Abstract
Recent preclinical studies have reported that pretreatment with the novel and highly-selective dopamine D3 receptor (D3R) antagonists R-VK4–40 or VK4–116 attenuates the abuse-related behavioral effects of oxycodone while enhancing its analgesic properties. However, whether these observed effects are generalizable to the broad class of D3R antagonists and/or extend to opioids other than oxycodone has not been extensively explored. The present study sought to assess the impact of pretreatment with another selective D3R antagonist, PG01037, on several behavioral effects of morphine in mice. C57Bl/6J mice were pretreated with PG01037 (0 – 10 mg/kg) and tested for 1) hyperlocomotion induced by acute morphine (5.6 – 56 mg/kg), 2) locomotor sensitization following repeated morphine (56 mg/kg), 3) antinociception following acute morphine (18 mg/kg), and 4) catalepsy following administration of PG01037 alone or in combination with morphine (56 mg/kg). PG01037 dose-dependently attenuated morphine-induced hyperlocomotion and morphine-induced antinociception at doses that did not alter basal locomotion or nociception alone, but did not prevent the induction of locomotor sensitization following repeated morphine administration. Moreover, PG01037 did not induce catalepsy either alone or in combination with morphine. These results suggest that attenuation of acute opioid-induced hyperactivity may be a behavioral effect shared among D3R-selective antagonists, thus supporting continued investigations into their use as potential treatments for opioid use disorder. However, PG01037 is unlike newer, highly-selective D3R antagonists in its capacity to reduce opioid-induced antinociception, indicating that modulation of opioid analgesia may vary across different D3R antagonists.
Keywords: morphine, dopamine D3 receptor, PG01037, locomotor activity, opioid analgesia, catalepsy
1. INTRODUCTION
The abuse of prescription and illicit opioids has culminated in a national healthcare crisis [1], prompting the search for novel pharmacotherapeutics that can safely and more effectively treat opioid use disorder (OUD) as compared to currently-available medications [2, 3]. The abuse-related behavioral effects of opioids are predominantly attributed to increased dopamine (DA) neurotransmission within the mesolimbic reward system [for review, see 4, 5, 6], a projection arising from DAergic neurons located in the ventral tegmental area (VTA) and terminating in the nucleus accumbens (NAc) [7, 8]. Opioids administered either systemically [9, 10] or directly into the VTA [10–13] produce increases in NAc DA levels by disinhibiting VTA DA neurons [14, 15]. Accordingly, the locomotor-activating, reinforcing, and reinstatement-inducing effects of opioids are each dampened following perturbation of NAc DA neurotransmission [16–23]. DA binds to five G protein-coupled receptor subtypes which are divided into two families. The D1-like receptor family includes the Gs-coupled D1 and D5 receptor subtypes (D1R and D5R) while the D2-like receptor family includes the Gi-coupled D2, D3, and D4 receptor subtypes (D2R, D3R, D4R) [24]. Administration of nonselective antagonists at either D1-like receptors or D2-like receptors reduces opioid-induced locomotor activation, opioid self-administration, and opioid seeking [for review, see 4, 5, 6, 25]. However, adverse side effects (e.g., extrapyramidal effects, hyperprolactinemia, somnolence, effects on blood pressure), poor retention rates, and/or loss of efficacy following chronic administration have hindered the potential clinical utility of these drug classes as treatments for substance use disorders [26–30]. Attention has therefore shifted towards receptor subtype-selective compounds that may retain pharmacotherapeutic efficacy while lacking undesirable behavioral effects.
The D3R has emerged as an appealing pharmacological target for the treatment of several neuropsychiatric diseases, including substance use disorders, as reviewed elsewhere [31–37]. Of most relevance to this report is preclinical evidence that D3R antagonism reliably attenuates opioid-induced hyperactivity, opioid self-administration, and opioid-seeking behavior, without producing adverse motoric effects associated with nonselective D2-like receptor antagonism [38–44]. Interestingly, two newly-developed and highly-selective D3R antagonists, R-VK4–40 and VK4–116 (247-fold and 1700-fold selectivity for D3R over D2R, respectively [45, 46]) have also been found to advantageously enhance, rather than attenuate, the analgesic effects of oxycodone while simultaneously blunting its abuse-related effects [42, 43]. However, these are the only studies to date to have investigated the impact of highly-selective D3R antagonists on opioid-induced analgesia [33], leaving unresolved whether the analgesia-enhancing effects observed with these compounds extends to other D3R antagonists and/or to analgesia induced by opioids other than oxycodone.
The present study therefore sought to determine the effects of pretreatment with another selective D3R antagonist, PG01037 (hD3R and hD2R Ki values = 0.7 nM and 93.3 nM, respectively; 133-fold selectivity for D3R over D2R [47]), on various unconditioned behavioral effects of morphine. PG01037 was selected for use in these studies for two major reasons. First, while PG01037 has been studied extensively in the context of psychostimulants [31], its impact on opioid-mediated effects has not previously been investigated [33]. Second, existing evidence already suggests that PG01037 may produce effects that are distinct from other selective D3R antagonists. For example, PG01037 pretreatment significantly enhances cocaine-induced hyperlocomotion [48], whereas other highly-selective D3R antagonists either attenuate or have no effect on this behavioral response [49]. We therefore reasoned that PG01037 would be an ideal test compound with which to assess whether modulations of the behavioral effects of opioids might also be dissimilar among D3R-selective antagonists.
Stimulation of locomotor activity in rodents is a useful and straightforward unconditioned behavioral response with which to interrogate NAc DA neurotransmission following systemic administration of many drugs of abuse, including opioids [50–54]. Previous work has demonstrated that pretreatment with the highly-selective D3R antagonists YQA14 or VK4–116 attenuates morphine- and oxycodone-induced hyperlocomotion respectively in mice [41, 46], but whether PG01037 similarly disrupts opioid-induced hyperlocomotion has not been investigated. We therefore first assessed the impact of PG01037 pretreatment on acute morphine-induced hyperactivity as well as the induction of locomotor sensitization to repeated morphine administration in mice. We next examined whether PG01037 modulates the antinociceptive effects of morphine. Finally, because nonselective blockade of D2-like receptors produces catalepsy alone and potentiates opioid-induced catalepsy [55–57], we investigated whether administration of PG01037 alone, or in combination with morphine, would induce cataleptic effects.
2. MATERIALS AND METHODS
2. 1. Subjects
Subjects used in this study were 72 adult male and female C57BL/6J mice (32/sex), 8–12 weeks old at the start of study. Mice were either acquired from Jackson Laboratory (Bar Harbor, ME; n = 48) or from a breeding colony at the National Institute on Drug Abuse (n = 24). Mice were housed in same-sex groups of 3–5 per cage in a climate-controlled vivarium with a 12-hr light cycle and had ad libitum access to food and water in the home cage. Procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Research Council and were approved by Institutional Animal Care and Use Committees at Emory University, Rowan University, or the National Institute on Drug Abuse of the National Institutes of Health. All behavioral testing was performed during the light cycle.
2.2. Locomotor Activity Apparatus
Locomotor activity was assessed in transparent polycarbonate cages (22 × 43 × 22 cm) that allowed passage of 8 infrared beams through the long wall and 4 infrared beams through the short wall of the enclosure at 4.9 cm intervals (San Diego Instruments; San Diego, California). Horizontal ambulations, defined as the sequential disruption of two adjacent infrared beams, were recorded in 5-min bins. The test chambers were prepared with a thin layer of clean bedding prior to each test session. Before the onset of experiments, mice were injected i.p. with saline and placed in the test chambers for 30 min for 3 consecutive days in order to habituate the mice to injections and the test apparatus.
