Abstract
Background:
Evidence evaluating the prospective association between low- to moderate-inorganic arsenic (iAs) exposure and cardiovascular disease in the general US population is limited. We evaluated the association between urinary arsenic concentrations in National Health and Nutrition Examination Survey (NHANES) 2003-2014 and heart disease mortality linked from the National Death Index through 2015.
Methods:
We modeled iAs exposure as urinary total arsenic and dimethylarsinate among participants with low seafood intake, based on low arsenobetaine levels (N=4,990). We estimated multivariable adjusted hazard ratios (HRs) for heart disease mortality per interquartile range (IQR) increase in urinary arsenic levels using survey-weighted, Cox proportional hazards models, and evaluated flexible dose-response analyses using restricted quadratic spline models. We updated a previously published relative risk of coronary heart disease mortality from a dose-response meta-analysis per a doubling of water iAs (e.g., from 10 to 20 μg/L) with our results from NHANES 2003-2014, assuming all iAs exposure came from drinking water.
Results:
A total of 77 fatal heart disease events occurred (median follow-up time 75 months). The adjusted HRs (95% CI) of heart disease mortality for an increase in urinary total arsenic and DMA corresponding to the interquartile range were 1.20 (0.83, 1.74) and 1.18 (0.68, 2.05), respectively. Restricted quadratic splines indicate a significant association between increasing urinary total arsenic and the HR of fatal heart disease for all participants at the lowest exposure levels <4.5 μg/L. The updated pooled relative risk of coronary heart disease mortality per doubling of water iAs (μg/L) was 1.16 (95% CI 1.07, 1.25).
Conclusions:
Despite a small number of events, relatively short follow-up time, and high analytical limits of detection for urinary arsenic species, iAs exposure at low- to moderate- levels is consistent with increased heart disease mortality in NHANES 2003-2014 although the associations were only significant in flexible dose-response models.
INTRODUCTION
Inorganic arsenic (iAs) is an established carcinogen for cancers of the skin, lung, and bladder, a possible carcinogen for cancers of the kidney, liver, and prostate (International Agency for Research on Cancer 2004), and has been associated with adverse birth outcomes (Milton et al. 2017), renal disease (Moody et al. 2018), diabetes (Kuo et al. 2017; Kuo et al. 2015), respiratory disease (Sanchez et al. 2018), and cardiovascular disease (Moon et al. 2018a). For cardiovascular disease, most epidemiological evidence comes from populations exposed to high concentrations of iAs (>50 μg/L) through drinking water (Chen et al. 2011; Moon et al. 2012; Yuan et al. 2007). Evidence evaluating the prospective association between low- to moderate- levels of iAs exposure (<50 μg/L in drinking water) and clinical cardiovascular disease, especially in US populations, are limited; a recent dose-response meta-analysis of populations with low- to moderate- iAs exposure found a positive association with clinical cardiovascular disease, but was limited by the small number of studies (N=5) (Moon et al. 2018a; Navas-Acien et al. 2019).
Drinking water and diet are the main sources of iAs exposure for the general US population. Drinking water arsenic exposure is a major concern for both communities reliant on domestic well water and communities reliant on regulated public drinking water systems (especially Hispanic and tribal communities, incarcerated populations in the Southwest, and rural communities in the Southwest and Central Midwest) (Ayotte et al. 2017; Nigra et al. 2020; Nigra and Navas-Acien 2020). Diet is the major source of iAs exposure when water arsenic concentrations are low (Kurzius-Spencer et al. 2014; Xue et al. 2010). Foods with high iAs levels include rice and rice products, cereals, other grains, vegetables, fruit and fruit juices, beers, and wines (Carignan et al. 2016; Davis et al. 2012; Navas-Acien et al. 2011; Nigra et al. 2016; Punshon et al. 2017; Rey deCastro et al. 2014; United States Food and Drug Administration 2014). Seafoods contain high levels of largely non-toxic organic arsenicals such as arsenobetaine and arsenocholine (Cubadda et al. 2017; Navas-Acien et al. 2011; Taylor et al. 2017; Taylor and Jackson 2016; Xue et al. 2010).
iAs exposure is typically modeled as the sum of inorganic (As+5, As+3) and methylated (MMA, DMA) arsenic species in urine. In general populations, however, evaluating exposure to iAs is complicated if seafood intake is common because recent seafood consumption contributes large concentrations of mostly non-toxic organic arsenicals (e.g. arsenobetaine, arsenocholine, arsenosugars, arsenolipids), some of which are further metabolized to DMA. Seafoods are also an important source of omega-3 polyunsaturated fatty acids protective of cardiovascular health (Mozaffarian and Wu 2011; Siscovick et al. 2017). Therefore, when estimating iAs exposure and internal dose, analyses of iAs exposure and cardiovascular disease in US populations must account for interindividual differences in iAs methylation capacity and remove the contribution of largely non-toxic organic arsenicals derived from seafood to eliminate large measurement error and confounding by recent seafood intake. Restriction to participants with undetectable arsenobetaine and regression recalibration of total arsenic and DMA concentrations for arsenobetaine are two common approaches for populations with frequent seafood intake (seafood consumption is the only source of arsenobetaine) (Jones et al. 2016).
The National Health and Nutrition Examination Survey (NHANES) is an on-going, nationally representative survey of the US population conducted by the National Center for Health Statistics (NCHS) which can address many limitations of the current literature evaluating iAs exposure and cardiovascular disease in the US population. NHANES participants are selected to be representative of multiple age and racial/ethnic groups and undergo a thorough examination including demographics and dietary questionnaires, physical examination, and laboratory testing of collected biospecimens, including the measurement of arsenic species in spot urine samples. Given the relevance of diet as an exposure source in the US population, urinary biomarkers of iAs exposure are preferred over water arsenic measures in epidemiologic analyses. The NCHS has also linked NHANES participants to death certificate records from the National Death Index (NDI) in publicly available datasets, enabling prospective analyses; NDI mortality data is available for NHANES 2003-2014 participants followed through December 2015.
Our objective was to evaluate the prospective association between baseline urinary iAs exposure, as reflected in urinary arsenic levels, and heart disease mortality through the end of 2015 for NHANES 2003-2014 participants. Analysis of urinary arsenic data from NHANES is limited by high limits of detection (LODs) for As+5, As+3, MMA, DMA, and arsenobetaine, which result in a large proportion of participants with concentrations below the LOD. Our primary biomarkers of iAs exposure are urinary total arsenic and DMA, restricted to participants with low/undetectable arsenobetaine (urinary arsenobetaine <1.2 μg/L, the highest LOD across all survey cycles). We also updated a previously published dose-response meta-analysis of the association between chronic low- to moderate- arsenic exposure and coronary heart disease mortality with our findings from NHANES 2003-2014 (Moon et al. 2018a).
METHODS
Study population and mortality follow-up
We evaluated participants 18 years and older with urinary arsenic data available from the six 2003-2014 cycles of NHANES and National Death Index (NDI) mortality follow-up data available through 2015. Participants from the 2003/2004 through the 2013/2014 NHANES cycle were eligible for mortality follow-up through December 2015 via the NDI review of death certificate records. Participants with insufficient identifying data were deemed ineligible for mortality follow-up by the National Center for Health Statistics (N= 66) (Office of Analysis and Epidemiology National Center for Health Statistics 2019). Among the participants with sufficient identifying data, those not identified in the NDI at the time of outcome ascertainment (December 2015) were assumed alive. For participants from all cycles, the underlying cause of death (considering both International Classification of Disease 9 and 10 codes) was available for diseases of the heart [I00-I09 (acute rheumatic fever and chronic rheumatic heart disease), I11 (hypertensive heart disease), I13 (hypertensive heart and chronic kidney disease), and I20-I51 (including angina pectoris, myocardial infarction, and other coronary heart disease)], malignant neoplasms (C00-C97), and all other causes (residual). We evaluated heart disease mortality (diseases of the heart) for all NHANES 2003-2014 participants. Cardiovascular disease mortality (diseases of the heart or cerebrovascular diseases, I60-I69) was not evaluated because data for cerebrovascular diseases was only available for NHANES 2003-2006 participants and the number of events was too small.
