Abstract
Background
Studies on the health effects of metal mixtures typically utilize biomarkers measured in a single biological medium, such as blood or urine. However, the ability to evaluate mixture effects are limited by the uncertainty whether a unified medium can fully capture exposure for each metal. Therefore, it is important to compare and assess metal mixtures measured in different media in epidemiology studies.
Objectives
The aim of this study was to examine the mixture predictive performance of urine and blood metal biomarkers and integrated multi-media biomarkers in association with birth outcomes.
Methods
In our analysis of 847 women from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) study, we measured 10 essential and non-essential metals in repeated and paired samples of urine and blood during pregnancy. For each metal, we integrated exposure estimates from paired urine and blood biomarkers into multi-media biomarkers (MMBs), using intraclass-correlation coefficient (ICC) and weighted quantile sum (WQS) approaches. Using Ridge regressions, four separate Environmental risk scores (ERSs) for metals in urine, blood, MMBICC, and MMBWQS were computed as a weighted sum of the 10 metal concentrations. We then examined associations between urine, blood, and multi-media biomarker ERSs and birth outcomes using linear and logistic regressions, adjusting for maternal age, maternal education, pre-pregnancy body mass index (BMI), and second-hand smoke exposure. The performance of each ERS was evaluated with continuous and tertile estimates and 95% confidence intervals of the odds ratio of preterm birth using area under the curve (AUC).
Results
Pb was the most important contributor of blood ERS as well as the two integrated multi-media biomarker ERSs. Individuals with high ERS (3rd tertile) showed increased odds of preterm birth compared to individuals with low ERS (1st tertile), with 2.8-fold (95% CI, 1.49 to 5.40) for urine (specific gravity corrected); 3.2-fold (95% CI, 1.68 to 6.25) for blood; 3.9-fold (95% CI, 1.72 to 8.66) for the multi-media biomarkers composed using ICC; and 5.2-fold (95% CI, 2.34 to 11.42) for multi-media biomarkers composed using WQS. The four ERSs had comparable predictive performances (AUC ranging from 0.64 to 0.68) when urine is examined with specific gravity corrected concentrations.
Conclusions
Within a practical metal panel, measuring metals in either urine or blood may be an equally good approach to evaluate the metals as a mixture. Applications in practical study design require validation of these methods with other cohorts, larger panels of metals and within the context of other adverse health effects of interest.
Keywords: Metals, prenatal stress, social support, manganese, Puerto Rico
INTRODUCTION
Exposure biomonitoring, which estimates human exposure by measuring chemical or other agents of interest or their metabolic products in different biologic media, such as blood and urine [1], has become a fundamental approach used in exposure assessment and environmental epidemiology [2]. With growing interest in the realistic scenario of studying the collective effects of environmental chemicals on humans, including metals [3–11], biomonitoring has become indispensable in studies of mixtures. Due to limiting factors such as financial cost and methodologic challenges, mixture studies based on biomarkers typically use a unified human specimen (i.e. blood, urine, etc.) to determine exposure to various chemicals [12–17]. While this approach may capture overall exposure to a class of chemicals with similar structure and pharmacokinetic properties, such as urinary phthalates and blood perfluorinated compounds (PFAS), it is more challenging to evaluate chemical classes such as metals. Because each metal possesses different pharmacokinetic properties, utilizing one medium for measuring metal mixtures may not represent exposure for each metal or accurately reflect overall human exposure. Moreover, for different metals, each medium may also represent a different window of exposure that provides important information in relation to the health outcome of interest.
A set of biomarkers reflecting integrated metal mixture information from multiple media not only reduces the error in the exposure estimation, but also captures different exposure sources and pathways. Thus, it may be appropriate to combine exposure from different media to assess human exposure to both single metal and metal mixtures. Previous studies have proposed different techniques to integrate biomarkers of exposure to single chemicals, including confirmatory factor analysis [18, 19], structural equation models [19–21], and the derivation of multi-media biomarkers (MMBs) through mixture methods: non-negative matrix factorization (NMF), independent component analysis (ICA) and weighted quantile sum (WQS) regression [22]. A few studies have modeled metal mixtures measured in multiple matrices simultaneously and demonstrated that a combination of different metal biomarker factors may improve the prediction of health outcomes [23, 24]. Those studies have validated techniques to select the most important biomarker for each exposure individually, which has provided useful information for recommending a more suitable biomarker for a single metal. To our knowledge, very few epidemiological studies have evaluated the overall performance of simple metal mixtures (up to 4 metals) measured in different media in association with health outcomes [25, 26]. Therefore, our goals were to assess whether data on a wide panel of metal mixture exposures measured using different media can be integrated and compare the performance of different matrices when assessing the relationship between metal mixtures and birth outcomes.
To achieve this goal, we conducted the following study. First, we proposed ways to integrate multi-media exposure information from biomarkers measured in different media. Second, we assessed the performance of metal mixtures measured in different media and the combined multi-media exposure as related to health outcomes. We chose adverse birth outcomes as our outcome of interest because exposure to metals impacts various biological pathways that contribute to adverse birth outcomes, including preterm delivery and low birthweight [4, 12, 27–39]. Limited mixture studies on this topic have mostly focused on metals measured in either urine or blood [4–8]. In the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) study, we measured a wide range of metals in paired urine and blood samples, which enabled us to compare the associations between adverse birth outcomes and urinary or blood metal mixtures, as well as integrated metal mixtures utilizing both matrices. We hypothesized that the use of urine, blood, and the integrated metal mixtures would demonstrate differing performance when modeling adverse birth outcomes, informing more efficient study designs for exposure assessment.
METHODS
2.1. Study population
This study used data collected from 847 pregnant women participating in the PROTECT study, an ongoing, prospective birth cohort [40–43]. The PROTECT study was launched in 2010 with funding from the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program to investigate Puerto Rico’s high preterm birth rate and the extent of hazardous waste contamination on the island. PROTECT aims to explore environmental exposures and other factors contributing to preterm birth risk and other adverse birth outcomes in Puerto Rico.
Study participants were recruited at approximately 14 ± 2 weeks of gestation at seven prenatal clinics and hospitals throughout Northern Puerto Rico and followed until delivery [40, 41]. Pregnant women included in the study were aged between 18 to 40 years, resided inside of the Northern Karst aquifer region, and were planning to deliver in the participating hospitals. Exclusion of participants included the use of oral contraceptives within the three months prior to pregnancy; use of in vitro fertilization to become pregnant; or any major medical or obstetrical complications, including pre-existing diabetes [44]. Each woman participated in a total of up to three study visits during 18 ± 2 weeks, 22 ± 2 weeks, and 26 ± 2 weeks of gestation. At the initial visit, detailed information on medical and pregnancy history was collected. Nurse-administered questionnaires were used to gather information on housing characteristics, employment status, and family situation at an in-home visit (22 ± 2 weeks). Spot urine samples were collected from women at up to three visits and blood samples were collected during the first and third visits. A total of 847 women who delivered a live singleton birth had available data on 10 paired urine and blood metal biomarkers (collected at the same time point) as well as information on covariates (Figure 1).
Figure 1.
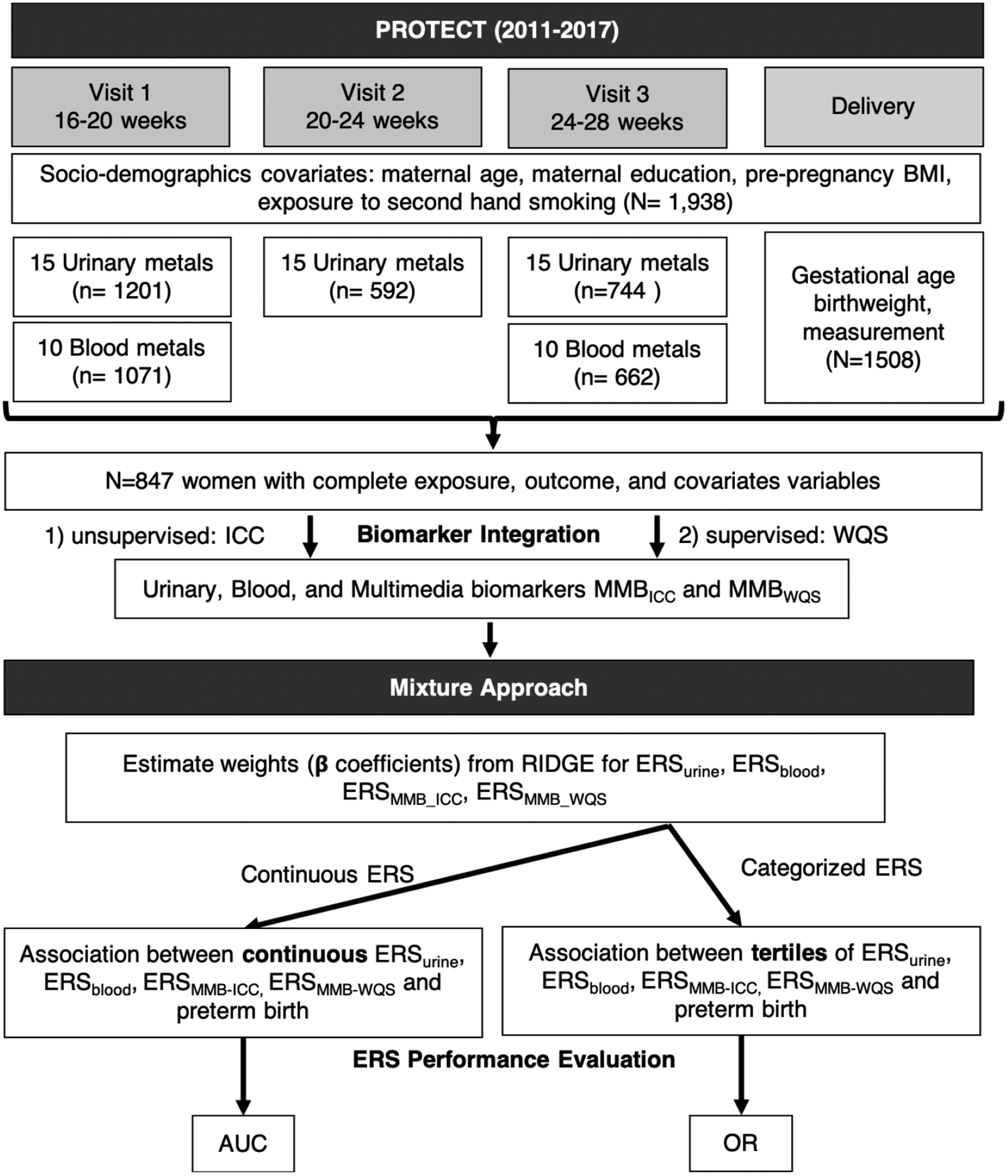
Schematic plot of study design, sample size, and statistical methods for constructing and evaluating multi-media biomarker (MMB) and Environmental Risk Score (ERS).
