Hispanic children, especially those with immigrant parents, are less likely to be exposed to tobacco smoke.26,27
Our data highlight the need for effective smoking cessation interventions for CF families. CF care teams can actively screen for TSE and deploy evidence-based approaches tailored to the needs of CF caregivers. Although tobacco dependence often requires repeated intervention, and tobacco users may have to attempt cessation several times before succeeding,24,28 evidence-based treatments can significantly increase the rates of long-term abstinence.29 The 2020 Report of the U.S. Surgeon General4 highlights such treatments that multiply quit rates and are effective across a variety of settings and populations.29,30 For example, behavioral counseling and pharmacotherapy are independently effective in increasing smoking cessation but are even more effective when used in combination.4 Treatment efficacy also improves with increased number of sessions and time in treatment. Determining what cessation strategies are most effective for CF families will be critical to improving CF lung health and maximizing the benefit from CFTR modulator therapies. Identifying smoke-exposed CF patients through active screening may also require changes to current CF clinical guidelines, such as adding annual nicotine testing.
CF caregivers who smoke face multiple barriers, from addiction and CF-related stress to unfavorable social norms or limited access to tobacco counseling and nicotine-replacement therapy and pharmacotherapy.31 Compounding this issue are clinical barriers in CF care centers, including limited staff trained to provide tobacco counseling, inadequate health system support and reimbursement for tobacco treatment services, and a general lack of consensus on undertaking an arduous effort with a perceived low level of success.31 Still, tobacco use is one of few modifiable risk factors for CF child health. It is essential that we make every effort to help CF caregivers to quit smoking in order to maximize the health potential of children and adolescents with CF.
The strengths of this study include using a national registry-based prospective cohort of CF patients to track periods of smoke exposure, cessation, and re-exposure over time, as well as the ability to control for sociodemographic variables that have a demonstrated relationship with TSE. The main limitation is the self-reported nature of the TSE data, known to underestimate actual exposure rates. The self-reported TSE also had a high proportion of missing data (22%), although missingness decreased substantially in later years, from 44% in 2006 to 30% in 2011, to 15% in 2012, to 11% in 2018. Additionally, since the CFFPR was not created with the intent to monitor smoke exposure, some information cannot be ascertained. For example, for part of the cohort, we do not know when the smoke exposure began because data on smoke exposure was not collected prior to 2006. This also means that we cannot determine if there is a greater beneficial impact of cessation when children are younger compared to cessation in older ages. Both these limitations point to the importance of improving the type and quality of data collection on TSE in CF patient registries.
Our major finding is that cessation of smoke exposure has the potential to recover nutritional and respiratory outcomes in children and adolescents with CF. Future research should identify and test effective strategies to reduce and eliminate exposure to tobacco smoke in this population in order to preserve lung health and mitigate poor health outcomes as early as possible.
Supplementary Material
Highlights.
Cessation of smoke exposure reduces the odds of a pulmonary exacerbation by 17% in the first year and by 6% in each additional year of cessation.
Cessation of smoke exposure is associated with 0.7% increase in ppFEV1 in the first year and 0.4% increase in each additional year of cessation.
Cessation of smoke exposure is associated with 1% increase in BMI percentile in the first year and 0.4% increase in each additional year of cessation.
Eliminating smoke exposure may improve CF respiratory and nutritional outcomes and should be prioritized in CF care.
Acknowledgments:
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Funding:
This study was supported by grants from the National Institutes of Health (P30DK72482, K08HL140190, HARRIS19A0-KB).
Abbreviations:
- BMI
body mass index
- CFFPR
CF Foundation Patient Registry
- ppFEV1
forced expiratory volume in 1 second, percent predicted
- TSE
tobacco smoke exposure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of competing interest: None
REFERENCES
- 1.O'Sullivan BP, Freedman SD. Cystic Fibrosis. Lancet. 2009;373(9678):1891–1904. [DOI] [PubMed] [Google Scholar]
- 2.Ong T, Schechter M, Yang J, et al. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children with Cystic Fibrosis. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oates GR, Baker E, Rowe SM, et al. Tobacco Smoke Exposure and Socioeconomic Factors Are Independent Predictors of Pulmonary Decline in Pediatric Cystic Fibrosis. J CystFibros. 2020;19(5):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smoking Cessation: A Report of the Surgeon General. In: Washington (DC)2020. [Google Scholar]
