Abstract
Background:
Bronchiolitis obliterans syndrome (BOS) is a highly morbid form of chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). Several plasma proteins have been identified as biomarkers for BOS after lung transplantation. Relevance of these biomarkers in BOS patients after allogeneic HCT has not been examined.
Objective:
We hypothesized that biomarkers associated with BOS after lung transplantation are also associated with BOS after allogeneic HCT.
Study Design:
We tested plasma samples from 33 adult HCT patients who participated in a phase II multicenter study of fluticasone, azithromycin and montelukast (FAM) treatment for new-onset BOS (NCT01307462), and matched control samples of HCT patients who had non-BOS chronic GVHD (n=31) and those who never experienced chronic GVHD (n=29) (NCT00637689 and NCT01902576). Candidate biomarkers included matrix metalloproteinase-9 (MMP-9), MMP-3 and chitinase-3-like1 glycoprotein (YKL-40).
Results:
MMP-9 concentrations were higher in BOS patients than those with non-BOS chronic GVHD (P=0.04) or no chronic GVHD (P<0.001). MMP-3 concentrations were higher in patients with BOS (P<0.001) or non-BOS chronic GVHD (P<0.001) than those with no chronic GVHD. YKL-40 concentrations did not differ statistically among the groups. MMP-9 concentrations before starting FAM therapy were higher in patients with treatment failure within 6 months than those with treatment success (P=0.006), while MMP-3 or YKL-40 concentrations did not differ statistically between the groups. Patients with MMP-9 concentrations ≥ 200,000 pg/ml before starting FAM therapy had worse overall survival compared to those with the lower MMP-9 concentrations.
Conclusions:
Plasma MMP-9 concentration could serve as a relevant biomarker at diagnosis of BOS after allogeneic HCT, for prognostication of survival and for prediction of treatment response. Further validation is required to confirm our findings.
Keywords: matrix metalloproteinase-9, matrix metalloproteinase-3, chitinase-3-like1 glycoprotein, bronchiolitis obliterans, allogeneic hematopoietic cell transplantation
Introduction
Bronchiolitis obliterans syndrome (BOS) is a highly morbid form of chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). BOS also occurs after lung transplantation and is characterized by peribronchial fibrosis and intraluminal obstruction of the small terminal airways, resulting in airflow obstruction.1 At least 3 plasma proteins, matrix metalloproteinase-9 (MMP-9), MMP-3 and chitinase-3-like1 glycoprotein (YKL-40), have been identified as biomarkers for BOS after lung transplantation.2–6 MMP family members are responsible for the turnover and degradation of the extracellular matrix through their capacity to cleave structural proteins such as collagens and elastin.7 MMP-9 contributes to the migration of neutrophils into lung tissues, leading to parenchymal destruction.8 MMP-3 was identified as a diagnostic biomarker for BOS after lung transplantation and after allogeneic HCT,6 and it was also identified a diagnostic biomarker for chronic GVHD.9 YKL-40 is a chitinase-like protein secreted by alveolar macrophages, neutrophils, and epithelial cells10 and functions as a growth factor for fibroblasts and vascular endothelial cells.11, 12 Animal models indicate that YKL-40 is a prominent regulator of lung inflammation, remodeling and fibrosis.13 The reported areas under the receiver operating characteristic curve (AUC) for association with diagnosis of BOS after lung transplantation were 0.79 (95% confidence interval [CI], 0.58–0.98) for MMP-9, 0.77 (95% CI, 0.68–0.86) for MMP-3 and 0.70 (95% CI, 0.60–0.83) for YKL-40.2, 4, 6
We hypothesized that biomarkers associated with BOS diagnosis after lung transplantation are also associated with BOS diagnosis after allogeneic HCT. We also hypothesized that these biomarkers may have prognostic relevance for BOS severity or subsequent survival, and predictive relevance for treatment failure. To test these hypotheses, we assessed these candidate biomarkers in enrollment plasma samples of patients who participated in a phase II multicenter study of fluticasone, azithromycin and montelukast (FAM) treatment for new-onset BOS after allogeneic HCT,14 and matched control samples of patients who had non-BOS chronic GVHD and patients who never experienced chronic GVHD.
