Abstract
Background:
Improved understanding of how prenatal exposure to environmental mixtures influences birth weight or other adverse outcomes is essential in protecting child health.
Objective:
We illustrate a novel exposure continuum mapping (ECM) framework that combines the self-organizing map (SOM) algorithm with generalized additive modeling (GAM) in order to integrate spatially-correlated learning into the study mixtures of environmental chemicals. We demonstrate our method using biomarker data on chemical mixtures collected from a diverse mother-child cohort.
Methods:
We obtained biomarker concentrations for 16 prevalent endocrine disrupting chemicals (EDCs) collected in the first-trimester from a large, ethnically/racially diverse cohort of healthy pregnant women (n=604) during 2009-2012. This included 4 organochlorine pesticides (OCPs), 4 polybrominated diphenyl ethers (PBDEs), 4 polychlorinated biphenyls (PCBs), and 4 perfluoroalkyl substances (PFAS). We applied a two-stage exposure continuum mapping (ECM) approach to investigate the combined impact of the EDCs on birth weight. First, we analyzed our EDC data with SOM in order to reduce the dimensionality of our exposure matrix into a two-dimensional grid (i.e., map) where nodes depict the types of EDC mixture profiles observed within our data. We define this map as the ‘exposure continuum map’, as the gridded surface reflects a continuous sequence of exposure profiles where adjacent nodes are composed of similar mixtures and profiles at more distal nodes are more distinct. Lastly, we used GAM to estimate a joint-dose response based on the coordinates of our ECM in order to capture the relationship between participant location on the ECM and infant birth weight after adjusting for maternal age, race/ethnicity, pre-pregnancy body mass index (BMI), education, serum cotinine, total plasma lipids, and infant sex. Single chemical regression models were applied to facilitate comparison.
Results:
We found that an ECM with 36 mixture profiles retained 70% of the total variation in the exposure data. Frequency analysis showed that the most common profiles included relatively low concentrations for most EDCs (~10%) and that profiles with relatively higher concentrations (for single or multiple EDCs) tended to be rarer (~1%) but more distinct. Estimation of a joint-dose response function revealed that lower birth weights mapped to locations where profile compositions were dominated by relatively high PBDEs and select OCPs. Higher birth weights mapped to locations where profiles consisted of higher PCBs. These findings agreed well with results from single chemical models.
Conclusions:
Findings from our study revealed a wide range of prenatal exposure scenarios and found that combinations exhibiting higher levels of PBDEs were associated with lower birth weight and combinations with higher levels of PCBs and PFAS were associated with increased birth weight. Our ECM approach provides a promising framework for supporting studies of other exposure mixtures.
Keywords: endocrine disrupting chemicals, environmental exposures, fetal growth, kohonen, mixtures, pregnancy
Introduction
Endocrine disrupting chemicals (EDCs) interfere with hormone action that may influence a broad range of health outcomes such as growth and development, organ function, metabolism, and reproduction (1, 2). In 2020, the Endocrine Society noted that thousands of manufactured chemicals in use today are EDCs and that exposures are ubiquitous (3). Early life exposures are of particular concern, as exposures during sensitive developmental windows can have lasting effects throughout the life course and exposures among pregnant women are common (4, 5).
Birth weight is an important indicator of fetal growth that has been shown to be a determinant of health risk later in life for a broad range of outcomes (6). Multiple studies of persistent EDCs have found links between maternal exposures and alterations of birth weight, with several finding that increased EDC exposure associates with lower birth weights (7–9). In Europe, studies of persistent EDCs, including polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), and poly- and perfluorinated alkyl substances (PFASs), found that even low-level exposures associated with lower birth weight (9, 10). In contrast, other studies in both Canada and the United States, which included polybrominated diphenyl ethers (PBDEs) as well as PCBs, OCPs, and PFASs, reported mostly null associations between EDCs and birth weight (11, 12). However, these studies evaluated EDCs independently, an approach that may not be appropriate for chemicals that behave like hormones (13). Studies that estimate independent effects may also underestimate health risks associated with combined exposures to EDCs (14). Thus, these conflicting findings may be due in part to the application of traditional analytic approaches to exposures that are chronic, low-dose, and involve multiple chemicals simultaneously. As such, the full scope of how prenatal exposures to EDCs influence children’s health remains largely unknown as limited assessment of “real-world” exposure mixtures results in an incomplete understanding of potential interactive or cumulative effects. This provides an impetus to develop new and innovative modeling approaches that can provide a more comprehensive understanding of complex exposure scenarios consisting of EDC mixtures.
The study of environmental mixtures presents a complex research area with broad ranging objectives (14). Here, we focus on one approach to the analysis of multiple exposures, namely dimension reduction. Dimension reduction involves the transformation of data from high-dimensional space to low-dimensional space, where meaningful properties of the original data are retained (15). A common technique is principal component analysis (PCA), which has been applied in studies involving large numbers of exposures in order to reduce dimensionality through identification of profiles (a.k.a., component loadings) that reflect primary modes of variance in the data (9, 16, 17). Here, the meaningful property is variance and the approach is well suited to address issues of multicollinearity; however, identifiability can be difficult (14). Another strategy has been to partition study populations into distinct subgroups using cluster analysis to assess measures of statistical distance between shared exposure attributes (17, 18). The meaningful property is grouping and interpretation is a key advantage as cluster profiles (a.k.a., centroids) reflect summaries of attributes and cluster assignments can be used as a categorical metric in subsequent analyses. However, parsimonious solutions provided by traditional techniques (e.g. k-means, hierarchical clustering) can be problematic for evaluation of dose-response relationships as outliers and intra-class heterogeneity within broadly defined groupings are of concern (17, 19). It is important to note that these techniques capture different features in the data, as PCA centers on explaining variation and clustering targets grouping structure, thus results may not always agree (17). Another key distinction is that PCA, or more broadly, factor analysis, is a continuous underlying measure whereas latent cluster analysis results in discrete structure. Both account for heterogeneity. Clusters partition the heterogeneity into within and between cluster heterogeneity. Application of either approach can construct exposure metrics that feature exposure estimates/measures that are accurate, precise, and capture a range of exposure levels in the population under study (20). However, moving forward, exposure data will continue to grow in complexity and therefore analytic techniques that can handle more complex, high-dimensional settings will be favored (21).