2.3. Acute Morphine-Induced Locomotion
The effects of PG01037 on acute morphine-induced locomotion were evaluated in 16 mice (8 males, 8 females) using a within-subjects design. The methods and timeline for locomotor studies were adapted from those used previously [48, 58–61]. Animals were initially placed in the center of the locomotor chamber and ambulations were recorded for a 90-min habituation period during which animals acclimated to the test chamber. Next, animals were briefly removed from the chamber, injected with PG01037 (vehicle, 0.1, 1, or 10 mg/kg i.p.), and returned to the locomotor chamber for 30 min. Finally, mice were again removed from the chamber, injected with morphine (vehicle, 5.6, 18, or 56 mg/kg i.p.), and placed back in the chamber for 120 min. The dose range and 30-min pretreatment time for PG01037 were carefully selected for use based on our previous work showing that doses up to 10 mg/kg do not appreciably disrupt basal locomotion but significantly modulate the locomotor-activating effects of cocaine in C57BL/6J mice [48]. The 5.6 – 56 mg/kg dose range of morphine was selected based on our own pilot studies showing that it captures both the ascending and descending limbs of the morphine dose-response curve. Dose administration of PG01037 was pseudorandomized and counterbalanced across animals within each dose of morphine. All doses of PG01037 were assessed for a given morphine dose before switching to a different morphine dose. The order of morphine dose testing was randomly selected as 18 mg/kg, 56 mg/kg, 5.6 mg/kg, vehicle. All test sessions were separated by at least 1 week to prevent the development of locomotor sensitization to morphine. All mice received all treatments.
2.4. Morphine-Induced Locomotor Sensitization
Sensitization induction took place over 5 consecutive days and was performed in 3 separate groups of mice (n = 8/group, 4 male and 4 female). Mice were initially placed in the center of the locomotor chamber, and locomotor activity was recorded for 90 min. They were then briefly removed from the chamber, injected with PG01037 (vehicle or 10 mg/kg, i.p.), and returned to the locomotor chamber for 30 min. Mice were again removed and injected with morphine (vehicle or 56 mg/kg i.p.), then placed back in the chamber for 120 min. Mice received the same dose-combination of PG01037 and morphine across each of the 5 induction days. Seven days following the last induction session, locomotor activity was again assessed as described above with the exception that all mice received vehicle as the pretreatment 30 min prior to challenge with 56 mg/kg morphine. The dosing regimen was selected based on our own pilot studies which showed that 5 daily injections of 56 mg/kg morphine resulted in robust and reliable sensitization.
2.5. Hot Plate Test for Thermal Nociception
Antinociception was assessed in mice using a hot plate system (Model 39, IITC Life Science Inc., Woodland Hills, CA, USA) set to 52 ± 0.2°C. Mice were placed on the platform surrounded by transparent Plexiglas walls and removed after the first sign of thermal distress (paw licking, jumping, hind paw stomping). The latency to the first indicator of pain was recorded. A maximal cutoff of 60 s was instituted to prevent tissue damage. The antinociceptive effects of PG01037 alone were assessed in one group of 8 mice (4 males, 4 females). Subjects were first placed on the hot plate prior to any drug treatment to measure baseline response latencies (time point 0). Next, mice were administered PG01037 (vehicle, 0.1, 1, or 10 mg/kg, i.p.) and tested on the hot plate at 30, 60, 90, and 120 min post-injection. PG01037 doses were counterbalanced across subjects. The effects of PG01037 pretreatment on morphine-induced antinociception were examined in a separate group of 16 mice (8 males, 8 females). Following baseline testing (time point 0), mice were administered PG01037 (vehicle, 0.1, 1, or 10 mg/kg, i.p.) followed 30 min later by morphine (18 mg/kg i.p.). Hot plate testing was assessed at 30, 60, 90, and 120 min post-morphine injection. Each mouse received 1–3 doses of PG01037 in a counterbalanced manner whereby all possible PG01037 x morphine dose combinations consisted of n = 8. For each experiment, hot plate test sessions were separated by 2–3 days.
2.6. Catalepsy
The capacity of PG01037 alone or in combination with morphine to produce catalepsy was assessed in 8 mice (4 males, 4 females). Mice were administered PG01037 (vehicle or 10 mg/kg, i.p.) followed 30 min later by morphine (vehicle or 56 mg/kg, i.p.). Catalepsy was evaluated using the “bar test” [62]. Each test was conducted by lifting the mouse by the tail and allowing it to grab by its forepaws a solid circular bar (0.5 mm diameter) secured horizontally 4.5 cm above a flat surface, then releasing the tail so that the mouse was positioned sitting upright on its hind legs. Upon assuming this position, the latency to remove at least one paw from the bar was recorded. The test was stopped if the subject failed to withdraw one paw within 60 s. Mice that could not be placed in the testing position after 3 attempts received a latency score of 0 s. In each test, catalepsy was measured 0, 15, 30, 60, and 120 min following administration of morphine. The order of dose-combinations was randomized across mice. Mice were tested once per week until each mouse received all treatment combinations. After PG01037/morphine testing was completed, all mice received a final catalepsy test in which they were administered risperidone (3 mg/kg, i.p.) followed by saline i.p. 30 min later. Catalepsy in these tests was measured up to 60 min following saline injection. The risperidone test was included as a positive control as it induces prominent catalepsy in mice [63]. The cataleptogenic effects of the risperidone vehicle were assessed in a separate cohort of 8 mice (4 male and 4 female).
2.7. Drugs
Morphine sulfate (National Institute on Drug Abuse Drug Supply Program, Bethesda, MD) was dissolved in sterile saline. PG01037 was synthesized by Ms. J. Cao in the Medicinal Chemistry Section, National Institute on Drug Abuse Intramural Research Program as described previously [47] and dissolved in sterile water. Risperidone (Sigma-Aldrich; St. Louis, MO) was dissolved in vehicle containing ethanol:CremophorEL (Sigma-Aldrich):saline (5:10:85 v/v). All drugs were administered i.p. at a volume of 10 ml/kg.
2.8. Statistical Analyses
For acute morphine-induced locomotion studies, total ambulations during the 2 h following morphine administration were analyzed via two-way ANOVA with repeated measures on both factors (PG01037 dose × morphine dose), followed by post hoc Dunnett’s multiple comparisons tests to compare each dose of PG01037 to its vehicle within each dose of morphine. Locomotor activity in the 30-min period after PG01037 administration (prior to morphine administration) was analyzed using one-way repeated measures ANOVA. Effects of vehicle or 10.0 mg/kg PG01037 alone on locomotor activity were assessed by paired t-test. For the induction phase of sensitization (days 1–5), total ambulations during the 2 h following morphine administration were analyzed via mixed two-way ANOVA with repeated measures on one factor (day) and independent measures on the other factor (PG01037-morphine dose combination). Dunnett’s multiple comparison tests were used to determine whether sensitization occurred within each group by comparing locomotion on each of days 2–5 vs. day 1, while Tukey’s tests were used to detect differences in locomotion between dosing conditions within each induction day. For the challenge day of sensitization studies (day 12), total ambulations during the 2 h following morphine administration were analyzed via one-way ANOVA followed by Tukey’s multiple comparisons tests. The antinociceptive effects of PG01037 alone or in combination with morphine were analyzed using a two-way ANOVA with repeated measures on one factor (time) and independent measures on the other factor (PG01037 dose), followed by Dunnett’s or Tukey’s multiple comparisons tests, as specified in the text. Latency scores in catalepsy experiments were analyzed using two-way ANOVA with repeated measures on both factors (treatment × time). The effect of 3 mg/kg risperidone + saline was excluded from statistical analyses because risperidone was included only as a positive control to validate the catalepsy detection procedure. All data were plotted and analyzed using GraphPad Prism v8.4 (GraphPad Software, La Jolla, CA, USA). Significance was set at p < 0.05 for all tests.