The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan, Northeastern University, and the University of Georgia. The study was described in detail to all participants, and informed consent was obtained prior to study enrollment.
2.2. Measurement of metals
Spot urine was collected in sterile polypropylene cups and aliquoted within one hour after collection, while blood samples were collected in metal-free whole blood tubes. All samples were frozen and stored at −80°C and shipped on dry ice. Analysis was performed at NSF International (Ann Arbor, MI, USA), where concentrations of 16 metals and metalloids were measured in both urine and blood: arsenic (As), barium (Ba), beryllium (Be), cadmium (Cd), cobalt (Co), chromium (Cr), cesium (Cs), copper (Cu), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb), titanium (Ti), uranium (U), vanadium (V), and zinc (Zn); an additional 5 metals and metalloids were measured in urine only: molybdenum (Mo), platinum (Pt), antimony (Sb), tin (Sn), and tungsten (W). Metal(loid) concentrations were measured using inductively coupled plasma mass spectrometry (ICPMS) as described previously [4]. The laboratory selected the appropriate isotopes for the requested elements to best avoid interferences from high levels of carbon and chloride in the biological sample matrix. The ICPMS was calibrated with a blank and a minimum of 4 standards for each element of interest. An R2 value of >0.995 was the minimum criterion for an acceptable calibration curve. The calibration curves were verified by initial checks at three calibration points within the curve. Continuing calibration checks and blanks after every 10 samples were also utilized throughout the analytical run to ensure the ICPMS system was maintaining acceptable performance. Urinary specific gravity (SG) was measured at the University of Puerto Rico Medical Sciences Campus using a hand-held digital refractometer (Atago Co., Ltd., Tokyo, Japan) as an indicator of urine dilution.
2.3. Preterm birth and auxiliary birth outcomes
All birth outcome data were extracted from medical records. We used the American Congress of Gynecologists (ACOG) recommendations to calculate gestational age at birth [45], as previously described in detail [46, 47]. In this study, preterm birth was defined as delivery < 37 completed weeks of gestation, as per common practice. Preterm birth can be classed into two groups, based on the clinical presentation of preterm delivery: medically indicated preterm birth (preterm births with preeclampsia, or with both artificial membrane rupture and induced labor) and spontaneous preterm birth (presentation of premature rupture of the membranes, spontaneous preterm labor, or both). In our analysis, the outcome of interest was overall and spontaneous preterm birth.
Other birth outcomes, including gestational age, and fetal growth outcomes [birthweight z-score, small for gestational age (SGA), and large for gestational age (LGA)], were also included in our analysis as auxiliary outcomes. INTERGROWTH-21st standard gestational age- and sex-specific birthweight z-scores were constructed and used in the analysis [48]. SGA and LGA births were defined as below the 10th percentile and above the 90th percentile of birthweight z-scores, respectively.
2.4. Statistical methods
To account for urinary dilution, metal concentrations in urine were corrected for SG using the equation: Pc = P[(SGp – 1)/(SGi – 1)]; where Pc is the SG-corrected biomarker concentration (ng/mL), P is the measured biomarker concentration, SGp is the median urinary specific gravity in this population (1.019), and SGi is the individual’s urinary specific gravity. Metal concentrations below the limit of detection (LOD) were replaced by LOD/√2. The geometric averages of participant concentrations across the visits were calculated for each SG-corrected urinary (visit 1=1201, visit 2=592, visit 3=744) and blood (visit 1=1071, visit 3=662) metal. In the case of a missing value at one of the study visits, the average for urine biomarkers was taken of the two remaining concentrations and the average for blood biomarker was equal to the single available concentration. In the case of two missing urine measurement, the “average” metal concentration was equal to the single available concentration. After these steps of preprocessing, metals that were measured in paired media (have both urinary and blood geometric average measurements) and had at least 50% of samples with concentrations above the LOD in both matrices were included in statistical analysis.
Descriptive statistics were calculated for all exposures and outcome variables. We applied natural logarithmic transformation to all urinary and blood metals because the distributions were right-skewed prior to transformation. Spearman’s rank correlations (rs) were used for the analysis of the relationships between paired urinary and blood metal average concentrations. Data were analyzed using R version 3.6.2. A schematic representation of the data accumulation and analytic procedure is also presented in Figure 1.
2.4.1. MMB composition
In this analysis, we proposed two approaches to integrate exposure information from urinary and blood metal measurements into a multi-media biomarker (MMB). In the first approach, inspired by the concept of cue combination in quantitative cognitive neurosciences (the minimum-variance unbiased estimator for multiple sources of information), we used an internal reliability indicator of the biomarkers to integrate MMB (unsupervised method). In the second approach, which is a supervised method, weighted quantile sum (WQS) models were used because the relative contribution of the individual biomarkers to the overall exposure and health outcome association is easier to interpret than many other mixtures methods. The details of the approaches are described below.
Integrating multi-media biomarker (MMB) using intraclass correlation coefficient (ICC)
Characterizing the within- and between-individual variation of measurements with parameters such as intraclass correlation coefficient (ICC) gives information on the reliability of the different media biomarkers. ICC is defined as the proportion of the total variance that is attributed to between-individual variability:
| [1] |
In epidemiological studies with repeated measurements, the ICC metric, ranging from 0 to 1, indicates reliability among multiple measurements of a quantity; when close to 0, ICC reflects large variations between repeated measures, a.k.a. poor ability to distinguish between individuals with high and low exposure levels; when close to 1, the repeated measures are close to each other which reflects a good ability to discriminate between individuals with high and low exposure levels. In this unsupervised machine learning approach, we utilized the ICCs calculated from repeated measurements of SG-corrected urinary (up to three visits) and blood metals (up to two visits) [17] as weighting parameters to construct an integrated MMB separately for each metal using equations [2], [3], [4]:
| [2] |
| [3] |
| [4] |
where is the weight of a medium (wurine[ICC] + wblood[ICC] = 1) expresses whether a certain medium (urine or blood) as a biomarker for a particular metal is a relatively reliable biomarker and C is the geometric average of metal concentration.
Integrating multi-media biomarker (MMB) using weighted quantile sum regression (WQS)
In addition to the unsupervised learning method, the amount of exposure information each biomarker carries can be quantified simultaneously based on the relationship of the exposure measured in a certain medium and health effect--a supervised approach. Therefore, the second approach for integrating urinary and blood biomarkers was weighted quantile sum regression (WQS), which models the body burden of quantiles of exposure. WQS estimates a set of weights, such that the linear combinations of the weights * quantile biomarkers have the highest association with the outcome [49] (equation [5]). Details of the WQS equation and annotations are previously described [50]. In our analysis, wi is the unknown weight for the ith medium (1=urine, 2=blood, c=2), z represents a vector of adjusted covariates, and ϕ is a vector of regression coefficients for those covariates. By placing the constraints of the weight (wi) estimates to be non-negative and sum to 1, the comparative values of urine and blood metals to multi-media biomarkers and the joint effect can be determined. The weights can then be used to quantify the contribution of each medium to the multi-media biomarker. In this supervised learning approach, we used WQS (100 bootstraps) to determine the association between each birth outcome and urinary and blood biomarkers of each metal, separately. A preliminary analysis was conducted before the construction of WQS models. As WQS assumes linearity, in an effort to detect potential non-linear relationships between individual metals and birth outcomes, we used generalized additive models (GAM) to graphically depict the metal and birth outcome relationship. Results from GAM including metal concentrations as splines and the GAM output graphics showed that when the smoothing estimator is significant the observed associations are linear (estimated degree of freedom=1). Therefore, we proceeded with WQS. All outcomes were regressed on the geometric averages of SG-corrected urinary and blood concentrations across all the available visits. Then we combined the weights to generate multi-media biomarkers using equation [6].
| [5] |
| [6] |
2.4.2. Single-pollutant analysis
Generalized linear models (GLM) were used to examine the associations between four types of metal biomarkers measured and composed (urinary, blood, MMBICC, and MMBWQS) and birth outcomes. Separate models were used for each metal biomarker and outcome (e.g., Mn: MMBICC and gestational age). As both MMBs described above are composed using the geometric mean of SG-corrected urinary and blood metal concentrations, geometric average concentrations across all the available visits were also used for individual urinary and blood models in this single-pollutant analysis. Geometric mean of urinary, blood and MMB biomarker concentrations were split in to tertiles (risk stratification purposes). The full models included the tertiles of metal biomarker concentrations and a final set of covariates that were selected based on a priori knowledge and whether their inclusion appreciably changed the effect estimates of metal exposure [51]. These covariates were maternal age, maternal education level, pre-pregnancy BMI, and exposure to second-hand smoking. Effect estimates and 95% confidence intervals were calculated for the highest versus the lowest tertiles of exposure to measure the risk stratification properties of individual metals and compare them to the collective effects of metal mixtures as described below.