Herein we demonstrate propose an ‘exposure continuum map (ECM)’ framework that seeks to enhance the study of complex chemical mixtures with by integrating intuitive clustering algorithms with novel spatially-correlated learning approaches to improve inferences. Conceptually, spatially-correlated learning improves statistical inferences by incorporating information from neighboring features in effort to improve estimation. In effect, this pooling of information may help compensate for limited sample size (e.g., rare exposures) and scenarios with high variation (e.g., outliers) often observed in environmental mixtures studies. Such strategies have a long and successful history outside of geography as a broad range of fields from text mining to cognitive neuroscience have realized the benefits of adopting such principles into the study of complex relationships (22–24).
We apply our ECM framework in a diverse birth cohort study of healthy pregnant women in order to analyze associations between prenatal exposure to EDC mixtures and birth weight. The motivating hypothesis is that variation in prenatal exposure to EDC mixtures will associate with variation in the birth weight. To address this question, we will identify profiles of EDC mixtures that occurred, their frequency distributions, and assess if certain combinations more strongly associate with birth weight than others.
Methods
Study population.
This study uses data originally collected from participants enrolled in the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD) Fetal Growth Studies – Singleton Cohort (11, 25). The primary objective of the Fetal Growth Studies was to establish fetal growth standards within the US using a diverse cohort of healthy women (25). The study ran from July 2009 through January 2013 at 12 clinical sites throughout the United States and included 2334 women, ages 18 to 40 years, with low-risk pregnancies. Women were recruited in the first trimester, carried a singleton gestation, and reported not to consume alcohol or tobacco products (25). Education, race/ethnicity, and pre-pregnancy body mass index (BMI), were self-reported at time of enrollment along with collection of blood samples. Full details on the Fetal Growth Studies cohort and data collection are available elsewhere (25). For this study, we use a subset of FGS participants who also participated in the Environmental Influences on Child Health Outcomes (ECHO) program (5). This population, which we define as the ECHO-FGS cohort, consisted of 1116 mother-child pairs recruited from May 2017 through April 2019 from ten of the original twelve study sites.
Exposure measures.
Maternal blood samples were collected from participants at 8-12 weeks gestation during FGS enrolment and plasma concentrations for 76 EDCs were determined at the Wadsworth Center New York State Department of Health (Albany, NY USA) as previously described in detail (11). A full description of findings have been published, with results revealing broad ranges of quantification among the targeted chemicals in the cohort (11). For this study, we desired our mixture data to be as balanced and complete as possible and thus selected the most prevalent exposures among each chemical class for analysis. This included four OCPs: hexachlorobenzene (HCB; 90%), oxychlordane (74%), trans-nonachlor (91%), and dichlorodiphenyldichloroethane (p,p’-DDE; 99%); four PBDE congeners: BDE47 (93%), BDE99 (62%), BDE100 (72%), and BDE153 (42%); four PCB congeners: PCB 138/158 (94%), PCB153 (94%), PCB170 (73%) and PCB180 (95%); and four PFAS substances: perfluorohexanesulfonic acid (PFHXS; 100%), perfluorononanoic acid (PFNA; 100%), perfluorooctanoic acid (PFOA; 100%), and perfluorooctanesulfonic acid (PFOS; 100%).
Birth weight and Maternal Covariates.
Infant birth weight was measured using an electronic infant scale or beam balance scale and was recorded in grams (g) (11). Sociodemographic, reproductive/obstetric, and lifestyle factors from the original study and the ECHO-FGS were selected a priori for adjustment as confounding variables based on literature evidence suggesting causal associations with both gestational EDC exposure and birth weight. We extracted covariate data from interviewer-administer study questionnaires, including maternal age at the time of biospecimen collection (years), self-reported pre-pregnancy BMI (kg/m2), race/ethnicity (non-Hispanic White (NHW), non-Hispanic Black (NHB), Hispanic, Asian/Pacific Islander (A & PI)), education (dichotomized as completion of secondary/high school or not) as an indicator of socioeconomic status, and infant sex (male, female). Plasma cotinine concentration (ng/ml) was used as an indicator of tobacco smoke exposure and total plasma lipids (ng/ml) were also included.
Statistical Analyses
The analyses below involved data from a subset of ECHO-FGS (n=604) participants with complete observations for all 16 EDCs. For all measures we used machine-read concentration values for EDCs without imputation of values below the LOQ (26). Data preparation for each variable involved log transformed (log (x+1)) to reduce skewness and then standardization to have a mean zero and standard deviation of one.
Estimation of Individual EDC Health Effects
In order to facilitate comparison with traditional approaches, we first performed single chemical analysis by fitting linear regression models between individual EDCs (p=16) and birth weight. This is expressed as:
| (1.0) |
where Yi is the subject birth weight for participant i; β is the vector of regression parameters for covariates Zi; γ is the coefficient of single chemical exposure for each EDC; and ϵi is an error term. Covariates included maternal age (years), pre-pregnancy BMI (kg/mg2), race/ethnicity, education, infant sex, log (x+1) transformed serum cotinine concentration (ng/mL), and total plasma lipids (ng/mL) were also included as a covariate (27). We corrected p-values for a false discovery rate using methods outlined elsewhere (28).
Estimation of EDC-Mixture Health Effects
Next, we used our two-stage exposure continuum mapping (ECM) approach to investigate the combined association of the EDCs with birth weight. In Stage 1, we analyzed our EDC data with a self-organizing map (SOM) (29) in order to reduce the dimensionality of our 604 x 16 exposure matrix into a two-dimensional grid (i.e., map) where nodes depict the types of EDC mixture profiles observed within our data. We define this map as the ‘exposure continuum map’, as the gridded surface reflects a continuous sequence of exposure profiles where adjacent nodes are composed of similar mixtures and extremes are more distinct. This ‘spatially correlated’ arrangement results in locations having neighborhood structure that can be leveraged into subsequent analysis. In addition, the compact, low-dimensional map is also useful for data exploration and visualization as it allows for larger numbers of profiles to be more easily understood. This is important because it offers users the ability to represent data as a high-resolution continuum of categorizations, a key distinction from the discrete clusters/factors produced with traditional methods (16, 18, 30). This work builds on previous studies of air pollution and chemical exposures and is the first study to leverage the organized structure of a SOM to explore health effects (Pearce, Waller et al. 2014, Pearce, Waller et al. 2015, Pearce, Waller et al. 2016, Doherty, Pearce et al. 2020).