Exclusion criteria
A total of 16,332 NHANES 2003-2014 participants had urinary arsenic measurements available. We excluded 279 participants who were pregnant, 237 missing body mass index, 1,061 with unreliable past 24-hour dietary recall status (because we relied on self-reported seafood/fish intake in our main models; unreliable dietary intake was determined by the National Center for Health Statistics when participants did not complete at least four of the five interview-recall steps), 220 missing urinary creatinine, 190 missing educational variables, 4,115 who were ineligible for mortality follow-up through the NDI, 95 missing urinary arsenobetaine, and 63 missing urinary total arsenic. To reduce the contribution of seafood-derived arsenicals to total arsenic and DMA concentrations, we further excluded 5,082 participants with arsenobetaine ≥1.2 μg/L (the highest arsenobetaine LOD across the 2003-2014 survey cycles) for a final sample size of 4,990 participants. Arsenobetaine has a half-life of 1-2 days, and excluding participants with detectable arsenobetaine levels in urine is a robust method to identify participants with seafood intake in the last few days (Navas-Acien et al. 2011).
Urine arsenic measurements
Total arsenic and speciated arsenic were measured via inductively coupled plasma-mass spectrometry in spot urine samples collected during the examination in a one-third random subsample of participants ≥ 6 years of age; laboratory methods have been previously described in detail (National Center for Health Statistics 2014, 2016). Briefly, recorded inter-assay coefficients of variation varied from 0.5 to 10.5% for As+5; from 0.0 to 12.1% for As+3; from 0.4 to 8.6% for monomethylarsonate (MMA); 0.0 to 11.4% for dimethylarsinate (DMA); 0.0 to 17.8% for arsenobetaine; and 0.7 to 19.4% for total arsenic. The limit of detection (LOD) varied from 0.79 to 1.0 μg/L for As+5; from 0.12 to 1.2 μg/L for As+3; from 0.2 to 0.9 μg/L for MMA; from 1.70 to 1.91 μg/L for DMA; from 0.4 to 1.19 μg/L for arsenobetaine; and from 0.26 to 0.88 μg/L for total arsenic (Supplemental Table 1). Values measured below the LOD were replaced by NCHS with the survey-specific LOD divided by the square root of two.
Estimation of inorganic arsenic exposure
Ideally, iAs internal dose is best estimated by summing urinary concentrations of As+5, As+3, MMA, and DMA to account for the substantial interindividual variability in the metabolism of inorganic arsenic species (As+5, As+3) to methylated metabolites (MMA, DMA). Because NHANES has relatively high limits of detection for some arsenic species and relatively low iAs exposures compared to some other epidemiological cohorts, the large majority of participants had undetectable concentrations of multiple arsenic species (especially As+5, As+3, and MMA, see Supplemental Table 1). Under these conditions, total arsenic and DMA (the main metabolite of iAs present in urine) are appropriate biomarkers of iAs internal dose when the contribution of organic arsenicals resulting from recent seafood intake which confound the estimation of iAs exposure are removed by either regression adjustment for arsenobetaine, regression recalibration for arsenobetaine, or restriction to participants with low arsenobetaine (Jones et al. 2016). For our primary analysis, we estimated iAs internal exposure as total arsenic and DMA as originally reported in NHANES, restricting to participants with low urinary arsenobetaine. To retain participants from all survey years while removing the contribution of seafood-derived arsenicals to total arsenic and DMA, we restricted our analysis to participants with arsenobetaine <1.2 μg/L (N = 4,990) and adjusted our models for self-reported past 24-hour intake of seafood/ fish, calculated from Food Commodity Index codes as previously reported (Nigra et al. 2016). Because the LOD for arsenobetaine was 1.19 and 1.16 μg/L in 2011/2012 and 2013/2014, respectively, this approach retains participants across all survey cycles (Supplemental Table 1).
Cox proportional hazard modeling
Urinary arsenic distributions were skewed and log-transformed for analysis. We used survey-weighted Cox proportional hazards models to evaluate progressively adjusted hazard ratios (HRs) of heart disease mortality by increasing quartile of urinary total arsenic and DMA concentrations using the ‘survey’ package in R (Lumley 2014). We used age as the time metric, with participants entering as late entries corresponding to age at baseline (NHANES examination). Participants were censored if they experienced a death recorded in the NDI and were assumed alive if no NDI record was available by the end of follow-up (December 2015). We also modeled the association per urinary arsenic increase corresponding to the difference between the 75th and 25th percentile (interquartile range, IQR). Model 1 was adjusted for age (age from baseline modeled as time metric), sex (male/female), race/ethnicity (Mexican American/non-Hispanic white/non-Hispanic Black/other, including multiple races), urinary creatinine (mg/dL, continuous, to account for urine dilution), and estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) formula because glomerular filtration rate influences arsenic excretion in urine (these are known confounders of the iAs-heart disease relationship) (Hsieh et al. 2019; Levey et al. 2009; Sweeney et al. 2010; Zheng et al. 2015). Model 2 (the main model of interest) was further adjusted for education (≤ 12 years / >12 years), body mass index (kg/m2, continuous), total blood cholesterol (mg/dL, continuous), serum cotinine (ng/mL, continuous), and self-reported past 24-hour intake of seafood and fish (g/kg bodyweight, log transformed continuous) (these are also confounders of the iAs-heart disease relationship). Model 3 (the mediation model) was further adjusted for hypertension (binary; defined as an average of three systolic blood pressure readings greater than or equal to 140 mmHg, an average of three diastolic blood pressure readings greater than or equal to 90 mmHg, self-reported use of hypertension medication, or self-reported physician diagnosis) and diabetes status (binary; defined as self-reported use of medication or self-reported physician diagnosis), because these may partially mediate the association between iAs exposure and fatal heart disease (Moon et al. 2013). We evaluated the proportional hazards assumption by visually assessing Schoenfeld residuals. As sensitivity analyses, we also adjusted Model 2 for urinary cadmium (μg/L, log transformed continuous) and blood lead (μg/dL, log transformed continuous) because these metals are also toxic to the cardiovascular system, and the NHANES survey cycle (categorical) to account for differences in urinary arsenic detection limits across cycles, with similar findings (data not shown). All covariates were measured once at baseline and treated as time fixed. To allow a more flexible dose-response analysis, we also modeled urinary total arsenic and DMA continuously using a restricted quadratic spline model with knots at the 10th, 50th, and 90th percentiles with the reference at the 10th percentile, with corresponding model adjustments for both Model 1 and Model 2 (main model of interest).
Sub-group analyses
In exploratory effect measure modification analyses, we evaluated whether the HRs for heart disease mortality per interquartile range increase in urinary total arsenic and DMA differed by the following subgroups: age (<70/≥ 70), sex (male/female), race/ethnicity (Mexican-American/non-Hispanic white/non-Hispanic Black/other, including multiple races), education (<12 yrs/≥12 yrs), self-reported smoking status (never/former/current), and BMI (<25/25-29/ ≥30 kg/m2). “Current” smokers were defined as having smoked greater than 100 cigarettes in their lifetime and currently smoking, “former” smokers were defined as smoking greater than 100 cigarettes in their lifetime but not currently smoking, and “never” smokers were defined as smoking less than 100 cigarettes in their lifetime. Cox proportional hazards models included interaction terms between the subgroup indicator and an increase in urinary total arsenic and DMA corresponding to the difference in the 75th versus 25th percentile. Because there are racial/ethnic differences in the consumption of arsenic-containing foods (e.g. rice) which contribute to urinary DMA and total arsenic concentrations, and potential differences in susceptibility to cardiovascular disease by racial/ethnic group, we hypothesized a priori that race/ethnicity may be an important effect measure modifier (Awata et al. 2017; Jones et al. 2018). We restricted this subgroup analyses to Mexican-American, non-Hispanic white, and non-Hispanic Black participants because of the small number of events experienced by participants in the Other Hispanic group (N= 3) and Other race, including multi-racial group (N= 4). Flexible restricted quadratic splines were modeled for all participants and also separately for non-Hispanic white participants because this was the only racial/ethnic group with an adequate number of events (N= 50) to evaluate spline models. Because the overall and stratum-specific number of events for heart disease mortality was low (N= 77 events overall), these effect estimates are purely hypothesis generating and should be interpreted with caution.
