Abstract
Background:
CD19 chimeric antigen receptor (CAR) T cell therapy with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) is approved for the standard of care treatment of relapsed or refractory large B cell lymphoma (LBCL). Patients with LBCL involving the gastrointestinal (GI) tract are at risk of perforation after lymphoma-directed therapy. The outcomes of CAR T cell therapy in patients with GI involvement have not been reported.
Objective:
To determine the safety and efficacy of CD19 CAR T cell therapy among patients with LBCL involvement of the GI tract.
Study Design:
A single-center retrospective study of 130 consecutive patients treated with standard of care or expanded access axi-cel or tisa-cel for LBCL. Of these, 24 patients had radiologic involvement of the GI tract prior to CAR T infusion. Incidence rates of severe immune effector cell-mediated toxicities and clinical outcomes were compared between the GI and non-GI involvement groups.
Results:
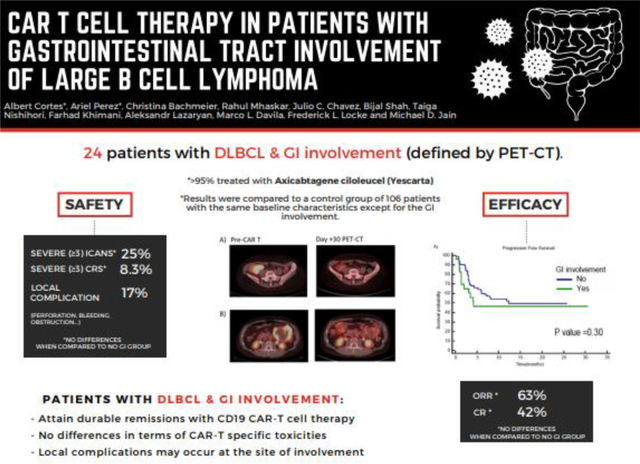
Of 24 patients with GI tract involvement, perforation occurred in 3 patients. One patient had a contained gastric perforation after leukapheresis while receiving bridging radiation therapy to the stomach. This patient was eventually able to proceed with lymphodepletion and product infusion. In the other 2 patients GI tract perforation occurred after CAR T infusion at day +13 and day +35. All 3 patients subsequently died while experiencing lymphoma progression. Upper GI bleeding occurred in 1 additional patient in the context of progressive disease 6 months after product infusion. Comparing the 24 patients with GI tract involvement to the 106 patients without GI tract involvement, the incidence of severe CRS and ICANS, length of hospitalization, use of anti-IL6, and steroids were similar. No significant differences between groups were found in the best overall response rate, progression-free survival, or overall survival.
Conclusions:
Outcomes of patients with GI tract involvement prior to CAR T cell therapy are similar to those without GI involvement, and durable remissions can be observed. However, patients with pre-existing GI tract involvement are at risk of perforation from disease progression before or after CAR T cell infusion.
Graphical Abstract
Introduction
Autologous CD19-directed chimeric antigen receptor (CAR) T cell therapy has demonstrated the ability to produce durable remissions in patients with relapsed or refractory large B cell lymphoma (LBCL). There are currently three commercially available CAR T products for LBCL, axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel), with the third product, lisocabtagene maraleucel, recently approved.1–3 In the pivotal ZUMA-1 trial for axi-cel, 83% of patients had an objective response, with 39% of patients with ongoing response at a median follow up of 27.1 months.4 In addition, patients treated with standard of care therapy outside of clinical trials show similar overall outcomes to the pivotal trials.5–7
LBCL may involve the gastrointestinal tract as either the primary site or with secondary involvement from systemic lymphoma. Such lymphomas carry a risk of perforation, bleeding or infection, whether due to lymphoma progression or due to devitalized tissue remaining after treatment.8,9 These patients are typically excluded from pivotal trials due to these risks, hence the safety and efficacy data on CAR-T cell therapy is lacking.
In this study, we aim to evaluate the impact of GI involvement on clinical outcomes for patients treated with standard of care CD19 targeted CAR T cell therapy.