To determine an appropriate number of profiles for our ECM, we fit maps of size 2 to 100 nodes and used Akaike’s Information Criterion (AIC) as well as the % of total variation explained (R2) to determine the final map size that adequately captures the variability (>70%) in the exposure data. Exposure profiles projected on the ECM were visualized using radial bar charts to enhance interpretation of the discovered patterns; profile classifications were used to assess the frequency distribution of participants within each type.
Next, we integrated the profile coordinates [U, V] from our ECM as a smooth bivariate interaction term within a generalized additive model (GAM) in order to assess whether location on our map associated with variation in our outcome after control of confounders. We employed GAMs (31) for this task as we desired penalization of our smoothers and it has proven robust in spatial settings where coordinate-based smooth terms have been used to model spatial effects (32). Here, the result is a 3-dimensional response surface that can be interpreted as a joint-dose response function for outcome to variability across the total mixture (33). In addition, the spatial smoothing alleviates challenges imposed by mixture profiles with smaller sample sizes. We express this model as:
| (1.1) |
where Yi is the subject outcome at locations Ui, Vi; β is the vector of regression parameters for covariates Zi; s(Ui, Vi) is a tensor product smooth term of the ECM coordinates; and ϵi is an error term. Smooth functions were developed through a combination of model selection and automatic smoothing parameter selection using penalized regression splines, which optimize the fit and make an effort to minimize the number of dimensions in the model (34). The choice of the smoothing parameters was made through restricted maximum likelihood (REML) and confidence intervals were estimated using an unconditional Bayesian method (34).
All analyses were conducted within the R environment for statistical computing (35) using the ECM R package available on Github: https://github.com/johnlpearce/.
Results
Study Population
Descriptive summaries for the 604 participants in our study population reveal that mothers were diverse across race/ethnic groups but generally similar in age and body composition (Table 1.) Geographically, the majority resided in the Northeast (n=289), followed by the Southeast (n=180), Midwest (n=79), and West (n=56), respectively. Modest variability in education, plasma cotinine, and total plasma lipids was also observed. The average birth weight for participant offspring was 3264 (SD=480) grams, with the lowest average observed for NHB and the highest for NHW. Gestational age at delivery was similar across the cohort. Distributions of the log transformed maternal serum concentrations for the 16 endocrine disrupting chemicals (EDCs) measured during 1st trimester for our study population were consistent with previous findings (11) (see APPENDIX).
Table 1.
Characteristics among mothers in our study population (n=604).
| Characteristics | Overall | NHW | NHB | Hispanic | A & PI |
|---|---|---|---|---|---|
| (n=604) | (n=186) | (n=184) | (n=137) | (n=97) | |
| 100% | 31% | 31% | 23% | 16% | |
| Mothers - | |||||
| Age (years), mean (SD) | 28.5 (5.8) | 31.2 (4.0) | 24.5 (5.5) | 28.6 (5.8) | 30.6 (4.5) |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 23.6 (3.0) | 23.4 (2.7) | 24.1 (3.4) | 24.0 (5.5) | 22.9 (2.8) |
| Education, n (%): | |||||
| Less than or up to high school | 162 (27%) | 7 (1%) | 80 (13%) | 58 (10%) | 17 (3%) |
| More than high school | 442 (73%) | 179 (29%) | 104 (17%) | 79 (13%) | 80 (13%) |
| Parity, n (%): | |||||
| Primiparous | 286 (47%) | 100 (17%) | 89 (15%) | 87 (14%) | 42 (7%) |
| Multiparous | 318 (53%) | 86 (14%)) | 95 (16%) | 50 (8%) | 55 (9%) |
| Region, n (%): | |||||
| Northeast | 289 (48%) | 78 (13%) | 55 (9%) | 98 (16%) | 58 (10%) |
| Southeast | 180 (30%) | 53 (9%) | 114 (18%) | 8 (1%) | 5 (1%) |
| Midwest | 79 (13%) | 44 (7%) | 11 (2%) | 12 (2%) | 12 (2%) |
| West | 56 (9%) | 11 (2%) | 4 (%) | 19 (3%) | 22 (4%) |
| Plasma cotinine (ng/mL), mean (SD) | 1.06 (10.4) | 0.7 (8.9) | 2.8 (16.5) | 0.1 (0.3) | 0.03 (0.1) |
| Total Plasma Lipids (ng/mL), mean (SD) | 607 (99.5) | 626 (107) | 569 (89) | 617 (97) | 627 (87) |
| Children - | |||||
| Sex, n (%) | |||||
| Femal | 290 (48%) | 82 (14%) | 95 (16%) | 69 (11%) | 44 (7%) |
| Male | 314 (52%) | 104 (17%) | 89 (14%) | 68 (11%) | 53 (8%) |
| Birth weight (g), mean (SD) | 3264 (480) | 3370 (496) | 3145 (479) | 3265 (448) | 3275 (447) |
| Gestational age at delivery (weeks), mean (SD) | 39.2 (1.6) | 39.1 (1.5) | 39.0 (1.7) | 39.3 (1.8) | 39.3 (1.5) |
NHW: non-Hispanic white; NHB: non-Hispanic black; A&PI: Asian and Pacific Islander
Statistical Analyses
Estimation of Individual EDC Health Effects
Results from single chemical analysis (Model 1.0) found no significant p-values for associations between infant birth weight and individual EDCs after FDR correction and adjustments. However, beta estimates (95% CIs) suggested lower birth weights were associated with increasing maternal exposure to both PBDE47 and PBDE100 (Figure 1). Furthermore, increasing birth weights were associated with all PCBs (Figure 1). Confidence intervals for all other EDCs included the null.
Figure 1.