2.4.3. Mixture analysis
Construction of Environmental Risk Scores (ERSs) using Ridge regression
Environmental Risk Score (ERS) was proposed as a potential summary measure for the effects of mixtures in epidemiologic research [52]. The concept of ERS is to build a predictive risk model as a weighted sum of the exposure levels from simultaneous assessment of the all the pollutants in the mixture. Weights are determined by the magnitudes (standardized regression coefficients) of the association between each exposure and the outcome of interest [52]. We constructed Environmental Risk Scores (ERSs) as weighted summary measures of the effects of metals where the weights were regression coefficients derived from models of the association between metal mixtures and the outcome of interest. We utilized Ridge regression to guide the weight of each metal in relation to preterm birth. Ridge regression is a regularized regression technique, and it is one of the commonly used supervised machine learning solutions [53]. Ridge is used to constrain the size of the estimated coefficients, and the objective function for a continuous outcome can be expressed as:
| [7] |
where i = 1, …, n indexes the subjects, is the vector of p covariates for the ith subject, and yi is the continuous health outcome for the ith subject. Ridge regression utilizes the regularization penalty parameter λ (λ ∈ [0, ∞)) to solve the multicollinearity problem and control the shrinkage of the L2 penalty. Ridge regression decreases the complexity of the models and enforces the β coefficients to be lower without forcing them to be zero [53, 54]. The individual associations are not constrained to be in the same direction in Ridge regression. Therefore, Ridge regression was ideal in this setting as our analytic purpose was to evaluate the same full set of metals as mixtures across different media and integrated biomarkers that have different directions of individual metal-birth outcome associations. Using Ridge regression with an underlying model including biomarkers of 10 metals and covariates, we performed 10-fold cross-validation and selected the value that minimized the cross-validated sum of squared residuals [52, 55]. Four separate ERSs for metals in urine, blood, MMBICC, and MMBWQS were computed as a weighted sum of the 10 metal concentrations (C):
| [8] |
ERS models and evaluations
In order to assess risk stratification power of the different ERSs, we further categorized ERS by its tertiles. We then refit the regression models with both continuous and categorical ERSs to examine its associations with preterm birth as well as the auxiliary birth outcomes. We conceptualized the ERSs as a weighted sum of metal exposure measured in urine, blood, and multi-media biomarkers composed by WQS and ICC methods, namely, ERSurine, ERSblood, ERSMMB-WQS, and ERSMMB-ICC. ROC (Receiver Operating Characteristics) curves were used to evaluate the preterm, spontaneous preterm birth, SGA, and LGA classification model performances of four ERSs. Specifically, the area under curve (AUC) of ROC were computed for quantifying and visualizing the biomarkers’ classification accuracy for the above-mentioned binary outcomes. We used a bootstrap resampling (2000 iterations) to compute 95% confidence intervals of AUCs for different models [56] and to test the difference between AUCs (the ci.auc() and roc.test() functions in the pROC package in R [57]). For binary outcome models with ERS tertiles, we also computed the odds ratio (OR) for the highest tertile versus the lowest tertile to measure the risk stratification properties of ERS.
RESULTS
Descriptive analysis
Demographic characteristics of the 847 women in this analysis are summarized in Table 1 and were described previously [43, 58]. Mothers included in our analysis had similar demographic characteristics to the overall PROTECT population [59]. Briefly, the cohort included women in their late 20s (median =27 years) and half of them had a BMI less than 25 kg/m2 prior to pregnancy. The majority of women (57%) had private medical insurance, were non-smokers (86%) and very few (6%) reported alcohol consumption within the last few months. More than half reported an annual household income of less than $30,000, while 44% had reported graduating from college or higher. Mean gestational age was 39.1 (standard deviation=2) weeks for 847 singleton births included in this analysis, among which 78 (9%) were preterm and 41 (5%) were spontaneous preterm. Supplementary Table 1 displays descriptive statistics, including geometric mean, geometric standard deviation, and selected percentiles, of 10 metal concentrations measured in the paired urine and blood samples, as well as Spearman correlation coefficients between the two media for each metal. Most of the paired metal concentrations in the two matrices had a low but significant correlation, with Spearman correlation coefficient ranging from 0.07 to 0.43, while Mn, Ni, and Zn concentrations measured in urine and blood were not correlated. All the following results on urinary metals are presented for SG-corrected concentrations unless described otherwise.
Table 1.
Demographic characteristics of study participants from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort (n=847).
| Variable | Mean (SD) |
|---|---|
| Maternal age at enrollment (years) | 26.9 (5.6) |
| gravidity (# pregnancies) | 2 (1) |
| Insurance type | N (%) |
| Private | 480 (57%) |
| Public (Mi Salud) | 281 (33%) |
| Missing | 86 (10%) |
| Maternal education (years) | |
| High school/GED | 185 (22%) |
| Some College or technical school | 293 (35%) |
| College degree | 268 (32%) |
| Masters degree or higher | 101 (12%) |
| Missing | 0 (0%) |
| Income status (US $) | |
| <$10,000 | 260 (31%) |
| ≥$10,000 to <$30,000 | 212 (25%) |
| ≥$30,000 to <$50,000 | 176 (21%) |
| ≥$50,000 | 104 (12%) |
| Missing | 95 (11%) |
| Marital status | |
| Single | 163 (19%) |
| Married or living together | 680 (80%) |
| Missing | 4 (0%) |
| Gravidity (# pregnancies) | |
| 0 | 361 (43%) |
| 1 | 304 (36%) |
| >1 | 181 (21%) |
| Missing | 1 (0%) |
| Pre-pregnancy BMI (kg/m2) | |
| ≤25 | 447 (53%) |
| >25 to ≤30 | 230 (27%) |
| >30 | 170 (20%) |
| Missing | 0 (0%) |
| Smoking Status | |
| Never | 726 (86%) |
| Ever | 109 (13%) |
| Current | 12 (1%) |
| Missing | 0 (0%) |
| Exposure to second hand smoking | |
| None | 772 (91%) |
| Up to 1 hour/day | 30 (4%) |
| More than 1 hour/day | 45 (5%) |
| Missing | 0 (0%) |
| Alcohol consumption | |
| None | 438 (52%) |
| Before pregnancy | 354 (42%) |
| Within the last few months | 50 (6%) |
| Missing | 5 (1%) |
| Infant Sex | |
| Female | 406 (48%) |
| Male | 437 (52%) |
| Missing | 4 (0%) |
MMB composition
The weights of urinary and blood metals in the composition of MMBs from ICC and WQS approaches are depicted in Figure 2. As the ICC approach is based on an unsupervised learning method, the metal biomarker weights are the same across the birth outcomes. In contrast, the WQS approach is a supervised learning method, therefore, the weights constructed for each of the metal biomarkers were different for the respective birth outcomes. The corresponding urinary and blood weights (WQS) for each birth outcome are presented in Supplementary Table 2, while Figure 2 focuses on weights constructed from WQS models regressing preterm birth. For the majority of metals, blood was the main contributor to the MMBs from both ICC and WQS approaches. The blood weights for those metals were higher from the WQS approach than the ICC approach, except for Mn and Pb where the blood weights were higher from the ICC approach (60% and 88%) than the WQS approach (56% and 72%). In contrast, MMB for As was mostly attributed to urine from the WQS approach (95%).
Figure 2.
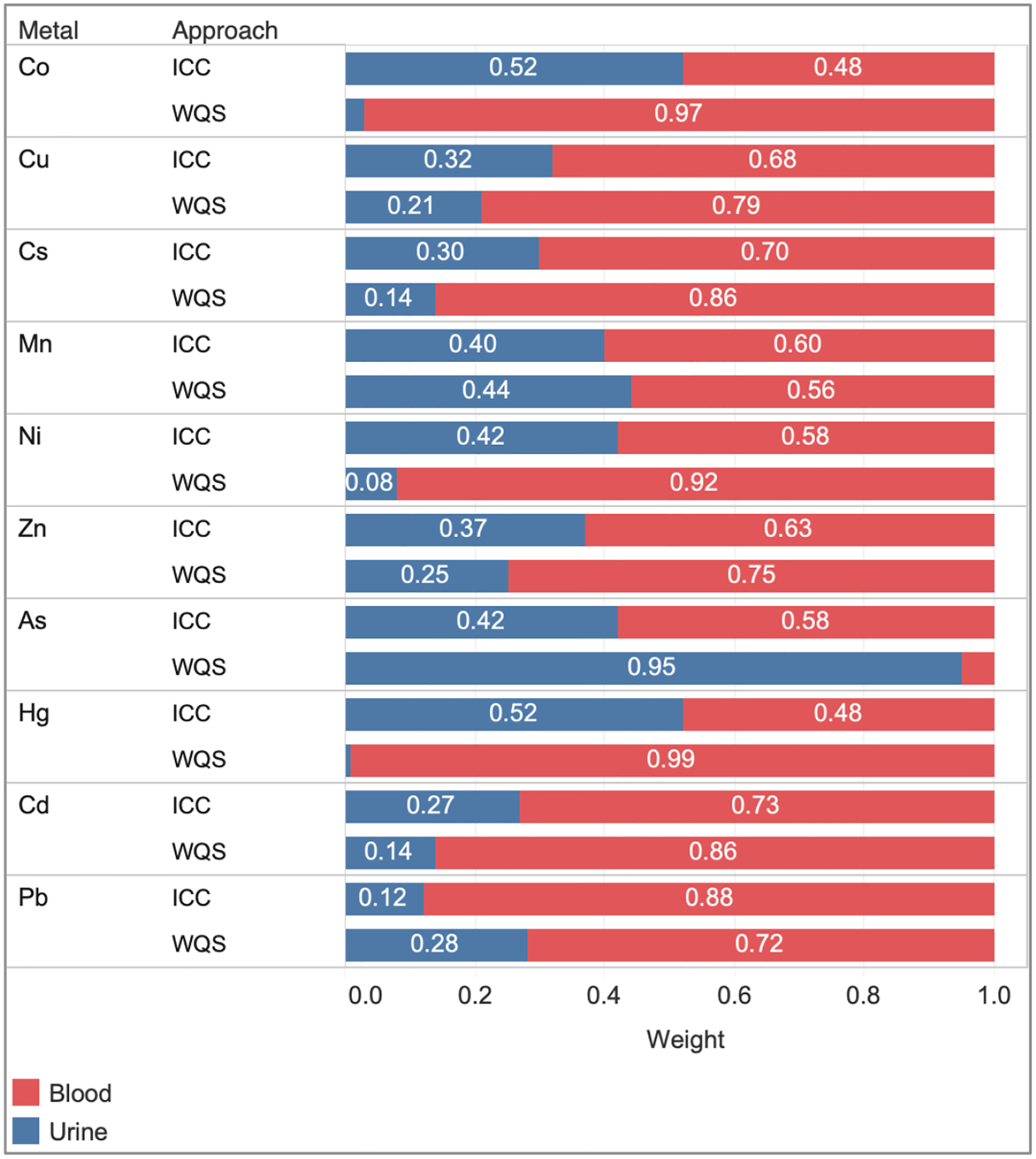
Bar graph of estimated urinary and blood biomarker weights for the MMBs using ICC approach and the WQS models of overall preterm birth. Larger weights indicate greater contributions of the original biomarkers to the MMBs.
