Abstract
7-Dehydrocholesterol reductase (DHCR7) catalyses the final step of cholesterol biosynthesis in the Kandutsch-Russel pathway, the reduction of 7-dehydrocholesterol (7DHC) to cholesterol. 7DHC can be acted on by a range of other enzymes including CYP27A1 and CYP11A1, as well as by UVB radiation, producing a number of derivatives including hydroxy-metabolites, some of which retain the C7–C8 double bond and are biologically active. These metabolites include lumisterol (L3) which is a stereoisomer of 7DHC produced in the skin by UVB radiation of 7DHC, as well as vitamin D3. The aim of this study was to test whether these metabolites could act as substrates or inhibitors of DHCR7 in rat liver microsomes. To initially screen the ability of these metabolites to interact with the active site of DHCR7, their ability to inhibit the conversion of ergosterol to brassicasterol was measured. Sterols that significantly inhibited this reaction included 7DHC (as expected), 20S(OH)7DHC, 27(OH) DHC, 8DHC, 20S(OH)L3 and 22(OH)L3 but not 7-dehydropregnenolone (7DHP), 25(OH)7DHC, L3 or vitamin D3 and its hydroxyderivatives. Sterols that inhibited ergosterol reduction were directly tested as substrates for DHCR7. 20S(OH)7DHC, 27(OH)DHC and 7-dehydrodesmosterol were confirmed to be substrates, giving the expected product with the C7–C8 double bond removed. No products were observed from 8DHC or 20S(OH)L3 indicating that these sterols are inhibitors and not substrates of DHCR7. The resistance of lumisterol and 7DHP to reduction by DHCR7 in cells will permit other enzymes to metabolise these sterols to their active forms retaining the C7–C8 double bond, conferring specificity to their biological actions.
Keywords: 7-dehydrocholesterol reductase, DHCR7, Lumisterol, Vitamin D3, 7-dehydropregnenolone
1. Introduction
7-Dehydrocholesterol reductase (DHCR7) catalyses the final step of cholesterol biosynthesis in the Kandutsch-Russel pathway, the reduction of the C7–C8 double bond of 7-dehydrocholesterol (7DHC) to produce cholesterol [1,2]. It carries out a similar reaction on 7-dehydrodesmosterol to produce desmosterol as the second last step in cholesterol biosynthesis by the Bloch pathway [3]. Different tissues display preference for either one of these pathways with the Kandutsch-Russel pathway predominating in the skin [4]. Thus, in the skin, 7DHC is not only the primary substrate for DHCR7 but also for vitamin D3 formation where it is converted to pre-vitamin D3 by UVB radiation, with subsequent thermal isomerisation to vitamin D3 [5]. DHCR7 in the skin therefore appears to be an important control point for vitamin D3 formation, regulating the availability of 7DHC for its photoconversion [6,7]. This is supported by several studies on SNPs in the 7DHCR gene which are associated with decreased levels of 25-hydroxyvitamin D3 (25(OH)D3) [6,8,9].
Homozygous or compound heterozygous mutations in the 7DHCR gene give rise to Smith-Lemi-Opitz Syndrome (SLOS), characterized by growth retardation, intellectual disability and congenital abnormalities [6,10]. Patients with this syndrome display low cholesterol levels and elevated 7DHC [11]. The low cholesterol in utero appears to be the prime cause of the developmental abnormalities but a role for 7DHC and its metabolites is also suspected [11,12]. While patients with SLOS might be predicted to have elevated 25(OH)D3 levels, this was not observed in the one study likely due to sun avoidance, because of the skin sensitivity to ultraviolet light associated with this syndrome [13].
DHCR7, comprising 475 amino acids, is embedded in the endoplasmic reticulum and uses NADPH as a cofactor [1,6]. Its crystal structure has not been determined but a homology model has been built based on the crystal structure MaSR1 [14], a sterol reductase form Methylomicrobium alcaliphilum with which it shares 37 % identity [6]. It is predicted to have 10 transmembrane domains, an NADPH binding site in the catalytic region and a sterol sensing domain [6]. The activity of DHCR7 in microsomes is commonly measured using the non-natural substrate, ergosterol, a Δ7 sterol from fungi which the reductase converts to brassicasterol [15,16]. Neither ergosterol nor brassicasterol are found in liver microsomes and endogenous cholesterol does not interfere with their measurement by chromatography. Sheffer et al. examined the substrate specificity of DHCR7 in rat liver microsomes using lathosterol, and non-endogenous sterols including ergosterol, 7-dehydrositosterol and 7-dehydroepicholesterol (3α OH group), as substrates and concluded that the presence of a C5–C6 double bond is required for the enzymatic reduction of the C7–C8 double bond [16].
A number of enzymatic and photochemical products of 7DHC metabolism have been characterized to date, summarised in Fig. 1. 7DHC can be converted to 8-dehydrocholesteol (8DHC) in a reversible reaction catalysed by 3β-hydroxysteroid-Δ8, Δ7-isomerase [17,18]. 7DHC is metabolized to 25(OH)7DHC and 27(OH)7DHC by CYP27A1 [19,20] and to 24- and 25-(OH)7DHC by CYP46A1 [20]. CYP11A1 removes the side chain from 7DHC producing 7-dehydropregnenolone (7DHP) via 22(OH)7DHC and 20,22(OH)27DHC intermediates [21–23]. All these sterols retain the delta 7-double bond. 7DHP can in turn be converted to some steroids which retain the Δ7 double bond [21,24–28]. UVB irradiation of 7DHC produces previtamin D3 from breakage of the sterol B-ring between carbons 9 and 10 (Fig. 1). This then undergoes spontaneous thermal isomerization to vitamin D3 [29]. Previtamin D3 can also undergo a further photochemical reaction producing lumisterol3 (L3) [30,31]. This involves resealing the B-ring, but in a 9β,10α orientation rather than the 9α,10β configuration seen in 7DHC, making lumisterol a stereoisomer of 7DHC. L3 can be converted to various hydroxy-derivatives by the actions of CYP11A1 and CYP27A1 (Fig. 1) [32–34]. The aim of the current study was to examine whether a range of these metabolites of 7DHC could act as either substrates or inhibitors of DHCR7 in rat liver microsomes.
Fig. 1.
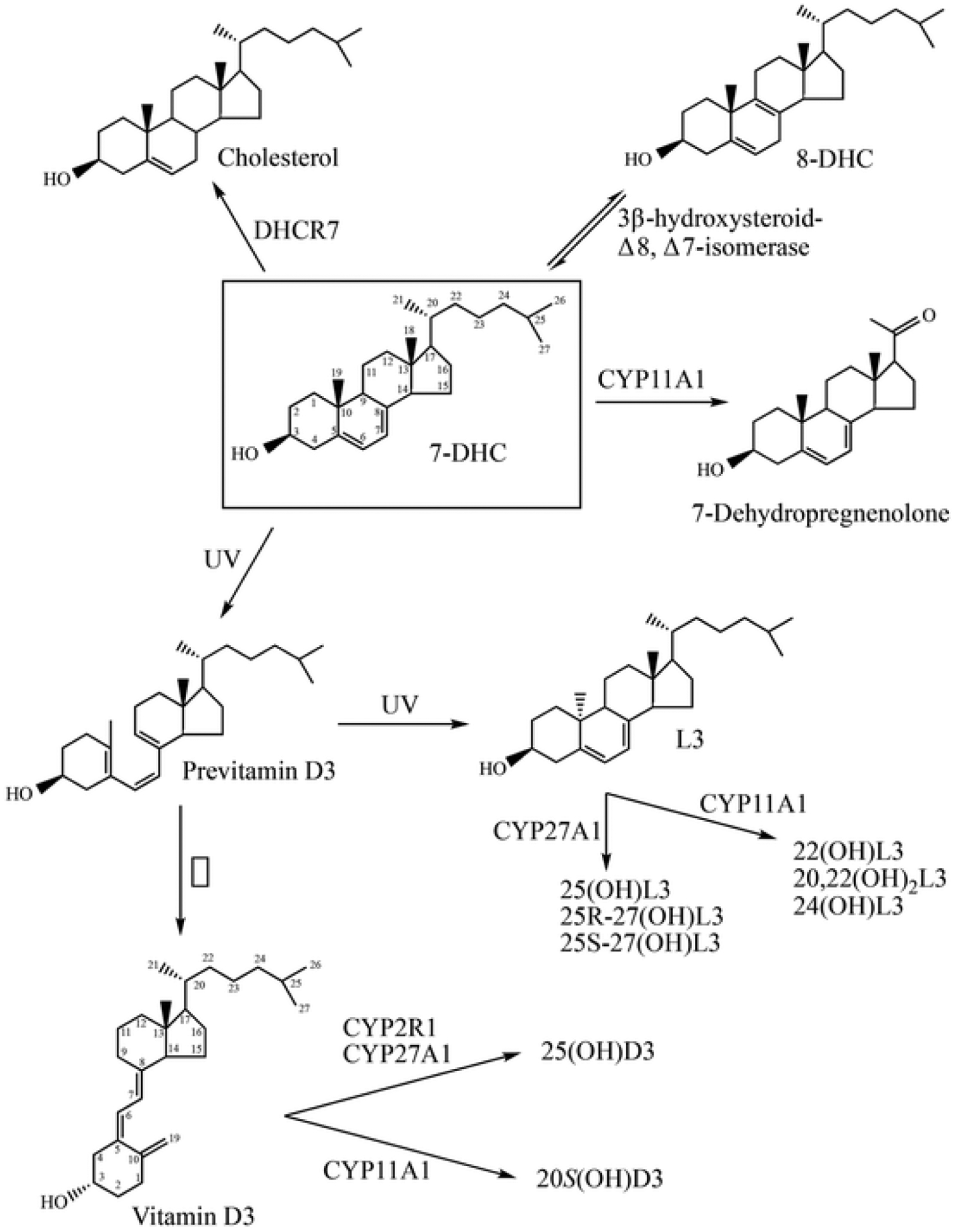
Pathways for the biosynthesis of the major metabolites of 7DHC used on this study. The 7DHC structure is boxed. See text for references.