Methods
Patients and sample collection
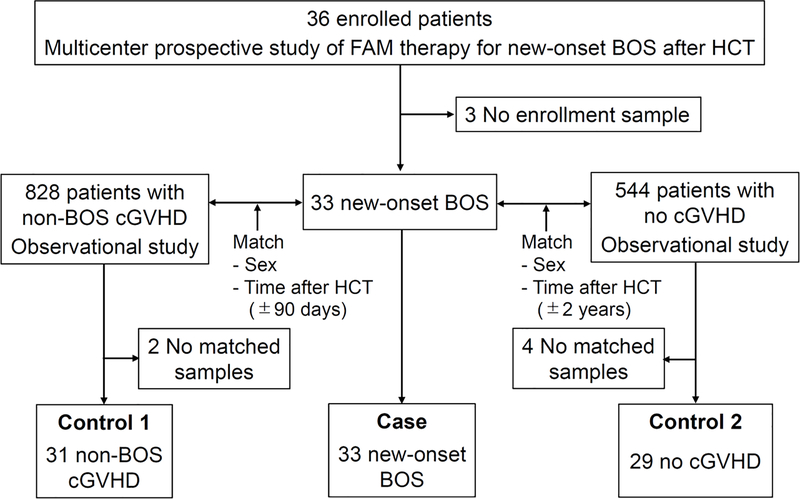
All samples were collected from patients aged 18 years or older. The study cohort included 93 patients (Figure 1): BOS cases (n=33) and two sets of controls, non-BOS chronic GVHD patients (n=31) and patients with no chronic GVHD (n=29). Cases included 33 patients with new-onset BOS, defined as diagnosis within 6 months of enrollment, who participated in a phase II multicenter study of FAM treatment (NCT01307462).14 We used enrollment plasma samples collected before starting FAM treatment and follow-up plasma samples collected at 3 and 6 months after starting FAM treatment. Three patients without enrollment samples were excluded from the original cohort of 36 patients. Controls were drawn from prospective, multicenter, longitudinal, observational studies of the Chronic GVHD Consortium (NCT00637689 and NCT01902576),15, 16 and were matched for patient sex and time after HCT (90-day window for non-BOS chronic GVHD and within 2 years for no chronic GVHD). Among the non-BOS chronic GVHD patients, absence of subsequent development of BOS after sample draw was confirmed. For 2 BOS cases, no non-BOS chronic GVHD patients met the matching criteria. Among the 29 control patients who never experienced chronic GVHD, absence of subsequent development of chronic GVHD after sample draw was confirmed. For 4 cases, no matches were found among patients without chronic GVHD. The study cohort had no overlap with one that was evaluated in previously published studies.6, 9 Blood was collected in EDTA, and plasma was stored in aliquots at −80°C within 4 hours of phlebotomy. Patients gave written informed consent allowing blood sample collection and the use of medical records for research in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of all participating centers.
Figure 1.
CONSORT diagram of the study cohort.
Acute GVHD was diagnosed and graded according to previously described criteria.17 Chronic GVHD was diagnosed and scored according to the 2014 National Institutes of Health consensus criteria.18 Organ involvement was defined as NIH organ score ≥1 except for the lungs. Lung involvement was defined for patients who met the 2014 NIH consensus criteria of BOS.18 Normative values for spirometric parameters (i.e., % predicted values) were calculated according to the NHANES III equations.19 Treatment failure was defined as ≥10% decrease in FEV1 % predicted at 6 months after starting FAM therapy or death within 6 months after starting FAM therapy.20 Treatment success was defined as absence of treatment failure within 6 months. The intensity of conditioning is defined as previously described.21
Luminex microbead assay
The Luminex microbead method (Luminex, Austin, TX) was used for measurement of plasma proteins, as previously described.22 Antibodies were purchased from R&D Systems, Minneapolis MN. The following antibodies were used for analysis: MMP-9 #MAB936 (capture) and #BAF911 (detection), MMP-3 #AF513 (capture) and #BAF513 (detection), and YKL-40 #MAB25991 (capture) and #BAF2559 (detection). The lower limits of detection were 10 pg/mL for MMP-9, 2000 pg/mL for MMP-3, and 32 pg/mL for YKL-40. No sample showed values below the lower limit of detection.
Statistical analysis
Protein concentrations were log-transformed for analysis and mean differences between the groups were calculated as fold differences. The Skewness/Kurtosis test was used to assess the normality assumption. Concentrations were compared between the groups using the t-test. Linear regression models were used to examine factors associated with protein concentrations. Model assumptions were evaluated with a residual by predicted plot. Factors with a P value < 0.05 in simple regression models were entered into a multiple regression model. A backward elimination procedure was used in developing a final model, using a P-value threshold of 0.05. Covariates included GVHD groups (main effect), patient age at sample draw, patient sex, race, stem cell source, conditioning intensity, donor type, a female donor to a male recipient, prior grade II-IV acute GVHD, duration from HCT to sample draw, steroid treatment at sample draw, NIH global severity excluding the lungs at sample draw and individual involved sites at sample draw. Overall survival was estimated using the Kaplan-Maier method. Two-sided P values < 0.05 were considered statistically significant. Correlations between biomarker concentrations and spirometric parameters were tested by Spearman’s correlation test. The receiver operating characteristic curves and related measures were estimated non-parametrically. Statistical analyses were performed using STATA version 12.1 (StataCorp, College Station, TX) and GraphPad Prism Version 6 (GraphPad Software, San Diego, CA).