Sensitivity analyses
We conducted several sensitivity analyses to further assess potential confounding by seafood intake and potential selection bias introduced by arsenobetaine restriction critera. First, we further restricted our analysis to participants who reported less than 15 grams of seafood/fish intake in the 24-hours preceeding examination and spot urine collection (N= 2,421 participants, N= 39 events) because we observed significant associations between self-reported seafood/fish intake and urine total arsenic and DMA above 16 grams of reported intake (Supplemental Table 2). Second, we repeated our main analyses restricting to participants with arsenobetaine <0.7 μg/L (N = 2,674) without further adjustment for self-reported intake of seafood/fish because at this cut-point reported seafood/fish intake was no longer associated with increasing quartiles of total arsenic and DMA (p>0.1) (Supplemental Table 2). This analysis excludes participants in survey cycles 2011/2012 and 2013/2014 because the LOD for arsenobetaine was above 0.7 μg/L in both survey cycles. Finally, we also evaluated observed distributions of total arsenic and DMA as reported for all NHANES participants regardless of urine arsenobetaine concentrations (N= 10,072 participants, N= 167 events), with regression recalibration for arsenobetaine as previously described (Jones et al. 2016). To evaluate the potential for selection bias resulting from restriction for urinary arsenobetaine concentrations, we assessed participant characteristics separately for participants with arsenobetaine <1.2 μg/L, participants with arsenobetaine <0.7 μg/L, and all NHANES participants without restriction for arsenobetaine by evaluating differences in participant characteristics across these various analytical study populations, including differences in mean age, serum cotinine, body mass index, total serum cholesterol, eGFR, self-reported seafood intake, urinary total arsenic, and urinary DMA, and differences in the weighted percentage of participants by sex, race/ethnicity, education, smoking status, hypertension status, and diabetes status.
Revised dose-response meta-analysis pooled relative risk for coronary heart disease mortality
Moon et al. previously reported a pooled relative risk (RR) for coronary heart disease mortality of 1.16 (95% CI 1.07, 1.26) per doubling of water arsenic (e.g., from 10 to 20 μg/L) across six prospective epidemiologic studies from populations with a range of low, moderate, and high iAs exposures. We updated this pooled RR by including the results from our primary NHANES 2003-2014 analysis (HR of heart disease mortality across quartiles of urinary total arsenic, restricted to participants with urinary arsenobetaine <1.2 μg/L, with Model 2 adjustments), following previously described methods (Moon et al. 2018a). We assumed that most fatal heart disease events experienced by NHANES participants were coronary heart disease events. Briefly, we assigned the median arsenic concentration within each quartile to all participants within each quartile, and assumed all urinary arsenic came from drinking water. Pooled analyses from a two-stage random-effects meta-analysis considered both log-linear and flexible restricted cubic splines of log-transformed arsenic relative risk models, as previously described (Moon et al. 2018a). We report RRs of coronary heart disease mortality per doubling of water arsenic from 10 μg/L (reference) to 20 μg/L. We assessed the amount of statistical heterogeneity across studies using the I2 statistic, which describes the proportion of total variation due to between-study heterogeneity, and Cochran’s Q-statistic (Higgins et al. 2003; Jackson et al. 2012).
RESULTS
For all 4,990 participants, the median (IQR) of total arsenic and DMA were 4.42 μg/L (2.52, 7.20) and 2.71 μg/L (1.35, 4.42), respectively. Participants were followed for 31,896 person-years, and a total of 438 fatal events occurred; 77 of these were fatal heart disease events (Table 1). At baseline, participants who later experienced a fatal heart disease event were older, more likely male, less likely Mexican-American, more likely former smokers, more likely to have hypertension, more likely to have diabetes, and more likely to have lower eGFR (Table 1). Compared to participants who did not experience a fatal heart disease event, those who later experienced a fatal heart disease event had lower baseline urinary total arsenic (median 3.50 μg/L versus 4.01 μg/L) and DMA concentrations (median μg/L 2.39 versus 2.48 μg/L) in unadjusted analyses (Table 1). Compared to participants in the lowest quartile of urinary total arsenic, those in the highest quartile of urinary total arsenic were younger, more likely male, more likely Mexican-American, other Hispanic, and non-Hispanic Black, were less likely to have hypertension, had higher eGFR, and reported consuming more seafood/fish in the previous 24-hours (Table 1).
Table 1.
Baseline participant characteristics by quartile of urinary total arsenic (μg/L) and heart disease mortality status (case versus non-case), restricted to participants with urinary arsenobetaine <1.2 μg/L (N=4,990).
| Quartile 1 (≤2.30 μg/L) | Quartile 2 (2.31- 4.00 μg/L) | Quartile 3 (4.01 – 6.50 μg/L) | Quartile 4 (> 6.51 μg/L) | Non-cases (N= 4,913) | Cases (N=77) | |
|---|---|---|---|---|---|---|
| Age (yr) - mean (SE) | 47.5 (0.6) | 45.2 (0.6) | 43.7 (0.6) | 42.3 (0.6) | 44.4 (0.4) | 72.2 (1.4) |
| Sex- N (%) male | 418 (35.8) | 531 (44.3) | 655 (52.0) | 838 (58.0) | 2,528 (47.4) | 2,385(61.9) |
| Race/ethnicity – N (%) | ||||||
| Mexican-American | 165 (6.5) | 200 (8.1) | 245 (8.9) | 390 (14.1) | 993 (9.4) | 7 (3.9) |
| Other Hispanic | 43 (2.3) | 87 (4.3) | 102 (4.4) | 172 (7.8) | 401 (4.7) | 3 (2.0) |
| Non-Hispanic white | 664 (80.8) | 644 (76.6) | 600 (71.1) | 533 (62.1) | 2,391 (72.6) | 50 (79.2) |
| Non-Hispanic Black | 144 (5.7) | 171 (7.2) | 275 (12.0) | 289 (11.4) | 866 (9.1) | 13 (9.1) |
| Other including multiple | 70 (4.7) | 66 (3.9) | 52 (3.6) | 78 (4.6) | 262 (4.2) | 4 (5.8) |
| Education – N (%) | ||||||
| < 12 years | 299 (17.8) | 302 (17.1) | 408 (20.7) | 487 (21.7) | 1,467 (19.2) | 29 (29.5) |
| ≥ 12 years | 787 (82.2) | 866 (82.9) | 866 (79.3) | 975 (78.3) | 3,446 (80.8) | 48 (70.5) |
| Smoking – N (%) | ||||||
| Never | 547 (51.5) | 603 (53.6) | 635 (53.5) | 694 (52.5) | 2,447 (52.9) | 32 (45.4) |
| Former | 230 (22.0) | 281 (25.4) | 274 (21.2) | 303 (21.7) | 1,058 (22.4) | 30 (40.8) |
| Current | 264 (26.5) | 232 (20.9) | 267 (25.2) | 324 (25.8) | 1,072 (24.7) | 15 (13.8) |
| Cotinine – mean (SE)a | 77.1 (6.3) | 65.3 (5.8) | 75.8 (4.7) | 77.8 (6.4) | 74.4 (3.3) | 36.8 (12.4) |
| BMI - mean (SE) | 27.9 (0.2) | 28.4 (0.2) | 29.0 (0.2) | 28.6 (0.2) | 28.5 (0.1) | 29.2 (0.9) |
| Cholesterol – mean (SE)b | 194.9 (1.6) | 194.6 (1.4) | 195.2 (1.6) | 192.9 (1.3) | 194.4 (0.8) | 196.7 (7.5) |
| Hypertension – N (%)c | 438 (36.7) | 452 (34.3) | 465 (33.7) | 490 (29.8) | 1,788 (33.2) | 57 (72.5) |
| Diabetes – N (%)d | 113 (9.7) | 138 (8.8) | 152 (8.9) | 153 (7.8) | 535 (8.6) | 21 (28.5) |
| eGFR - mean (SE)e | 92.8 (0.8) | 93.8 (0.8) | 93.4 (0.7) | 96.3 (0.8) | 94.6 (0.4) | 62.7 (3.1) |
| Seafood intake – mean (SE)f | 10.9 (1.5) | 14.4 (2.2) | 16.0 (2.8) | 16.3 (2.1) | 14.4 (1.1) | 17.1 (6.1) |
| Urinary total arsenic, μg/L g | 1.60 (1.15, 1.98) | 3.09 (2.70, 3.51) | 5.06 (4.56, 5.75) | 9.22 (7.65, 11.90) | 4.01 (2.30, 5.54) | 3.50 (2.04, 5.37) |
| Urinary DMA, μg/L g | 1.27 (1.20, 1.35) | 1.98 (1.27, 2.30) | 3.17 (2.69, 3.72) | 5.72 (4.57, 7.52) | 2.48 (1.35, 4.00) | 2.39 (1.23, 3.23) |
Analyses conducted using ‘survey’ package to account for NHANES complex sampling design and weights; percentages are weighted.