ERSs
ERS weights derived from Ridge models regressing preterm and spontaneous preterm birth on metal mixtures are shown in Figure 3. The values of the weights for all the outcomes are provided in Supplementary Table 3. The largest contributors to preterm birth ERS from urine mixture were Cd (−0.01), Ni (−0.008), and As (−0.006). For preterm birth ERSs constructed from the blood, MMBICC, and MMBWQS mixtures, Pb and Mn were the largest positive weight contributors for all three. A similar weight distribution was observed for spontaneous preterm birth. The preterm birth ERSs from each biomarker mixture were normally distributed and ranged from −0.06 to 0.04 for urine; −0.13 to 0.88 for blood; −0.001 to 0.11 for MMBICC; and 0.08 to 0.48 for MMBWQS. For preterm birth ERSs, pairwise correlations among urine ERS and other ERSs were weak (r<0.2), whereas blood ERS had relatively higher correlations with the ERSs for MMBICC (r=0.88) and MMBWQS (r=0.53). The weight distribution for spontaneous preterm birth ERSs were similar to overall preterm birth ERS weights.
Figure 3.
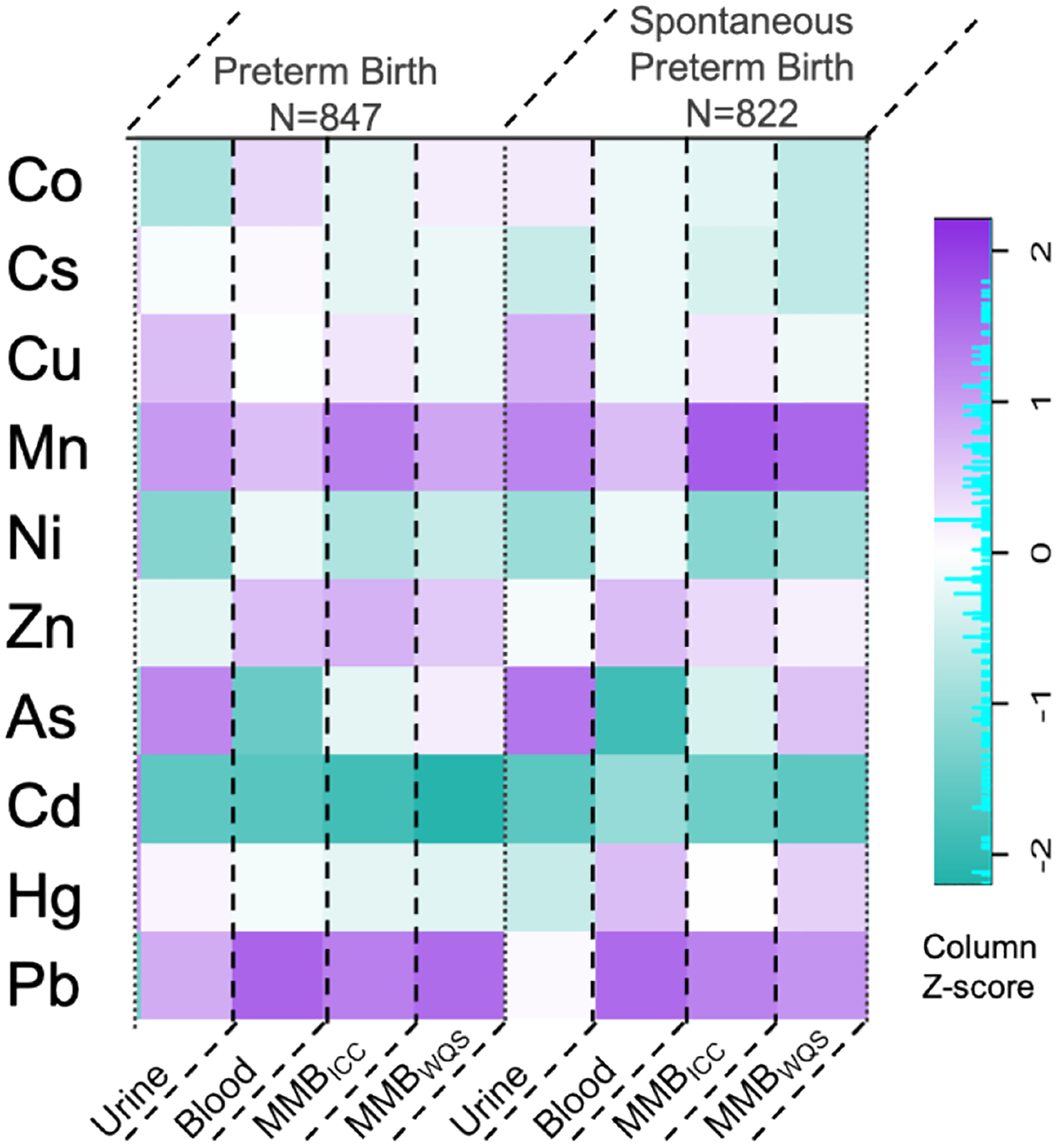
Heat map of weights for each individual metal biomarker extracted by the Ridge regression models regressing birth outcomes on urinary, blood, and two integrated multi-media biomarkers (MMB).
Continuous ERSs and birth outcomes
The result from our primary analyses of continuous ERSs and preterm birth are presented in Figure 4. All the ERSs were significantly associated with increased odds of preterm birth, with odds ratios ranging from 1.83 (95% CI: 1.27 to 2.59) to 2.00 (95% CI: 1.45 to 2.75). Changes in auxiliary birth outcomes associated with ERSs are shown in Supplementary Table 4. When spontaneous preterm birth is regressed on the four ERSs, odds ratios were generally higher compared to the overall preterm birth models, ranging from 2.34 (95% CI: 1.53, 3.56) to 2.56 (95% CI: 1.58, 4.14). For fetal growth outcomes, all ERSs were significantly associated with lower birthweight z-scores; the associations between ERSs and SGA were stronger (OR: 1.54 to 1.99) than the associations between ERSs and LGA (OR: 1.32 to 1.53) (Supplementary Table 4).
Figure 4.
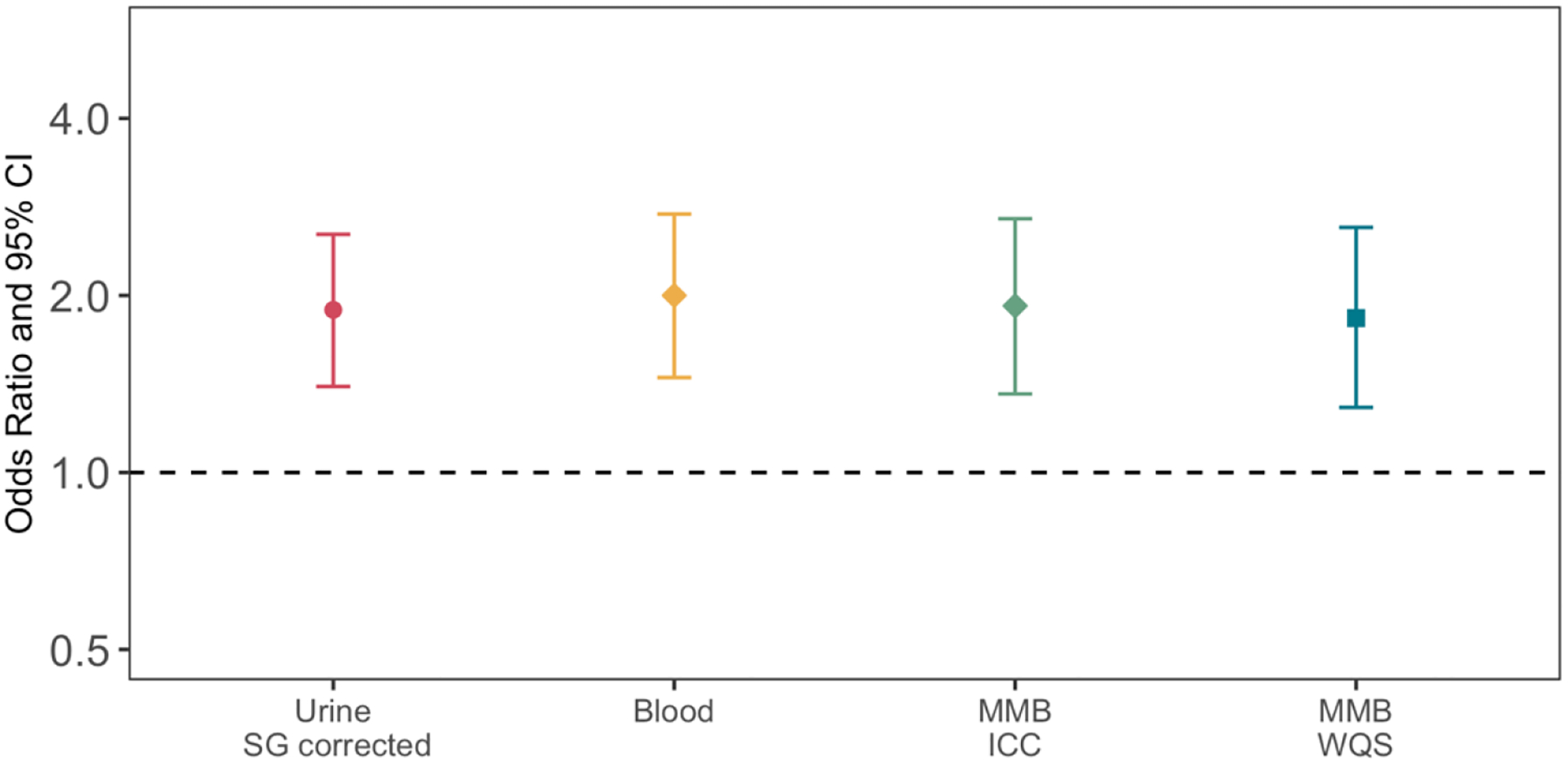
Odds ratio (OR) of preterm birth associated with Environmental Risk Scores (ERSs) constructed for urine (SG-corrected), blood, and two integrated multi-media biomarkers (MMB). Effect estimates presented as OR for IQR increase in average exposure biomarker concentration. Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
Figure 5 visualizes the AUC plots depicting the performance of different ERSs and preterm birth models and shows that there are no obvious differences in the AUC between urine, blood, and two MMB ERSs. Area under the curves for the four ERSs also did not differ statistically, indicating that urine, blood, and two MMB ERSs had comparable predictive performance in these models. Predictive performances of ERS on other binary outcomes followed similar patterns; performances of urine, blood, and MMB biomarkers were comparable, with AUC ranging from 0.66 to 0.69 for spontaneous preterm birth, from 0.60 to 0.65 for SGA, and from 0.60 to 0.62 for LGA.