Materials and Methods
This retrospective single-center cohort study included all consecutive patients receiving CD19 targeted CAR T cell therapy with axi-cel or tisa-cel as a standard of care therapy according to the FDA label as of October 2019, or who were treated on the expanded access programs for axi-cel (NCT03153462) and tisa-cel (NCT03601442) for the provision of CAR T when products fall outside of manufacturing specifications. This study was approved by the local Institutional Review Board and conducted per Good Clinical Practice. We conducted review of radiographic reports and images from restaging CT and/or PET/CT done for all patients as part of work-up within one month before CAR T cell infusion. Gastrointestinal tract involvement was considered to be present when a malignant mass appeared to be in contact with any part of the gastrointestinal tract from the proximal esophagus to distal rectum. Endoscopic and or histologic confirmation of gastrointestinal tract involvement was not required. Evidence of mechanical obstruction or perforation was obtained from clinical documentation. Cytokine Release Syndrome (CRS) and Immune Effector Cell Associated Neurotoxicity Syndrome (ICANS) were graded by CARTOX or the ASTCT Consensus Guidelines.10, 11 Secondary organ toxicities were classified according to the National Cancer Institute Common Toxicity Criteria (CTCAE) v5.0.
Clinical outcomes were determined for all subjects and compared between groups: patients with GI involvement versus patients without GI involvement. The association between patients’ GI involvement status with best overall response rate (ORR), best complete response rate (CR), or toxicities were evaluated via chi-square and Fisher’s exact test. Overall survival (OS) was calculated from axi-cel or tisa-cel infusion time until death, or the last date patient was known to be alive. Progression-free survival (PFS) was calculated from the date of axi-cel or tisa-cel infusion to lymphoma progression, death, or the last date patient was known to be alive and without progression. Kaplan Meier analysis was conducted to report median OS, and PFS estimates with 95% confidence intervals. The log-rank test was used to investigate the difference between median OS and PFS estimates across patients with and without GI involvement. Multivariate Cox regression analysis adjusting for gender and R-IPI score was used to assess the differences in OS and PFS estimates between patients with and without GI involvement. P < 0.05 was defined as significant in all analyses. All statistical analyses were conducted using SPSS version 26 software.
Results
Patient characteristics
Of 130 patients with LBCL undergoing CAR T cell therapy, 24 (18.4%) had involvement of the gastrointestinal tract. Altogether, 6 had multiple sites of the GI tract involved, 3 involved the esophagus only, 5 had gastric involvement, 4 with colon involvement, 3 with small intestine involvement, and 3 involving the rectum. Among patients with GI involvement, the median age at the time of infusion was 65 years (range, 25 – 79 years). Twenty (83.3%) patients had DLBCL, 3 (12.5%) had Transformed Follicular Lymphoma, and 1 (4.2%) patient had Primary Mediastinal B-cell Lymphoma (PMBCL). The median number of prior treatment lines were 4 (range, 2 – 9). Most patients were treated with axi-cel (95.8%). The cell of origin assessment was available in 20 cases, of which 14 (70%) had germinal center B-cell (GCB)-like, and 6 (30%) had non-GCB-like disease. Of the 12 patients with pre-treatment fluorescence in situ hybridization (FISH) studies, 5 (29.4%) had double or triple hit biology. (Supplementary Table S1).
In the group with GI involvement, the median time from last PET/CT to infusion was 8.5 days (range, 1 – 67 days). Most patients (75%) received bridging therapy before CAR-T infusion. The median time from the start of bridging therapy to product infusion was 23.5 days (range, 8 – 58 days) and 11 (61.1%) patients completed bridging before the last PET/CT was performed.
There were no significant baseline differences between the 106 patients without GI involvement compared to the 24 patients with GI involvement. Main baseline characteristics are summarized in Table 1.
Table 1.