3. RESULTS
3.1. Effects of PG01037 on Acute Morphine-Induced Hyperlocomotion
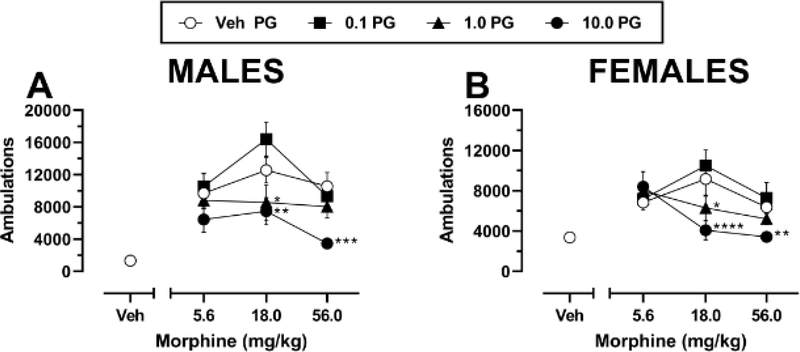
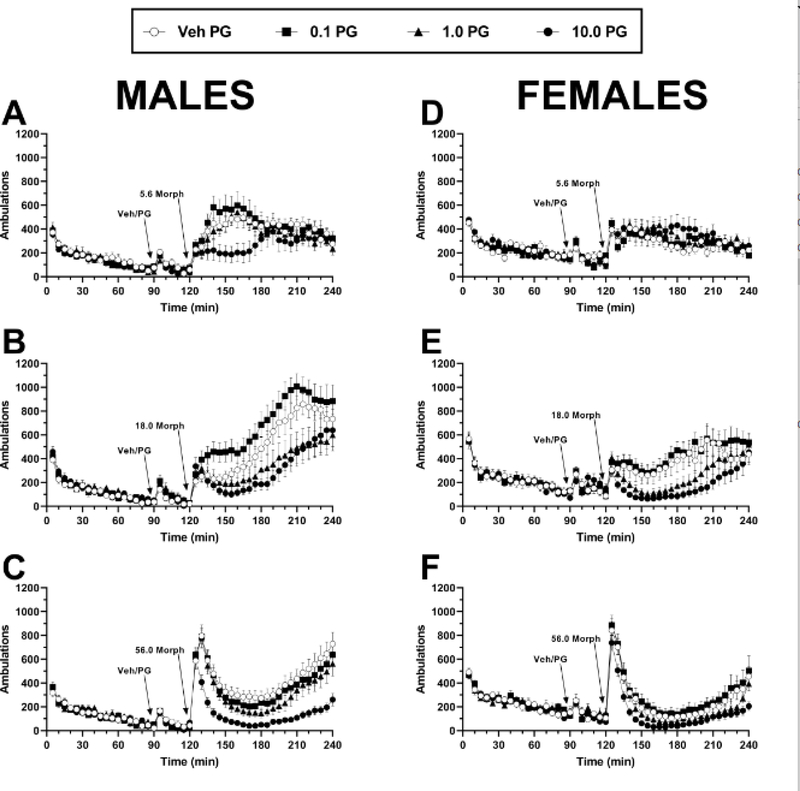
Administration of morphine after vehicle pretreatment resulted in increased locomotor activity with a typical inverted U-shaped dose-response function in both male (n = 8) and female (n = 8) mice (Fig. 1A–B). Two-way repeated measures ANOVA of PG01037 in combination with 5.6 – 56 mg/kg morphine in males indicated significant main effects of morphine dose (F(2,14) = 5.87, p = 0.014), PG01037 dose (F(3,21) = 24.44, p < 0.0001), and a significant morphine × PG01037 interaction (F(6,42) = 2.33, p = 0.049). Post hoc comparisons revealed that pretreatment with 1 or 10 mg/kg significantly attenuated the locomotor-activating effects of 18 mg/kg morphine, while the highest dose of PG01037 also significantly attenuated the locomotor-activating effects of 56 mg/kg morphine. Two-way repeated measures ANOVA of PG01037 in combination with 5.6 – 56 mg/kg morphine in females indicated a significant main effect of PG01037 dose (F(3,21) = 13.18, p < 0.0001) but not of morphine dose (F(2,14) = 1.92, p = 0.184) and a significant morphine × PG01037 interaction (F(6,42) = 7.27, p < 0.0001). Post hoc comparisons revealed a pattern of effects that was nearly identical to that of males, whereby 1 or 10 mg/kg PG01037 significantly reduced the effects of 18 mg/kg morphine and 10 mg/kg PG01037 significantly reduced the effects of 56 mg/kg morphine. The inhibitory actions of PG01037 on morphine-induced locomotion were observable within 5–15 min following morphine administration and persisted for the duration of the 120-min observation period (males, Fig. 2A–C; females, Fig. 2D–F).
Fig. 1.
Effects of pretreatment with PG01037 on acute morphine-induced locomotor activity in A male (n = 8) and B female (n = 8) C57BL/6J mice. Mice were pretreated with vehicle or 0.1 – 10 mg/kg PG01037, followed 30 min later by vehicle or 5.6 – 56 mg/kg morphine. Shown are mean ± SEM total ambulations in the 120-min period following morphine administration. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared to vehicle at the same dose of morphine. All mice received all treatments. “Veh” = vehicle; “PG” = PG01037. Doses on the abscissa are plotted along a log scale. Absence of error bars indicates that SEM values did not extend beyond the limits of the depicted symbol.
Fig. 2.
Time course of changes in locomotor activity following pretreatment with PG01037 and subsequent administration of morphine in A-C male (n = 8) and D-F female (n = 8) C57BL/6J mice. PG01037 (vehicle, 0.1 – 10 mg/kg) was administered 30 min prior to A, D 5.6 mg/kg morphine, B, E 18 mg/kg morphine, or C, F 56 mg/kg morphine. Each data point represents mean ± SEM ambulations recorded in 5-min bins. Arrows indicate time of pretreatment injection (“Veh/PG”, i.e. vehicle or 0.1 – 10 mg/kg PG01037 respectively) or time of morphine injection. All mice received all treatments. “Veh” = vehicle; “PG” = PG01037; “Morph” = morphine.
PG01037 administration did not significantly alter locomotor activity in the 30 min following its administration but prior to morphine injection in either males (one-way repeated measures ANOVA, (F(3,21) = 0.81, p = 0.504) or females (one-way repeated measures ANOVA, (F(3,21) = 0.19, p = 0.908) (Fig. S1). To further confirm a lack of effect by PG01037 alone, we administered vehicle or 10 mg/kg PG01037 followed 30 min later by saline and locomotion was monitored for 120 min. 10 mg/kg PG01037 pretreatment did not significantly alter total ambulations during this longer observation period in either males (paired t-test, t(7) = 1.05, p = 0.33) or females (paired t-test, t(7) = 2.23, p = 0.06) (Fig. S2). Because sex differences were not observed for PG01037’s impact on either basal locomotion or acute morphine-induced hyperlocomotion, the remaining experiments were carried out using treatment groups comprised of equal numbers of male and female mice.