Cotinine measured in serum (ng/mL).
Total cholesterol measured in serum (mg/dL).
Hypertension defined as an average of three systolic blood pressure readings greater than or equal to 140 mmHg, an average of three diastolic blood pressure readings greater than or equal to 90 mmHg, self-reported use of hypertension medication, or self-reported physician diagnosis.
Diabetes defined as self-reported use of medication or self-reported physician diagnosis.
Estimated glomerular filtration rate (eGFR) using CKD-Epi formula.
Self-reported past 24-hour intake in g/kg bodyweight.
Values are median (interquartile range).
Hazard ratios of heart disease mortality
The median (range) of follow-up time was 6.3 (0.1, 13.1) years. In our main model adjusting for age, sex, race/ethnicity, urinary creatinine, and eGFR (Model 2), the HR (95% CI) of heart disease mortality comparing the highest to the lowest quartile of urinary total arsenic and urinary DMA were 1.21 (0.46, 3.14) and 1.09 (0.37, 3.25) respectively (Model 2, Table 2). Further adjustment for diabetes and hypertension status (potential mediators) somewhat attenuated effect estimates for total arsenic (1.18, 95% CI 0.44, 3.14, although 95% CIs for Model 2 and Model 3 overlap) but not for DMA (1.09, 95% CI 0.35, 3.36) Model 3, Table 2). Adjusted HRs of heart disease mortality for an increase in log urinary total arsenic and DMA corresponding to the IQR (1.05 μg/L and 1.19 μg/L, respectively) were 1.20 (0.83, 1.74) and 1.18 (0.68, 2.05), respectively (Model 2, Table 2). Schoenfeld residuals indicated proportional hazards for all models evaluating urine total arsenic and DMA, except for the model assessing increasing quartiles of DMA for which proportionality may not hold. In flexible dose-response analyses, restricted quadratic spline models showed a positive and significant association between log-scale urinary total arsenic and heart disease mortality at the lowest ends of the distribution for both all participants and non-Hispanic white participants (Figure 1).
Table 2.
Hazard ratios (95% CIs) for heart disease mortality by quartiles of urinary total arsenic and DMA (μg/L) and per increase corresponding to the interquartile range (IQR), restricted to participants with arsenobetaine <1.2 μg/L (N=4,990).
| Urinary total arsenic | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per IQR a | |
| No. cases/ non-cases | 77 / 4,913 | 19 / 1,067 | 25 / 1,143 | 17 / 1,257 | 16 / 1,446 | |
| Person-years | 31,896 | 6,366 | 7,073 | 8,407 | 10,049 | |
| HRs (95% CI) | ||||||
| Model 1 | 1 (reference) | 1.24 (0.60, 2.58) | 1.45 (0.64, 3.29) | 1.24 (0.45, 3.46) | 1.21 (0.81, 1.79) | |
| Model 2 (main model) | 1 (reference) | 1.24 (0.58, 2.68) | 1.44 (0.65, 3.21) | 1.21 (0.46, 3.14) | 1.20 (0.83, 1.74) | |
| Model 3 (mediation) | 1 (reference) | 1.20 (0.55, 2.59) | 1.38 (0.63, 3.05) | 1.18 (0.44, 3.14) | 1.17 (0.79, 1.73) | |
|
| ||||||
| Urinary DMA | ||||||
|
| ||||||
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per IQR a | |
| No. cases/ non-cases | 77 / 4,913 | 25 / 1,398 | 27 / 837 | 22 / 1,245 | 13 / 1,433 | |
| Person-years | 31,896 | 8,191 | 5,757 | 8,640 | 9,309 | |
| HRs (95% CI) | ||||||
| Model 1 | 1 (reference) | 1.28 (0.56, 2.88) | 1.81 (0.86, 3.82) | 1.14 (0.38, 3.42) | 1.25 (0.70, 2.23) | |
| Model 2 (main model) | 1 (reference) | 1.28 (0.55, 2.99) | 1.77 (0.84, 3.70) | 1.09 (0.37, 3.25) | 1.18 (0.68, 2.05) | |
| Model 3 (mediation) | 1 (reference) | 1.26 (0.53, 3.00) | 1.77 (0.84, 3.70) | 1.09 (0.35, 3.36) | 1.18 (0.68, 2.08) | |
Model 1 adjusts for age, sex, race/ethnicity (Mexican American/non-Hispanic white/non-Hispanic Black/other, including multiple races), urinary creatinine (mg/dL, and eGFR (CKD-Epi formula)
Model 2 further adjusts for education (binary, less than high school/high school or greater), body mass index, total cholesterol (mg/dL), serum cotinine (ng/mL), and past 24-hr reported intake of fish and seafood (g/kg bodyweight)
Model 3 further adjusts for diabetes and hypertension, potential mediators
Hazard ratio (95% confidence interval) of heart disease mortality by an increase in log transformed urinary total arsenic and DMA concentration corresponding to the interquartile range (75th to 25th percentile). Interquartile range values were 1.05 μg/L for total arsenic and 1.19 μg/L for DMA.
Figure 1. Restricted quadratic spline models of hazard ratios (HRs) and 95% confidence intervals of heart disease mortality for a one-unit increase in urinary total arsenic and DMA concentrations (μg/L), restricted to all participants (Panel A; N=4,990, cases= 77) and non-Hispanic white participants (Panel B; N=2,441, cases = 50) with arsenobetaine <1.2 μg/L, NHANES 2003-2014.