Figure 5.
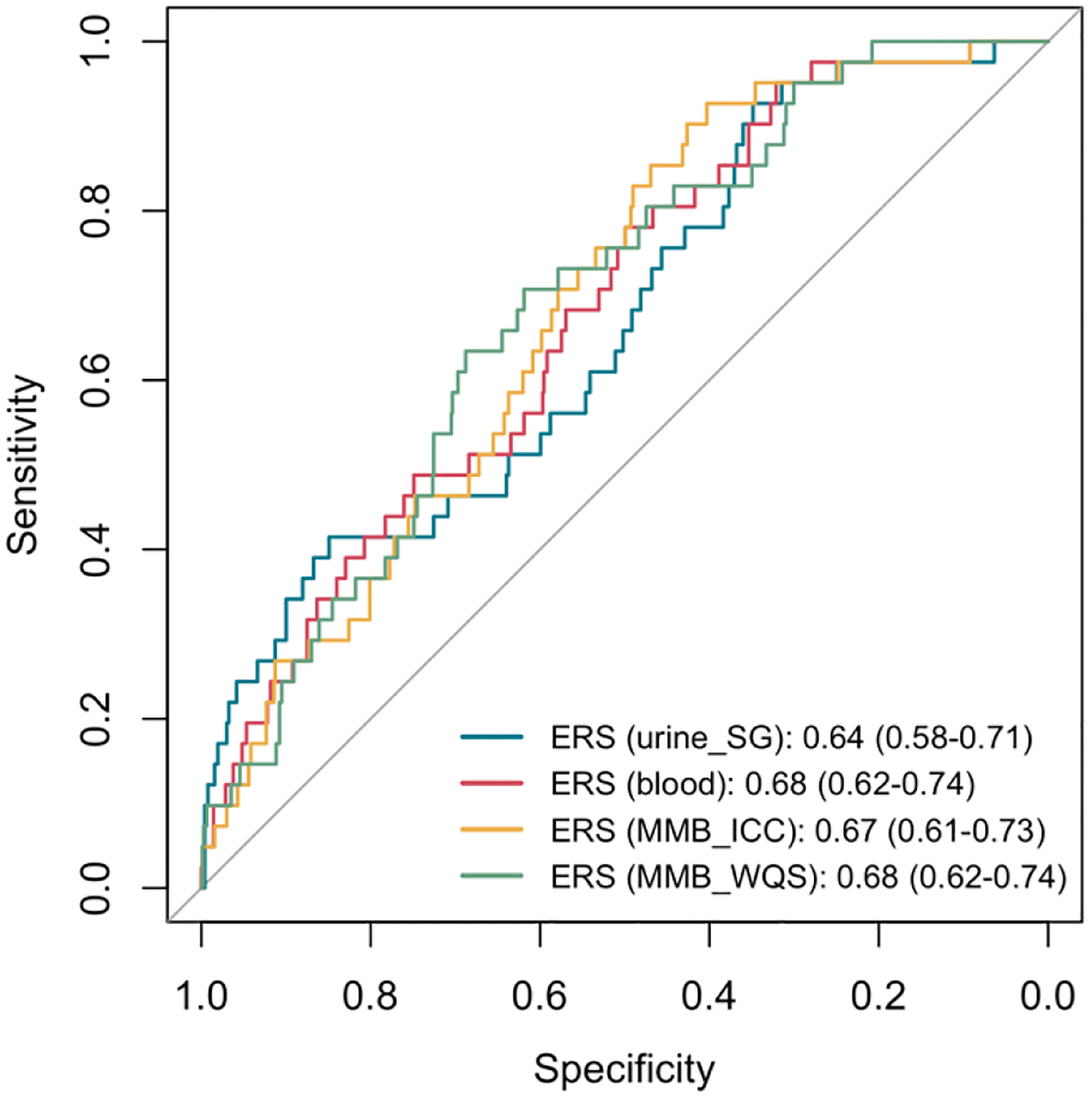
Area under the curves for preterm birth according to environmental risk score (ERS) constructed for urinary (SG corrected), blood, and two integrated multi-media biomarkers (MMB).
In an additional analysis, to illustrate the difference in the urine biomarker performance between disregarding versus accounting for urine dilution, we reported the odds ratio associated with both uncorrected and SG-corrected urine ERSs. Therefore, the performance was compared between metal mixtures measured in uncorrected urine, SG-corrected urine, blood, MMBICC, and MMBWQS. The results are presented in Supplementary Table 4 and Supplementary Figure 1. There was no significant association between uncorrected urine ERS and preterm birth, and the odds ratios for other ERS associations were greater than the odds ratio for the uncorrected urine metal ERS. The p values in Supplementary Figure 1 represent the significance of the test results for comparing the predictive performances (preterm birth) of different ERSs using AUC. The uncorrected urine ERS showed lower prediction performance than SG-corrected urine (p=0.04), blood ERS (p=0.02), MMBICC ERS (p=0.003), and MMBWQS ERS (p=0.07). This indicates that the area under the uncorrected urine ERS curve (AUC = 0.61; 95%CI = 0.54–0.68) is significantly smaller than the other AUCs shown in Figure 5: SG-corrected urine ERS (AUC = 0.64; 95%CI = 0.58–0.71), the blood ERS (AUC = 0.68; 95%CI = 0.62–0.74), MMBICC ERS (AUC = 0.67; 95%CI = 0.61–0.73), and MMBWQS (AUC = 0.68; 95%CI = 0.62–0.74).
Because Pb was the most important contributor of preterm birth blood ERS as well as the two MMB ERSs, we conducted another additional analysis excluding Pb from Ridge models while constructing and evaluating the performance of the ERSs. The effect estimates from this analysis for all four (urine, blood, MMBICC, MMBWQS) ERS were attenuated compared to the primary analyses (Supplementary Table 5). Continuous blood ERS was no longer significantly associated with preterm birth (OR/IQR=1.03, 95% CI 0.77 to 1.37, p=0.83). The effect estimates for urine ERS was 1.73 (95% CI 1.22 to 2.43, p=0.002), MMBICC was 1.83 (95% CI 1.23 to 2.54, p<0.001), and MMBWQS was 1.76 (95% CI 1.29 to 2.41, p<0.001).
Tertile metals, ERSs, and birth outcomes
ORs of preterm birth comparing the highest versus the lowest tertiles of individual metal biomarkers and ERSs are shown in Figure 6. After adjusting for covariates, individual associations for Mn (MMBs), Ni (urine), Zn (blood, MMBs), Cd (urine), Pb (blood, MMBs), and odds of preterm were significant. Ni and Cd biomarkers were associated with lower odds of preterm birth while Mn, Zn, and Pb were associated with higher odds of preterm birth. For example, a subject in Ni tertile 3 had 0.76 times lower odds of preterm birth (95% CI, 0.57 to 1) compared with a subject in Ni tertile 1. In contrast, a subject in Pb tertile 3 had 1.53 times higher odds of preterm birth (95% CI 1.14 to 2.06) compared with a subject in Pb tertile 1. As for ERS models, ORs of preterm birth ranged from 2.83 (95% CI, 1.49 to 5.40) for urine; 3.24 (95% CI, 1.68 to 6.25) for blood; 3.86 (95% CI, 1.72 to 8.66) for MMBICC; and 5.17 (95% CI, 2.34 to 11.42) for MMBWQS, after controlling for the same set of covariates. These ORs from the mixture analysis were considerably stronger than those for individual metals. Both individual and mixture analysis results for spontaneous preterm birth and auxiliary outcomes can be found in Supplementary Tables 4 and 6.
Figure 6.