2. Materials and methods
2.1. Materials
Glucose 6-phosphate and glucose-6-phosphate dehydrogenase (from Leuconostoc mesenteroides), NADPH, ergosterol, vitamin D3 and 7-dehydrocholesterol (7DHC) were from Sigma-Aldrich (Sydney, Australia). 25(OH)D3 was from Carbosynth Ltd (Compton, UK); lumisterol (L3) and 8-dehydrocholesteol (8DHC) was from Toronto Research Chemicals (Toronto, Canada), and 7-dehydodesmosterol (7DHD) was from Kerafast Inc (Boston, MA). 20S(OH)D3 was produced enzymatically from the action of bovine CYP11A1 on vitamin D3 as described before [29]. 27(OH)7DHC and 25(OH)7DHC were produced enzymatically from the action of human CYP27A1 on 7DHC [19] while 7-dehydropregnenolone (7DHP) was produced by the action of human CYP11A1 on 7DHC [21,22]. 20S(OH)L3, 22(OH)L3, 24(OH)L3 and 20,22(OH)2L3 were produced from the metabolism of lumisterol by bovine CYP11A1 [34] while 25(OH)L3, (25R)-27(OH)L3 and (25S)-27 (OH)L3 were produced from the action of human CYP27A1 on lumisterol [33]. 20S(OH)-7DHC was produced chemically [35]. Ergosterol and 7DHD were purified prior to use by HPLC using a C18 Alltima column (250 × 4.6 mm, 5 μm) (Grace, Hichrom, Berkshire, UK) with a 15 min 64–100 % methanol gradient followed by 55 min with 100 % methanol, at a flow rate of 0.5 mL/min.
2.2. Preparation of rat liver microsomes
Livers from Wister rats (12 weeks old) were obtained through the UWA Program for use of Unwanted Tissues from Other Studies. Finely cut tissue fragments were homogenised using a Potter-Elvehjem homogeniser in 5 vol 0.25 M sucrose and microsomes prepared by differential centrifugation as described before [36,37].
2.3. Incubations of microsomes to measure DHCR7 activity
The activity of DHCR7 in rat liver microsomes was measured from the conversion of ergosterol to brassicasterol, measured at 205 nm by reverse-phase HPLC. The incubation procedure was based on that described by Shefer et al. [16]. Ergosterol (or other potential substrates) was initially dissolved in 45 % (by wt) 2-hydroxypropyl-β-cyclodextrin at final concentration of 600 μM. Liver microsomes (0.5 mg) were incubated with 30 μM ergosterol and 1.0 mM NADPH in 100 mM potassium phosphate buffer (pH 7.5) in a final volume of 0.5 mL for 1 h at 37 °C. Reactions were stopped by adding 0.5 mL 20 % (w/v) KOH in 50 % methanol, then stigmasterol (20 nmol) was added as an internal standard. Samples were saponified by incubating samples at 37 °C for 1 h. Sterols were then extracted 3 times with 2 mL of hexane, extracts combined and dried under a stream of nitrogen at room temperature. Samples were made up in 0.35 mL 90 % methanol in water for HPLC analysis.
To screen for other possible substrates or inhibitors of DHCR7, competition experiments were carried out using ergosterol as substrate, as above, but with a competing sterol or secosteroid (30 μM) also present. Competing sterols or secosteroids were initially made up as 600 μM stocks in 45 % 2-hydroxypropyl-β-cyclodextrin. The final 2-hydroxypropyl-β-cyclodextrin concentration was kept at 3.27 % for all experiments.
2.4. HPLC analysis of incubation products
Samples were analysed on a Perkin-Elmer HPLC system (PE LC-250B Pump with Series 200 Autosampler) with a UV detector set at 205, equipped with a C18 Alltima column (250 × 4.6 mm, 5 μm) (Grace, Hichrom, Berkshire, UK). Metabolites were separated using a 15 min 64–100 % methanol gradient, followed by 65 min with 100 % methanol at a flow rate of 0.5 mL/min. The injection volume was set to 0.2 mL.
3. Results
3.1. Measuring DHCR7 activity with ergosterol as substrate
Ergosterol, only available from the diet, is a well characterized substrate for DHCR7 and was used in the current study. Both itself and its product (brassicasterol) are not detectable in rat liver microsomes making it more suitable than using 7DHC as substrate [15,16]. This is because cholesterol, which is the product of the reductase acting on 7DHC, is abundant in microsomes (Fig. 2) making it difficult to measure low rates of 7DHC metabolism. The metabolism of ergosterol by rat liver microsomes was measured by HPLC at 205 nm. Chromatograms of the extracted sterols displayed a curved baseline at 205 nm for the first 15 min due to the methanol gradient used, but the baseline was flat during the times that sterol substrates and products eluted facilitating peak area measurement (Fig. 2). Almost complete conversion of ergosterol, added dissolved in 2-hydroxypropyl-β-cyclodextrin, to brassicasterol was observed in the test incubation, with no conversion in the control lacking NADPH. 7DHC, the natural substrate for the enzyme, at the same concentration as ergosterol (both 30 μM) was able to inhibit the conversion of ergosterol to brassicasterol by 93 %, confirming that the ergosterol and 7DHC compete for the active site of the reductase, with 7DHC having the higher affinity (Fig. 2; Table 1).
Fig. 2.