3.2. Effects of PG01037 on Morphine-Induced Locomotor Sensitization
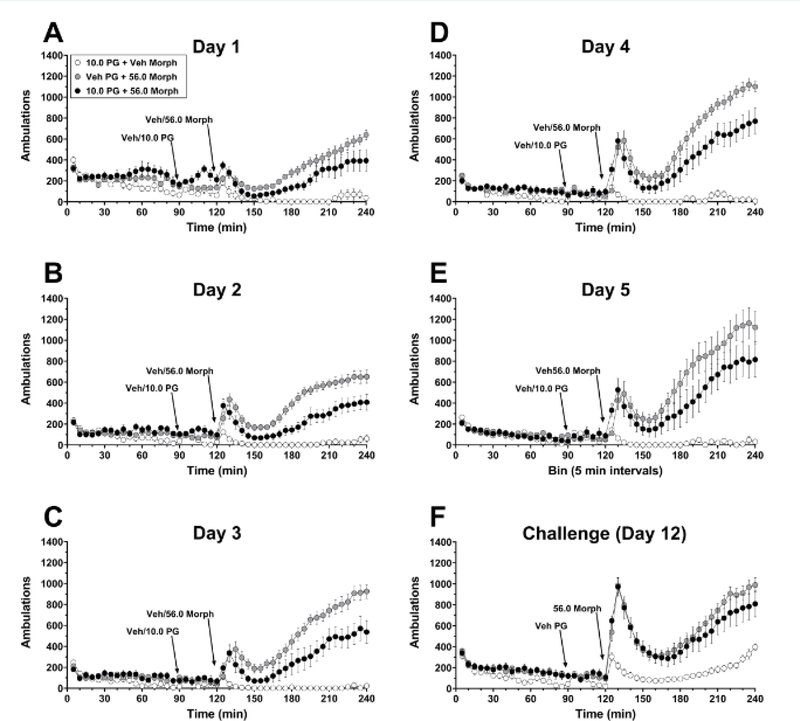
To test the impact of selective D3R antagonism on the development of morphine-induced locomotor sensitization, mice were injected daily for 5 consecutive days with PG01037 (vehicle or 10 mg/kg, i.p.) 30 min prior to morphine (vehicle or 56 mg/kg, i.p.). 10 mg/kg PG01037 was selected for use in this experiment because it produced the greatest attenuation of morphine’s acute locomotor activity and did not disrupt basal locomotion in the preceding experiments, while 56 mg/kg morphine was selected based on pilot studies demonstrating a more robust sensitized response to this dose as compared to lower doses. Two-way mixed factors ANOVA revealed significant main effects of induction day (F(4, 84) = 17.13, p < 0.0001) and PG01037-morphine dose combination (F(2, 21) = 25.23, p < 0.0001) and a significant day × PG01037-morphine interaction (F(8, 84) = 4.92, p < 0.0001). Post hoc tests indicated that mice pretreated with either vehicle or 10 mg/kg PG01037 prior to 56 mg/kg morphine showed significant sensitization of morphine-induced hyperactivity that peaked on day 5 at ~2.0-fold the level of activity on day 1 (Fig. 3; time course, Fig. 4A–E). However, the onset of sensitization was temporally delayed in PG01037-treated vs. vehicle-treated mice (day 4 vs. day 3, respectively), and morphine-induced hyperlocomotion was significantly attenuated during induction days 3–5 in PG01037-treated mice as compared to vehicle-pretreated mice by an average reduction of ~34.8% (Fig. 3). Mice treated daily with 10 mg/kg PG01037 prior to saline did not show any significant changes in locomotion across the induction phase (Fig. 3; time course, Fig. 4A–E).
Fig. 3.
Effects of pretreatment with PG01037 on morphine-induced locomotor sensitization. Mice received a combination of either vehicle + 56 mg/kg morphine, 10 mg/kg PG01037 + 56 mg/kg morphine, or 10 mg/kg PG01037 + vehicle of morphine daily for 5 days. One week later (day 12), all mice received vehicle pretreatment prior to a challenge with 56 mg/kg morphine. Shown are mean ± SEM total number of ambulations in the 120-min period following injection of morphine (vehicle or 56 mg/kg), which was administered 30 min after PG01037 pretreatment. *** p < 0.001, **** p < 0.0001, significant difference compared to Day 1 within the same treatment group. # p < 0.05, ## p < 0.01, #### p < 0.0001, compared to vehicle + 56 mg/kg morphine treatment group within the same day. $$$ p < 0.001, $$$$ p < 0.0001, compared to 10 mg/kg PG01037 + saline group on Day 12. n = 8/group (4 male, 4 female). “Veh” = vehicle; “PG” = PG01037; “Morph” = morphine. Absence of error bars indicates that SEM values did not extend beyond the limits of the depicted symbol.
Fig. 4.
Time course of locomotor activity following pretreatment with PG01037 (vehicle or 10 mg/kg) and subsequent administration of morphine (vehicle or 56 mg/kg) during induction days 1–5 of sensitization and challenge test with morphine alone one week later. Experimental details are as described for Figure 3. Shown are mean ± SEM ambulations recorded in 5-min bins on A induction day 1, B induction day 2, C induction day 3, D induction day 4, and E induction day 5 of sensitization induction, or F challenge day. Arrows indicate time of pretreatment injection (“Veh/PG”, i.e. vehicle or 10 mg/kg PG01037 respectively) or time of morphine injection (“Veh/56. 0 Morph”, i.e. vehicle or 56 mg/kg morphine respectively). n = 8/group (4 male, 4 female). “Veh” = vehicle; “PG” = PG01037; “Morph” = morphine.
One week after the final induction session, all mice received vehicle pretreatment followed by a morphine challenge (56 mg/kg, i.p.). One-way ANOVA (F(2, 21) = 16.45, p < 0.0001) with post hoc Tukey’s tests revealed that the PG01037-morphine and vehicle-morphine groups exhibited sensitized locomotor responses to morphine (evidenced by significantly greater locomotion as compared to the PG01037-vehicle group) that were not significantly different from each other (p > 0.05, PG01037-morphine vs. vehicle-morphine) (Fig. 3; time course, Fig. 4F).
3.3. Effects of PG01037 on Morphine-Induced Antinociception
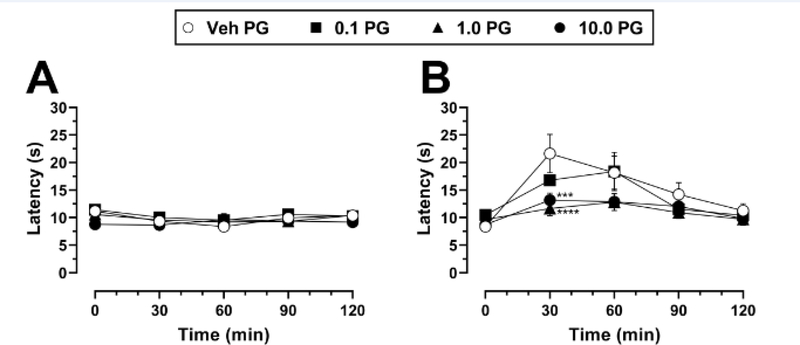
The effects of PG01037 administration alone on nociception in the hot plate test are shown in Fig. 5A. Two-way mixed-factors ANOVA indicated a significant main effect of time (F(4, 112) = 4.02, p = 0.004) but not of PG01037 dose (F(3, 28) = 0.82, p = 0.49) or a time × PG01037 interaction (F(12, 112) = 0.18, p = 0.18). Because of the lack of a significant main effect of PG01037 treatment or of the time × PG01037 interaction, post hoc analyses were performed solely on the factor of time. Latency scores following each of the four PG01037 pretreatment doses were averaged together within each time point, creating 5 total means for post hoc comparisons (i.e., latency scores at 0, 30, 60, 90, and 120 min time points). Tukey’s tests then revealed that reaction latencies decreased slightly but significantly at the 30-min and 60-min time points as compared to 0 min (p < 0.05), while latencies at the 90-min and 120-min time points were not significantly different from 0 min (p > 0.05).
Fig. 5.
Thermal nociception in mice following pretreatment with PG01037 alone or in combination with morphine. A Mice were administered PG01037 (vehicle, 0.1 – 10 mg/kg) and thermal nociception was assessed over 120 min following PG01037 injection. B Mice were administered PG01037 (vehicle, 0.1 – 10 mg/kg) 30 min prior to 18 mg/kg morphine, and thermal nociception was assessed over 120 min following morphine injection. Each data point represents mean ± SEM latency in seconds to the first indicator of nociception. *** p < 0.001, **** p < 0.0001, compared to vehicle at the same dose of morphine. n = 8/group (4 male, 4 female). “Veh” = vehicle; “PG” = PG01037. Absence of error bars indicates that SEM values did not extend beyond the limits of the depicted symbol.