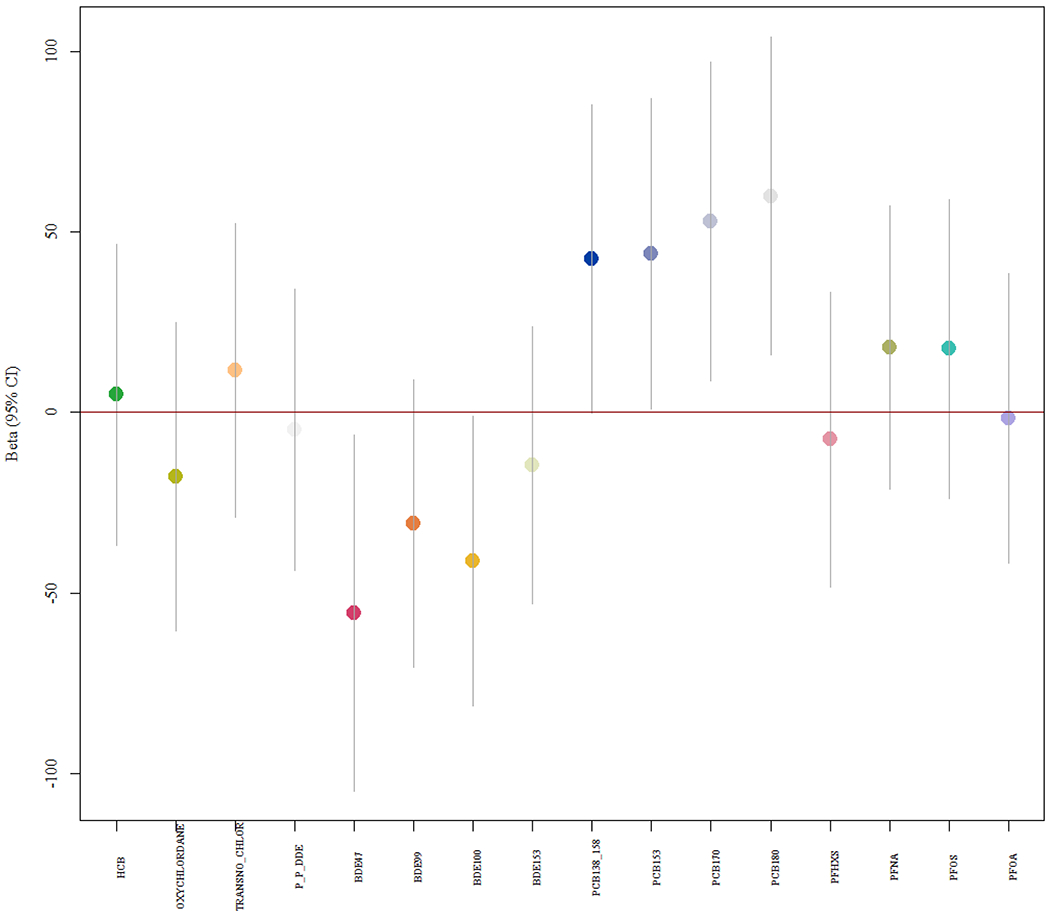
Model 1.0 single chemical model beta coefficients (95% CIs) for associations between EDCs and birth weight. (Format: color)
Estimation of Mixture Health Effects
Exposure Continuum Map.
Results from our map size evaluation revealed that a 6X6 ECM with 36 profiles had the lowest AIC and explained > 70% of the variation in our exposure data. Broad examination revealed the ECM consisted of a range of exposure scenarios, with rarer extremes located in the corners and more common scenarios near the center of the map (Figure 2). More specifically, we found the right central section of the map captured EDC profiles that were most common (~27%) and exhibited patterns with relatively low concentrations (i.e., below average). The upper right side of the ECM illustrates a collection of rarer profiles where participants’ samples contained relatively higher concentrations for p,p’-DDE and HCB, both OCPs. Moving towards the upper left, we find modestly frequent profiles where participants’ samples contained the highest PCBs within our cohort. In the left central section, we find profiles with smaller numbers of participants whose samples contained relatively higher levels of certain OCPs. The bottom left corner identifies participants of the cohort with samples that contained the highest reported PFAS concentrations. The bottom right corner reveals participants with samples containing the highest PBDE concentrations. Overall, the ECM reveals that the ECHO-FGS participants experienced widespread exposure to multiple classes of EDCs, but that concentrations between chemical classes do not seem to be highly correlated. This agrees with similar studies that have reported that exposure to high levels of one EDC class does not appear to be consistently predictive of exposure levels within other EDC classes (36).
Figure 2.
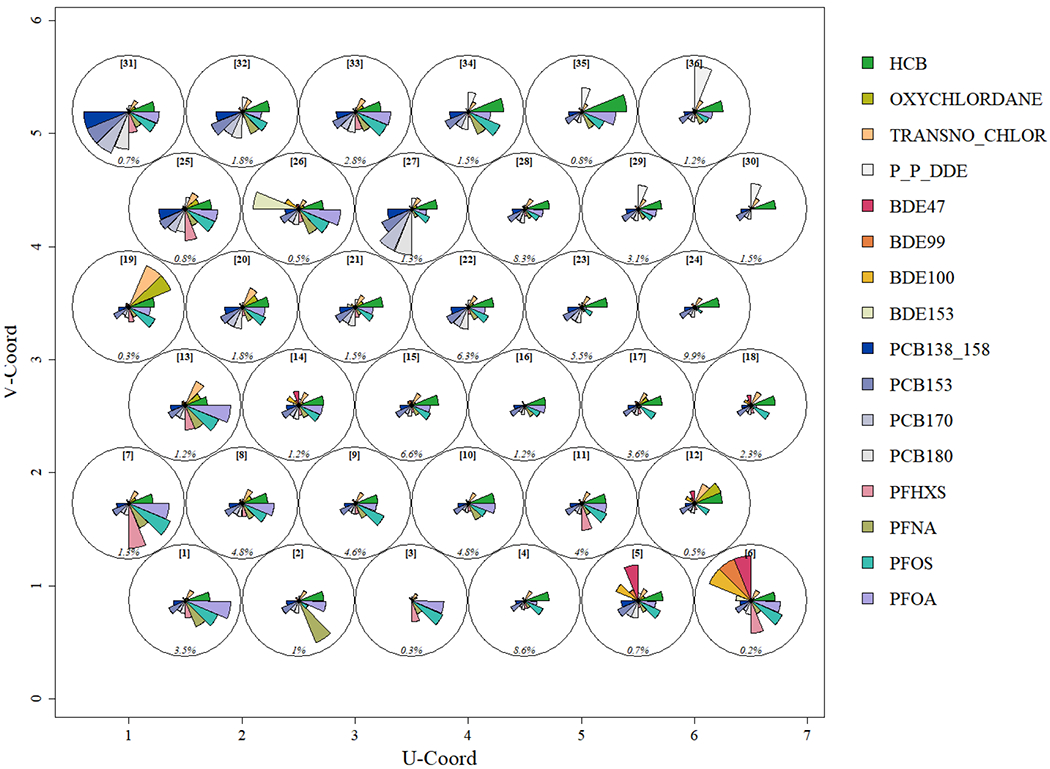
An Exposure Continuum Map (ECM) that illustrates the types of chemical mixture profiles measured in biomarkers collected among mothers (n=604) in our study population in 2009-2012. Pie segments reflect the normalized mean value of concentrations among participants assigned to each profile. Node [IDs] provided in order to reference profiles. Relative frequencies (%) are provided on the bottom of each profile. (Format: color)
Joint-Dose Response.