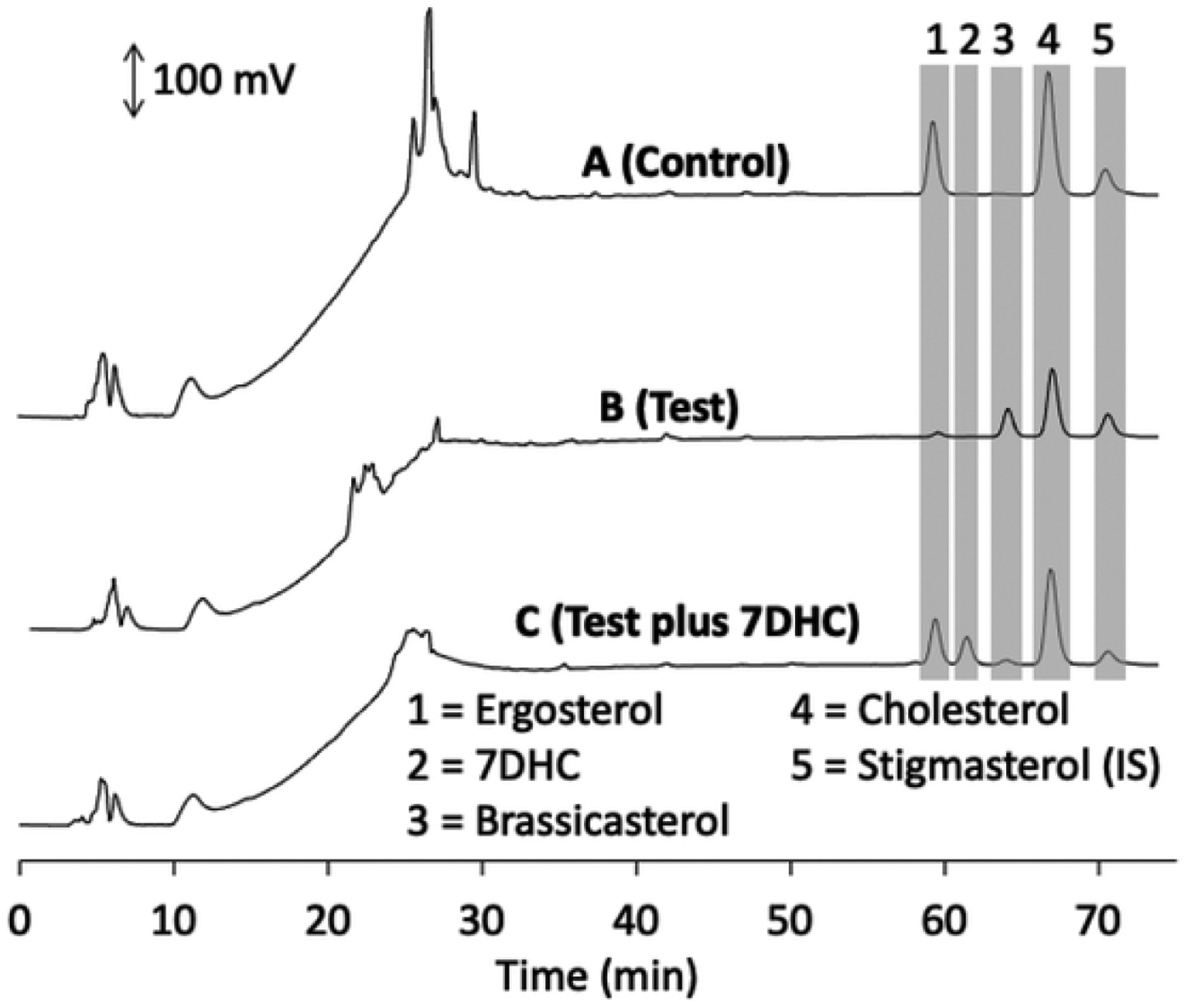
HPLC analysis of microsomal DHCR7 activity towards ergosterol in the absence and presence of 7DHC. Rat microsomes were incubated with 30 μM substrates for 1 h at 37 °C, saponified and extracted 3 times with hexane. Stigmasterol was added prior to saponification as an internal standard. Samples were analysed by reverse phase HPLC at 205 nm on a C18 Alltima column using a methanol-water solvent system as described in the Methods. (A), Incubation with ergosterol in the absence of NADPH (negative control); (B), test incubation with ergosterol; (C), test incubation with ergosterol in the presence of 30 μM 7DHC. Relevant peaks are highlighted with grey bars and numbered for identification from standards run separately.
Table 1. Competition of sterols and secosteroids for reduction of ergosterol by DHCR7.
The reduction of ergosterol to brassicasterol was measured with both ergosterol and the competing substrate at a final concentration of 30 μM. The amount of brassicasterol produced in a 1 h incubation was measured by HPLC as in Fig. 1. Data are mean ± SE of three replicates. Statistical significance for the difference in activity for each sterol tested to the normalized control (ergosterol only) for each experiment was evaluated by the Student’s t-test. ns, not significant p > 0.05. Data were obtained from 7 separate experiments as indicated by the different SE for the normalised controls. The stereochemistry of the competing substrates is provided, where known.
| Competing Substrate | Normalised activity Control (% control) | Normalised Activity Test (% control) | p-value | |
|---|---|---|---|---|
| 7DHC-like | 7DHC | 100 ± 15.7 | 6.6 ± 2.6a | p < 0.01 |
| 7DMD | 100 ± 15.7 | 42.6 ± 9.2a | p < 0.05 | |
| 20S(OH)7DHC | 100 ± 4.2 | 5.84 ± 2.36 | p < 0.001 | |
| 27(OH)7DHC | 100 ± 1.0 | 75.4 ± 2.8 | p < 0.001 | |
| 25(OH)7DHC | 100 ± 1.5 | 109.3 ± 7.5 | ns | |
| 7DHP | 100 ± 5.8 | 105.7 ± 7.3 | ns | |
| 8DHC | 100 ± 6.3 | 66.0 ± 7.2 | p < 0.05 | |
| Secosteroids | vitamin D3 | 100 ± 5.8 | 92.3 ± 9.2 | ns |
| 20S(OH)D3 | 100 ± 6.3 | 96.5 ± 3.4 | ns | |
| 25(OH)D3 | 100 ± 6.3 | 92.3 ± 3.8 | ns | |
| Lumisterols | L3 | 100 ± 5.8 | 106.8 ± 9.8 | ns |
| 20S(OH)L3 | 100 ± 4.5 | 33.8 ± 15.6 | p < 0.05 | |
| 22(OH)L3 | 100 ± 1.0 | 88.0 ± 5.0 | p < 0.05 | |
| 20,22(OH)2L3 | 100 ± 5.8 | 102.6 ± 8.5 | ns | |
| 24(OH)L3 | 100 ± 4.2 | 100.4 ± 4.5 | ns | |
| 25(OH)L3 | 100 ± 5.8 | 93.8 ± 9.2 | ns | |
| 25R27(OH)L3 | 100 ± 5.8 | 84.5 ± 10.2 | ns | |
| 25S27(OH)L3 | 100 ± 5.8 | 92.0 ± 8.8 | ns | |
Significantly different from each other, p < 0.001.
Using ergosterol as substrate, product formation was linear with time at least up to 70 min and linear with microsomal protein concentration up to 1.5 mg/mL under the conditions of our assay (Fig. 3). The Km for NADPH measured at an ergosterol concentration of 30 μM was 0.28 mM. We routinely assayed DHCR activity using a 1.0 h incubation with 1.0 mg/mL microsomal protein and a NADPH concentration of 1.0 mM (see Methods).
Fig. 3.