In mice pretreated with vehicle of PG01037, 18 mg/kg morphine increased reaction latency ~2.5-fold 30 min after morphine administration, with the effect gradually returning to near-baseline levels by the 120-min time point (Fig. 5B). Two-way mixed factors ANOVA indicated a significant main effect of time (F(4, 112) = 23.41, p < 0.0001), no main effect of PG01037 dose (F(3, 28) = 2.29, p = 0.10), and a significant time × PG01037 interaction (F(12, 112) = 2.77, p = 0.003). Post hoc Dunnett’s tests revealed that compared to vehicle pretreatment, administration of 1 or 10 mg/kg PG01037 significantly attenuated the antinociceptive effects of morphine at the 30-min time point when morphine’s effects were maximal, reducing them by 40% and 54%, respectively. This attenuating effect of PG01037 fell just short of statistical significance at the 60-min time point (p = 0.06 for both 1 and 10 mg/kg PG01037 compared to vehicle).
3.4. Effects of PG01037 Alone or in Combination with Morphine on Catalepsy
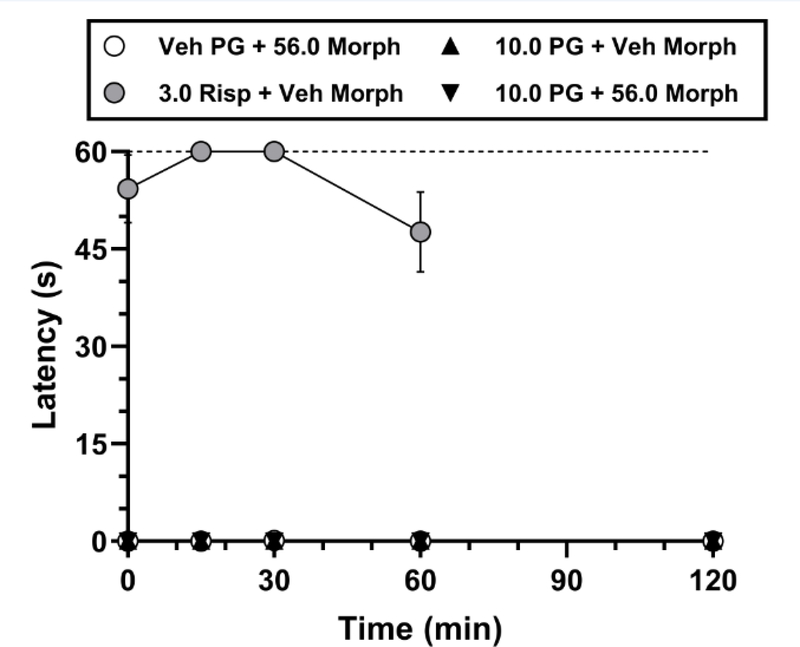
To determine whether selective D3R antagonism induces catalepsy either alone or in combination with morphine, mice were administered PG01037 (vehicle or 10 mg/kg, i.p.) 30 min prior to morphine (vehicle or 56 mg/kg, i.p.). We purposely selected the highest doses administered of each compound in our locomotor and nociception experiments in order to maximize the potential detection of catalepsy. Neither administration of PG01037 alone, morphine alone, nor their combination resulted in catalepsy (Fig. 6). Analysis of these treatment conditions using two-way repeated measures ANOVA showed no significant main effect of treatment (F(2,14) = 1.00, p = 0.39), time (F(4,28) = 1.00, p = 0.42), or a treatment × time interaction (F(8,56) = 1.00, p = 0.45). For all mice tested, the latency to withdraw a forepaw in any of the aforementioned conditions did not exceed 1 s. Administration of risperidone’s vehicle similarly did not produce paw withdrawal latencies > 1 s (data not shown), whereas administration of 3 mg/kg risperidone produced a robust increase in catalepsy across the 60-min test period, with paw withdrawal latencies ranging on average from ~48 s up to the procedural maximum time allowed of 60 s (Fig. 6).
Fig. 6.
Catalepsy following administration of PG01037 alone or in combination with morphine. Mice were pretreated with PG01037 (vehicle or 10 mg/kg) followed 30 min later by administration of vehicle or 56 mg/kg morphine, or 3 mg/kg risperidone followed 30 min later by administration of vehicle morphine. Each data point represents mean ± SEM latency in seconds to withdraw a paw in the bar test. Latencies were measured at 0, 15, 30, 60, and 120 min relative to the second injection. The dotted line represents the 60-s maximal time allowed for paw withdrawal. All mice received all treatments (n = 8; 4 male, 4 female). “Veh” = vehicle; “PG” = PG01037; “Risp” = risperidone; “Morph” = morphine. Absence of error bars indicates that SEM values did not extend beyond the limits of the depicted symbol.
4. DISCUSSION
In the present study, pretreatment with PG01037 dose-dependently attenuated acute morphine-induced hyperactivity in mice. This finding is in agreement with previous reports demonstrating that pretreatment with several other selective D3R antagonists similarly produces significant reductions in the locomotor-activating effects of morphine or oxycodone [41, 46, 64]. Collectively, these results suggest that D3R antagonism reliably attenuates the locomotor-activating effects of opioids regardless of the specific compound used, indicating a D3R antagonist class effect. Because the locomotor-activating effects of opioids are most often attributed to increased DA neurotransmission within the mesolimbic system [17, 50, 52], the attenuated locomotor response to opioids that is produced by D3R antagonists may reflect as-yet unidentified modulations in mesolimbic DA signaling and/or NAc output that are likely to also mediate their concomitant reductions in opioid reward. It is interesting to note that while various D3R antagonists all appear to attenuate opioid-induced hyperlocomotion, their impact on psychostimulant-induced hyperlocomotion is more variable. We and others have reported that PG01037 and the selective D3R antagonist NGB294 enhance the locomotor-activating effects of cocaine or amphetamine respectively [48, 65], whereas other D3R antagonists either reduce or do not affect psychostimulant-induced hyperlocomotion [49, 66, 67]. The reasons as to why D3R antagonists reliably attenuate opioid-induced hyperlocomotion but exhibit more diverse effects on stimulant-induced hyperlocomotion remain unclear and will require further research to resolve.
In contrast to its effects on acute morphine-induced hyperactivity, PG01037 did not disrupt the induction of locomotor sensitization in the present study, since mice that were treated with PG01037 and morphine throughout induction days 1–5 displayed a sensitized response to morphine challenge that was comparable to that displayed by mice treated with the vehicle of PG01037 and morphine throughout induction. Although the NAc contributes to the locomotor-activating effects of acute systemic opioid administration [68–70], it is generally accepted that neuroadaptations within the VTA, and not the NAc, underlie the development of opioid-induced locomotor sensitization [71–73]. The finding that PG01037 pretreatment attenuates acute morphine-induced hyperactivity but not its sensitization may therefore indicate that PG01037 reduces opioid-induced hyperlocomotion via actions in the NAc that effectively “mask” the overt appearance of sensitization, whereas the VTA-dependent adaptations underlying sensitization are unaltered by PG01037 and can be “unmasked” when subjects are challenged with morphine alone. Additional studies assessing the impact of intra-VTA or intra-NAc administration of D3R antagonists on the induction of opioid locomotor sensitization will be required to test the veracity of this hypothetical schema. It is noteworthy that our sensitization results with PG01037 seem to contradict a report that pretreatment with the highly-selective D3R antagonist VK4–116 attenuated the induction of oxycodone-induced locomotor sensitization [46]. However, some key procedural differences may underlie these discrepant findings including the use of different opioids (morphine vs. oxycodone), imposition of 7 days vs. 2 days between the final induction session and the expression test, or most notably, use of different D3R antagonists (PG01037 vs. VK4–116) which may themselves exert different qualitative effects on opioid-induced locomotor sensitization for reasons not yet understood.