Results from Model 1.1 identified that our joint-dose response function to the total EDC mixture had marginally significant (F=1.8, p=0.07) association with birth weight after covariate adjustment. Looking at the function in 3-dimensions (Figure 3) shows adjusted birth weights were higher on the upper left, modest across the center and upper right, and lowest towards the bottom right corner (Figure 3. Overlaying the ECM (Figure 2) reveals higher birth weights occurred where profiles exhibited higher PCB, OCP, and PFAS concentrations. The peak response occurred where PCB exposures were also the highest. The lowest birth weights occurred where participants were found to have the highest PBDE exposures. The flatter central section corresponds to areas of the ECM representative of less extreme exposures.
Figure 3.
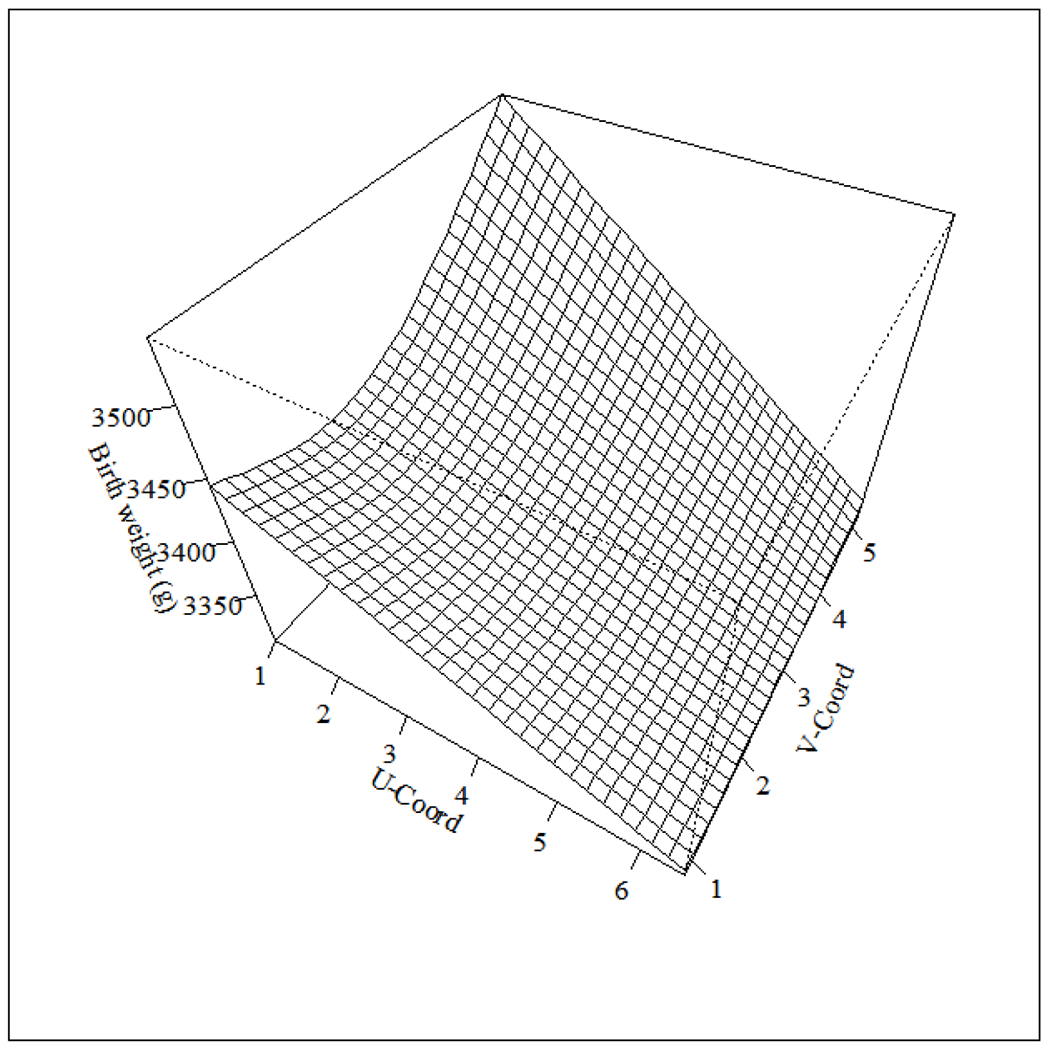
The estimated joint-dose response function from Model 1.1 that illustrates the relationship between birth weight and location on our exposure continuum map after controlling for select confounders. (Format: bw)
Discussion
The primary objective of this study was to demonstrate how an exposure continuum mapping (ECM) framework that integrates self-organizing maps with generalized additive modeling can be used for health investigations involving exposure to complex mixtures. We found a key benefit of ECM was the ability to construct a joint-dose response function that allowed us to visualize how our response varied across changes in combinations observed across the total mixture. This novel tool relies on spatially correlated learning to assess mixture effects as the organizational structure provide by SOM is modeled as bivariate smooth term within a generalized additive model. We illustrated our ECM approach through application to biomarker data collected from an ethnically/racially diverse cohort of healthy pregnant women (n=604) in order examine associations between prenatal exposure to EDC mixtures and birth weight (25). These data are well-suited for our work as they represent validated biomarker measures for multiple EDCs and measures of birth weight that have been assessed in prior studies (11, 37). Our results revealed a varying response of birth weight to variation in prenatal exposure to EDC mixtures, a finding that supports growing evidence that EDC mixtures have different associations with fetal growth than individual EDCs (37, 38). Overall, we found the ECM offers opportunity to enhance understanding of the often-nuanced behavior of complex environmental exposures by providing a novel framework for characterizing exposure and exploring of joint health effects to the overall mix.