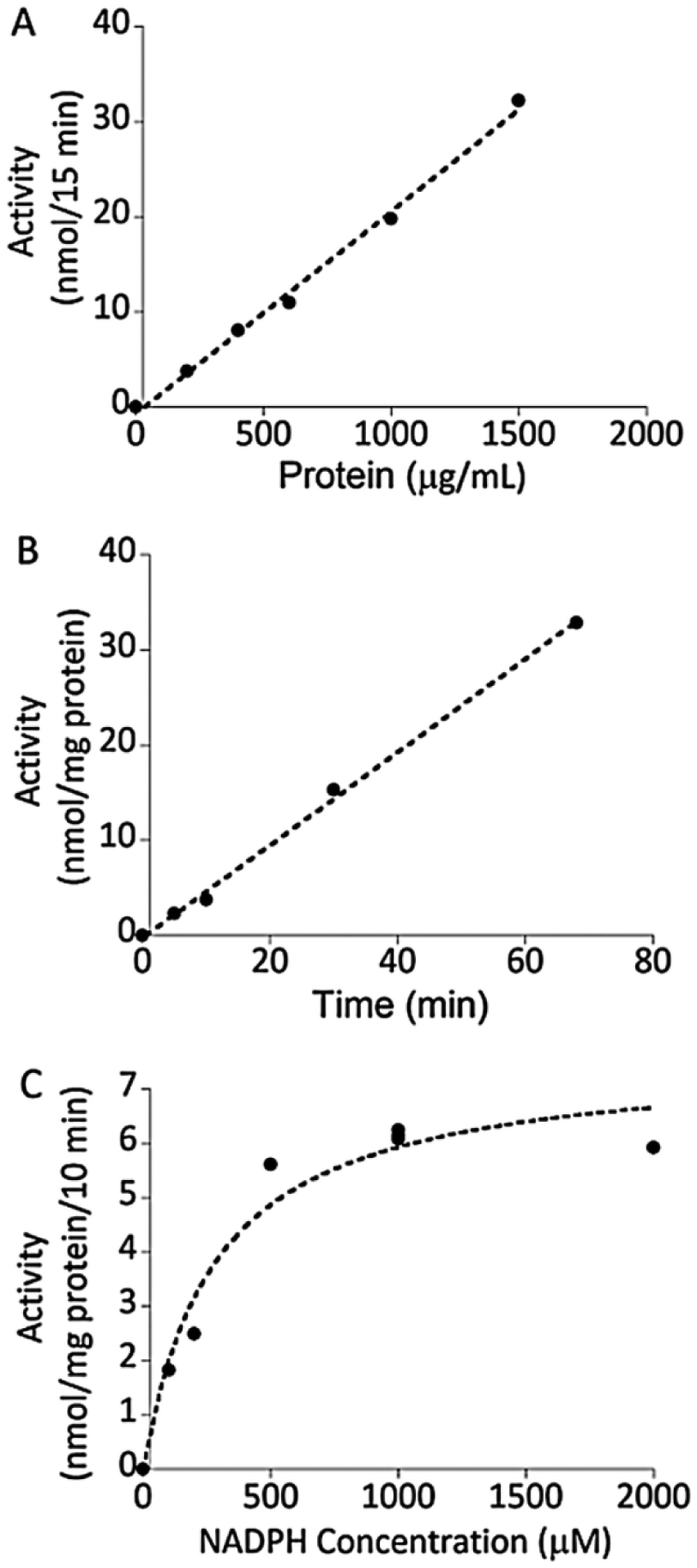
Characteristics of the assay of DHCR7 in microsomes using ergosterol as substrate. (A), Time dependence of DHCR7 activity measured with 30 μM ergosterol and a microsomal protein concentration of 1.0 mg/mL, as in Fig. 2. (B), Linearity of DHCR7 activity with microsomal protein concentration measured with 30 μM ergosterol and an incubation time of 15 min. (C) Km for NADPH measured with 30 μM ergosterol, 0.5 mg/mL microsomal protein and an incubation time of 10 min.
3.2. Competition of sterols and secosteroids with ergosterol
Competition experiments using ergosterol as substrate, similar to that described in Fig. 2 for ergosterol and 7DHC, were carried out with a range of different sterols and secosteroids to screen for other possible substrates or inhibitors of DHCR7 (Table 1). All these sterols and secosteroids are produced endogenously in humans or are predicted to be present based on known enzymatic reactions (see Introduction). 7DHD is the natural substrate for DHCR7 in the Bloch pathway (where C7–C8 double bond reduction precedes reduction of the C24–C25 double bond) where it is converted to desmosterol. 7DHD inhibited ergosterol reduction by 57 % (Table 1). This was significantly less inhibition than was observed for 7DHC (93 %) in the same experiment (Table 1). Incubation of 7DHD alone with the microsomes resulted in substantial conversion to desmosterol from the action of DHCR7 with no 7DHC, the product of DHCR24 acting on 7DHD, being detected (Fig. 4).
Fig. 4.
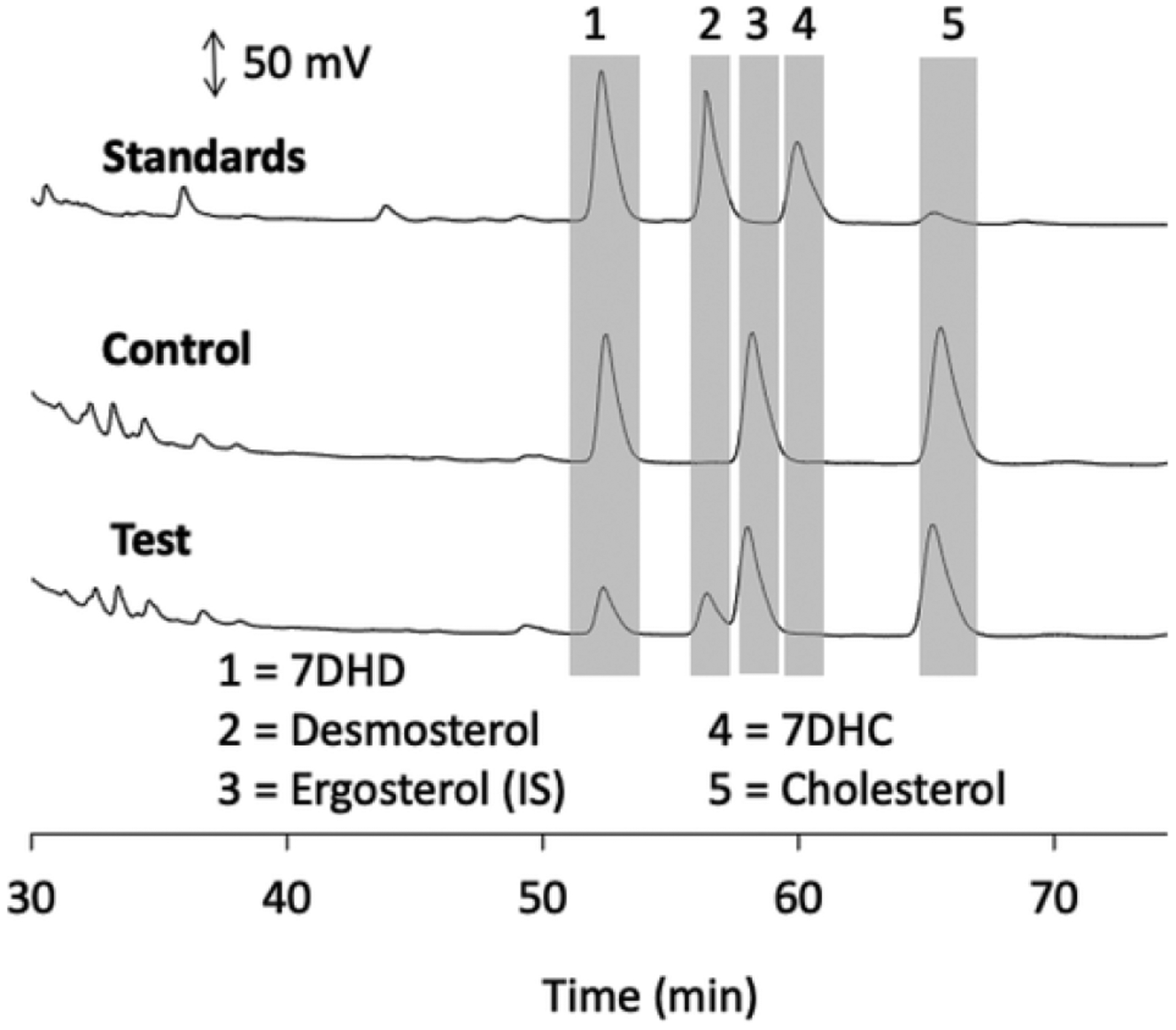
Metabolism of 7DMD by rat liver microsomes. Microsomes were incubated with 30 μM 7DMD for 20 min at 37 °C and products analysed by HPLC as in Fig. 2. In this experiment however, ergosterol (15 nmol) was added as an internal standard at the end of the incubation, prior to extraction. (A), Chromatograms of standards, (B), control reaction lacking NADPH and (C), test reaction with NADPH. Chromatogram traces up to 30 min were similar to those in Fig. 2 and are not shown as no reactants nor products eluted during this period.
3.3. Interaction of 7DHC-like metabolites with DHCR7
Analysis of hydroxy-derivatives of 7DHC revealed that 20S(OH) 7DHC inhibited ergosterol metabolism to a similar extent to 7DHC. In contrast, 27(OH)7DHC significantly inhibited brassicasterol formation by 25 %, much less than that observed with 7DHC, while no significant inhibition was observed for 25(OH)7DHC. Similarly, 7DHP which has the same B-ring configuration as 7DHC but lacks 6-carbons of the side chain, did not significantly inhibit brassicasterol formation.