Results
Patient characteristics
The median ages were 56 years (range, 31–72 years) in BOS patients, 53 years (range, 21–68 years) in non-BOS patients, and 53 years (range, 23–75 years) in patients with no chronic GVHD. Other patient characteristics are summarized in Table 1. Patients with BOS more frequently had steroid treatment at sample draw and eye involvement compared to control patients. Other characteristics did not differ statistically between the groups.
Table 1.
Patient characteristics
| Characteristic | New-onset BOS | Non-BOS chronic GVHD | No chronic GVHD | P for BOS vs. non-BOS chronic GVHD | P for BOS vs. no chronic GVHD |
|---|---|---|---|---|---|
| Total number | 33 | 31 | 29 | ||
| Median age at sample draw (range), y | 56 (31–72) | 53 (21–68) | 53 (23–75) | 0.16 | 0.43 |
| Sex | 1.0 | 1.0 | |||
| Male | 19 (58) | 17 (55) | 16 (55) | ||
| Female | 14 (42) | 14 (45) | 13 (45) | ||
| Race | 0.70 | 0.28 | |||
| White | 30 (91) | 27 (87) | 23 (79) | ||
| Others | 3 (9) | 4 (13) | 6 (21) | ||
| Primary diagnosis | 0.26 | 0.69 | |||
| Acute leukemia | 13 (39) | 10 (32) | 15 (52) | ||
| Chronic leukemia | 6 (18) | 1 (3) | 3 (10) | ||
| MDS/MPN | 9 (27) | 14 (45) | 5 (17) | ||
| Lymphoma | 4 (12) | 4 (13) | 4 (14) | ||
| Other | 1 (3) | 2 (6) | 2 (7) | ||
| Stem cell source | 0.83 | 0.19 | |||
| Peripheral blood stem cell | 30 (91) | 28 (90) | 24 (83) | ||
| Bone marrow | 3 (9) | 2 (6) | 2 (7) | ||
| Cord blood | 0 (0) | 1 (3) | 3 (10) | ||
| Conditioning intensity | 1.0 | 0.80 | |||
| Myeloablative | 20 (61) | 18 (58) | 16 (55) | ||
| Reduced intensity | 13 (39) | 13 (42) | 13 (45) | ||
| Donor type | 0.14 | 0.15 | |||
| HLA-matched relative | 18 (55) | 10 (32) | 9 (31) | ||
| HLA-mismatched relative | 1 (3) | 1 (3) | 2 (7) | ||
| Unrelated donor | 14 (42) | 20 (65) | 18 (62) | ||
| A female donor to a male recipient | 11 (33) | 6 (19) | 6 (21) | 0.26 | 0.39 |
| Prior grade II-IV acute GVHD | 17 (52) | 22 (71) | 18 (62) | 0.13 | 0.45 |
| Median months from HCT to sample draw (range) | 18 (5–132) | 18 (5–72) | 13 (9–64) | 0.81 | 0.17 |
| Median months from chronic GVHD to sample draw (range) | 7 (0–90) | 7 (0–66) | NA | 0.55 | NA |
| Steroid treatment at sample draw | 30* (94) | 20 (65) | 3 (10) | 0.005 | <0.001 |
| NIH global severity excluding the lungs | 0.25 | NA | |||
| None or mild | 13 (39) | 19 (61) | NA | ||
| Moderate | 14 (42) | 9 (29) | NA | ||
| Severe | 6 (18) | 3 (10) | NA | ||
| Involved sites other than the lungs | |||||
| Skin | 14 (42) | 11 (35) | NA | 0.62 | |
| Mouth | 15 (45) | 17 (55) | NA | 0.62 | |
| Eye | 27(82) | 16 (52) | NA | 0.016 | |
| Gastrointestinal tract | 5 (15) | 5 (16) | NA | 1.0 | |
| Liver | 3 (9) | 2 (6) | NA | 1.0 | |
| Joint/fascia | 7 (21) | 5 (16) | NA | 0.75 | |
| Genital | 3 (9) | 2 (6) | NA | 1.0 |
One patient is excluded due to absence of information.
BOS, bronchiolitis obliterans; NA, not applicable.