Baseline patient characteristics of patients in the GI involvement and non-GI involvement groups
| Characteristic | GI involvement group | Non-GI involvement group | p value | |
|---|---|---|---|---|
| n(%) | n(%) | |||
|
| ||||
| Age, years | Median (Range) | 65 (25–79) | 64 (19–78) | |
| Gender | Male | 15 (62.5) | 62 (58.5) | 0.82 |
| Female | 9 (37.5) | 44 (41.5) | ||
| Performance status by ECOG | 0–1 | 16 (69.6)* | 84 (79.2) | 0.40 |
| ≥2 | 7 (30.4)* | 22 (20.8) | ||
| Disease type | DLBCL | 20 (83.3) | 74 (69.8) | 0.21 |
| TFL | 3 (12.5) | 28 (26.4) | ||
| PMBCL | 1 (4.2) | 4 (3.8) | ||
| Double or triple hit | 5 (29.4)* | 18 (24.7)* | 0.76 | |
| Ann Arbor Stage** | I/II | 4 (16.7) | 22 (20.8) | 0.78 |
| III/IV | 20 (83.3) | 84 (79.2) | ||
| IPI score** | 0–2 | 3 (13) * | 36 (34) | 0.08 |
| 3–5 | 20 (87)* | 70 (66) | ||
| Cell of origin | GCB-like | 14 (58.3) | 60 (56.6) | 0.99 |
| Non-GCB | 6 (25) | 27 (25.5) | ||
| Unknown | 4 (16.7) | 19 (17.9) | ||
| Product | Axi-cel | 1 (4.2) | 10 (9.4) | 0.69 |
| Tisa-cel | 23 (95.8) | 96 (90.6) | ||
Percentages based on number of patients with available data.
At the time of leukapheresis
ECOG, Eastern Cooperative Group performance status grade; DLBCL, diffuse large B cell lymphoma; TFL, transformed follicular lymphoma; GCB, germinal center B, axi-cel, axicabtagene ciloleucel, tisa-cel, tisagenlecleucel
Safety
In the GI involvement group, the median duration of hospital stay was 17 days (range, 8 – 59 days). Severe CRS (Grade ≥3) occurred in 2 (8.3%) patients. Severe ICANS (Grade ≥3) occurred in 6 (25%) patients. Twelve (50%) patients required anti-IL6 therapy with Tocilizumab and 12 patients required steroids. Twelve out of 24 patients died in the GI involvement group, all of whom had lymphoma progression at the time of death except for one patient who died in an auto accident.
In terms of organ-specific adverse events, 4 (17%) patients experienced local complications from gastrointestinal tract involvement. Three of them suffered a GI perforation. One patient had a contained gastric perforation after leukapheresis while receiving bridging radiation therapy to the stomach. This patient was eventually able to proceed with lymphodepletion and product infusion. In the other 2 patients GI tract perforation occurred after CAR T infusion at day +13 and day +35. All three patients subsequently died of progressive lymphoma. Upper GI bleeding occurred in 1 patient in the context of progressive disease 6 months after product infusion. Two patients had single-site gastric involvement, one had small bowel involvement, and one had multifocal disease (both stomach and small bowel).