Whereas PG01037 administration alone did not disrupt thermal nociception in the present study, it dose-dependently attenuated the antinociceptive effects of acute morphine, evidenced by an apparent downward shift of morphine’s efficacy over time. This result is in opposition to recent studies using the newer and highly-selective D3R antagonists VK4–116 and R-VK4–40, as pretreatment with these compounds enhances the antinociceptive effects of oxycodone in rats [42, 43]. Why PG01037 produces an opposite effect on opioid-mediated antinociception in the present study remains unclear, although VK4–40 and VK4–116 notably exhibit some other effects that are discordant with older D3R antagonists; for example, they do not potentiate the cardiovascular effects of cocaine as compared to older-generation D3R antagonists [49]. It is also noteworthy that our present study examined the antinociceptive effects of morphine rather than oxycodone, and did so in mice rather than rats. These procedural differences aside, our present findings highlight the need for more research to clarify the mechanisms by which highly-selective D3R antagonists modulate the analgesic properties of clinically-utilized opioid analgesics [33], and to ascertain why different antagonists may yield different results. Possible explanations may include the greater selectivity for D3R over D2R exhibited by R-VK4–40 (hD3R and hD2R Ki values = 0.89 and 219 nM, respectively; 247-fold selective for D3R over D2R [45]) and VK4–116 (hD3R and hD2R Ki values = 6.8 and 11,400 nM, respectively; 1700-fold selective for D3R over D2R [46] as compared to PG01037 (hD3R and hD2R Ki values = 0.7 nM and 93.3 nM, respectively; 133-fold selectivity for D3R over D2R, [47]), and/or potential biases in partial agonism or antagonism of specific D3R-mediated intracellular signaling pathways, similar to what has previously been reported among other D2R-selective and D3R-selective ligands [74, 75].
Combined administration of nonselective D2-like receptor antagonists with opioids induces catalepsy in mice that is substantially greater than that produced by either drug alone [55, 56]. It is generally believed that D2-like receptor antagonists exert these effects via actions at the D2R subtype because selective D2R blockade alone produces catalepsy in mice [76], rats [77], and nonhuman primates [78], whereas catalepsy has not been detected following treatment with D3R antagonists [77, 79, 80]. In agreement with these latter findings, PG01037 in the present study showed no evidence of inducing cataleptic effects alone at a behaviorally-active dose that significantly modulated morphine-induced hyperactivity and antinociception. More importantly, the present study is the first to demonstrate that concurrent administration of high doses of a selective D3R antagonist (PG01037) and an opioid (morphine) does not induce catalepsy. Given that D3R antagonists are being considered as potential pharmacotherapeutics for several neuropsychiatric and neurological disorders including OUD, the lack of cataleptic effects following D3R antagonism alone or in combination with morphine adds to accumulating evidence that D3R antagonists exhibit a desirable safety profile and lack adverse motoric effects as compared to nonselective D2-like receptor antagonists, even when opioids are concurrently administered.
In summary, we show that pretreatment with the selective D3R antagonist PG01037 attenuates acute morphine-induced hyperactivity similar to other selective D3R antagonists, indicating that reduction of this behavioral effect of opioids may be a feature shared by all compounds in this drug class. Furthermore, the absence of cataleptic effects following administration of a D3R antagonist, alone or in combination with morphine, lends further support to their potential use and safety as treatments for OUD. Recently-developed, highly-selective D3R antagonists such as R-VK4–40 and VK4–116 exhibit the most desirable behavioral profiles for clinical investigation because they reduce the abuse-related behavioral effects of opioids and simultaneously do not disrupt their analgesic efficacy [33]. However, our present results with PG01037 provide a cautionary note that the potentiation of opioid-induced analgesia observed with R-VK4–40 and VK4–116 may not be universal for all D3R antagonists. Additional research will be needed to elucidate the neurobiological mechanisms by which highly-selective D3R antagonists such as R-VK4–40 and VK4–116 favorably alter the abuse-related and analgesic effects of opioids.
Supplementary Material
HIGHLIGHTS.
PG01037 attenuates acute morphine-induced hyperactivity
PG01037 attenuates morphine-induced antinociception
PG01037 does not produce catalepsy alone or in combination with morphine
ACKNOWLEDGEMENTS
The authors thank Ms. J. Cao in the Medicinal Chemistry Section, NIDA-IRP for synthesis of PG01037.
FUNDING
This work was supported by the National Institutes of Health grants from the National Institute on Drug Abuse [DA044726 (SLF), DA038453, DA049257 (DW), DA039991 (DFM)] and the Intramural Research Program of the National Institutes of Health [National Institute on Drug Abuse; DA000424 (ZXX/AHN)].
ABBREVIATIONS
- D1R
D1 receptor
- D2R
D2 receptor
- D3R
D3 receptor
- D4R
D4 receptor
- D5R
D5 receptor; receptor
- DA
dopamine
- NAc
nucleus accumbens
- OUD
opioid use disorders
- VTA
ventral tegmental area
Footnotes
DECLARATION OF INTEREST
Declarations of interest: none.
DATA STATEMENT
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Volkow ND, Blanco C, The changing opioid crisis: development, challenges and opportunities, Mol Psychiatry 26(1) (2021) 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blanco C, Volkow ND, Management of opioid use disorder in the USA: present status and future directions, Lancet 393(10182) (2019) 1760–1772. [DOI] [PubMed] [Google Scholar]
- [3].Kreek MJ, Reed B, Butelman ER, Current status of opioid addiction treatment and related preclinical research, Sci Adv 5(10) (2019) eaax9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Di Chiara G, North RA, Neurobiology of opiate abuse, Trends Pharmacol Sci 13(5) (1992) 185–93. [DOI] [PubMed] [Google Scholar]
- [5].Pierce RC, Kumaresan V, The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse?, Neurosci Biobehav Rev 30(2) (2006) 215–38. [DOI] [PubMed] [Google Scholar]
- [6].Wise RA, Opiate reward: sites and substrates, Neurosci Biobehav Rev 13(2–3) (1989) 129–33. [DOI] [PubMed] [Google Scholar]
- [7].Bjorklund A, Dunnett SB, Dopamine neuron systems in the brain: an update, Trends Neurosci 30(5) (2007) 194–202. [DOI] [PubMed] [Google Scholar]
- [8].Moore RY, Bloom FE, Central catecholamine neuron systems: anatomy and physiology of the dopamine systems, Annu Rev Neurosci 1 (1978) 129–69. [DOI] [PubMed] [Google Scholar]
- [9].Chefer VI, Kieffer BL, Shippenberg TS, Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice, Eur J Neurosci 18(7) (2003) 1915–22. [DOI] [PubMed] [Google Scholar]
- [10].Gysling K, Wang RY, Morphine-induced activation of A10 dopamine neurons in the rat, Brain Res 277(1) (1983) 119–27. [DOI] [PubMed] [Google Scholar]
- [11].Devine DP, Leone P, Pocock D, Wise RA, Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies, J Pharmacol Exp Ther 266(3) (1993) 1236–46. [PubMed] [Google Scholar]
- [12].Spanagel R, Herz A, Shippenberg TS, Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway, Proc Natl Acad Sci U S A 89(6) (1992) 2046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leone P, Pocock D, Wise RA, Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release, Pharmacol Biochem Behav 39(2) (1991) 469–72. [DOI] [PubMed] [Google Scholar]