While most previous studies examined class-specific EDC mixture effects independently (37, 38), we focused on examining the effect of joint exposure to multiple EDC classes. This a distinguishing feature that differs from previous studies that examined class specific EDC mixture effects independently (14). Another advantage is that our framework provided an exposure characterization (Figure 2) that allowed us to understand which EDC mixture combinations occurred in our cohort and their frequency distributions. For example, application of ECM allowed us to see that lower birth weights were associated with exposures exhibiting higher concentrations of PBDEs and p,p’-DDE and that these exposures occurred in ~9 percent of the study population (Figures 2&3). This finding is generally consistent with recent literature (37, 38); however, our results have the benefit of showing that the strongest response with lower birth weight occurred for rarer exposure patterns that contained multiple classes of EDCs. Such findings improved our understanding of how complex EDC exposures occurred in our study population and allowed us to identify that relatively low exposures are quite common and that profiles comprised of high exposures are distinct and rare (Figures 2 & 3). Another important aspect of this study is the finding of a total mixture effect when associations identified by our single chemical models were null (Figure 1). Findings across the two approaches were generally consistent but results suggest that our mixture approach may have greater sensitivity than single chemical models. Overall, we found our results to be informative and interpretable, characteristics we feel are useful for investigations involving exposure to complex environmental mixtures.
There is a wide range of settings in which the ECM approach, coupled with spatial learning, might be preferred for investigations involving complex mixtures, involving exposure to EDCs. To begin, we believe that exposure characterization and estimation of joint-dose response relationships presents a comprehensive suite of information that can enhance epidemiologic investigations and risk assessments involving complex mixtures (39, 40). An important aspect of our approach is that the development of our exposure metric involves an unsupervised dimension reduction as we seek to identify patterns using information within the exposure matrix only, a strategy that differs from supervised approaches such as Bayesian kernel machine regression (BKMR) and weighted quantile sum (WQS) regression, where health outcome information is incorporated into the mixtures models. While supervised approaches may have the benefit of targeting outcome-relevant combinations, formulating important contrasts in exposure features based on the response of the outcome does raise some concerns about bias and computation can be difficult. On the other hand, unsupervised learning approaches may suffer from a lack of health specificity but have the benefit of identifying patterns based on contrasts within the observed data. Identification of features in the data based on contrasts in exposure can support identification of prevalent exposures that are useful for subsequent analyses across multiple health outcomes and for identifying exposure reduction interventions. Another distinction to note is that our application of SOM focused on capturing distance-based profiles of mixtures that occur in the data rather than identifying correlations among multiple variables. This differs from dimension reduction methodologies like PCA, as chemical combinations that occur may not always contain constituents that correlate (17). Of course, other methodologies seek to identify distance-based patterns of occurrence, such as k-means clustering, but are often applied using strategies that focus on parsimonious solutions that emphasize distinction (17, 30). Such strategies are ideal if broad groupings are desired; however, an inability to construct dose-response relationships in subsequent analyses has been a point of criticism (14). Our approach provides improvement to this particular aspect of mixtures research, as implementation of our higher resolution ECM into a GAM offers a highly flexible tool for using exposure groupings to explore a joint dose-response (Figure 3). The results are highly intuitive and the smoothing of the risk surface across neighboring units results in a pooling across regions, a strategy often applied in small area health statistics to investigate rare events (41).
However, this analysis has limitations. First, we note that our study cohort included only healthy pregnant women with full term pregnancies without major chronic conditions before pregnancy. The results may not be generalizable to all pregnant women, as enrollment did not include women with comorbidities who may be more susceptible to EDCs. We suspect that our null findings may be partially explained by a ‘healthy cohort’ effect, as our population may have been more resilient to environmental exposures than others given enrollment of low-risk pregnancies only. Studying the effect of EDC mixtures in populations that are high risk for a low/high birth weight delivery is an important future direction. Another possible limitation relates to our exposure data. While we chose to analyze highly prevalent EDCs (36), we may have missed less prevalent EDCs that could have been important to our health outcomes. Integration of more sophisticated variable selection strategies would likely improve identification of health-relevant exposure profiles and is the anticipated focus of future work. There is also the possibility of confounding from unmeasured variables (e.g., social determinants, diet) that could have influenced our findings. Another concern was that we combined multiple exposure variables that have varying degrees of measurement error, a problem that may result in bias towards the null (42).
Conclusion
In summary, we found our ECM approach provides a promising framework for supporting studies of exposure mixtures. Results from our ‘real world’ application revealed that a wide range of prenatal exposure mixtures occurred and that rarer combinations exhibiting higher levels of PBDEs and p,p’-DDE were associated with lower birth weights. The method is applicable to other environmental mixtures and settings.
Acknowledgements
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), U2COD023375 (Pearce), UG30D023316 (Vena/Wapner), and UH30D023290 (Perera/Herbstman). Resources provided by the NICHD Fetal Growth Studies was supported under award numbers HHSN275200800028C and HHSN275201000009C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
APPENDIX
AP_Figure_1.