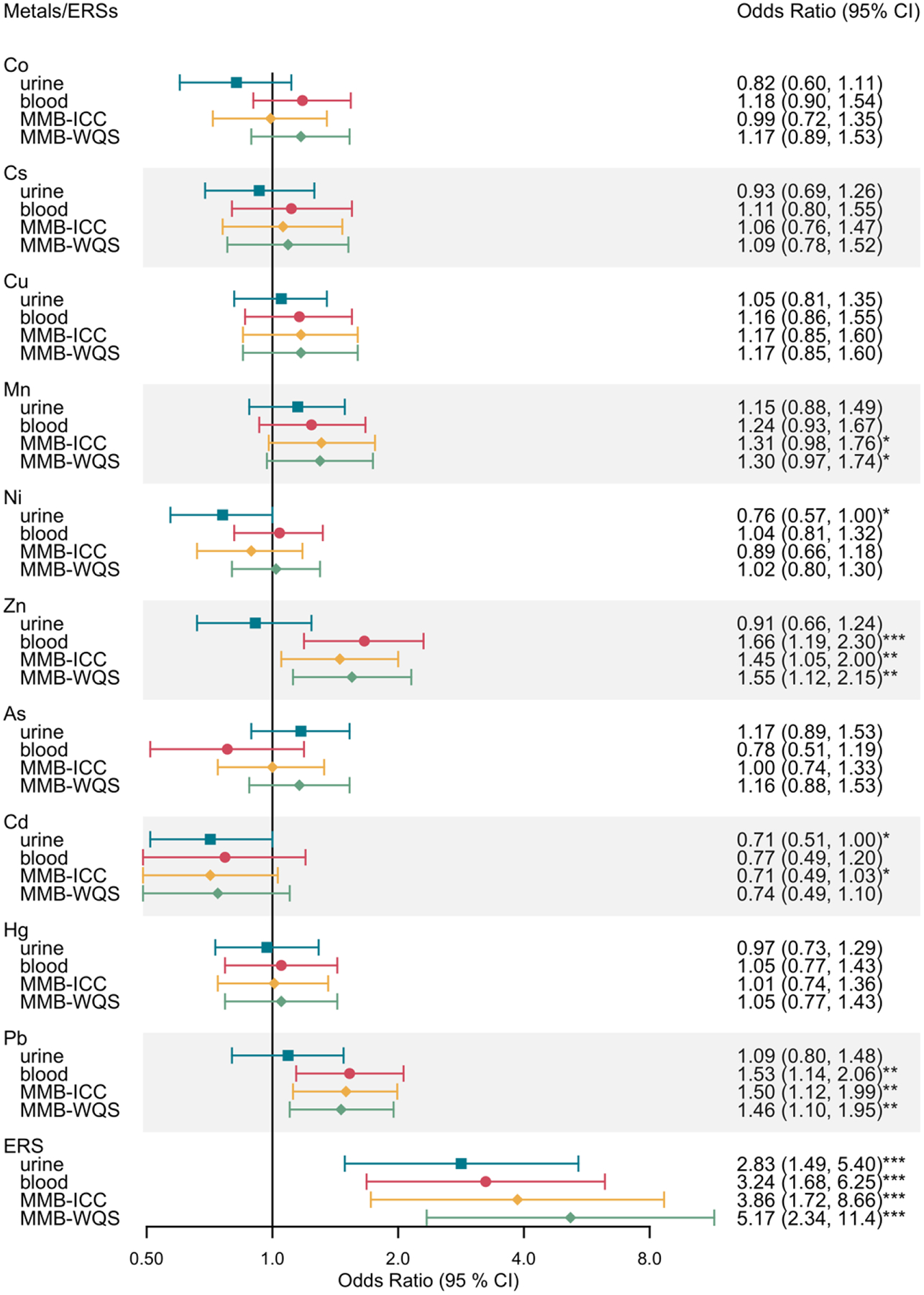
Odds ratio (OR) of preterm birth comparing the highest versus the lowest tertiles of individual metals and environmental risk scores (ERSs) constructed for urine, blood, and two integrated multi-media biomarkers (MMB) mixtures. Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
DISCUSSIONS
Epidemiologic studies aiming to determine the effects of environmental chemical mixtures on human health are growing rapidly. Due to limiting factors such as the financial cost and methodologic challenges, mixture studies based on biomarkers typically use a unified human specimen, such as blood or urine to determine exposure to various chemicals [12–17]. Although this approach applies well to chemicals with similar structure and pharmacokinetics, it is challenging to accurately describe metal mixtures using one unified medium. Each metal exhibits unique physiochemical properties and toxicokinetics, such as half-life, storage, or elimination rate from the body. As such, the preference for either blood or urine concentration as a better indicator is different across metals, often determined by the half-life of each metal and cost of measurement. For example, urinary concentration of As has often been used as an indicator of recent exposure because urine is the main route of excretion of most arsenic species [60, 61]. In contrast, blood is the preferred specimen for Pb, as blood Pb has a longer half-life and subsequently lower variability in the body compared to urine [62]. As for other metals such as Mn, Cu, and Cr, there is a lack of consensus in the literature as to which biomarker is the most consistent and valid. Previous mixture studies on prenatal metal exposures and birth outcomes measured metals in different media including urine [4, 63–65], whole blood [7, 66], cord blood [67], toenails [5], and teeth [68]. As mentioned above, each medium depicts biomarker levels in a particular body compartment that may have differential biological relevance and may not fully represent the best measure of internal dose for all the metals. Therefore, it is imperative that we understand how the choice of different media can impact the performance of analyzing chemical mixtures in relation to a specific health outcome. In this paper, we evaluated metals measured in urine and blood, as well as two integrated multi-media biomarker indices, in relation to birth outcomes among pregnant women.
We first applied supervised and unsupervised approaches to combine exposure information from multi-media (urine and blood) metal biomarkers. For each individual metal, the weights for urine and blood from both approaches were generally similar with a few exceptions, most notably with As. When applying the supervised approach with WQS where the relationship of urine and blood biomarkers with the health outcome (preterm birth) is considered, approximately 95% of the As association was driven by urinary As. This result indicates that urinary As is the more important predictor than the blood As in modeling preterm birth. However, the weight for urinary As from the ICC approach was much lower (42%). This difference is mainly due to a lower ICC (0.21) for the repeated measurements of urinary As in this study [17]. To our knowledge, only one previous study on pregnant women reported an ICC (0.16) for urinary As during pregnancy, which is similar to the ICC calculated in the present study [69]. These ICCs indicate weak reliability of urinary As during pregnancy, while reports on the general population demonstrated fair reliability of urinary As (ICC ranging from 0.45 to 0.49) over a longer period of time (1–2 years) [70, 71]. The discrepancy was possibly due to the physiological changes related to the pregnancy (i.e. metabolic changes, plasma volume expansion) [72–75], and unique environmental and behavioral factors such as dietary habits unique to this population.
Once we constructed MMBs, we used Ridge models to guide the weights of each metal biomarker in constructing the ERSs. Four ERSs (SG-corrected urine, blood, and the integrated MMBs) had comparable level of performances. Examining uncorrected metal mixture concentrations resulted in a significantly lower performance in urine ERS compared to the other four ERSs. These results indicate that consideration of measuring metal mixtures in either urine or blood may be an equally good approach when correcting urinary metals for SG. Although the optimal urine concentration adjustment approach for metals remains uncertain [76–78], our findings underline the importance of considering urinary dilution when evaluating the health effects of a metal mixture measured in urine. This conclusion is supported by previous literature validating the improved robustness and reliability of physiological measures for correcting variation in urinary output [79–83].
In Ridge models using blood, MMBICC, and MMBWQS, Pb was most strongly associated with preterm birth. This is consistent with our previous finding within this population where concentrations of blood Pb among pregnant women in Puerto Rico (average=0.33 μg/dL) were well below the level of concern set by CDC, a blood level of 5 μg/dL for pregnant women [84]. Yet Pb was the most significant predictor of preterm birth [51]. Therefore, when we conducted the same analysis for all the biomarker mixtures excluding Pb, the association between blood ERS and preterm birth was no longer significant. These findings shed light on the importance of studying Pb in metal mixtures, especially blood biomarkers, as the performance of blood ERS was mainly driven by the strong effect of Pb. The findings also warrant further studies of different metal panels in regard to mixture performance.
Ridge regression and tertile analysis of individual metals also revealed other important predictors of overall and spontaneous preterm birth, including essential metals such as Cu, Mn, and Zn and non-essential metal As. In our previous work, we compared our findings on the effect of Cu, Mn, Zn, Ni, and Pb on adverse birth outcomes with previous animal and human studies and discussed potential mechanism of action in detail [51, 85]. Briefly, consistent with our findings, previous studies have suggested that elevated levels of essential metals Mn, Cu, and Ni may be associated with increased risk of intrauterine growth restriction [86], preterm delivery [4, 31, 87], and low birthweight [32, 33]. However, our findings of increased risk in relation to Zn are contrary to animal studies, observational and randomized trials on humans where maternal Zn deficiency is often associated with adverse birth outcomes, including preterm birth [88, 89]. We proposed that blood Zn levels may reflect the state of various processes in the body, including inflammation, oxidative stress and other key functions [90–92] that can play a role in gestation length. Several important mechanisms of action were suggested for the abovementioned metals, including oxidative stress and inflammation [93–103], disruption of reproductive hormones [104–115], and epigenetic changes [116, 117], but need to be investigated more closely in future longitudinal studies with data on molecular markers of these potential mediators.
After comparing individual and ERS tertiles in relation to preterm birth, we observed a few significant but overall smaller effects corresponding to individual metals and stronger effects corresponding to the four combined ERSs, indicating the cumulative impact of the individual metals (Figure 6). This is consistent with previous literature where stronger cumulative effects of metals and environmental chemicals were reported when analyzed as mixtures [6, 118, 119]. After adjusting for covariates, the odds ratio of preterm birth comparing a subject in the higher end of overall metals exposure (ERS tertile 3) with a subject in the lower end (ERS tertile 1) were 2.8 for urine (95% CI, 1.49 to 5.40), 3.2 for blood (95% CI, 1.68 to 6.25), 3.9 for MMBICC (95% CI, 1.72 to 8.66), and 5.2 for MMBWQS (95% CI, 2.34 to 11.42). Assuming these odds ratios quantify the potential for risk stratification of preterm birth, the integrated multi-media biomarker models resulted in a higher risk of preterm birth associated with overall metal exposure. From a risk stratification perspective, integration of urine and blood biomarkers that were derived from both ICC and WQS approaches improved the model performance in the mixture models compared to the sole urine or blood biomarker models. Although the confidence intervals for these odds ratio estimates were wider, the MMB integration, especially using the WQS approach, resulted in substantially higher effect estimate. This finding supports that while multiple measurements of the exposure mixture may measure metal body burden differentially, there may still be room for improvement for exposure measurement error structure and effect estimate when incorporating urinary and blood biomarker information.