Comparing the patients with no GI involvement to those with GI involvement, no differences were found in rates of severe CRS (10.5% vs 8.3%, p=0.75), severe ICANS (25% in both groups, P=1), anti-IL6 therapy use (50% vs 47.2%, p=0.92), steroid use (50% vs 44.3%, p=0.65), or length of hospital stay (median 17 vs 15 days, P=0.09).
Efficacy
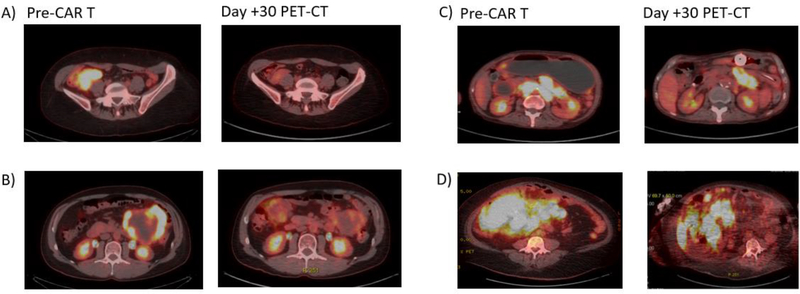
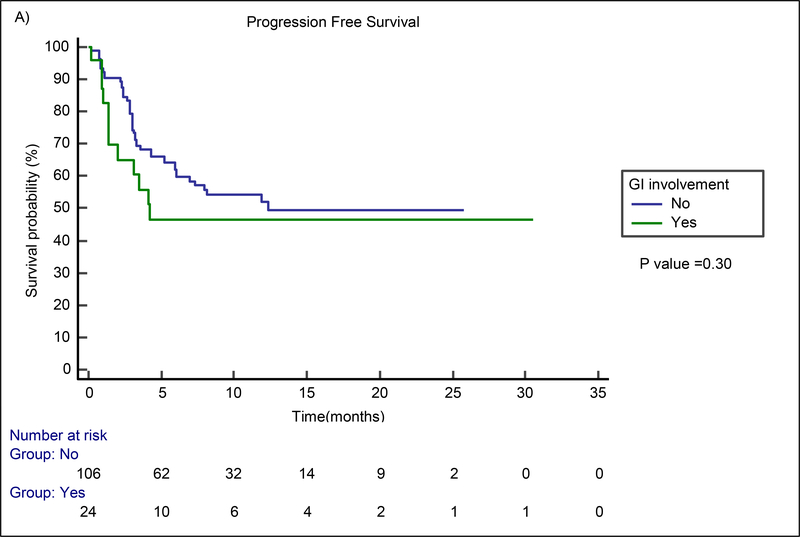
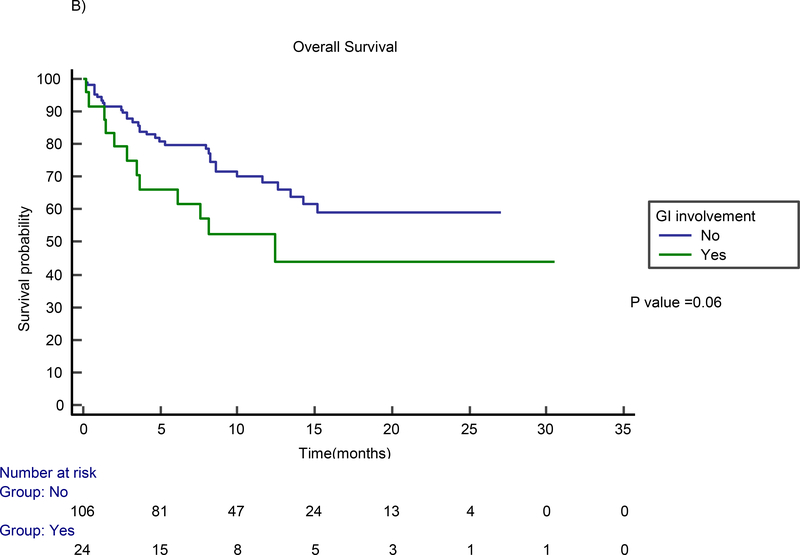
The median follow-up time for the entire cohort of 130 patients was 8.2 months (range, 0.2 – 30.5 months). The median follow-up time for survivors (n=85) was 10.9 months (range, 1.3 – 30.5 months). In the GI involvement group, best overall response and CR rates were 63% and 42%, respectively. Figure 1 shows examples of patients with GI involvement who were responders (A-B) and non-responders (C-D) at one month after CAR T therapy. No difference in best ORR (p=0.27) or best CR rate (p=0.24) between the GI and non-GI involvement groups was found. The median PFS for the GI involvement group was 4.2 months (95% CI not estimable) and the median survival was 12.4 months (95% CI 4.1–20.7). No statistically significant difference in PFS (p=0.30) or OS (p=0.06) was found between patients with and without GI involvement via the Kaplan Meier analysis (Figure 2). Similarly, on multivariable Cox proportional hazards regression analysis, the presence of GI involvement did not significantly affect PFS (HR=1.28; 95% CI 0.61 to 2.51; p=0.29) or OS (HR=1.44; 95% CI 0.72 to 2.87; p=0.46). Despite no significant differences in toxicity or safety outcomes overall, four patients experienced significant morbidity attributed to GI lymphoma involvement, which we present in detail here.
Figure 1. Examples of responders (A and B) and non-responders (C and D).
A) Right lower quadrant mass with cecal involvement (CR at D+30)
B) Left upper quadrant centrally necrotic mass with gastric and bowel involvement (PR at D+30)
C) Extensive intraabdominal disease, small bowel, and gastric involvement (PD at D+30 with worsening pleural disease and lung nodules)
D) Large abdominal mass involving the colon and small bowel (PD at D+30 with increase uptake in celiac and peripancreatic nodes)
Figure 2.