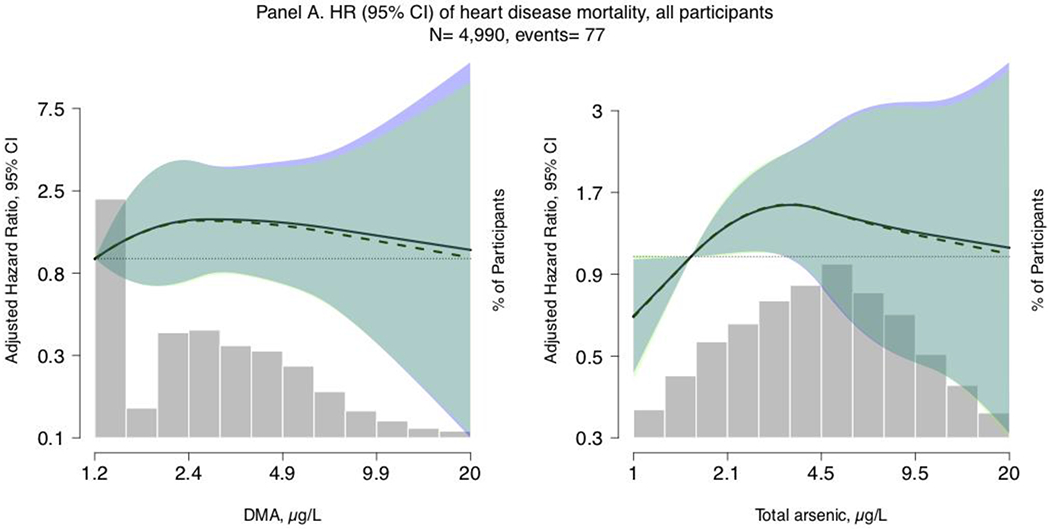
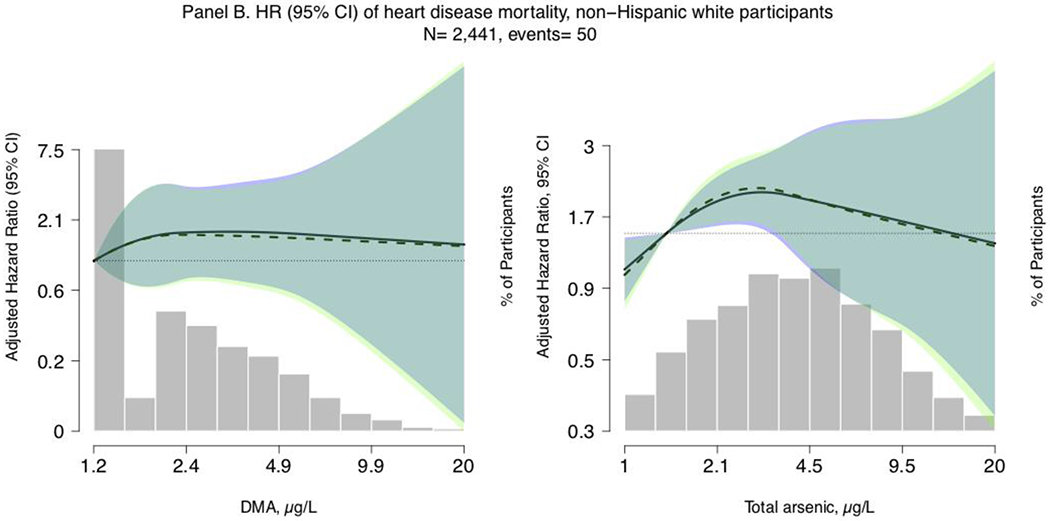
Lines represent the hazard ratio (left y-axis) based on restricted quadratic spline models with knots at the 10th, 50th, and 90th percentiles of urinary arsenic concentrations (x-axis), with the reference set at the 10th percentile. Shaded rectangles represent the proportion of the population (right y-axis) with urinary arsenic concentrations at a given point on the urinary arsenic distribution (x-axis). Solid lines (HRs) and blue areas surrounding the solid lines (95% confidence interval) correspond to Model 1, which is adjusted for age (age from baseline modeled as time metric), sex (male/female), race/ethnicity (Mexican American/Non-Hispanic White/Non-Hispanic Black/Other, including Multiple Races), estimated glomerular filtration rate (continuous, using CKD-Epi formula), and urinary creatinine (mg/dL, continuous, to account for urine dilution). Dashed lines (HRs) and light green areas surrounding the dashed lines (95% confidence interval) correspond to Model 2, which is further adjusted for education (≤ 12 years / >12 years), body mass index (continuous), total blood cholesterol (mg/dL, continuous), serum cotinine (ng/mL, continuous), and self-reported past 24-hour intake of seafood and fish (g/kg bodyweight). Dark green areas represent overlap in the 95% confidence intervals between Models 1 and 2.
Sensitivity analyses considering alternate inorganic arsenic exposure metrics
To identify potential selection bias issues related to our sensitivity analyses which assessed alternative restriction criteria, Supplemental Table 3 presents participant characteristics for all NHANES participants, participants with arsenobetaine <1.2 μg/L, and participants with arsenobetaine <0.7 μg/L. Compared to all NHANES participants, those with arsenobetaine < 1.2 μg/L were slightly more likely Mexican-American (9.4% versus 8.4%) and slightly less likely non-Hispanic Black (9.1% versus 11.2%), reported lower seafood/fish intake (14.4 g/kg bodyweight versus 34.0 g/kg bodyweight), and had lower median urine total arsenic (4.00 μg/L versus 7.49 μg/L) and DMA (2.48 μg/L versus 3.11 μg/L). Similar patters were found among participants with arsenobetaine <0.7 μg/L. In sensitivity analyses further restricting to participants with past 24-hour self-reported intake of seafood/fish <15 grams (N = 2,421 participants and N = 39 events), the HRs of heart disease mortality for an increase in urine total arsenic and DMA corresponding to the IQR were 1.20 (0.82, 1.77) and 1.18 (0.68, 2.07), respectively (Model 2, Supplemental Table 4). In sensitivity analyses restricting to participants with urinary arsenobetaine <0.7 μg/L (participants from 2003-2010 survey cycles only, N = 2,674 participants and N= 55 events), the HRs of heart disease mortality for an increase in log urinary total arsenic and DMA corresponding to the IQR were 1.39 (0.92, 2.11) and 1.48 (0.82, 2.67), respectively (Model 2, Supplemental Table 4). In analyses without restriction by arsenobetaine status that recalibrated urine arsenic concentrations for arsenobetaine via regression recalibration (N= 10,072 participants and N= 167 events), HRs of heart disease mortality for an increase in log urinary total arsenic and DMA corresponding to the IQR were 1.00 (0.79, 1.28) and 1.00 (0.71, 1.39) respectively (Model 2, Supplemental Table 4).
Sub-group analyses
In exploratory sub-group analyses for the main model of interest (Model 2), adjusted HRs of heart disease mortality by an increase in log scale urinary total arsenic corresponding to the IQR were significantly different by racial/ethnic group (p=0.002, Supplemental Table 5). Although sub-group specific effect estimates were unstable due to the small number of events in each stratum, the largest effect estimates were seen for Mexican-American participants (HR 5.09, 95% CI 2.00, 12.94), and non-Hispanic white participants (1.17, 95% CI 0.76, 1.80). In contrast, the HR for non-Hispanic Black participants indicated a significant inverse association (0.42, 95% CI 0.23, 0.65). We also observed larger effect estimates for participants <70 years of age (1.58, 95% CI 0.72, 3.43) compared to those >= 70 years (1.08, 95% CI 0.75, 1.56), for never smokers (1.57, 95% CI 0.89, 2.75) compared to current smokers (0.63, 95% CI 0.30, 1.35), and for participants with body mass index >= 30 (1.77, 95% CI 0.90, 3.47) compared to participants with body mass index <25 (0.79, 95% CI 0.43, 1.45). None of these interactions were statistically significant and they are reported only for descriptive purposes.
Revised pooled relative risk of coronary heart disease mortality
In pooled log-linear dose-response association models evaluating the RR of coronary heart disease mortality, adding the results from NHANES 2003-2014 for heart disease (presented in Table 3) to six prior studies (Chen et al. 1996; Chen et al. 2011; D’Ippoliti et al. 2015; Farzan et al. 2015; Moon et al. 2013; Wade et al. 2009) resulted in a pooled RR of 1.88 (95% CI 1.34, 2.62) at 200 μg/L, 1.62 (1.25, 2.10) at 100 μg/L, 1.40 (1.17, 1.68) at 50 μg/L, and 1.16 (1.07, 1.25) at 20 μg/L compared to 10 μg/L (reference) (Table 3). RR estimates were similar in revised models including NHANES 2003-2014 findings compared to the original meta-analysis, the I2 value decreased from 51 (95% CI 0, 83) to 45 (95% CI 0, 79), and the p-value for heterogeneity increased from 0.087 to 0.14. In non-linear restricted cubic spline models, including results from NHANES 2003-2014 resulted in a pooled RR of 1.96 (1.25, 3.06) at 200 μg/L, 1.66 (1.23, 2.25) at 100 μg/L, 1.42 (1.18, 1.71) at 50 μg/L, and 1.16 (1.07, 1.25) at 20 μg/L compared to 10 μg/L (reference).
Table 3.