Comparison of biomarker concentration among the groups
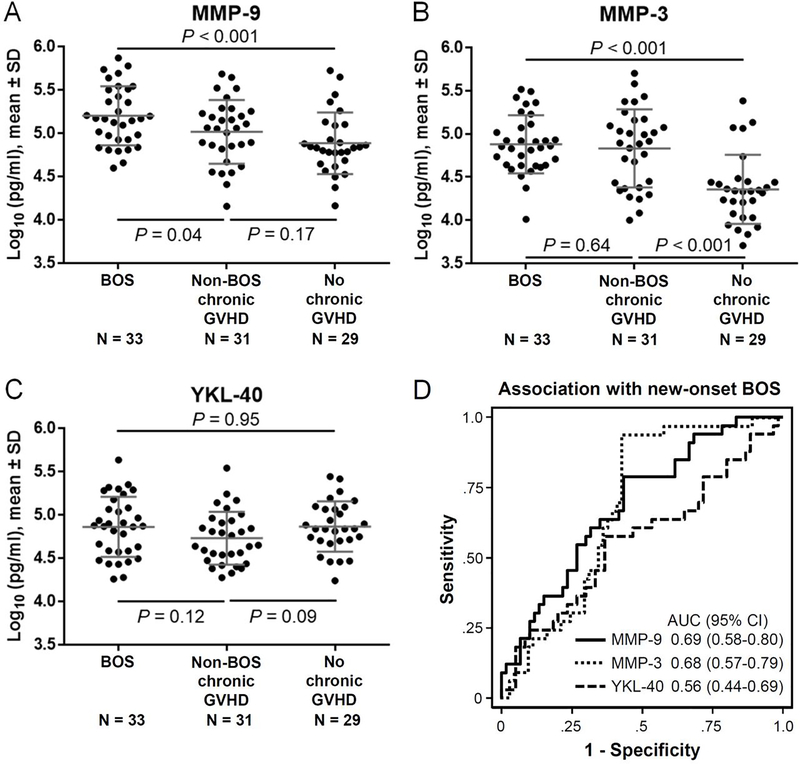
Log-transformed values of all tested biomarkers did not violate the Skewness/Kurtosis test for normality. MMP-9 concentrations were higher in BOS patients compared with those with non-BOS chronic GVHD (P = 0.04) or those with no chronic GVHD (P < 0.001) (Figure 2A). MMP-3 concentrations were higher in patients with BOS or those with non-BOS chronic GVHD compared with those with no chronic GVHD (P < 0.001 for both comparisons) and were similar between patients with BOS and those with non-BOS chronic GVHD (Figure 2B). YKL-40 concentrations did not differ statistically among the groups (Figure 2C). The AUCs of the association of biomarker concentrations with new-onset BOS among all patients were 0.69 (95% CI, 0.58–0.80, P = 0.003) for MMP-9, 0.68 (95% CI, 0.57–0.79, P = 0.005) for MMP-3, and 0.56 (95% CI, 0.44–0.69, P = 0.32) for YKL-40 (Figure 2D). We further compared biomarker concentrations according to severity of BOS (i.e., 2014 NIH PFT score). Concentrations did not differ statistically according to BOS severity for any of the biomarkers. Correlations between biomarker concentrations and FEV1 % predicted or FEV1/FVC ratios were also low (Spearman rank correlation values ranged from −0.18 to 0.20).
Figure 2. Diagnostic relevance.
Plasma concentrations were compared in BOS cases and controls for (A) MMP-9, (B) MMP-3 and (C) YKL-40. (D) Receiver operating characteristic curves for association with diagnosis of new-onset BOS. The horizontal lines indicate means, and error bars indicate standard deviations. P values were derived from t-test. SD, standard deviation; AUC, area under the curve; CI, confidence interval.