Kaplan Meier estimates for PFS (A) and OS (B) by the presence of GI Tract involvement
Case #1: Contained gastric perforation in the setting of pre-existing gastric outlet obstruction
A 66 year old male diagnosed with relapsed/ refractory DLBCL with extensive intraabdominal disease, gastric and small bowel involvement. Prior lines of therapy included R-CHOP, HD-MTX, R-DICEP, and external beam radiation therapy to the gastric region. He had a GJ tube in place due to gastric outlet obstruction. He underwent leukapheresis, received bridging therapy with dexamethasone followed by Fludarabine Cyclophosphamide (Flu-Cy) lymphodepletion, and axicabtagene ciloleucel infusion. His post-CAR T course was complicated by a maximum grade 3 CRS and grade 1 ICANS. On day +13, a follow-up abdominal CT scan with intravenous and oral contrast was performed to assess for improvement of gastric outlet syndrome and ability to resume diet. On imaging an air and fluid level was detected posterior to the stomach tracking towards the spleen, concerning for gastric perforation in the area of the cardia. The surgical consultation service recommended conservative management. The patient’s condition slowly improved. He was able to tolerate tube feeds and a liquid diet at the time of discharge. He returned to his country of residence on day +31. He died on day +110 with biopsy proven lymphoma progression and concurrent empyema.
Case #2: Gastric perforation prior to CAR T infusion with subsequent aero-digestive fistulization.
A 32 year old male was diagnosed with relapsed/ refractory DLBCL with gastric involvement. Prior lines of therapy included R-CHOP, R-GDP. He underwent leukapheresis and, while receiving bridging radiation therapy to the stomach, was admitted for severe abdominal pain with CT scan imaging showing a contained gastric perforation. The surgical consultation service recommended conservative management. After extensive risk-benefit discussion with patient and multidisciplinary evaluation, a consensus was reached to proceed with Flu-Cy lymphodepletion and axi-cel administration. After CAR T cell infusion, he experienced maximum Grade 1 CRS and Grade 1 ICANS. On day +15, after product infusion patient was noted to have persistent fevers with an upward trend in his inflammatory markers. CT imaging showed a gastro-bronchial fistula, which was later confirmed by bronchoscopy. His clinical condition worsened, requiring intubation and vasopressor support with multiple agents for septic shock. His disease exhibited characteristics of progression with malignant lymphocytes found in pleural fluid, ascites, and peripheral blood. He was transitioned to comfort measures and expired on day +41.
Case #3: Incidental finding of small bowel perforation
A 52 year old male was diagnosed with relapsed/refractory DLBCL with a large abdominal mass involving the hepatic flexure, extension into the mesentery, and involvement of adjacent small bowel. Prior lines of therapy included: R-CHOP with Bortezomib, R-GEMOX, and R-ICE. He underwent leukapheresis, bridging with R-GEMOX and dexamethasone, followed by Flu-Cy lymphodepletion and axicabtagene ciloleucel infusion. His post-CAR T course was complicated by Grade 2 CRS and Grade 4 ICANS. On day +35, free intraperitoneal gas concerning for perforated viscus was reported on restaging CT imaging with evidence of disease progression of mesenteric and peri-pancreatic masses. He had stable chronic abdominal pain with no clinical signs of peritonitis. The surgical consultation service recommended conservative management. He was eventually discharged home on D+57 in stable condition. The patient was not deemed a candidate for additional cancer-specific therapies with eventual transition to hospice care and died on day +103.
Case #4: Hemodynamically significant upper gastrointestinal bleed secondary to tumor progression
A 66 year old male diagnosed with relapsed/refractory Transformed Follicular Lymphoma with gastric involvement. Prior lines of therapy included radiation therapy to the gastric region and single-agent rituximab (before transformation), R-CHOP, and R-ICE. He was evaluated for CAR T cell therapy, underwent leukapheresis, bridging with R-GEMOX, followed by Flu-Cy lymphodepletion and axicabtagene ciloleucel infusion. His post-CAR T course was complicated by Grade 2 CRS and Grade 1 ICANS. PET-CT at day +90 after product infusion showed disease progression. On day +182 he was admitted with severe anemia and hemorrhagic shock secondary to upper gastrointestinal bleed. Hemorrhage was controlled with endoscopic measures as well as subsequent embolization of the left gastric and gastroepiploic artery. The patient was discharged home in stable condition. The patient received several additional lines of therapy for progressive disease but ultimately died in hospice on day +244.