To confirm that 20S(OH)7DHC and 27(OH)7DHC could serve as substrates for DHCR7, these were incubated with the enzyme in rat liver microsomes in the absence of ergosterol (Fig. 5). 20S(OH)7DHC was converted to the expected 20S-hydroxycholesterol, based on an identical HPLC retention time to authentic standard, by rat liver microsomes in the presence but not the absence of NADPH (Fig. 4A). 20S (OH)-7DHC was converted to the expected 20S-hydroxycholesterol based on its identical HPLC retention time to authentic standard, in the presence but not the absence of NADPH (Fig. 5A). Similarly, 27(OH) 7DHC was converted to the expected 27-hydroxycholesterol product by the microsomes in the presence but not the absence of NADPH (Fig. 5B). A small amount of 27(OH)7DHC product was also seen in competition with ergosterol (not shown), consistent with the small but significant inhibition of ergosterol reduction seen with this sterol (Table 1). Thus, 27(OH)7DHC is a substrate for DHCR7, but the reduction rate is lower than for ergosterol. 7DHP, missing six carbons from its side chain compared to 7DHC, was not converted to pregnenolone by DHCR7 (Fig. 6A), consistent with its lack of inhibition of ergosterol reduction (Table 1).
Fig. 5.
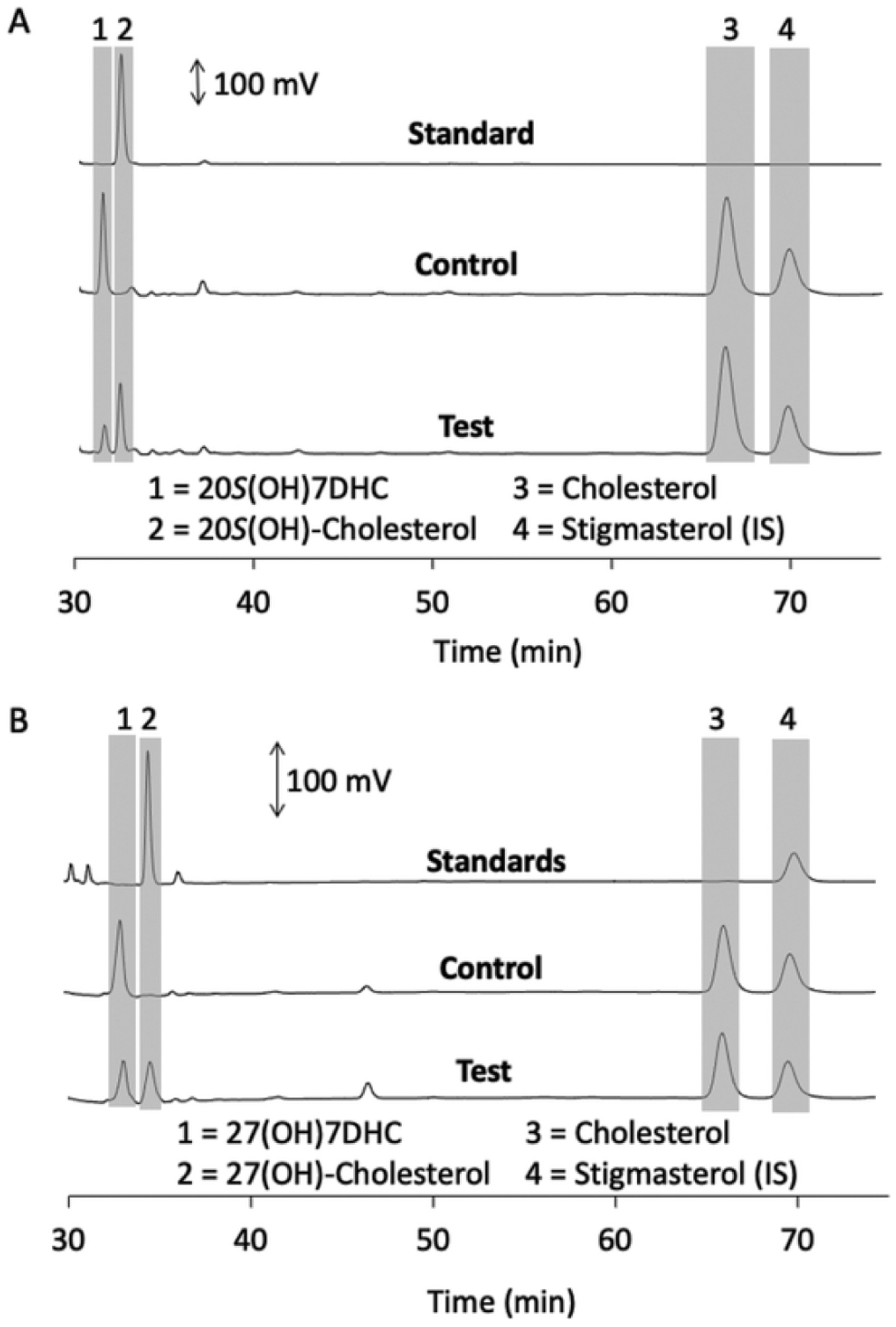
Metabolism of 20S(OH)7DHC and 27(OH)7DHC by DHCR7 in microsomes.
Microsomes were incubated with 60 μM 20S(OH)7DHC (A) or 27(OH)7DHC (B) for 1 h at 37 °C and products analysed by HPLC as in Fig. 2. Each panel shows chromatograms of standard(s), control reaction lacking NADPH and the test reaction with NADPH. Chromatogram traces up to 30 min were similar to those in Fig. 2 and are not shown as no reactants nor products eluted during this period.
Fig. 6.
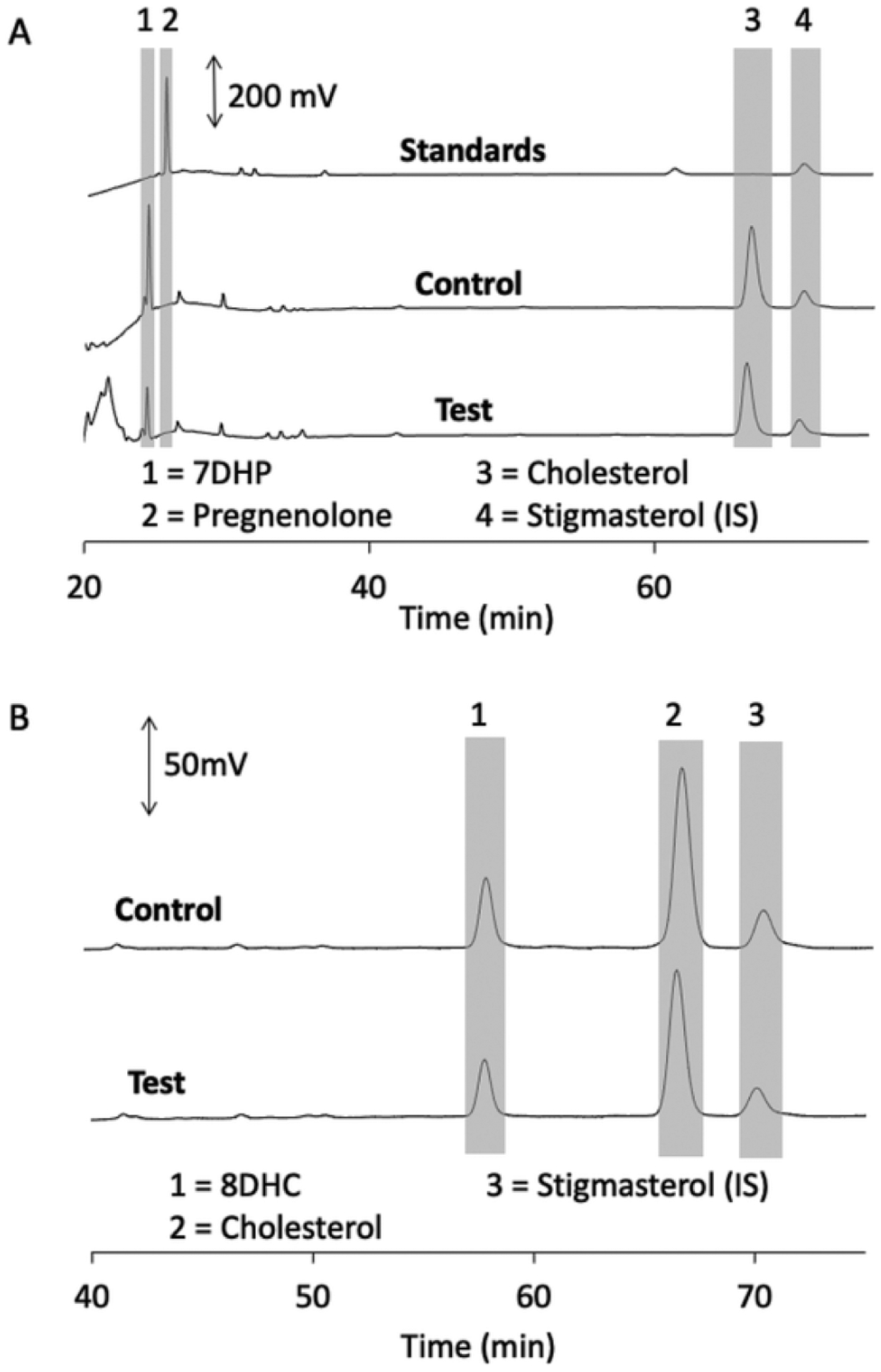
7DHP and 8DHC are not metabolised by DHCR7 in microsomes. Microsomes were incubated with 60 μM 7-dehydropregnenolone (A) or 8DHC (B) for 1 h at 37 °C and products analysed by HPLC as in Fig. 2. Each panel shows chromatograms of standard(s), control reaction lacking NADPH and the test reaction with NADPH.