Strengths and Limitations
Specific strengths of our study include its longitudinal design in which repeated urinary and blood biomarkers provided more accurate exposure information during pregnancy. This allowed us to quantify the temporal reliability of the two media measurements (ICCs), which were further used as weights to integrate the multi-media measure of exposure. Secondly, this study utilized data-driven machine learning approaches to 1) inform the composition of multi-media metal biomarkers measured in different media and 2) guide the construction of environmental risk scores for each medium reflecting the overall exposure to the metal mixture. Finally, this study evaluated the health effects of multiple metals simultaneously and compared the performance of different media biomarkers and integrated biomarkers in relation to adverse birth outcomes in the context of a mixture. The results lay the groundwork for future epidemiological studies on biomarker selection when examining the mixture effects of metals.
However, our study has several limitations. The relatively small sample size did not allow cross-validation within this population on ERS, which may have caused overfitting of ERS. Future studies with a larger sample size should implement training and test datasets to cross-validate ERSs. Studies with larger sample sizes are also needed to address potential improvement of models by including non-linear terms and interactions between metals and covariates that were not accounted for in the current analysis. Depending on the pharmacokinetics of each chemical within a family, mixture studies, in general, are challenged by the complications of combining chemical biomarkers where each may be representing a unique window of exposure. While our ERS estimates also suffered from this limitation, by combining multi-media exposure using both urine and blood biomarkers, we were able to reduce the measurement error in the mixture analysis to an extent. Because the average concentrations of metal biomarkers across three visits were used in this analysis, windows of vulnerability during pregnancy were not evaluated. Given that associations between metal mixtures and birth outcomes may depend on developmental time windows, it is important for future longitudinal studies to evaluate the temporality of the associations. In addition, while we evaluated a mixture of 10 essential and non-essential metals, it is possible that other metals that were not assessed in our study affect the performance of urine or blood biomarkers. Future work is needed to expand the mixture evaluation to include more metals and other biospecimens including hair and saliva, as well as other adverse health outcomes because our results may not be generalizable to other outcomes of interest. Finally, while the mixture of metals in this study is representative of exposures experienced by Puerto Rican populations, it may not accurately reflect exposure profiles for other populations.
CONCLUSIONS
Our study used innovative methodology to provide new detailed insights into the individual and integrated associations of urinary and blood mixture biomarkers of metal exposure with birth outcomes. Our investigation demonstrates, within practical metal analytical panels, that measuring metals in either urine or blood may be an equally good approach to evaluate the metals as a mixture. The results of our study elucidate the importance of considering the overall mixture performance of a certain medium. Future studies are needed to expand to evaluate the performance with different metal panels, media, health outcomes of interest, and methods to integrate exposure information, to further address how to most effectively study the health impacts of exposure to mixtures.
Supplementary Material
Acknowledgements
We would like to extend our gratitude to all PROTECT study participants and their families. The authors also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc. and the Delta OBGyn Group in Manati, as well as the Manati Medical Center and the Metro Pavia Hospital in Arecibo.
Funding
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (grants P42ES017198, R01ES031591, and P50ES026049). Additional support was provided from NIEHS grant number P30ES017885 and the Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health. This study was also partly supported by Award Number U54 MD007600 from the National Institute on Minority Health and Health Disparities.
Abbreviations:
- Co
cobalt
- Cs
cesium
- Cu
copper
- Mn
manganese
- Ni
nickel
- Zn
zinc
- As
arsenic
- Cd
cadmium
- Hg
mercury
- Pb
lead
- SGA
small for gestational age
- LGA
large for gestational age
- MMB
multi-media biomarker
- ICC
intraclass correlation coefficient
- WQS
weighted quantile sum regression
- OR
odds ratio
- AUC
area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no actual or potential competing financial interest
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCE
- 1.Strimbu K and Tavel JA, What are biomarkers? Current Opinion in HIV and AIDS, 2010. 5(6): p. 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke T, et al. , Human Biomonitoring for Environmental Chemicals. 2006, National Research Council of the National Academies, Washington, DC. [Google Scholar]
- 3.Taylor KW, et al. , Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environmental health perspectives, 2016. 124(12): p. A227–A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SS, et al. , Urinary trace metals individually and in mixtures in association with preterm birth. Environ. Int, 2018. 121(Pt 1): p. 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Signes-Pastor AJ, et al. , Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environ Epidemiol, 2019. 3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govarts E, et al. , Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. Int J Environ Res Public Health, 2016. 13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y, et al. , Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health, 2017. 17(1): p. 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, et al. , Combined effects of multiple prenatal exposure to pollutants on birth weight: The Mothers and Children’s Environmental Health (MOCEH) study. Environ Res, 2019: p. 108832. [DOI] [PubMed] [Google Scholar]
- 9.Braun JM, et al. , What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environmental health perspectives, 2016. 124(1): p. A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger K, et al. , Prenatal phthalate, paraben, and phenol exposure and childhood allergic and respiratory outcomes: Evaluating exposure to chemical mixtures. Science of The Total Environment, 2020: p. 138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalloo G, et al. , Exposures to chemical mixtures during pregnancy and neonatal outcomes: the home study. Environment international, 2020. 134: p. 105219. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, et al. , Heavy metals and living systems: An overview. Indian J. Pharmacol, 2011. 43(3): p. 246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin S and Griswold W, Human health effects of heavy metals. Environmental Science and Technology briefs for citizens, 2009. 15: p. 1–6. [Google Scholar]
- 14.Borowska S and Brzoska MM, Metals in cosmetics: implications for human health. J Appl Toxicol, 2015. 35(6): p. 551–72. [DOI] [PubMed] [Google Scholar]
- 15.Rehman K, et al. , Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem, 2018. 119(1): p. 157–184. [DOI] [PubMed] [Google Scholar]
- 16.Gorman Ng M, et al. , Field Measurements of Inadvertent Ingestion Exposure to Metals. Ann Work Expo Health, 2017. 61(9): p. 1097–1107. [DOI] [PubMed] [Google Scholar]
- 17.Ashrap P, et al. , Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ. Res, 2020. 183: p. 109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budtz-Jørgensen E, et al. , Consequences of exposure measurement error for confounder identification in environmental epidemiology. Statistics in medicine, 2003. 22(19): p. 3089–3100. [DOI] [PubMed] [Google Scholar]
- 19.Grandjean P and Budtz-Jørgensen E, Total imprecision of exposure biomarkers: implications for calculating exposure limits. American journal of industrial medicine, 2007. 50(10): p. 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim B-M, et al. , Effect of hemoglobin adjustment on the precision of mercury concentrations in maternal and cord blood. Environmental research, 2014. 132: p. 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heilmann C, et al. , Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med, 2006. 3(8): p. e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin-Schwartz Y, et al. , Multi-media biomarkers: Integrating information to improve lead exposure assessment. Environmental Research, 2020. 183: p. 109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rechtman E, et al. , Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Translational psychiatry, 2020. 10(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer JA, et al. , Associations of a Metal Mixture Measured in Multiple Biomarkers with IQ: Evidence from Italian Adolescents Living near Ferroalloy Industry. Environ Health Perspect, 2020. 128(9): p. 97002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin-Schwartz Y, et al. , Integrated measures of lead and manganese exposure improve estimation of their joint effects on cognition in Italian school-age children. Environ Int, 2021. 146: p. 106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders AP, et al. , Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ Int, 2019. 131: p. 104993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamanti-Kandarakis E, et al. , Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev, 2009. 30(4): p. 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Coster S and van Larebeke N, Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J. Environ. Public Health, 2012. 2012: p. 713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendiola J, et al. , Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ. Health, 2011. 10(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom MS, et al. , Toxic trace metals and human oocytes during in vitro fertilization (IVF). Reprod. Toxicol, 2010. 29(3): p. 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, et al. , Maternal exposure to nickel in relation to preterm delivery. Chemosphere, 2018. 193: p. 1157–1163. [DOI] [PubMed] [Google Scholar]
- 32.Zota AR, et al. , Maternal blood manganese levels and infant birth weight. Epidemiology, 2009. 20(3): p. 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashley-Martin J, et al. , Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int J Hyg Environ Health, 2018. 221(6): p. 876–882. [DOI] [PubMed] [Google Scholar]
- 34.Mikelson CK, et al. , Placental concentrations of essential, toxic, and understudied metals and relationships with birth outcomes in Chattanooga, TN. Environ Res, 2018. 168: p. 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eum JH, et al. , Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environ Health, 2014. 13(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas S, et al. , Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environmental Research, 2015. 140: p. 430–439. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DW, Mettil W, and Schneider JS, Effects of low level lead exposure on associative learning and memory in the rat: Influences of sex and developmental timing of exposure. Toxicol Lett, 2016. 246: p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S, et al. , Exposure to Low Levels of Lead in Utero and Umbilical Cord Blood DNA Methylation in Project Viva: An Epigenome-Wide Association Study. Environ Health Perspect, 2017. 125(8): p. 087019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silver MK, et al. , Low-level prenatal lead exposure and infant sensory function. Environ Health, 2016. 15(1): p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantonwine DE, et al. , Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ. Int, 2014. 62: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeker JD, et al. , Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ. Sci. Technol, 2013. 47(7): p. 3439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watkins DJ, et al. , Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health, 2015. 218(2): p. 212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashrap P, et al. , Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environ. Int, 2018. 121(Pt 1): p. 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashrap P, et al. , Maternal Urinary Metal and Metalloid Concentrations in Association with Oxidative Stress Biomarkers. Antioxidants (Basel), 2021. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Committee on Obstetric Practice, t.A.I.o.U.i.M., and and t.S.f.M.-F. Medicine;, Committee Opinion No 700: Methods for Estimating the Due Date. 2017.