Discussion
To our knowledge, this is the largest reported series of CD19 targeted CAR T patients with GI involvement by LBCL. The presence of radiographic infiltration of the GI tract in our series was not associated with increased CAR T toxicity or worse clinical outcomes. However, a small subset of patients (3/24), who experienced GI tract perforations in the context of progressive disease, had complicated post-CAR T courses and poor outcomes. Overall, CAR T associated toxicities including CRS and ICANS were not increased in patients with GI involvement. In terms of efficacy, no significant differences in PFS or OS were noted between patients with GI involvement compared to patients without GI involvement.
Local complications are well described in patients with GI involvement by lymphoma, either as a consequence of disease progression or treatment effect. DLBCL is the most frequent histology (39%) with GI involvement, and it is also associated with the highest frequency of perforations (13.2%).8 Among different sites of extranodal disease, GI involvement is associated with worse OS rates according to an extensive Surveillance, Epidemiology, and End Results database analysis.12
There are limited data on the impact of GI tract involvement for patients treated with CAR T cell therapy. Zeng et al. reported 14 patients with GI involvement of B cell malignancies (mostly LBCL) treated on a clinical trial with sequential infusion of anti-CD22 and anti-CD19 CAR T cells. The reported CR rate in these patients was 53.8% and no perforations were observed.13
There are several limitations to our study. First, GI involvement was defined in our study based on radiographic criteria. We did not uniformly assess patients by endoscopy, and patients with GI involvement by endoscopy may be at a higher risk of local complications compared to those with radiographic involvement alone. It is possible that patients with transmural lymphoma involvement of the GI tract may have poorer outcomes than patients with only geographically close radiographic involvement. Conversely, patients with occult GI involvement may have been incorrectly classified as not involving the GI tract by radiographic criteria. Second, the Kaplan-Meier analysis showed a non-significant trend (p=0.06) towards decreased OS in patients with GI involvement. Our study may be underpowered to detect a true decrease in OS in patients with GI involvement due to a limited sample size. Finally, we have recently shown that a high metabolic tumor volume (MTV) is associated with higher systemic inflammation and poorer outcomes in patients undergoing CAR-T cell therapy for LBCL.14, 15 We did not have MTV measurements for all of the patients in this study and were therefore not able to assess this important covariate. It is possible that patients with disseminated involvement including the GI tract may have poorer outcomes than patients with localized or small-volume GI tract involvement.
In conclusion, patients with GI involvement may attain durable remissions after CD19 targeted CAR T cell therapy. However, we observed perforations in patients with GI involvement in the context of disease refractoriness. Overall, no differences in toxicity or clinical outcomes were observed in patients with GI involvement when compared to a similar cohort of patients without GI infiltration. We continue to advocate practitioners take caution in selecting patients for CAR T cell therapy, in particular use caution in patients with known transmural disease or a history of perforation.
Supplementary Material
HIGHLIGHTS.
Patients with DLBCL with gastrointestinal involvement can attain durable remissions with CD19 CAR-T cell therapy.
No differences in terms of clinical outcomes and CAR-T specific toxicities are seen when compared to a similar cohort without GI involvement.
Extra caution must be taken in those patients with transmural disease for the risk of local complications such as perforation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. [DOI] [PubMed] [Google Scholar]
- 4.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. 2020:JCO1902104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol. 2020:JCO1902103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidya R, Habermann TM, Donohue JH, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24:2439–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vzrdiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008. [Google Scholar]
- 10.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Santomasso BD, Locke FL, et al. ASBMT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638.. [DOI] [PubMed] [Google Scholar]
- 12.Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol. 2014;89(3):310–314. [DOI] [PubMed] [Google Scholar]
- 13.Zeng C, Cheng J, Li T, et al. Efficacy and toxicity for CD22/CD19 chimeric antigen receptor T-cell therapy in patients with relapsed/refractory aggressive B-cell lymphoma involving the gastrointestinal tract. Cytotherapy. 2020;22(3):166–171. [DOI] [PubMed] [Google Scholar]
- 14.Jain MD, Zhao H, Wang X, et al. Tumor interferon signaling and suppressive myeloid cells associate with CAR T cell failure in large B cell lymphoma [published online ahead of print, 2021 Jan 15]. Blood. 2021; blood.2020007445.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Advances. 2020; 14(4): 3268–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.