The reversible isomerization of 7DHC to 8DHC is catalysed by 3β-hydroxysteroid-Δ8, Δ7-isomerase [17,18]. 8DHC, like 7DHC, accumulates in SLOS patients [6,11]. 8DHC decreased ergosterol reduction by DHCR7 by 34 % (Table 1). However, no product was detected by HPLC when it was tested alone, suggesting that it is a competitive inhibitor of DHCT7 but not a substrate, consistent with its lack of a double bond at C7–C8 for reduction (Fig. 6B).
3.4. Interaction of vitamin D3, lumisterol and their hydroxyderivatives with DHCR7
Vitamin D3 which has a broken B-ring did not significantly compete with ergosterol for binding to the reductase, nor did its hydroxyderivatives, 20S(OH)D3 or 25(OH)D3 (Table 1). L3 is a stereoisomer of 7DHC and is formed from pre-vitamin D3 by the UV-driven resealing of the B-ring (Fig. 1). It has the C5–C6 and C7–C8 double bonds present as in 7DHC but the opposite configuration (9β,10α orientation) for the reformed C9–C10 bond (Fig. 1). L3 did not significantly compete with ergosterol in the DHCR7 assay, nor did 20,22(OH)2L3, 24(OH)L3, 25(OH) L3 or the two isomers of 27(OH)L3. However, both 22(OH)L3 and 20S (OH)L3 significantly inhibited ergosterol reduction, by 22 % and 64 % respectively.
To confirm that L3 is not a substrate for DHCR7, it was incubated with rat liver microsomes in the absence of ergosterol. No product was detected in the resulting chromatogram (Fig. 7A). No standard was available for the predicted product, 7-8-dihydrolumisterol, but based on the change in retention times for the other similar Δ7 sterols upon reduction of the C7–C8 double bond, it would be expected to have a slightly longer retention time (2–4 min) than lumisterol. In a similar experiment testing 20S(OH)L3 as a substrate, no product was observed from this sterol based on the absence of any peak with a retention time 1–2 min longer than the 20S(OH)L3 that was not present in the control (Fig. 7B). Thus, we conclude that lumisterol is not a competitive inhibitor of ergosterol metabolism by DHCR7 nor can it act as a substrate for the enzyme, while 20S(OH)L3 is an inhibitor of DHCR7 but cannot serve as a substrate.
Fig. 7.
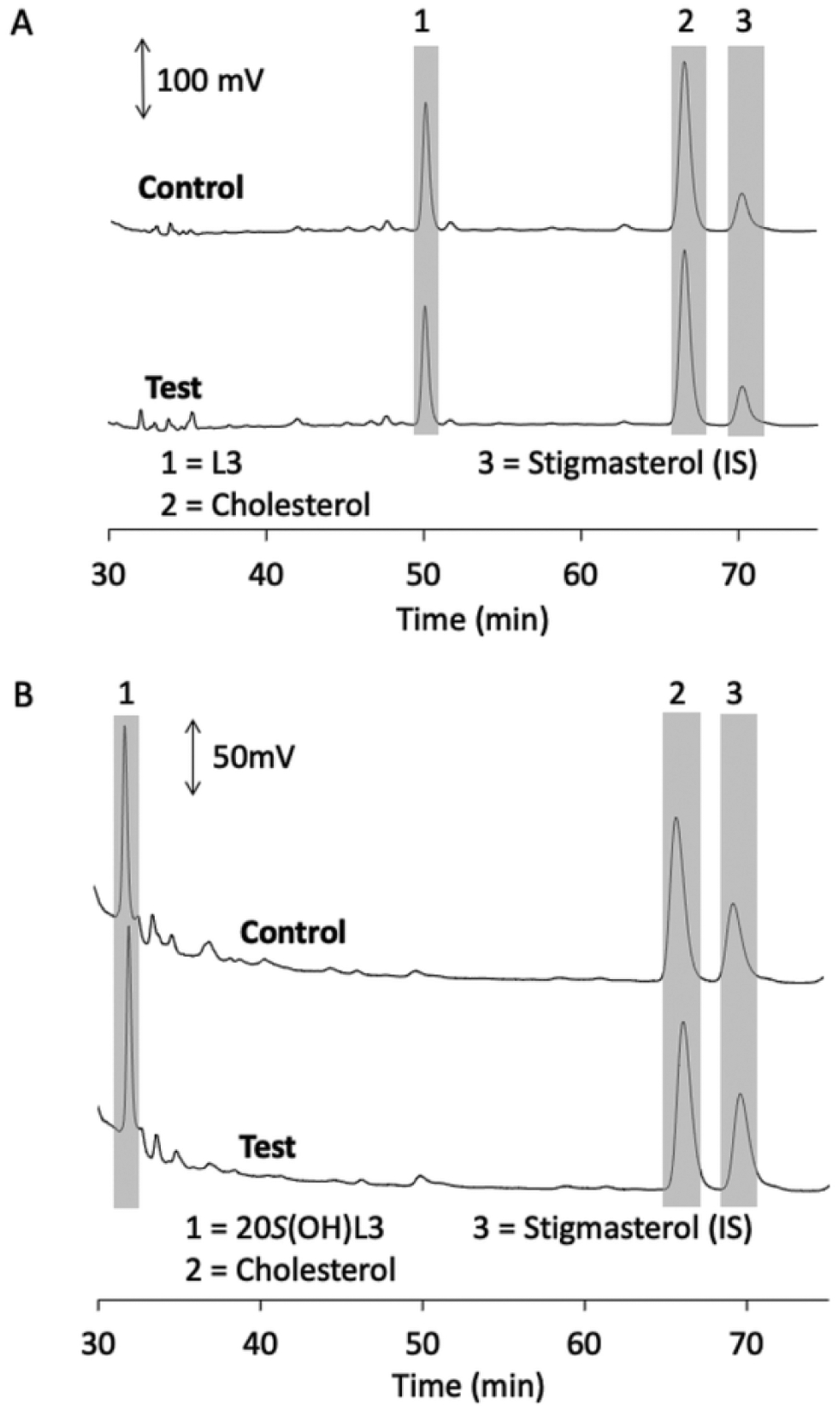
Lumisterol (L3) and 20S(OH)L3 are not metabolised by DHCR7 in microsomes. Microsomes were incubated with 60 μM L3 (A) or 30 μM 20S(OH) L3 for 1 h at 37 °C and products analysed by HPLC as in Fig. 2. Each panel shows chromatograms of the control reaction lacking NADPH and the test reaction with NADPH. Relevant peaks are highlighted with grey bars and numbered for identification from standards run separately.