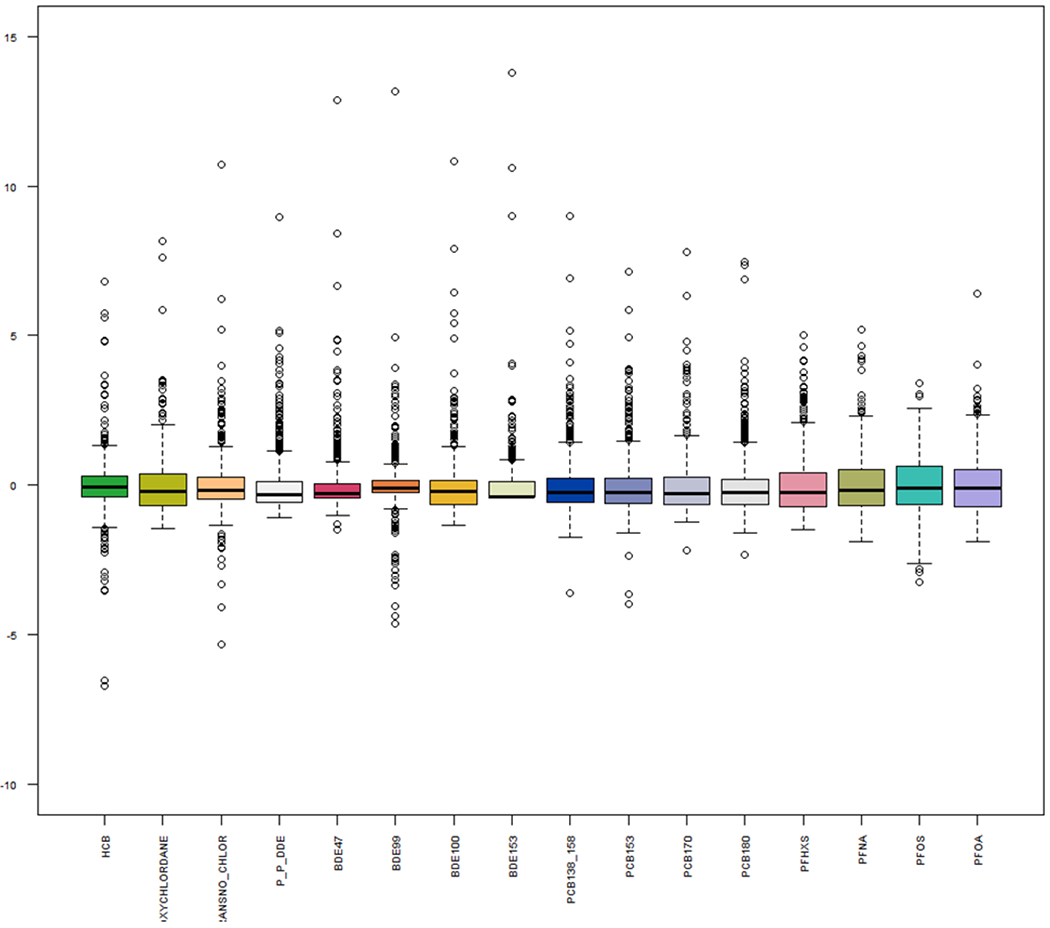
Standardized boxplot distributions of log transformed maternal serum concentrations for the 16 endocrine disrupting chemicals (EDCs) measured during 1st trimester for our cohort. Standardized concentrations provide a sense of relative variability across chemicals measured on different scales. Abbreviations: OCPs, organochlorine pesticides; HCB, hexachlorobenzene; oxychlordane; trans-Nonachlor, p,p-DDE, dichlorodiphenyldichloroethane; (P)BDE, (poly)brominated diphenyl ether; PCBs, polychlorinated biphenols; PFASs, poly- and perfluoroalkyl substances; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate (Format: color).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bergman A, Heindel JJ, Kasten T, Kidd KA, Jobling S, Neira M, Zoeller RT, Becher G, Bjerregaard P, Bomman R, Brandt I, Kortenkamp A, Muir D, Drisse MN, Ochieng R, Skakkebaek NE, Bylehn AS, Iguchi T, Toppari J, Woodruff TJ. The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect. 2013;121(4):A104–6. Epub 2013/04/04. doi: 10.1289/ehp.1205448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–E150. Epub 2015/11/07. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaws JD P; Patisaul HB; Gore A; Ratzman L; Vandenberg LN Plastics, EDCs & Health. www.endocrine.org: 2020 12/01/2020. Report No.
- 4.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878–85. Epub 2011/01/15. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillman MW, Blaisdell CJ. Environmental influences on Child Health Outcomes, a Research Program of the National Institutes of Health. Curr Opin Pediatr. 2018;30(2):260–2. Epub 2018/01/23. doi: 10.1097/MOP.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Class QA, Rickert ME, Lichtenstein P, D’Onofrio BM. Birth Weight, Physical Morbidity, and Mortality A Population-based Sibling-Comparison Study. American Journal of Epidemiology. 2013;179(5):550–8. doi: 10.1093/aje/kwt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Street ME, Bernasconi S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int J Mol Sci. 2020;21(4). Epub 2020/02/26. doi: 10.3390/ijms21041430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods MM, Lanphear BP, Braun JM, McCandless LC. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environmental Health. 2017;16(1): 115. doi: 10.1186/s12940-017-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, De Boer M, Chevrier C, Eggesbø M, Guxens M, Krämer U. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environmental health perspectives. 2012;120(2):162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenters V, Portengen L, Rignell-Hydbom A, Jänsson BA, Lindh CH, Piersma AH, Toft G, Bonde JP, Heederik D, Rylander L. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environmental health perspectives. 2016;124(3):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck Louis GM, Zhai S, Smarr MM, Grewal J, Zhang C, Grantz KL, Hinkle SN, Sundaram R, Lee S, Honda M, Oh J, Kannan K. Endocrine disruptors and neonatal anthropometry, NICHD Fetal Growth Studies - Singletons. Environ Int. 2018;119:515–26. Epub 2018/07/29. doi: 10.1016/j.envint.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serme-Gbedo YK, Abdelouahab N, Pasquier J-C, Cohen AA, Takser L. Maternal levels of endocrine disruptors, polybrominated diphenyl ethers, in early pregnancy are not associated with lower birth weight in the Canadian birth cohort GESTE. Environmental Health. 2016;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudriault P, Mazaud-Guittot S, Lavoue V, Coiffec I, Lesne L, Dejucq-Rainsford N, Scholze M, Kortenkamp A, Jegou B. Endocrine Disruption in Human Fetal Testis Explants by Individual and Combined Exposures to Selected Pharmaceuticals, Pesticides, and Environmental Pollutants. Environ Health Perspect. 2017;125(8):087004. Epub 2017/08/11. doi: 10.1289/EHP1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarevic N, Barnett AG, Sly PD, Knibbs LD. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ Health Perspect. 2019;127(2):26001. Epub 2019/02/06. doi: 10.1289/EHP2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. 2nd ed. New York, NY: Springer; 2009. xxii, 745 p. p. [Google Scholar]
- 16.Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagana X, Robinson O, Casas M, Sunyer J, Vrijheid M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ Health Perspect. 2015;123(10):1030–7. Epub 2015/05/09. doi: 10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalloo G, Wellenius GA, McCandless L, Calafat AM, Sjodin A, Karagas M, Chen A, Yolton K, Lanphear BP, Braun JM. Profiles and Predictors of Environmental Chemical Mixture Exposure among Pregnant Women: The Health Outcomes and Measures of the Environment Study. Environ Sci Technol. 2018;52(17):10104–13. Epub 2018/08/09. doi: 10.1021/acs.est.8b02946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg V, Nost TH, Pettersen RD, Hansen S, Veyhe AS, Jorde R, Odland JO, Sandanger TM. Persistent Organic Pollutants and the Association with Maternal and Infant Thyroid Homeostasis: A Multipollutant Assessment. Environ Health Perspect. 2017; 125(1):127–33. Epub 2016/05/25. doi: 10.1289/EHP152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanobetti A, Austin E, Coull BA, Schwartz J, Koutrakis P. Health effects of multi-pollutant profiles. Environ Int. 2014;71:13–9. Epub 2014/06/21. doi: 10.1016/j.envint.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieuwenhuijsen MJ. Exposure assessment in environmental epidemiology: Oxford University Press, USA; 2015. [Google Scholar]