Pooled relative risks (95% confidence intervals) for coronary heart disease mortality in relation to water arsenic concentrations, as published in Moon et al. 2018(a) before and after including results from NHANES 2003-2014.
| # Studies (# relative risks) | Original estimates published by Moon et al. 2018(a) 6 (16) |
Updated estimates including NHANES 2003-2014 7 (19) |
|---|---|---|
| Log-linear or constant dose-response association model (log-transformed arsenic) | ||
| 10 μg/L arsenic | 1.00 (Reference) | 1.00 (Reference) |
| 20 μg/L arsenic | 1.16 (1.07, 1.26) | 1.16 (1.07, 1.25) |
| 50 μg/L arsenic | 1.41 (1.17, 1.71) | 1.40 (1.17, 1.68) |
| 100 μg/L arsenic | 1.64 (1.25, 2.15) | 1.62 (1.25, 2.10) |
| 200 μg/L arsenic | 1.90 (1.34, 2.70) | 1.88 (1.34, 2.62) |
| p-linear trend | <0.001 | <0.001 |
| I2 (95% CI)* | 51 (0, 83) | 45 (0, 79) |
| p-heterogeneity# | 0.087 | 0.14 |
| Non-linear or flexible dose-response association model (restricted cubic splines of log-transformed arsenic) | ||
| 10 μg/L arsenic | 1.00 (Reference) | 1.00 (Reference) |
| 20 μg/L arsenic | 1.16 (1.05, 1.27) | 1.16 (1.07, 1.25) |
| 50 μg/L arsenic | 1.40 (1.13, 1.73) | 1.42 (1.18, 1.71) |
| 100 μg/L arsenic | 1.62 (1.15, 2.28) | 1.66 (1.23, 2.25) |
| 200 μg/L arsenic | 1.87 (1.13, 3.10) | 1.96 (1.25, 3.06) |
| p-nonlinear trend^ | 0.99 | 0.84 |
DISCUSSION
In this analysis of NHANES 2003-2014 participants with low/undetectable urinary arsenobetaine, urinary total arsenic concentrations, reflecting iAs exposure, were positively and significantly associated with heart disease mortality in adjusted restricted quadratic spline models at lower levels of iAs exposure. In dose-response analyses comparing participants in the fourth versus first quartile of iAs exposure, the HR of heart disease mortality ranged from 1.21 (0.46, 3.14) for total arsenic to 1.09 (0.37, 3.25) for DMA for all participants. These positive but non-significant associations were similar to analyses evaluating the association per IQR increase (1.20, 95% CI 0.83, 1.74 for total arsenic, and 1.18, 95% CI 0.68, 2.05 for DMA), were adjusted for a number of known cardiovascular risk factors, and were attenuated for total arsenic with adjustment for likely mediators between iAs exposure and cardiovascular disease (diabetes and hypertension status), as expected.
Our findings also support potential effect measure modification of the association between iAs exposure and heart disease mortality by race/ethnicity. Flexible restricted quadratic spline models indicated a positive and significant association between an increase in urinary total arsenic corresponding to the IQR and fatal heart disease for non-Hispanic white participants, also at lower levels of iAs exposure. Although we were unable to assess flexible restricted quadratic spline models for Mexican-American and non-Hispanic Black participants because of the small number of events in these subgroups, stratified linear models evaluating the HR per increase in total arsenic corresponding to the IQR were markedly higher and significant for Mexican-American participants (5.09, 95% CI 2.00, 12.94) and inverse and significant for non-Hispanic Black participants (0.42, 95% CI 0.23, 0.65). While these findings could suggest potential effect measure modification across racial/ethnic groups, they need to be interpreted carefully due to limitations in sample size and should be further evaluated in cohorts with a larger number of events. Because dietary sources of arsenic exposure differ greatly by racial/ethnic subgroup, these results could reflect selection bias introduced by our arsenobetaine restriction criteria. Although participant characteristics were similar across arsenobetaine restriction criteria, restriction to participants with low seafood/fish intake may also introduce differential selection bias by race/ethnicity if the correlation between seafood/fish consumption and other foods beneficial to the cardiovascular system or other cardiovascular disease risk factors are also differential by racial/ethnic subgroup. In restricting to participants with low arsenobetaine, we may have also removed the cardioprotective impact of seafood/fish consumption. Unmeasured confounding by some other factor that might be relevant and differential by race/ethnicity could also explain the apparent effect measure modification.
Our primary sensitivity analyses excluded participants with urinary arsenobetaine < 0.7 μg/L. Although this analysis was limited by fewer events (N= 55 events) and to NHANES 2003-2010, effect estimates for this analytic sample were stronger than those reported in our main analysis, indicating that stronger restriction by arsenobetaine likely reduced bias from seafood-derived arsenicals while also reducing the precision of our effect estimates. Removing participants in the last two NHANES survey cycles (2011-2014) also removed participants with the shortest follow-up time who contributed person-time to the denominator but were probably less likely to contribute to fatal heart disease events. Although self-reported 24-hour intake of seafood/fish remained associated with increasing quartiles of urinary total arsenic even after restriction to arsenobetaine concentrations <1.2 μg/L, analyses further restricting to participants with reported seafood/fish intake <15 grams were similar to our main effect estimates, indicating that further adjustment for reported seafood intake was likely successful in eliminating confounding by seafood intake (Model 2). Because we relied on arsenic measured in spot urine samples, which reflect exposure from the previous 1-4 days, it is possible that other seafood-derived arsenicals could be contributing to total arsenic concentrations even after restriction to participants with urinary arsenobetaine < 1.2 μg/L and adjustment for self-reported past 24-hour intake of seafood/fish, although this would likely result in an underestimation of the true effect. This may explain why restricted quadratic spline models indicated a significant association between urine total arsenic and heart disease mortality at lower levels of urine total arsenic concentrations only (<4.5 μg/L). It remains unclear why the association plateaus at higher exposure levels, although this phenomenon has been described for a number of environmental exposures and may be related to depletion of susceptible participants with higher exposures levels, measurement error, or other challenges in exposure ascertainment (Stayner et al. 2003; Steenland et al. 2015). Moreover, urinary arsenic is relatively stable within-persons over time in many populations and is a suitable proxy for chronic exposure in the absence of changes in drinking water sources or dietary patterns (Navas-Acien et al. 2009). In contrast, using regression calibration for arsenobetaine yielded null results, possibly indicating that the high limits of detection for arsenobetaine (especially in later survey years, which were not included in the original method development paper) resulted in residual confounding because the complete removal of seafood arsenicals to urine total arsenic and DMA concentrations was not possible. Restriction to participants with low arsenobetaine and further adjustment for reported seafood intake may be the strongest epidemiological tool to address such confounding and the high analytical LODs in NHANES urinary arsenic analyses, especially when arsenobetaine LODs are high.
Increased susceptibility to iAs related cardiovascular disease may also result from differences in iAs methylation capacity (AS3MT) (Balakrishnan et al. 2017; Lindberg et al. 2007; Pierce et al. 2013), underlying nutritional status (especially B-vitamins relevant for one-carbon metabolism) (Bozack et al. 2018; Spratlen et al. 2017), or other sociodemographic and social factors unequally distributed across racial/ethnic subgroups. The potentially higher risk for Mexican-American participants compared to non-Hispanic white and Black participants warrants follow-up, and needs to be confirmed in future studies with longer follow-up time and a higher number of participants and events. This is particularly important because the large majority of Mexican-Americans in the US lived in the Southwestern US during the study period (2003-2014), where concentrations of arsenic in drinking water (from both domestic wells and public water systems) are high relative to the rest of the United States (Ayotte et al. 2017; Gonzalez-Barrera and Lopez 2013; Nigra et al. 2020). Additional high-quality studies across diverse Hispanic/Latino communities are needed, especially because of the great diversity across Hispanic/Latino communities, and the observed lower cardiovascular disease risk among Hispanic groups despite a higher prevalence of certain cardiovascular disease risk factors (Balfour et al. 2016). Due to the small number of events and standardized reporting of race/ethnicity in NHANES from 2003-2014, we were unable to assess the association for participants in the Other Hispanic, non-Hispanic Asian, and Other race, including multi-racial groups.