- [14].Johnson SW, North RA, Opioids excite dopamine neurons by hyperpolarization of local interneurons, J Neurosci 12(2) (1992) 483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matsui A, Williams JT, Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons, J Neurosci 31(48) (2011) 17729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hand TH, Franklin KB, 6-OHDA lesions of the ventral tegmental area block morphine-induced but not amphetamine-induced facilitation of self-stimulation, Brain Res 328(2) (1985) 233–41. [DOI] [PubMed] [Google Scholar]
- [17].Kelley AE, Stinus L, Iversen SD, Interactions between D-ala-met-enkephalin, A10 dopaminergic neurones, and spontaneous behaviour in the rat, Behav Brain Res 1(1) (1980) 3–24. [DOI] [PubMed] [Google Scholar]
- [18].Phillips AG, LePiane FG, Fibiger HC, Dopaminergic mediation of reward produced by direct injection of enkephalin into the ventral tegmental area of the rat, Life Sci 33(25) (1983) 2505–11. [DOI] [PubMed] [Google Scholar]
- [19].Smith JE, Guerin GF, Co C, Barr TS, Lane JD, Effects of 6-OHDA lesions of the central medial nucleus accumbens on rat intravenous morphine self-administration, Pharmacol Biochem Behav 23(5) (1985) 843–9. [DOI] [PubMed] [Google Scholar]
- [20].Spyraki C, Fibiger HC, Phillips AG, Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system, Psychopharmacology (Berl) 79(2–3) (1983) 278–83. [DOI] [PubMed] [Google Scholar]
- [21].Stinus L, Koob GF, Ling N, Bloom FE, Le Moal M, Locomotor activation induced by infusion of endorphins into the ventral tegmental area: evidence for opiate-dopamine interactions, Proc Natl Acad Sci U S A 77(4) (1980) 2323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang B, Luo F, Ge X, Fu A, Han J, Effect of 6-OHDA lesions of the dopaminergic mesolimbic system on drug priming induced reinstatement of extinguished morphine CPP in rats, Beijing Da Xue Xue Bao Yi Xue Ban 35(5) (2003) 449–52. [PubMed] [Google Scholar]
- [23].Shippenberg TS, Bals-Kubik R, Herz A, Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors, J Pharmacol Exp Ther 265(1) (1993) 53–9. [PubMed] [Google Scholar]
- [24].Beaulieu JM, Gainetdinov RR, The physiology, signaling, and pharmacology of dopamine receptors, Pharmacol Rev 63(1) (2011) 182–217. [DOI] [PubMed] [Google Scholar]
- [25].Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y, Relapse to opioid seeking in rat models: behavior, pharmacology and circuits, Neuropsychopharmacology 44(3) (2019) 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cho DI, Zheng M, Kim KM, Current perspectives on the selective regulation of dopamine D(2) and D(3) receptors, Arch Pharm Res 33(10) (2010) 1521–38. [DOI] [PubMed] [Google Scholar]
- [27].Millan MJ, Peglion JL, Vian J, Rivet JM, Brocco M, Gobert A, Newman-Tancredi A, Dacquet C, Bervoets K, Girardon S, et al. , Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: 1. Activation of postsynaptic D3 receptors mediates hypothermia, whereas blockade of D2 receptors elicits prolactin secretion and catalepsy, J Pharmacol Exp Ther 275(2) (1995) 885–98. [PubMed] [Google Scholar]
- [28].Haney M, Ward AS, Foltin RW, Fischman MW, Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans, Psychopharmacology (Berl) 155(4) (2001) 330–7. [DOI] [PubMed] [Google Scholar]
- [29].Kishi T, Matsuda Y, Iwata N, Correll CU, Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials, J Clin Psychiatry 74(12) (2013) e1169–80. [DOI] [PubMed] [Google Scholar]
- [30].Grabowski J, Shearer J, Merrill J, Negus SS, Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence, Addict Behav 29(7) (2004) 1439–64. [DOI] [PubMed] [Google Scholar]
- [31].Heidbreder CA, Newman AH, Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders, Ann N Y Acad Sci 1187 (2010) 4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sokoloff P, Le Foll B, The dopamine D3 receptor, a quarter century later, Eur J Neurosci 45(1) (2017) 2–19. [DOI] [PubMed] [Google Scholar]
- [33].Galaj E, Newman AH, Xi ZX, Dopamine D3 receptor-based medication development for the treatment of opioid use disorder: Rationale, progress, and challenges, Neurosci Biobehav Rev 114 (2020) 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Joyce JN, Millan MJ, Dopamine D3 receptor antagonists as therapeutic agents, Drug Discov Today 10(13) (2005) 917–25. [DOI] [PubMed] [Google Scholar]
- [35].Joyce JN, Millan MJ, Dopamine D3 receptor agonists for protection and repair in Parkinson’s disease, Curr Opin Pharmacol 7(1) (2007) 100–5. [DOI] [PubMed] [Google Scholar]
- [36].Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F, Drago F, Dopamine D(3) receptor as a new pharmacological target for the treatment of depression, Eur J Pharmacol 719(1–3) (2013) 25–33. [DOI] [PubMed] [Google Scholar]
- [37].Torrisi SA, Laudani S, Contarini G, De Luca A, Geraci F, Manago F, Papaleo F, Salomone S, Drago F, Leggio GM, Dopamine, Cognitive Impairments and Second-Generation Antipsychotics: From Mechanistic Advances to More Personalized Treatments, Pharmaceuticals (Basel) 13(11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boateng CA, Bakare OM, Zhan J, Banala AK, Burzynski C, Pommier E, Keck TM, Donthamsetti P, Javitch JA, Rais R, Slusher BS, Xi ZX, Newman AH, High Affinity Dopamine D3 Receptor (D3R)-Selective Antagonists Attenuate Heroin Self-Administration in Wild-Type but not D3R Knockout Mice, J Med Chem 58(15) (2015) 6195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Galaj E, Manuszak M, Babic S, Ananthan S, Ranaldi R, The selective dopamine D3 receptor antagonist, SR 21502, reduces cue-induced reinstatement of heroin seeking and heroin conditioned place preference in rats, Drug Alcohol Depend 156 (2015) 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hu R, Song R, Yang R, Su R, Li J, The dopamine D(3) receptor antagonist YQA14 that inhibits the expression and drug-prime reactivation of morphine-induced conditioned place preference in rats, Eur J Pharmacol 720(1–3) (2013) 212–7. [PubMed] [Google Scholar]
- [41].Lv Y, Hu RR, Jing M, Zhao TY, Wu N, Song R, Li J, Hu G, Selective dopamine D3 receptor antagonist YQA14 inhibits morphine-induced behavioral sensitization in wild type, but not in dopamine D3 receptor knockout mice, Acta Pharmacol Sin 40(5) (2019) 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, Newman AH, Dopamine D3R antagonist VK4–116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects, Neuropsychopharmacology 44(8) (2019) 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jordan CJ, Humburg B, Rice M, Bi GH, You ZB, Shaik AB, Cao J, Bonifazi A, Gadiano A, Rais R, Slusher B, Newman AH, Xi ZX, The highly selective dopamine D3R antagonist, R-VK4–40 attenuates oxycodone reward and augments analgesia in rodents, Neuropharmacology 158 (2019) 107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O, Dopamine D3 Receptor Antagonism Reverses the Escalation of Oxycodone Self-administration and Decreases Withdrawal-Induced Hyperalgesia and Irritability-Like Behavior in Oxycodone-Dependent Heterogeneous Stock Rats, Front Behav Neurosci 13 (2019) 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shaik AB, Kumar V, Bonifazi A, Guerrero AM, Cemaj SL, Gadiano A, Lam J, Xi ZX, Rais R, Slusher BS, Newman AH, Investigation of Novel Primary and Secondary Pharmacophores and 3-Substitution in the Linking Chain of a Series of Highly Selective and Bitopic Dopamine D3 Receptor Antagonists and Partial Agonists, J Med Chem 62(20) (2019) 9061–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kumar V, Bonifazi A, Ellenberger MP, Keck TM, Pommier E, Rais R, Slusher BS, Gardner E, You ZB, Xi ZX, Newman AH, Highly Selective Dopamine D3 Receptor (D3R) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment, J Med Chem 59(16) (2016) 7634–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH, Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor, J Med Chem 48(3) (2005) 839–48. [DOI] [PubMed] [Google Scholar]