- 21.Lioy PJ, Smith KR. A discussion of exposure science in the 21st century: a vision and a strategy. Environ Health Perspect. 2013;121(4):405–9. Epub 2013/02/06. doi: 10.1289/ehp.1206170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poeppel D The maps problem and the mapping problem: two challenges for a cognitive neuroscience of speech and language. Cogn Neuropsychol. 2012;29(1-2):34–55. doi: 10.1080/02643294.2012.710600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise JA, Thomas JJ, Pennock K, Lantrip D, Pottier M, Schur A, Crow V, editors. Visualizing the non-visual: Spatial analysis and interaction with information from text documents. Proceedings of Visualization 1995 Conference; 1995: IEEE. [Google Scholar]
- 24.Waller LA, Särkkä A, Olsbo V, Myllymäki M, Panoutsopoulou IG, Kennedy WR, Wendelschafer-Crabb G. Second- order spatial analysis of epidermal nerve fibers. Statistics in Medicine. 2011;30(23):2827–41. [DOI] [PubMed] [Google Scholar]
- 25.Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, DAlton ME, Skupski D, Nageotte MP, Ranzini AC, Owen J, Chien EK, Craigo S, Albert PS, Kim S, Hediger ML, Buck Louis GM. Cohort Profile: NICHD Fetal Growth Studies-Singletons and Twins. Int J Epidemiol. 2018;47(1):25–1. Epub 2017/10/13. doi: 10.1093/ije/dyx161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163(4):374–83. Epub 2006/01/06. doi: 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005; 113(7):853–7. Epub 2005/07/09. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995;57(l):289–300. [Google Scholar]
- 29.Kohonen T Essentials of the self-organizing map. Neural Netw. 2013;37:52–65. Epub 2012/10/17. doi: 10.1016/j.neunet.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Austin E, Coull B, Thomas D, Koutrakis P. A framework for identifying distinct multipollutant profiles in air pollution data. Environ Int. 2012;45:112–21. Epub 2012/05/16. doi: 10.1016/j.envint.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie T, Tibshirani R. Generalized additive models. 1st ed. London ; New York: Chapman and Hall; 1990. xv, 335 p. p. [Google Scholar]
- 32.Young RL, Weinberg J, Vieira V, Ozonoff A, Webster TF. A power comparison of generalized additive models and the spatial scan statistic in a case-control setting. International Journal of Health Geographies. 2010;9(1):37. doi: 10.1186/1476-072X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, Heindel JJ, Rider CV, Webster TF, Carlin DJ. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology: Lessons from an Innovative Workshop. Environ Health Perspect. 2016; 124(12): A227–A9. Epub 2016/12/03. doi: 10.1289/EHP547. child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning. None of these activities were directly related to the present study. The other authors declare they have no actual or potential competing financial interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood SN. Generalized additive models: an introduction with R: CRC press; 2017. [Google Scholar]
- 35.Team RC. R: A language and environment for statistical computing. Vienna, Austria; 2013. [Google Scholar]
- 36.Mitro SD, Johnson T, Zota AR. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr Environ Health Rep. 2015;2(4):367–78. Epub 2015/09/06. doi: 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouidir M, Buck Louis GM, Kanner J, Grantz KL, Zhang C, Sundaram R, Rahman ML, Lee S, Kannan K, Tekola-Ayele F, Mendola P. Association of Maternal Exposure to Persistent Organic Pollutants in Early Pregnancy With Fetal Growth. JAMA Pediatrics. 2020; 174(2): 149–61. doi: 10.1001/jamapediatrics.2019.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu JMY, Arbuckle TE, Janssen P, Lanphear BP, Zhuang LH, Braun JM, Chen A, McCandless LC. Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: A Bayesian analysis using kernel machine regression. Environmental Research. 2021; 195:110749. doi: 10.1016/j.envres.2021.110749. [DOI] [PubMed] [Google Scholar]
- 39.Drakvik E, Altenburger R, Aoki Y, Backhaus T, Bahadori T, Barouki R, Brack W, Cronin MTD, Demeneix B, Hougaard Bennekou S, van Klaveren J, Kneuer C, Kolossa-Gehring M, Lebret E, Posthuma L, Reiber L, Rider C, Rüegg J, Testa G, van der Burg B, van der Voet H, Warhurst AM, van de Water B, Yamazaki K, Oberg M, Bergman A. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environment International. 2020;134:105267. doi: 10.1016/j.envint.2019.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamra GB, Buckley JP. Environmental exposure mixtures: questions and methods to address them. Current epidemiology reports. 2018;5(2):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickle LW, Waller LA, Lawson AB. Current practices in cancer spatial data analysis: a call for guidance. Int J Health Geogr. 2005;4(1):3. Epub 2005/01/15. doi: 10.1186/1476-072X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dionisio KL, Baxter LK, Chang HH. An empirical assessment of exposure measurement error and effect attenuation in bipollutant epidemiologic models. Environ Health Perspect. 2014; 122(11):1216–24. Epub 2014/07/09. doi: 10.1289/ehp.1307772. [DOI] [PMC free article] [PubMed] [Google Scholar]