Although the current analysis was limited by the small number of events, short follow-up time (median time from NHANES examination to end of NDI follow-up 75 months), and high analytical limits of detection for urinary arsenic species, our reported effect estimates are similar to those estimated in other prospective US cohorts for incident fatal and non-fatal cardiovascular disease with low- to moderate- levels of iAs exposure (Moon et al. 2013; Moon et al. 2018a). Across the studies included in the original meta-analysis by Moon et al., relative risk estimates for fatal coronary heart disease comparing arsenic exposure at 20 μg/L to 10 μg/L in drinking water ranged from 1.06 (0.99, 1.13) (Wade et al. 2009) to 1.27 (1.09, 1.48) (D’Ippoliti et al. 2015), with a pooled relative risk across six studies of 1.16 (1.07, 1.26). In two prospective US-based studies included in this meta-analysis, iAs exposure at low- to moderate- levels in drinking water was significantly associated with coronary heart disease (James et al. 2015; Moon et al. 2013). In a prospective analysis of a case-control study of adults from New Hampshire, the association between estimated water arsenic concentrations and fatal coronary heart disease was positive but non-significant (relative risk 1.14, 95% CI 0.87, 1.50, N= 154 events) (Farzan et al. 2015). Similarly, our current study found a HR of fatal heart disease per increase in urinary total arsenic corresponding to the interquartile range of 1.20 (0.83, 1.74, N= 77 events) for all participants. Pooling our study results with those included in Moon et al.’s recent meta-analysis resulted in similar RR estimate per doubling of water arsenic from 10 to 20 μg/L (1.16, 95% CI 1.07, 1.25), with lower estimates of heterogeneity, providing further support for the association between low- to moderate- exposure to iAs and cardiovascular disease at exposure levels relevant for the general US population. In pooling our study results, we assumed that all iAs exposure came from drinking water (as in a previously published dose-response meta analysis), which is not true in the US population where diet is the major source for those with water arsenic concentrations below 10 μg/L (Moon et al. 2018a). The main rationale for doing this is to enable pooling across studies with different metrics of arsenic exposure. Water and dietary arsenic may have different associations with cardiovascular disease because of differences in absorption, metabolism, and the arsenic species present in water versus food. Because low- to moderate- iAs exposure is relevant for the entire US population (Nachman et al. 2017; Nigra et al. 2020), even modest effect estimates of the association between iAs exposure and cardiovascular disease indicate that elevated iAs exposure may result in high burden of cardiovascular disease for the US population.
Possible mechanisms by which iAs exposure impacts cardiovascular disease development include platelet aggregation (Lee et al. 2002), inflammation (Simeonova et al. 2003), oxidative stress (Kumagai and Pi 2004), endothelial dysfunction (Barchowsky et al. 1999), and atherosclerosis (Makhani et al. 2018; Negro Silva et al. 2017). Recent epidemiological studies in US populations also indicate that iAs exposure is associated with increased left ventricular wall thickness and hypertrophy (Pichler et al. 2019), prolonged QTc interval (Moon et al. 2018b), diabetes (Grau-Perez et al. 2017), and biomarkers of endothelial dysfunction (Farzan et al. 2017). The association between chronic, high iAs exposure (>50 μg/L in drinking water) and clinical cardiovascular disease is well established (Chen et al. 2011; Moon et al. 2012; Yuan et al. 2007). The current study adds to a growing body of evidence evaluating the prospective association between low iAs exposure in US populations (<10 μg/L in drinking water) and clinical cardiovascular disease (Moon et al. 2018a; Navas-Acien et al. 2019).
Despite the significant challenges and limitations in estimating iAs exposure from NHANES participants, our study contributes important evidence of the adverse impact of chronic iAs exposure at low- to moderate levels relevant to the general US population. The updated pooled meta-analysis evaluating the RR of coronary heart disease per doubling of water arsenic should be an important consideration for the US Environmental Protection Agency’s (EPA) ongoing iAs risk assessment. Although public drinking water arsenic exposure has declined in some areas of the US over time in accordance with a EPA lowering the maximum contaminant level for arsenic in public water systems, many communities in the US remain exposed to arsenic concentrations greater than 5 and 10 μg/L (Nigra et al. 2020).
Our findings further support a potential association between low- to moderate- iAs exposure and cardiovascular disease in the general US population. Future studies should replicate this analysis when additional NDI follow-up data becomes publicly available for additional cycles of NHANES participants beyond 2013/2014, and should separately evaluate the association between low- to moderate- iAs exposure in high quality prospective cohorts of Mexican-American participants to assess potential increased susceptibility to iAs-related cardiovascular disease.
Supplementary Material
Funding acknowledgments:
This study was supported by NIEHS grants P42ES010349, P30ES009089, R01ES028758, and R21ES029668. Anne Nigra is also supported by NIEHS grants 2T32ES007322 and F31ES029799.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Awata H, Linder S, Mitchell LE, Delclos GL. 2017. Association of dietary intake and biomarker levels of arsenic, cadmium, lead, and mercury among Asian populations in the United States: NHANES 2011–2012. Environmental Health Perspectives 125:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayotte JD, Medalie L, Qi SL, Backer LC, Nolan BT. 2017. Estimating the high-arsenic domestic-well population in the conterminous United States. Environmental Science & Technology 51:12443–12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan P, Vaidya D, Franceschini N, Voruganti VS, Gribble MO, Haack K, et al. 2017. Association of cardiometabolic genes with arsenic metabolism biomarkers in American Indian communities: The Strong Heart Family Study (SHFS). Environmental Health Perspectives 125:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour PC Jr., Ruiz JM, Talavera GA, Allison MA, Rodriguez CJ. 2016. Cardiovascular disease in Hispanics/Latinos in the United States. J Lat Psychol 4:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. 1999. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radical Biology & Medicine 27:1405–1412. [DOI] [PubMed] [Google Scholar]
- Bozack AK, Saxena R, Gamble MV. 2018. Nutritional influences on one-carbon metabolism: Effects on arsenic methylation and toxicity. Annu Rev Nutr 38:401–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Punshon T, Karagas MR, Cottingham KL. 2016. Potential exposure to arsenic from infant rice cereal. Annals of Global Health 82:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-J, Chiou H-Y, Chiang M-H, Lin L-J, Tai T-Y. 1996. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arteriosclerosis, Thrombosis, and Vascular Biology 16:504–510. [DOI] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. 2011. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 342:d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubadda F, Jackson BP, Cottingham KL, Van Horne YO, Kurzius-Spencer M. 2017. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Science of the Total Environment 579:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P. 2015. Arsenic in drinking water and mortality for cancer and chronic diseases in central Italy, 1990-2010. PloS One 10:e0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. 2012. Rice consumption and urinary arsenic concentrations in US children. Environmental Health Perspectives 120:1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR. 2015. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol Appl Pharmacol 287:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Howe CG, Zens MS, Palys T, Channon JY, Li Z, et al. 2017. Urine arsenic and arsenic metabolites in U.S. adults and biomarkers of inflammation, oxidative stress, and endothelial dysfunction: A cross-sectional study. Environmental Health Perspectives 125:127002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Barrera A, Lopez MH. 2013. A demographic portrait of Mexican-origin Hispanics in the United States. Washington, DC: Pew Hispanic Center. [Google Scholar]
- Grau-Perez M, Kuo CC, Gribble MO, Balakrishnan P, Jones Spratlen M, Vaidya D, et al. 2017. Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. Environmental Health Perspectives 125:127004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CY, Wang SL, Fadrowski JJ, Navas-Acien A, Kuo CC. 2019. Urinary concentration correction methods for arsenic, cadmium, and mercury: A systematic review of practice-based evidence. Current Environmental Health Reports 6:188–199. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. 2004. Some drinking-water disinfectants and contaminants, including arsenic. Available: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Drinking-Water-Disinfectants-And-Contaminants-Including-Arsenic-2004 84]. [PMC free article] [PubMed]
- Jackson D, White IR, Riley RD. 2012. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 31:3805–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA. 2015. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environmental Health Perspectives 123:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, et al. 2016. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: Evidence from MESA and NHANES. American Journal of Epidemiology 184:590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Tellez-Plaza M, Vaidya D, Grau-Perez M, Post WS, Kaufman JD, et al. 2018. Ethnic, geographic and dietary differences in arsenic exposure in the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of Exposure Science & Environmental Epidemiology 29(3):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y, Pi J. 2004. Molecular basis for arsenic-induced alteration in nitric oxide production and oxidative stress: Implication of endothelial dysfunction. Toxicol Appl Pharmacol 198:450–457. [DOI] [PubMed] [Google Scholar]
- Kuo C-C, Moon KA, Wang S-L, Silbergeld E, Navas-Acien A. 2017. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environmental Health Perspectives 125(8):087001–087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, et al. 2015. Arsenic exposure, arsenic metabolism, and incident diabetes in the Strong Heart Study. Diabetes Care 38(4):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, et al. 2014. Contribution of diet to aggregate arsenic exposures: An analysis across populations. Journal of Exposure Science & Environmental Epidemiology 24(2):156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Bae ON, Chung SM, Kang KT, Lee JY, Chung JH. 2002. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: A contributing factor to cardiovascular disease. Toxicol Appl Pharmacol 179:83–88. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, et al. 2007. Metabolism of low-dose inorganic arsenic in a central European population: Influence of sex and genetic polymorphisms. Environmental Health Perspectives 115:1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T 2014. survey: Analysis of complex survey samples. R package version 330.