Biomarker concentration according to 6-month treatment failure and survival according to biomarker concentrations
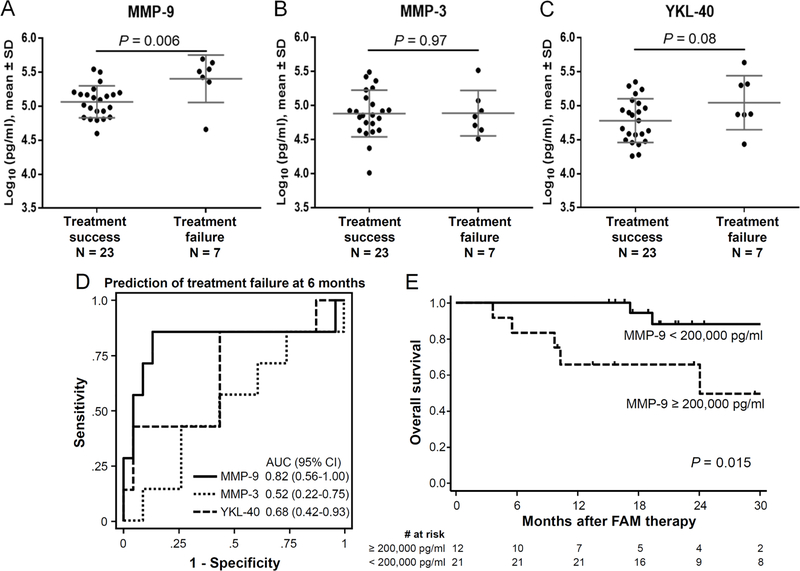
In examining the predictive relevance of the biomarkers for treatment failure, we compared concentrations of each biomarker according to 6-month treatment failure (Figure 3). Three patients with missing pulmonary function test results at 6 months were excluded from analysis. MMP-9 concentrations before starting FAM therapy were higher in patients with treatment failure compared with those with treatment success (P = 0.006) (Figure 3A), while MMP-3 and YKL-40 concentrations did not differ statistically between the two groups (Figures 3B and C). The AUC for the association of biomarker concentrations with treatment failure at 6 months after FAM therapy were 0.82 (95% CI, 0.56–1.00, P = 0.01) for MMP-9, 0.52 (95% CI, 0.22–0.75, P = 0.90) for MMP-3, and 0.68 (95% CI, 0.42–0.93, P = 0.16) for YKL-40 (Figure 3D). Sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) of MMP-9 for treatment failure according to different thresholds are shown in Table 2. Concentrations of MMP-9, MMP-3 and YKL-40 were similar between responders and non-responders at 6 months according to the 2014 NIH overall response criteria.
Figure 3. Predictive and prognostic relevance.
Plasma concentrations were compared between patients with treatment success and those with treatment failure at 6 months after FAM therapy for (A) MMP-9, (B) MMP-3 and (C) YKL-40. (D) Receiver operating characteristic curves in predicting treatment failure at 6 months after FAM therapy. (E) Overall survival was compared according to MMP-9 concentrations before FAM therapy. The horizontal lines indicate means, and error bars indicate standard deviations. P values were derived from t-test. SD, standard deviation; AUC, area under the curve; CI, confidence interval.
Table 2.
Sensitivity, specificity, PPV, and NPV of MMP-9 for predicting treatment failure at 6 months after FAM therapy
| Threshold, log10 pg/ml | # of patients ≥ threshold | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| 4.6 | 29 | 1.00 | 0.04 | 0.24 | 1.00 |
| 4.7 | 28 | 0.86 | 0.04 | 0.21 | 0.50 |
| 4.8 | 27 | 0.86 | 0.09 | 0.22 | 0.67 |
| 4.9 | 23 | 0.86 | 0.26 | 0.26 | 0.86 |
| 5.0 | 19 | 0.86 | 0.43 | 0.32 | 0.91 |
| 5.1 | 17 | 0.86 | 0.52 | 0.35 | 0.92 |
| 5.2 | 11 | 0.86 | 0.78 | 0.55 | 0.95 |
| 5.3 | 9 | 0.86 | 0.87 | 0.67 | 0.95 |
| 5.4 | 7 | 0.71 | 0.91 | 0.71 | 0.91 |
| 5.5 | 6 | 0.57 | 0.91 | 0.67 | 0.88 |
| 5.6 | 2 | 0.29 | 1.00 | 1.00 | 0.82 |
PPV, positive predictive value; NPV, negative predictive value; NA, not applicable.
We further examined the prognostic relevance of these biomarkers in predicting overall survival after FAM therapy for patients with new-onset BOS. In exploring multiple thresholds, patients with MMP-9 concentrations ≥ 200,000 pg/ml before FAM therapy had worse overall survival compared with those with the lower MMP-9 concentrations (Figures 3E). Probabilities of overall survival after the sample draw did not differ statistically according to any threshold concentrations of MMP-3 or YKL-40. The combination of MMP-9 and YKL-40 concentrations did not improve the predictive values in treatment failure or survival (data not shown). Longitudinal changes in MMP-9 concentrations at 3 and 6 months after FAM therapy did not correlate with treatment failure or changes in FEV1 % predicted (data not shown).
Factors associated with MMP-9 concentrations
We used linear regression models to identify factors associated with MMP-9 concentrations in all samples (Table 3). Simple regression models showed that higher MMP-9 concentrations were associated with 5 factors: BOS compared with no chronic GVHD, male patients, a female donor to a male recipient, longer duration from HCT to sample draw, and steroid treatment at sample draw. Patient age, stem cell source, conditioning intensity, donor type, prior acute GVHD, NIH global severity excluding the lungs, and individual involved sites other than the lungs were not statistically associated with MMP-9 concentrations. Multiple regression models showed that higher MMP-9 concentrations were associated with BOS compared with no chronic GVHD and with male patient sex. Constant variance and normal distributions of the residuals in the final model were confirmed with a residual by predicted plot and the Skewness/Kurtosis test. The results of multiple regression analysis were similar after adjustment for duration from HCT to sample draw, steroid treatment at sample draw, or eye involvement.