4. Discussion
To date there have been limited studies on the range of sterols that can be reduced by DHCR7 and hence its substrate specificity. What has been reported is that DHCR7 requires a sterol with a double bond between C5 and C6 for it to be able to do the reduction at C7 and C8, converting the double bond in this position to a single bond [16]. Given that there are many natural metabolites of 7DHC (Fig. 1), particularly in skin cells [28,32], we were interested in whether any of these could act as substrates or inhibitors of DHCR7.
Modification of the side chain of 7DHC dramatically influenced the ability of DHCR7 to interact with the sterol, in a manner dependent on the site of modification. The presence of a C24–C25 double bond, as seen for 7DHD, reduced the ability of the sterol to compete with ergosterol compared to that seen for 7DHC. This indicates that 7DHC is the tighter binding substrate for the reductase, however relative maximum velocities for the enzyme acting on 7DHC and 7DHD remain to be established. Rat liver microsomes converted 7DHD to the expected product of DHCR7 action, desmosterol, with no detectable conversion to 7DHC suggesting low DHCR24 activity in the microsomes. DHCR7 and DHCR24 have been reported to physically interact in the endoplasmic reticulum [38].
The presence of the side chain of 7DHC is necessary for DHCR7 activity as cleavage of 6 carbons catalysed by CYP11A1, producing 7DHP, removed its ability to compete with ergosterol for reduction or to be acted on as a substrate. Hydroxylation of the side chain at C27 or C25 by CYP27A1 dramatically reduced or removed its ability to compete with ergosterol, but some product (27(OH)cholesterol) was observed for 27(OH)7DHC. The active site of DHCR7 can readily accommodate a hydroxyl group at C20, with 20S(OH)7DHC competing with ergosterol to a similar degree as 7DHC and being converted to the expected product, 20S(OH)cholesterol. Thus, metabolism of 7DHC by CYP11A1 or other enzymes hydroxylating the side chain at C20 does not prevent metabolism by DHCR7, but removal of the side chain by CYP11A1 does. It should be noted that 20S(OH)7DHC has been detected in the human epidermis [32]. Hydroxylation near the end of the side chain by CYP27A1 [19,20] dramatically reduces the ability of the sterol to interact with DHCR7. In the case of 7DHP, it has been shown that it can be made from 7DHC by CYP11A1 in isolated mitochondria from rat skin [22] as well as in adrenal glands, human placenta and epidermal keratinocytes incubated ex vivo [21,28]. Furthermore, 7DHP has been detected in the human epidermis and serum [39]. Thus, in addition to SLOS patients, 7DHP can be produced under non-pathological conditions not only in the skin but also on the systemic level and can be hydroxylated to produce a number of unsaturated steroids so far detected in SLOS patients [12,25–27,40] and under ex-vivo physiological conditions [21,28,39], or in vitro [41]. 7DHP and some of its derivatives such as 20(OH)7DHP, 7,20(OH)27DHP have anti-proliferative activities in human keratinocytes, melanocytes and melanoma cells cultured in vitro [21,42–44]. The lack of activity of DHCR7 on7DHP, and presumably its metabolites, enables them to maintain their Δ7 configuration without conversion to the corresponding more abundant steroids lacking the C7–C8 double bond. Thus, they retain their sensitivity to UVB which can potentially transform them to their corresponding secosteroid or lumisterol derivatives which have been reported to have biological activity on normal and malignant cells [24,28,42–46].
The favourable binding of sterols possessing a hydroxyl group on the side chain close to the d-ring, to DHCR7, was also seen with 20S (OH)L3 and to a lesser degree by 22(OH)L3, which both inhibited (presumably competitively) ergosterol metabolism by the enzyme. 20S(OH) L3 did not appear to be metabolized by DHCR7, indicating that sterol substrates require the 9α,10β conformation in the B-ring, as seen in 7DHC and ergosterol, to carry out the C7–C8 reduction, with the 9β,10α conformation present in lumisterol being unsuitable, even when binding is enhanced by the presence of a 20-hydroxyl group. L3 is produced from previtamin D3 in a UVB-mediated photochemical reaction (Fig. 1) and its formation was believed to be a mechanism to prevent excessive production of vitamin D3 [30]. More recent studies indicate that it is a prohormone converted to biologically active hydroxylumisterols by CYP11A1 and CYP11A1 [32,33,47–49]. Lumisterol is present in human skin at approximately 1% of the 7DHC concentration but is present in human serum at a concentration approximately equal to 7DHC [32]. Thus, its inability to be acted on by DHCR7 should be of physiological relevance, conversion of 7DHC to lumisterol protects it against the action of DHCR7, providing increased metabolic stability. Lumisterol can then be activated either by CYP11A1 or CYP27A1 to produce biologically active metabolites regulating epidermal functions [49]. 20S(OH) L3 has also been detected in human skin and serum and displays biological activity on normal and transformed skin cells [32]. In skin, its concentration is approximately 3 % of that of 7DHC so it is unlikely to be a major inhibitor of DHCR7 in vivo. However, it remains to be established if human DHCR7 has similar substrate/inhibitor specificity to the rat enzyme.
Vitamin D3, one of the major metabolites of 7DHC, does not appear to interact with DHCR7 based on its lack of inhibition of ergosterol reduction. This is not surprising given the alteration in structure that occurs with B-ring opening and isomerisation reactions involved in vitamin D synthesis (Fig. 1). This lack of interaction was also seen for major monohydroxy-derivatives of vitamin D3, 20(OH)D3 and 25(OH)D3, so even the addition of the 20-hydroxyl group to the former did not facilitate binding to DHCR7. It has been reported that vitamin D decreases DHCR7 activity rapidly in keratinocytes, without altering the protein level [15]. Our data suggest that it is not due to competitive inhibition of the enzyme, consistent with their observation that there was no inhibition when vitamin D was added to cell lysates.
In conclusion, our identification of 8DHC and 20(OH)L3 as inhibitors of DHCR may aid in the further studies on the enzyme providing a structural basis for the development of tight binding inhibitors. Furthermore, resistance of lumisterol and 7DHP to DHCR7 enzymatic activity in cells will permit these compounds to be further metabolized to their active forms retaining the C7–C8 double bond which may confer specificity to their local and systemic actions.
Acknowledgements
This work was supported by the National Institutes of Health, United States (grant numbers 1R01AR073004-01A1, R01AR071189-01A1, R21 AI149267-01A1) and VA merit grant (no. 1I01BX004293-01A1) to A.T.S. and by the University of WA.