- [48].Manvich DF, Petko AK, Branco RC, Foster SL, Porter-Stransky KA, Stout KA, Newman AH, Miller GW, Paladini CA, Weinshenker D, Selective D2 and D3 receptor antagonists oppositely modulate cocaine responses in mice via distinct postsynaptic mechanisms in nucleus accumbens, Neuropsychopharmacology 44(8) (2019) 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jordan CJ, Humburg BA, Thorndike EB, Shaik AB, Xi ZX, Baumann MH, Newman AH, Schindler CW, Newly Developed Dopamine D3 Receptor Antagonists, R-VK4–40 and R-VK4–116, Do Not Potentiate Cardiovascular Effects of Cocaine or Oxycodone in Rats, J Pharmacol Exp Ther 371(3) (2019) 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Broekkamp CL, Phillips AG, Cools AR, Stimulant effects of enkephalin microinjection into the dopaminergic A10 area, Nature 278(5704) (1979) 560–2. [DOI] [PubMed] [Google Scholar]
- [51].Delfs JM, Schreiber L, Kelley AE, Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat, J Neurosci 10(1) (1990) 303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kalivas PW, Widerlov E, Stanley D, Breese G, Prange AJ Jr., Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity, J Pharmacol Exp Ther 227(1) (1983) 229–37. [PubMed] [Google Scholar]
- [53].Kelly PH, Iversen SD, Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats, Eur J Pharmacol 40(1) (1976) 45–56. [DOI] [PubMed] [Google Scholar]
- [54].Kelly PH, Seviour PW, Iversen SD, Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum, Brain Res 94(3) (1975) 507–22. [DOI] [PubMed] [Google Scholar]
- [55].Kiritsy-Roy JA, Standish SM, Terry LC, Dopamine D-1 and D-2 receptor antagonists potentiate analgesic and motor effects of morphine, Pharmacol Biochem Behav 32(3) (1989) 717–21. [DOI] [PubMed] [Google Scholar]
- [56].Rodriguez-Arias M, Broseta I, Aguilar MA, Minarro J, Lack of specific effects of selective D(1) and D(2) dopamine antagonists vs. risperidone on morphine-induced hyperactivity, Pharmacol Biochem Behav 66(1) (2000) 189–97. [DOI] [PubMed] [Google Scholar]
- [57].Wilcox RE, Bozarth M, Levitt RA, Reversal of morphine-induced catalepsy by naloxone microinjections into brain regions with high opiate receptor binding: a preliminary report, Pharmacol Biochem Behav 18(1) (1983) 51–4. [DOI] [PubMed] [Google Scholar]
- [58].Porter-Stransky KA, Petko AK, Karne SL, Liles LC, Urs NM, Caron MG, Paladini CA, Weinshenker D, Loss of beta-arrestin2 in D2 cells alters neuronal excitability in the nucleus accumbens and behavioral responses to psychostimulants and opioids, Addict Biol 25(6) (2020) e12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Missale C, Nash SR, Robinson SW, Jaber M, Caron MG, Dopamine receptors: from structure to function, Physiol Rev 78(1) (1998) 189–225. [DOI] [PubMed] [Google Scholar]
- [60].Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D, Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine, Neuropsychopharmacology 31(10) (2006) 2221–30. [DOI] [PubMed] [Google Scholar]
- [61].Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD, Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals, Proc Natl Acad Sci U S A 99(21) (2002) 13873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sanberg PR, Bunsey MD, Giordano M, Norman AB, The catalepsy test: its ups and downs, Behav Neurosci 102(5) (1988) 748–59. [DOI] [PubMed] [Google Scholar]
- [63].Fink-Jensen A, Schmidt LS, Dencker D, Schulein C, Wess J, Wortwein G, Woldbye DP, Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor, Eur J Pharmacol 656(1–3) (2011) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].You ZB, Gao JT, Bi GH, He Y, Boateng C, Cao J, Gardner EL, Newman AH, Xi ZX, The novel dopamine D3 receptor antagonists/partial agonists CAB2–015 and BAK4–54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats, Neuropharmacology 126 (2017) 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA, Xu M, Zhang J, Richtand NM, The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion, Pharmacol Biochem Behav 86(4) (2007) 718–26. [DOI] [PubMed] [Google Scholar]
- [66].Galaj E, Ananthan S, Saliba M, Ranaldi R, The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats, Psychopharmacology (Berl) 231(3) (2014) 501–10. [DOI] [PubMed] [Google Scholar]
- [67].Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL, YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice, Addict Biol 17(2) (2012) 259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schildein S, Agmo A, Huston JP, Schwarting RK, Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity, Brain Res 790(1–2) (1998) 185–94. [DOI] [PubMed] [Google Scholar]
- [69].Vaccarino FJ, Corrigall WA, Effects of opiate antagonist treatment into either the periaqueductal grey or nucleus accumbens on heroin-induced locomotor activation, Brain Res Bull 19(5) (1987) 545–9. [DOI] [PubMed] [Google Scholar]
- [70].Teitelbaum H, Giammatteo P, Mickley GA, Differential effects of localized lesions of n. accumbens on morphine- and amphetamine-induced locomotor hyperactivity in the C57BL/6J mouse, J Comp Physiol Psychol 93(4) (1979) 745–51. [DOI] [PubMed] [Google Scholar]
- [71].Kalivas PW, Duffy P, Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons, J Pharmacol Exp Ther 241(1) (1987) 204–12. [PubMed] [Google Scholar]
- [72].Vanderschuren LJ, Kalivas PW, Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies, Psychopharmacology (Berl) 151(2–3) (2000) 99–120. [DOI] [PubMed] [Google Scholar]
- [73].Vezina P, Kalivas PW, Stewart J, Sensitization occurs to the locomotor effects of morphine and the specific mu opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens, Brain Res 417(1) (1987) 51–8. [DOI] [PubMed] [Google Scholar]
- [74].Taylor M, Grundt P, Griffin SA, Newman AH, Luedtke RR, Dopamine D3 receptor selective ligands with varying intrinsic efficacies at adenylyl cyclase inhibition and mitogenic signaling pathways, Synapse 64(3) (2010) 251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Weiwer M, Xu Q, Gale JP, Lewis M, Campbell AJ, Schroeder FA, Van de Bittner GC, Walk M, Amaya A, Su P, L DO, Sacher JR, Skepner A, Fei D, Dennehy K, Nguyen S, Faloon PW, Perez J, Cottrell JR, Liu F, Palmer M, Pan JQ, Hooker JM, Zhang YL, Scolnick E, Wagner FF, Holson EB, Functionally Biased D2R Antagonists: Targeting the beta-Arrestin Pathway to Improve Antipsychotic Treatment, ACS Chem Biol 13(4) (2018) 1038–1047. [DOI] [PubMed] [Google Scholar]
- [76].Hattori K, Uchino S, Isosaka T, Maekawa M, Iyo M, Sato T, Kohsaka S, Yagi T, Yuasa S, Fyn is required for haloperidol-induced catalepsy in mice, J Biol Chem 281(11) (2006) 7129–35. [DOI] [PubMed] [Google Scholar]
- [77].Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M, S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626, J Pharmacol Exp Ther 293(3) (2000) 1063–73. [PubMed] [Google Scholar]
- [78].Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD, Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys, J Pharmacol Exp Ther 334(2) (2010) 556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DN, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AK, Hagan JJ, Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A, J Pharmacol Exp Ther 294(3) (2000) 1154–65. [PubMed] [Google Scholar]
- [80].Torrisi SA, Salomone S, Geraci F, Caraci F, Bucolo C, Drago F, Leggio GM, Buspirone Counteracts MK-801-Induced Schizophrenia-Like Phenotypes through Dopamine D3 Receptor Blockade, Front Pharmacol 8 (2017) 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.