Table 3.
Linear regression analysis of factors associated with MMP-9 concentrations
| Simple regression | Multiple regression | ||||
|---|---|---|---|---|---|
|
|
|||||
| Factor | N | Fold difference* (95% CI) | P | Fold difference* (95% CI) | P |
| Main comparison | |||||
| No chronic GVHD | 29 | 1.00 (reference) | 1.00 (reference) | ||
| BOS | 33 | 2.07 (1.37–3.13) | 0.001 | 2.05 (1.37–3.07) | 0.001 |
| Non-BOS chronic GVHD | 31 | 1.35 (0.89–2.06) | 0.16 | 1.35 (0.90–2.04) | 0.15 |
| Patient age at sample draw, continuous (per decade) | 93 | 0.97 (0.83–1.13) | 0.69 | ||
| Patient sex | |||||
| Female | 41 | 1.00 (reference) | 1.00 (reference) | ||
| Male | 52 | 1.52 (1.07–2.16) | 0.019 | 1.50 (1.08–2.09) | 0.016 |
| Race | |||||
| White | 80 | 1.00 (reference) | |||
| Others | 13 | 0.69 (0.41–1.14) | 0.15 | ||
| Stem cell source | |||||
| Peripheral blood stem cell | 82 | 1.00 (reference) | |||
| Bone marrow | 7 | 1.33 (0.67–2.62) | 0.41 | ||
| Cord blood | 4 | 0.90 (0.37–2.19) | 0 82 | ||
| Conditioning intensity | |||||
| Myeloablative | 54 | 1.00 (reference) | |||
| Reduced intensity | 39 | 0.76 (0.53–1.09) | 0.13 | ||
| Donor type | |||||
| HLA-matched relative | 37 | 1.00 (reference) | |||
| HLA-mismatched relative | 4 | 0.68 (0.27–1.69) | 0.40 | ||
| Unrelated donor | 52 | 0.92 (0.63–1.33) | 0.65 | ||
| A female donor to a male recipient | 23 | 1.64 (1.10–2.45) | 0.016 | ||
| Prior grade II-IV acute GVHD | 57 | 0.74 (0.52–1.06) | 0.10 | ||
| Duration from HCT to sample draw per year | 93 | 1.14 (1.03–1.27) | 0.009 | ||
| Steroid treatment at sample draw † | 53 | 1.69 (1.19–2.39) | 0.003 | ||
| NIH global severity excluding the lungs ‡ | |||||
| None or mild | 32 | 1.00 (reference) | |||
| Moderate | 23 | 1.30 (0.82–2.05) | 0.27 | ||
| Severe | 9 | 0.97 (0.51–1.82) | 0.91 | ||
| Involved sites ‡ | |||||
| Skin | 25 | 0.92 (0.60–1.42) | 0.71 | ||
| Mouth | 32 | 1.15 (0.75–1.75) | 0.51 | ||
| Eye | 43 | 1.18 (1.75–1.84) | 0.47 | ||
| Gastrointestinal tract | 10 | 0.98 (0.55–1.76) | 0.95 | ||
| Liver | 5 | 0.89 (0.41–1.96) | 0.78 | ||
| Joint/fascia | 12 | 1.30 (0.76–2.22) | 0.33 | ||
| Genital | 5 | 1.80 (0.83–3.89) | 0.14 | ||
Fold difference values represent the anti-log10 of the regression coefficient.
One patient is excluded due to absence of information.
Analysis was performed in 64 patients with BOS or non-BOS chronic GVHD.
Discussion
By testing samples collected in prospective studies, our results showed that plasma MMP-9 concentration was associated with a diagnosis of BOS after allogeneic HCT and might also predict subsequent survival and treatment failure after FAM therapy. Our results showed that plasma MMP-3 concentration was associated with diagnosis of chronic GVHD after allogeneic HCT, not specific for BOS. In contrast to prior lung transplant BOS studies, our results did not support plasma YKL-40 as a biomarker for BOS after allogeneic HCT.