Abbreviations:
- 7DHC
7-dehydrocholesterol
- 8DHC
8-dehydrocholesterol
- 7DHD
7-dehydrodesmosterol
- 7DHP
7-dehydropregnenolone
- L3
lumisterol3
- SLOS
Smith-Lemi-Opitz Syndrome
- 20S(OH)D3
20S-hydroxyvitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- [1].Kandutsch AA, Enzymatic reduction of the delta7 bond of 7-dehydrocholesterol, J. Biol. Chem 237 (1962) 358–362. [PubMed] [Google Scholar]
- [2].Kandutsch AA, Russell AE, Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol, J. Biol. Chem 235 (1960) 2256–2261. [PubMed] [Google Scholar]
- [3].Bloch K, The biological synthesis of cholesterol, Science 150 (1965) 19–28. [DOI] [PubMed] [Google Scholar]
- [4].Mitsche MA, McDonald JG, Hobbs HH, Cohen JC, Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways, Elife 4 (2015) e07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr., Anderson RR, Blank IH, Parrish JA, Elias P, Photosynthesis of previtamin D3 in human skin and the physiologic consequences, Science 210 (1980) 203–205. [DOI] [PubMed] [Google Scholar]
- [6].Prabhu AV, Luu W, Li D, Sharpe LJ, Brown AJ, DHCR7: A vital enzyme switch between cholesterol and vitamin D production, Prog. Lipid Res 64 (2016) 138–151. [DOI] [PubMed] [Google Scholar]
- [7].Prabhu AV, Luu W, Sharpe LJ, Brown AJ, Cholesterol-mediated degradation of 7-Dehydrocholesterol reductase switches the balance from cholesterol to vitamin D synthesis, J. Biol. Chem 291 (2016) 8363–8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D, Genome-wide association study of circulating vitamin D levels, Hum. Mol. Genet 19 (2010) 2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD, Common genetic determinants of vitamin D insufficiency: a genome-wide association study, Lancet 376 (2010) 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nowaczyk MJ, Irons MB, Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology, Am. J. Med. Genet. C Semin. Med. Genet 160c (2012) 250–262. [DOI] [PubMed] [Google Scholar]
- [11].Tint GS, Salen G, Batta AK, Shefer S, Irons M, Elias ER, Abuelo DN, Johnson VP, Lambert M, Lutz R, et al. , Correlation of severity and outcome with plasma sterol levels in variants of the Smith-Lemli-Opitz syndrome, J. Pediatr 127 (1995) 82–87. [DOI] [PubMed] [Google Scholar]
- [12].Griffiths WJ, Abdel-Khalik J, Crick PJ, Ogundare M, Shackleton CH, Tuschl K, Kwok MK, Bigger BW, Morris AA, Honda A, Xu L, Porter NA, Bjorkhem I, Clayton PT, Wang Y, Sterols and oxysterols in plasma from Smith-Lemli-Opitz syndrome patients, J. Steroid Biochem. Mol. Biol 169 (2017) 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rossi M, Federico G, Corso G, Parenti G, Battagliese A, Frascogna AR, Della Casa R, Dello Russo A, Strisciuglio P, Saggese G, Andria G, Vitamin D status in patients affected by Smith-Lemli-Opitz syndrome, J. Inherit. Metab. Dis 28 (2005) 69–80. [DOI] [PubMed] [Google Scholar]
- [14].Li X, Roberti R, Blobel G, Structure of an integral membrane sterol reductase from Methylomicrobium alcaliphilum, Nature 517 (2015) 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zou L, Porter TD, Rapid suppression of 7-dehydrocholesterol reductase activity in keratinocytes by vitamin D, J. Steroid Biochem. Mol. Biol 148 (2015) 64–71. [DOI] [PubMed] [Google Scholar]
- [16].Shefer S, Salen G, Honda A, Batta AK, Nguyen LB, Tint GS, Ioannou YA, Desnick R, Regulation of rat hepatic 3beta-hydroxysterol delta7-reductase: substrate specificity, competitive and non-competitive inhibition, and phosphorylation/dephosphorylation, J. Lipid Res 39 (1998) 2471–2476. [PubMed] [Google Scholar]
- [17].Paik YK, Billheimer JT, Magolda RL, Gaylor JL, Microsomal enzymes of cholesterol biosynthesis from lanosterol. Solubilization and purification of steroid 8-isomerase, J. Biol. Chem 261 (1986) 6470–6477. [PubMed] [Google Scholar]
- [18].Long T, Hassan A, Thompson BM, McDonald JG, Wang J, Li X, Structural basis for human sterol isomerase in cholesterol biosynthesis and multidrug recognition, Nat. Commun 10 (2019) 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pikuleva I, Javitt NB, Novel sterols synthesized via the CYP27A1 metabolic pathway, Arch. Biochem. Biophys 420 (2003) 35–39. [DOI] [PubMed] [Google Scholar]
- [20].Ačimovič J, Goyal S, Košir R, Goličnik M, Perše M, Belič A, Urlep Ž, Guengerich FP, Rozman D, Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis, Sci. Rep 6 (2016) 28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC, Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes, Int. J. Biochem. Cell Biol 44 (2012) 2003–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC, A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin, Eur. J. Biochem. / FEBS 271 (2004) 4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW, A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1), Proc. Natl. Acad. Sci. U. S. A 100 (2003) 14754–14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC, Novel vitamin D photoproducts and their precursors in the skin, Dermatoendocrinol 5 (2013) 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD, Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome), J. Steroid Biochem. Mol. Biol 82 (2002) 225–232. [DOI] [PubMed] [Google Scholar]
- [26].Shackleton CH, Marcos J, Palomaki GE, Craig WY, Kelley RI, Kratz LE, Haddow JE, Dehydrosteroid measurements in maternal urine or serum for the prenatal diagnosis of Smith-Lemli-Opitz syndrome (SLOS), Am. J. Med. Genet. A 143A (2007) 2129–2136. [DOI] [PubMed] [Google Scholar]
- [27].Shackleton CH, Roitman E, Kelley R, Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency, Steroids 64 (1999) 481–490. [DOI] [PubMed] [Google Scholar]
- [28].Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC, Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin, PLoS One 4 (2009) e4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A, Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells, Drug Metab. Dispos 39 (2011) 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Holick MF, MacLaughlin JA, Doppelt SH, Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator, Science 211 (1981) 590–593. [DOI] [PubMed] [Google Scholar]
- [31].MacLaughlin JA, Anderson RR, Holick MF, Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin, Science 216 (1982) 1001–1003. [DOI] [PubMed] [Google Scholar]
- [32].Slominski AT, Kim TK, Hobrath JV, Janjetovic Z, Oak ASW, Postlethwaite A, Lin Z, Li W, Takeda Y, Jetten AM, Tuckey RC, Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols, Sci. Rep 7 (2017) 11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tuckey RC, Li W, Ma D, Cheng CYS, Wang KM, Kim TK, Jeayeng S, Slominski AT, CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites, J. Steroid Biochem. Mol. Biol 181 (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tuckey RC, Slominski AT, Cheng CY, Chen J, Kim TK, Xiao M, Li W, Lumisterol is metabolized by CYP11A1: discovery of a new pathway, Int. J. Biochem. Cell Biol 55 (2014) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A, Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity, Steroids 75 (2010) 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cheng CY, Slominski AT, Tuckey RC, Metabolism of 20-hydroxyvitamin D3 by mouse liver microsomes, J. Steroid Biochem. Mol. Biol 144 (Pt B) (2014) 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tuckey RC, Tang EKY, Maresse SR, Delaney DS, Catalytic properties of 25-hydroxyvitamin D3 3-epimerase in rat and human liver microsomes, Arch. Biochem. Biophys 666 (2019) 16–21. [DOI] [PubMed] [Google Scholar]
- [38].Luu W, Hart-Smith G, Sharpe LJ, Brown AJ, The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally, J. Lipid Res 56 (2015) 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY, Tuckey RC, Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland, Sci. Rep 5 (2015) 14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo LW, Wilson WK, Pang J, Shackleton CH, Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome, Steroids 68 (2003) 31–42. [DOI] [PubMed] [Google Scholar]
- [41].Sushko TA, Gilep AA, Yantsevich AV, Usanov SA, Role of microsomal steroid hydroxylases in Δ7-steroid biosynthesis, Biochemistry (Mosc.) 78 (2013) 282–289. [DOI] [PubMed] [Google Scholar]
- [42].Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF, Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity, PLoS One 5 (2010) e9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT, Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity, Steroids 76 (2011) 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT, Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells, Steroids 74 (2009) 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE, Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity, J. Invest. Dermatol 131 (2011) 1167–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Piotrowska A, Wierzbicka J, Slebioda T, Wozniak M, Tuckey RC, Slominski AT, Zmijewski MA, Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes, Steroids 110 (2016) 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chaiprasongsuk A, Janjetovic Z, Kim TK, Schwartz CJ, Tuckey RC, Tang EKY, Raman C, Panich U, Slominski AT, Hydroxylumisterols, photoproducts of previtamin D3, protect human keratinocytes against UVB-induced damage, Int. J. Mol. Sci 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chaiprasongsuk A, Janjetovic Z, Kim TK, Jarrett SG, D’Orazio JA, Holick MF, Tang EKY, Tuckey RC, Panich U, Li W, Slominski AT, Protective effects of novel derivatives of vitamin D(3) and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms, Redox Biol. 24 (2019) 101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Slominski AT, Chaiprasongsuk A, Janjetovic Z, Kim TK, Stefan J, Slominski RM, Hanumanthu VS, Raman C, Qayyum S, Song Y, Song Y, Panich U, Crossman DK, Athar M, Holick MF, Jetten AM, Zmijewski MA, Zmijewski J, Tuckey RC, Photoprotective properties of vitamin d and lumisterol hydroxyderivatives, Cell Biochem. Biophys 78 (2020) 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]