- 5.Sanders DB, Emerson J, Ren CL, et al. Early Childhood Risk Factors for Decreased Fev1 at Age Six to Seven Years in Young Children with Cystic Fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega-Garcia JA, Lopez-Fernandez MT, Llano R, et al. Smoking Prevention and Cessation Programme in Cystic Fibrosis: Integrating an Environmental Health Approach. J Cyst Fibros. 2012;11(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaco JM, Vanscoy L, Bremer L, et al. Interactions between Secondhand Smoke and Genes That Affect Cystic Fibrosis Lung Disease. Jama-Journal of the American Medical Association. 2008;299(4):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PW 3rd, Parker RA, Roberts, Krishnamani MR, Phillips JA 3rd. Association of Poor Clinical Status and Heavy Exposure to Tobacco Smoke in Patients with Cystic Fibrosis Who Are Homozygous for the F508 Deletion. J Pediatr. 1992;120(2 Pt 1):261–264. [DOI] [PubMed] [Google Scholar]
- 9.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the Relative Contribution of Environmental and Genetic Factors to Variation in Cystic Fibrosis Lung Function. J Pediatr. 2010;157(5):802–807 e801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassanzad M, Eslampanah S, Modaresi M, Tashayoie-Nejad S, Velayati AA. Pulmonary Function and Hospital Admission in Patients with Cystic Fibrosis Based on Household Second-Hand Smoking. Tanaffos. 2018;17(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Baker E, Harris WT, Rowe SM, Rutland SB, Oates GR. Tobacco Smoke Exposure Limits the Therapeutic Benefit of Tezacaftor/Ivacaftor in Pediatric Patients with Cystic Fibrosis. J Cyst Fibros. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courville CA, Raju SV, Liu B, Accurso FJ, Dransfield MT, Rowe SM. Recovery of Acquired Cystic Fibrosis Transmembrane Conductance Regulator Dysfunction after Smoking Cessation. Am J Respir Crit Care Med. 2015;192(12):1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis BW, Sultana R, Sharma R, et al. Early Postnatal Secondhand Smoke Exposure Disrupts Bacterial Clearance and Abolishes Immune Responses in Muco-Obstructive Lung Disease. J Immunol. 2017;199(3):1170–1183. [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between Cigarette Smoking, Pipe/Cigar Smoking, and Smoking Cessation, and Haemostatic and Inflammatory Markers for Cardiovascular Disease. Eur Heart J. 2005;26(17):1765–1773. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000Cdc Growth Charts for the United States: Methods and Development. Vital Health Stat 11. 2002(246):1–190. [PubMed] [Google Scholar]
- 16.Allison P Missing Data: Quantitative Applications in the Social Sciences. 2001. In: Sage: Thousand Oaks. [Google Scholar]
- 17.Cogen J, Emerson J, Sanders DB, et al. Risk Factors for Lung Function Decline in a Large Cohort of Young Cystic Fibrosis Patients. Pediatr Pulmonol. 2015;50(8):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical Use of Ibuprofen Is Associated with Slower Fev1 Decline in Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2007;176(11):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Keogh R, Clancy JP, Szczesniak RD. Flexible Semiparametric Joint Modeling: An Application to Estimate Individual Lung Function Decline and Risk of Pulmonary Exacerbations in Cystic Fibrosis. Emerg Themes Epidemiol. 2017;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt JM. Treatment of Pulmonary Exacerbations in Cystic Fibrosis. Eur Respir Rev. 2013;22(129):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp BT, Ortega-Garcia JA, Sadreameli SC, et al. The Impact of Secondhand Smoke Exposure on Children with Cystic Fibrosis: A Review. Int J Environ Res Public Health. 2016;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vital Signs: Disparities in Nonsmokers’ Exposure to Secondhand Smoke—United States, 1999–2012. Morbidity and Mortality Weekly Report 2015;64(4):103–108. Accessed November 29, 2020. [PMC free article] [PubMed] [Google Scholar]
- 23.Creamer M, Wang T, Babb S, et al. Tobacco Product Use and Cessation Indicators among Adults — United States, 2018. MMWR Morb Mortal Wkly Rep 2019. 2019;68(45):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotz D, Batra A, Kastaun S. Smoking Cessation Attempts and Common Strategies Employed. Dtsch Arztebl Int. 2020;117(1-2):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotz D, West R. Explaining the Social Gradient in Smoking Cessation: It's Not in the Trying, but in the Succeeding. Tob Control. 2009;18(1):43–46. [DOI] [PubMed] [Google Scholar]
- 26.Sexton K, Adgate JL, Church TR, et al. Children's Exposure to Environmental Tobacco Smoke: Using Diverse Exposure Metrics to Document Ethnic/Racial Differences. Environmental Health Perspectives. 2004;112(3):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merianos AL, Jandarov RA, Choi K, Mahabee-Gittens EM. Tobacco Smoke Exposure Disparities Persist in Us Children: Nhanes 1999–2014. Preventive medicine. 2019;123:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West R, McEwen A, Bolling K, Owen L. Smoking Cessation and Smoking Patterns in the General Population: A 1-Year Follow-Up. Addiction. 2001;96(6):891–902. [DOI] [PubMed] [Google Scholar]
- 29.Fiore M, United States. Tobacco Use and Dependence Guideline Panel. Treating Tobacco Use and Dependence : 2008 Update. 2008 update ed. Rockville, Md.: U.S. Dept. of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 30.Prochaska JJ, Benowitz NL. The Past, Present, and Future of Nicotine Addiction Therapy. Anna Rev Med. 2016;67:467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oates GR, Harris WT, Gutierrez HH, et al. Tobacco Smoke Exposure in Pediatric Cystic Fibrosis: A Qualitative Study of Clinician and Caregiver Perspectives on Smoking Cessation. Pediatr Pulmonol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.