MMP-9 is present in low quantities in the healthy adult lung but is much more abundant in the lungs of patients suffering from chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis.23, 24 MMP-9 contributes to the remodeling processes leading to airway obstruction.5 Since bronchial neutrophils are suggested to be a major source of MMP-9,25 azithromycin, a component in FAM therapy that impedes neutrophil activity, may reduce bronchial neutrophil infiltration, and some studies have shown improved survival after lung transplantation.26 The results of the current study suggest that similar mechanisms are relevant in BOS after allogeneic HCT. In addition, our results indicated the potential of plasma MMP-9 concentrations in predicting treatment failure of FAM therapy. Concentrations of plasma MMP-9 may be affected by many factors. Multiple regression analysis showed that only BOS and male patient sex were independently associated with higher plasma concentrations of MMP-9.
A comprehensive proteomic analysis identified plasma MMP-3 as a diagnostic biomarker of BOS after allogeneic HCT and after lung transplantation.6 MMP-3 promotes epithelial-mesenchymal transition leading to tissue fibrosis.27 In a proteomic study, diagnostic relevance was derived mostly from comparison between patients with BOS and those with no chronic GVHD, and the difference in MMP-3 concentrations between patients with BOS and those with non-BOS chronic GVHD was small.6 We observed similar results in the current study of an independent cohort.
YKL-40 is a prominent regulator of lung inflammation and remodeling, prompting us to include it in the panel tested for biomarkers of BOS after HCT.28 YKL-40 is associated with chronic obstructive pulmonary disease, asthma and lung fibrosis.10, 29–31 High serum YKL-40 levels are associated with poor survival in patients with idiopathic pulmonary fibrosis.29 In the context of lung transplantation, pretransplant serum YKL-40 concentrations predicted development of BOS,3 and serum YKL-40 concentrations showed diagnostic relevance in distinguishing between post-infectious BOS and acute bronchiolitis in children,4 while they were not useful for diagnosing BOS in adults.2 YKL-40 concentrations in adult studies including ours were much higher compared with those in the pediatric study. Our study may not have been able to detect differences because the study included only adult patients or because other characteristics differ between HCT and lung transplantation.
This study has several limitations. Although this was a hypothesis-driven analysis based on prior literature, the sample size was small, and we did not have an independent cohort to validate the results. Our results should be interpreted with caution since there were only 7 patients who had treatment failure with FAM therapy. Our prognostic and predictive thresholds require further validation. Second, this study includes only adult patients, and the results might not apply to pediatric patients. Third, the matching windows for time after HCT had to be large in order to find adequate numbers of control samples. Although duration from HCT to sample draw was associated with MMP-9 concentrations in simple regression analysis, it was dropped in multiple regression analysis. The results were similar when duration from HCT to sample draw was forced in the multiple regression analysis, indicating that differences in sample timing had only a minor influence on the results. Lastly, we did not have bronchoalveolar lavage samples, although several studies have shown that MMP-9 and YKL-40 concentrations in bronchoalveolar lavage performed better than serum or plasma concentrations as biomarkers for BOS diagnosis after lung transplantation.25, 32
Although prognostic and predictive biomarkers are an urgent unmet need in the field,33 little data has been reported so far in BOS patients. Our results may indicate that plasma MMP-9 concentrations could serve as such a biomarker and that the therapeutic potential of MMP inhibitors could be tested in patients with BOS, since many MMP inhibitors are currently under development.34 Further studies are necessary to determine whether high plasma MMP-9 concentrations occur with impending BOS.
Highlights.
Plasma MMP-9 is a diagnostic and prognostic biomarker of BOS after allogeneic HCT.
Plasma MMP-9 concentration is predictive of treatment response after FAM therapy.
MMP-9 concentrations were higher in BOS patients than those with other situations.
MMP-9 concentrations were higher in patients who failed to FAM therapy.
BOS patients with MMP-9 ≥ 200,000 pg/ml had worse overall survival.
Acknowledgments
We thank all members in the Chronic GVHD Consortium (U54 CA163438) which was funded as part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Disease Research (ORDR), NCATS, funded through collaboration between NCATS and the National Cancer Institute.
Financial disclosure: This study was supported by grants from the National Cancer Institute (CA118953, CA163438), and from the Japan Society for the Promotion of Science (18K08345).