- 46.Aker AM, et al. , The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ Res, 2019. 169: p. 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson KK, et al. , Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int, 2019. 132: p. 105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villar J, et al. , International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. The Lancet, 2014. 384(9946): p. 857–868. [DOI] [PubMed] [Google Scholar]
- 49.Carrico C, et al. , Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat, 2015. 20(1): p. 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yorita Christensen KL, et al. , Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int J Hyg Environ Health, 2013. 216(6): p. 703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashrap P, et al. , Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ. Int, 2020. 138: p. 105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SK, et al. , Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PLoS One, 2014. 9(6): p. e98632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoerl AE and Kennard RW, Ridge regression: applications to nonorthogonal problems. Technometrics, 1970. 12(1): p. 69–82. [Google Scholar]
- 54.Tibshirani R, Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society: Series B (Methodological), 1996. 58(1): p. 267–288. [Google Scholar]
- 55.Park SK, Zhao Z, and Mukherjee B, Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environmental Health, 2017. 16(1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpenter J and Bithell J, Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Statistics in medicine, 2000. 19(9): p. 1141–1164. [DOI] [PubMed] [Google Scholar]
- 57.Robin X, et al. , pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics, 2011. 12(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aker AM, et al. , The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ. Res, 2019. 169: p. 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson KK, et al. , Demographic risk factors for adverse birth outcomes in Puerto Rico in the PROTECT cohort. PLoS One, 2019. 14(6): p. e0217770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchet JP, Lauwerys R, and Roels H, Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health, 1981. 48(1): p. 71–9. [DOI] [PubMed] [Google Scholar]
- 61.Kubota R, Kunito T, and Tanabe S, Chemical speciation of arsenic in the livers of higher trophic marine animals. Mar Pollut Bull, 2002. 45(1–12): p. 218–23. [DOI] [PubMed] [Google Scholar]
- 62.Barbosa F Jr., et al. , A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect, 2005. 113(12): p. 1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howe CG, et al. , Prenatal Metal Mixtures and Birth Weight for Gestational Age in a Predominately Lower-Income Hispanic Pregnancy Cohort in Los Angeles. Environ Health Perspect, 2020. 128(11): p. 117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howe CG, et al. , Prenatal metal mixtures and fetal size in mid-pregnancy in the MADRES study. Environ Res, 2020: p. 110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SS, et al. , Urinary trace metals in association with fetal ultrasound measures during pregnancy. Environ Epidemiol, 2020. 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SS, et al. , Birth outcomes associated with maternal exposure to metals from informal electronic waste recycling in Guiyu, China. Environ Int, 2020. 137: p. 105580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Govarts E, et al. , Early-life exposure to multiple persistent organic pollutants and metals and birth weight: Pooled analysis in four Flemish birth cohorts. Environ Int, 2020. 145: p. 106149. [DOI] [PubMed] [Google Scholar]
- 68.Cassidy-Bushrow AE, et al. , In utero metal exposures measured in deciduous teeth and birth outcomes in a racially-diverse urban cohort. Environ Res, 2019. 171: p. 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song L, et al. , Exposure to arsenic during pregnancy and newborn mitochondrial DNA copy number: A birth cohort study in Wuhan, China. Chemosphere, 2020. 243: p. 125335. [DOI] [PubMed] [Google Scholar]
- 70.Steinmaus C, et al. , Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomarkers Prev, 2005. 14(4): p. 919–24. [DOI] [PubMed] [Google Scholar]
- 71.Kile ML, et al. , Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Perspect, 2009. 117(3): p. 455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.King JC, Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr, 2000. 71(5): p. 1218S–1225S. [DOI] [PubMed] [Google Scholar]
- 73.Weaver VM, et al. , Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res, 2014. 132: p. 226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheung KL and Lafayette RA, Renal physiology of pregnancy. Adv. Chronic Kidney Dis, 2013. 20(3): p. 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hytten FE and Paintin DB, Increase in plasma volume during normal pregnancy. J. Obstet. Gynaecol. Br. Emp, 1963. 70: p. 402–7. [DOI] [PubMed] [Google Scholar]
- 76.Barr DB, et al. , Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect, 2005. 113(2): p. 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boeniger MF, Lowry LK, and Rosenberg J, Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J, 1993. 54(10): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 78.Suwazono Y, et al. , Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers, 2005. 10(2–3): p. 117–26. [DOI] [PubMed] [Google Scholar]
- 79.Pearson MA, et al. , Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J Expo Sci Environ Epidemiol, 2009. 19(3): p. 336–42. [DOI] [PubMed] [Google Scholar]
- 80.Haddow JE, et al. , Replacing creatinine measurements with specific gravity values to adjust urine cotinine concentrations. Clin Chem, 1994. 40(4): p. 562–4. [PubMed] [Google Scholar]
- 81.Meeker JD, et al. , The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environmental Health Perspectives, 2004. 112(17): p. 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller RC, et al. , Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clinical Chemistry, 2004. 50(5): p. 924–932. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda M, et al. , Bias induced by the use of creatinine-corrected values in evaluation of β2-microgloblin levels. Toxicology letters, 2003. 145(2): p. 197–207. [DOI] [PubMed] [Google Scholar]
- 84.CDC, Guidelines for the identification and management of lead exposure in pregnant and lactating women. 2010, National Center for Environmental Health: Washington, DC, USA. [Google Scholar]
- 85.Ashrap P, et al. , Psychosocial status modifies the effect of maternal blood metal and metalloid concentrations on birth outcomes. Environ Int, 2021. 149: p. 106418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vigeh M, et al. , Blood manganese concentrations and intrauterine growth restriction. Reprod Toxicol, 2008. 25(2): p. 219–23. [DOI] [PubMed] [Google Scholar]
- 87.Bakouei S, et al. , High Intake of Manganese During Second Trimester, Increases the Risk of Preterm Delivery: A Large Scale Cohort Study. Glob J Health Sci, 2015. 7(5): p. 226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Osendarp SJ, et al. , The need for maternal zinc supplementation in developing countries: an unresolved issue. J Nutr, 2003. 133(3): p. 817S–827S. [DOI] [PubMed] [Google Scholar]
- 89.Jamilian M, et al. , The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth, 2019. 19(1): p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galloway SP, McMillan DC, and Sattar N, Effect of the inflammatory response on trace element and vitamin status. Annals of clinical biochemistry, 2000. 37(3): p. 289–297. [DOI] [PubMed] [Google Scholar]
- 91.Bonaventura P, et al. , Zinc and its role in immunity and inflammation. Autoimmunity reviews, 2015. 14(4): p. 277–285. [DOI] [PubMed] [Google Scholar]
- 92.Guo C-H, et al. , Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clinical biochemistry, 2011. 44(4): p. 275–280. [DOI] [PubMed] [Google Scholar]
- 93.Gaetke LM and Chow CK, Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology, 2003. 189(1–2): p. 147–63. [DOI] [PubMed] [Google Scholar]
- 94.Desole MS, et al. , Glutathione deficiency potentiates manganese toxicity in rat striatum and brainstem and in PC12 cells. Pharmacol Res, 1997. 36(4): p. 285–92. [DOI] [PubMed] [Google Scholar]
- 95.Li L and Yang X, The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev, 2018. 2018: p. 7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farina M, et al. , Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochemistry international, 2013. 62(5): p. 575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.González A, Steffen KL, and Lynch JP, Light and excess manganese: implications for oxidative stress in common bean. Plant Physiology, 1998. 118(2): p. 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srivastava S and Dubey R, Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regulation, 2011. 64(1): p. 1–16. [Google Scholar]
- 99.Culotta VC and Daly MJ, Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxidants & redox signaling, 2013. 19(9): p. 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen P, Bornhorst J, and Aschner M, Manganese metabolism in humans. 2019. [DOI] [PubMed]
- 101.Sarkar S, et al. , Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology, 2018. 64: p. 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim SS, et al. , Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers. Environ. Res, 2019. 179(Pt B): p. 108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thounaojam TC, et al. , Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Biochem, 2012. 53: p. 33–39. [DOI] [PubMed] [Google Scholar]
- 104.Diamanti-Kandarakis E, et al. , Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev, 2009. 30(4): p. 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Coster S and van Larebeke N, Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health, 2012. 2012: p. 713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mendiola J, et al. , Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health, 2011. 10(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bloom MS, et al. , Toxic trace metals and human oocytes during in vitro fertilization (IVF). Reprod Toxicol, 2010. 29(3): p. 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng X, et al. , Alterations of serum hormone levels in male workers occupationally exposed to cadmium. J Toxicol Environ Health A, 2002. 65(7): p. 513–21. [DOI] [PubMed] [Google Scholar]
- 109.Jurasovic J, et al. , Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals, 2004. 17(6): p. 735–43. [DOI] [PubMed] [Google Scholar]
- 110.Zeng X, et al. , Impact of cadmium exposure on male sex hormones: a population-based study in China. Environ Res, 2004. 96(3): p. 338–44. [DOI] [PubMed] [Google Scholar]
- 111.Telisman S, et al. , Reproductive toxicity of low-level lead exposure in men. Environ Res, 2007. 105(2): p. 256–66. [DOI] [PubMed] [Google Scholar]
- 112.Menke A, et al. , The association of urinary cadmium with sex steroid hormone concentrations in a general population sample of US adult men. BMC Public Health, 2008. 8: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meeker JD, et al. , Environmental exposure to metals and male reproductive hormones: circulating testosterone is inversely associated with blood molybdenum. Fertil Steril, 2010. 93(1): p. 130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagata C, et al. , Urinary cadmium and serum levels of estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol Biomarkers Prev, 2005. 14(3): p. 705–8. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Morales P, et al. , Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem, 1994. 269(24): p. 16896–901. [PubMed] [Google Scholar]
- 116.Bitto A, et al. , Epigenetic modifications due to heavy metals exposure in children living in polluted areas. Curr Genomics, 2014. 15(6): p. 464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burris HH, et al. , Epigenetics: linking social and environmental exposures to preterm birth. Pediatr Res, 2016. 79(1–2): p. 136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee S, et al. , Combined effects of multiple prenatal exposure to pollutants on birth weight: The Mothers and Children’s Environmental Health (MOCEH) study. Environ Res, 2020. 181: p. 108832. [DOI] [PubMed] [Google Scholar]
- 119.Kalloo G, et al. , Exposures to chemical mixtures during pregnancy and neonatal outcomes: The HOME study. Environ Int, 2020. 134: p. 105219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