- Makhani K, Chiavatti C, Plourde D, Negro Silva LF, Lemaire M, Lemarie CA, et al. 2018. Using the apolipoprotein E knock-out mouse model to define atherosclerotic plaque changes induced by low dose arsenic. Toxicol Sci 166:213–218. [DOI] [PubMed] [Google Scholar]
- Milton AH, Hussain S, Akter S, Rahman M, Mouly TA, Mitchell K. 2017. A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. International Journal of Environmental Research and Public Health 14(6):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody EC, Coca SG, Sanders AP. 2018. Toxic metals and chronic kidney disease: A systematic review of recent literature. Current Environmental Health Reports 5(4):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A. 2012. Arsenic exposure and cardiovascular disease: An updated systematic review. Curr Atheroscler Rep 14:542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Annals of Internal Medicine 159:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, et al. 2018a. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. International Journal of Epidemiology 46(6):1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Zhang Y, Guallar E, Francesconi KA, Goessler W, Umans JG, et al. 2018b. Association of low-moderate urine arsenic and QT interval: Cross-sectional and longitudinal evidence from the Strong Heart Study. Environmental pollution (Barking, Essex : 1987) 240:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. 2011. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology 58:2047–2067. [DOI] [PubMed] [Google Scholar]
- Nachman KE, Ginsberg GL, Miller MD, Murray CJ, Nigra AE, Pendergrast CB. 2017. Mitigating dietary arsenic exposure: Current status in the United States and recommendations for an improved path forward. The Science of the Total Environment 581-582:221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. 2014. Laboratory Procedure Manual: Urine arsenic speciation. Available: https://www.cdc.gov/Nchs/Data/Nhanes/Nhanes_13_14/UAS_UASS_H_MET.pdf.
- National Center for Health Statistics. 2016. 2013-2014 Data Documentation, Codebook, and Frequencies: Urinary speciated arsenics. Available: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UAS_H.htm.
- Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. 2009. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: The Strong Heart Study. Environmental Health Perspectives 117(9):1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. 2011. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental Research 111:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Sanchez TR, Mann K, Jones MR. 2019. Arsenic exposure and cardiovascular disease: Evidence needed to inform the dose-response at low levels. Current Epidemiology Reports 6:81–92. [Google Scholar]
- Negro Silva LF, Lemaire M, Lemarié CA, Plourde D, Bolt AM, Chiavatti C, et al. 2017. Effects of inorganic arsenic, methylated arsenicals, and arsenobetaine on atherosclerosis in the mouse model and the role of As3MT-mediated methylation. Environmental Health Perspectives 125:077001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Nachman KE, Love DC, Grau-Perez M, Navas-Acien A. 2016. Poultry consumption and arsenic exposure in the US population. Environmental Health Perspectives 125:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Chen Q, Chillrud SN, Wang L, Harvey D, Mailloux B, et al. 2020. Inequalities in public water arsenic concentrations in counties and community water systems across the United States, 2006-2011. Environmental Health Perspectives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Navas-Acien A. 2020. Arsenic in US correctional facility drinking water, 2006-2011. Environmental Research 188:109768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Analysis and Epidemiology National Center for Health Statistics. 2019. The Linkage of National Center for Health Statistics Survey Data to the National Death Index – 2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. Available: https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm.
- Pichler G, Grau-Perez M, Tellez-Plaza M, Umans J, Best L, Cole S, et al. 2019. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circ Cardiovasc Imaging 12:e009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Tong L, Argos M, Gao J, Farzana J, Roy S, et al. 2013. Arsenic metabolism efficiency has a causal role in arsenic toxicity: Mendelian randomization and gene-environment interaction. International Journal of Epidemiology 42:1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punshon T, Jackson BP, Meharg AA, Warczack T, Scheckel K, Guerinot ML. 2017. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. The Science of the Total Environment 581-582:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey deCastro B, Caldwell KL, Jones RL, Blount BC, Pan Y, Ward C, et al. 2014. Dietary sources of methylated arsenic species in urine of the United States population, NHANES 2003–2010. PloS One 9:e108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez TR, Powers M, Perzanowski M, George CM, Graziano JH, Navas-Acien A. 2018. A meta-analysis of arsenic exposure and lung function: Is there evidence of restrictive or obstructive lung disease? Current Environmental Health Reports 5:244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Hulderman T, Harki D, Luster MI. 2003. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environmental Health Perspectives 111:1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. 2017. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlen MJ, Gamble MV, Grau-Perez M, Kuo C-C, Best LG, Yracheta J, et al. 2017. Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: Evidence from the Strong Heart Study. Food and Chemical Toxicology 105:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayner L, Steenland K, Dosemeci M, Hertz-Picciotto I. 2003. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scandinavian Journal of Work, Environment & Health 29:317–324. [DOI] [PubMed] [Google Scholar]
- Steenland K, Karnes C, Darrow L, Barry V. 2015. Attenuation of exposure-response rate ratios at higher exposures: a simulation study focusing on frailty and measurement error. Epidemiology 26:395–401. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Takimoto C, Wood L, Porter JM, Tracewell WG, Darwish M, et al. 2010. A pharmacokinetic and safety study of intravenous arsenic trioxide in adult cancer patients with renal impairment. Cancer Chemother Pharmacol 66:345–356. [DOI] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, et al. 2017. Human exposure to organic arsenic species from seafood. Science of the Total Environment 580:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VF, Jackson BP. 2016. Concentrations and speciation of arsenic in New England seaweed species harvested for food and agriculture. Chemosphere 163:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Food and Drug Administration. 2014. Total Diet Study: Elements Results Summary Statistics, Market Baskets 2006 through 2011. Available: http://www.fda.gov/downloads/Food/FoodScienceResearch/TotalDietStudy/UCM184301.pdf.
- Wade TJ, Xia Y, Wu K, Li Y, Ning Z, Le XC, et al. 2009. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. International Journal of Environmental Research and Public Health 6:1107–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Wang S-W, Liu SV, Georgopoulos P. 2010. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environmental Health Perspectives 118:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, et al. 2007. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. American Journal of Epidemiology 166:1381–1391. [DOI] [PubMed] [Google Scholar]
- Zheng LY, Umans JG, Yeh F, Francesconi KA, Goessler W, Silbergeld EK, et al. 2015. The association of urine arsenic with prevalent and incident chronic kidney disease: evidence from the Strong Heart Study. Epidemiology 26:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