Conflict of interest statement: Y.I. reports personal fees from Novartis, Janssen, and Meiji Seika Pharma outside the submitted work. P.J.M. reports personal fees from Pfizer, Pediatric Transplantation and Cellular Therapy Consortium, Genentech, Pharmacyclics, Neovii, Enlivex Therapeutics, Mesoblast, Rigel, Talaris, Janssen, and Mount Sinai School of Medicine, non-financial support from Janssen, and others from AbGenomics (now AltruBio) outside the submitted work. S.J.L. reports grants from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda, and steering committee of Incyte. Other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kastelijn EA, van Moorsel CH, Ruven HJ, Korthagen NM, Kwakkel-van Erp JM, van de Graaf EA, et al. YKL-40 and matrix metalloproteinases as potential biomarkers of inflammation and fibrosis in the development of bronchiolitis obliterans syndrome. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:28–35. [PubMed] [Google Scholar]
- 3.Jaksch P, Taghavi S, Klepetko W, Salama M. Pretransplant serum human chitinase-like glycoprotein YKL-40 concentrations independently predict bronchiolitis obliterans development in lung transplant recipients. J Thorac Cardiovasc Surg. 2014;148:273–81. [DOI] [PubMed] [Google Scholar]
- 4.Jang YY, Park HJ, Chung HL. Serum YKL-40 levels may help distinguish exacerbation of post-infectious bronchiolitis obliterans from acute bronchiolitis in young children. Eur J Pediatr. 2017;176:971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pain M, Royer PJ, Loy J, Girardeau A, Tissot A, Lacoste P, et al. T Cells Promote Bronchial Epithelial Cell Secretion of Matrix Metalloproteinase-9 via a C-C Chemokine Receptor Type 2 Pathway: Implications for Chronic Lung Allograft Dysfunction. Am J Transplant. 2017;17:1502–14. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Yue Z, Yu J, Daguindau E, Kushekhar K, Zhang Q, et al. Proteomic Characterization Reveals That MMP-3 Correlates With Bronchiolitis Obliterans Syndrome Following Allogeneic Hematopoietic Cell and Lung Transplantation. Am J Transplant. 2016;16:2342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riise GC, Ericson P, Bozinovski S, Yoshihara S, Anderson GP, Lindén A. Increased net gelatinase but not serine protease activity in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2010;29:800–7. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Storer BE, Kushekhar K, Abu Zaid M, Zhang Q, Gafken PR, et al. Biomarker Panel for Chronic Graft-Versus-Host Disease. J Clin Oncol. 2016;34:2583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–73. [DOI] [PubMed] [Google Scholar]
- 11.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285:926–31. [DOI] [PubMed] [Google Scholar]
- 12.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–73. [DOI] [PubMed] [Google Scholar]
- 13.Kang MJ, Yoon CM, Nam M, Kim DH, Choi JM, Lee CG, et al. Role of Chitinase 3-Like-1 in Interleukin-18-Induced Pulmonary Type 1, Type 2, and Type 17 Inflammation; Alveolar Destruction; and Airway Fibrosis in the Murine Lung. Am J Respir Cell Mol Biol. 2015;53:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams KM, Cheng GS, Pusic I, Jagasia M, Burns L, Ho VT, et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chronic GC. Rationale and design of the chronic GVHD cohort study: improving outcomes assessment in chronic GVHD. Biol Blood Marrow Transplant. 2011;17:1114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chronic GC. Design and Patient Characteristics of the Chronic Graft-versus-Host Disease Response Measures Validation Study. Biol Blood Marrow Transplant. 2018;24:1727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 18.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21:984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen JA, Hanash SM, Tabellini L, Baik C, Lawler RL, Grogan BM, et al. A novel soluble form of Tim-3 associated with severe graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells JM, Parker MM, Oster RA, Bowler RP, Dransfield MT, Bhatt SP, et al. Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPDGene. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez IE, Amarie OV, Mutze K, Königshoff M, Yildirim A, Eickelberg O. Systematic phenotyping and correlation of biomarkers with lung function and histology in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;310:L919–27. [DOI] [PubMed] [Google Scholar]
- 25.Smith GN Jr., Mickler EA, Payne KK, Lee J, Duncan M, Reynolds J, et al. Lung transplant metalloproteinase levels are elevated prior to bronchiolitis obliterans syndrome. Am J Transplant. 2007;7:1856–61. [DOI] [PubMed] [Google Scholar]
- 26.Jain R, Hachem RR, Morrell MR, Trulock EP, Chakinala MM, Yusen RD, et al. Azithromycin is associated with increased survival in lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2010;29:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korthagen NM, van Moorsel CH, Barlo NP, Ruven HJ, Kruit A, Heron M, et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med. 2011;105:106–13. [DOI] [PubMed] [Google Scholar]
- 30.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–27. [DOI] [PubMed] [Google Scholar]
- 31.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taghavi S, Krenn K, Jaksch P, Klepetko W, Aharinejad S. Broncho-alveolar lavage matrix metalloproteases as a sensitive measure of bronchiolitis obliterans. Am J Transplant. 2005;5:1548–52. [DOI] [PubMed] [Google Scholar]
- 33.Paczesny S, Hakim FT, Pidala J, Cooke KR, Lathrop J, Griffith LM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant. 2015;21:780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors - Novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res. 2017;1864:1927–39. [DOI] [PubMed] [Google Scholar]