Abstract
Background:
Exposures to per- and polyfluoroalkyl substances (PFASs) may affect metabolic outcomes, including lipid concentrations in the blood. However, few studies have evaluated potential associations between PFASs and lipids longitudinally.
Objectives:
We estimated associations between PFAS and lipid concentrations at birth and at several points in childhood.
Methods:
We measured concentrations of five major PFASs in cord serum and in serum collected at 18 months, five years and nine years in 490 children from a prospective cohort in the Faroe Islands. Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) concentrations were measured at birth, 18 months and nine years. We estimated associations between PFAS and lipid concentrations and evaluated possible effect modification by sex. We also tested whether PFAS associations with age-nine lipids varied by exposure period.
Results:
Serum PFAS concentrations at ages five and nine were positively associated with lipid concentrations at age nine. Cross-sectional associations between PFASs and lipids at age nine were the strongest, with increases in serum concentrations of perfluorodecanoic acid (PFDA), perfluorononanoic acid (PFNA) and perfluorooctanesulfonic acid (PFOS) associated with increases in TC, HDL-C and LDL-C. We found statistically significant differences in estimated PFAS effects by sex, where girls had stronger positive associations between PFASs and TC and LDL-C and boys had stronger positive associations with HDL-C. In repeated measure models, exposure period was a significant modifier of PFAS effects.
Conclusions:
Our findings suggest that childhood PFAS exposures may be associated with elevated serum lipid concentrations. This is a public health concern, as a detrimental lipid profile in childhood is a risk factor for later development of hyperlipidemia and cardiovascular disease.
Keywords: per- and polyfluoroalkyl substances, PFAS, lipids, childhood, cardiometabolic
1. INTRODUCTION
Per- and polyfluoroalkyl substances (PFASs) are a class of synthetic chemicals widely used in industry and commercial products because of their unique physical and chemical characteristics (ATSDR 2018; Lindstrom et al., 2011; Schultz et al., 2003). Their persistence and mobility in water has led to widespread environmental contamination, and they are now detectable in most human blood samples (Fromme et al., 2009; Lindstrom et al., 2011; Sunderland et al., 2019). Increasing evidence suggests that PFAS exposures are associated with metabolic health outcomes, including detrimental changes in lipid profiles and metabolic diseases such as diabetes and obesity (EFSA 2020; Sunderland et al., 2019). Of these outcomes, a positive association with lipids, including total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), has been most consistently identified in both occupational and nonoccupational cohorts (ATSDR 2018; Sunderland et al., 2019).
Prenatal and childhood exposures to PFASs could be particularly important. Early developmental stages are vulnerable to chemical disruption, and prenatal and childhood factors, including chemical exposures, have been linked to cardiovascular and metabolic disease incidence in adults (Barouki et al., 2012; La Merrill and Birnbaum, 2011; Makri et al., 2004; Mcmillen and Robinson, 2005). Early exposures to endocrine disruptors like PFASs may impact the endocrine systems involved in growth and metabolism, thereby altering metabolic homeostasis and potentially leading to obesity and metabolic syndrome later in life (Braun, 2017; La Merrill and Birnbaum, 2011).
Recent studies have evaluated associations between childhood lipid profiles and PFAS exposures during pregnancy and childhood. While associations with prenatal exposures have been inconsistent (Jensen et al., 2020; Maisonet et al., 2015; Manzano-Salgado et al., 2017; Matilla-Santander et al., 2017; Spratlen et al., 2019; Starling et al., 2014), several studies in children and young adults have found positive associations between PFASs, including PFOS and PFOA, and TC and LDL-C (Geiger et al., 2014; Jain and Ducatman, 2018; Khalil et al., 2018; Koshy et al., 2017; Zeng et al., 2015). However, these studies measured PFAS concentrations at only one point in time, and therefore could not evaluate the relative importance of different periods of exposure. In addition, these studies were cross-sectional and potentially subject to reverse causation (EFSA, 2018; EFSA, 2020). Only one existing study, conducted in the Boston-based Project Viva cohort, measured PFAS exposures both prenatally and in childhood. This study found significant cross-sectional associations between childhood PFAS concentrations and elevated TC and LDL-C in females, but not males (Mora et al., 2018). While one other study of prenatal PFAS exposures also found suggestive evidence of sex-specific effects (Jensen et al., 2020), most existing studies have not evaluated the role of sex as a potential effect-modifier of any childhood PFAS-lipid association.
The present study addresses these research gaps by estimating associations between repeated measures of prenatal and childhood PFAS exposures and lipid concentrations in a longitudinal cohort of children from the Faroe Islands. We hypothesized that exposures to PFASs would be associated with a detrimental lipid profile, including elevated TC and LDL-C. Because several studies of PFASs and adiposity have found stronger positive effects in females than in males, we also hypothesized that any observed associations would be modified by sex, with greater effects in females (Halldorsson et al., 2012; Jensen et al., 2020).
2. METHODS
2.1. Study Population
Our study was conducted in the Faroese Cohort 5, which includes 490 mother-child pairs recruited between October 2007 and April 2009 at the National Hospital in Tórshavn, Faroe Islands. Details on this cohort are included elsewhere (Eryasa et al., 2019; Grandjean et al., 2017; Weihe et al., 2016). Study participants were limited to full-term singleton children. Standard questionnaires were used to record medical history, current health, and social factors during and before pregnancy. Relevant obstetric information was abstracted from medical records, including parity and pre-pregnancy weight and height. We obtained umbilical cord blood samples immediately after birth. All cohort members were invited for follow-up at ages 18 months, five years and nine years. Each follow-up visit included a maternal interview, pediatric examination, and blood sampling. We assessed the duration of exclusive and mixed breastfeeding by questionnaire at the 18-month visit. The Harvard T.H. Chan institutional review board and Faroese ethical review committee approved the study protocol (IRB16–1225), and written informed consent was obtained from all mothers. A study selection flow chart is included in the Supplementary Material as Figure S1.
2.2. Serum PFAS concentrations
We assessed exposures to five common PFAS compounds: perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexanesulfonic acid (PFHxS), perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA). Serum was obtained from cord blood at birth and at the 18-month, five-year and nine-year clinical examinations, and was frozen shortly after separation. Samples were stored at −80°C until analyses were performed at the University of Southern Denmark, with a maximum storage time of three years. We measured PFAS concentrations using online solid-phase extraction followed by high-pressure liquid chromatography with tandem mass spectrometry (LC-MS/MS) (Haug et al., 2009). Extraction was conducted with a Thermo Scientific EQuan MAX system (Thermo Scientific, San Jose CA). The LC-MS/MS system consisted of a Dionex 3000 liquid chromatographic system coupled to a Thermo Quantiva Triple Quadrupole tandem mass spectrometer with heated electrospray ionization (Thermo Scientific, San Jose CA). Each analytical series included quality control serum samples, calibration standards (e.g. NIST SRM 1957), and reagent and serum blanks. The laboratory is certified for analysis of PFASs by the Human Biomonitoring Monitoring program for the European Union (HBM4EU), and regularly participates in the German External Quality Assessment Scheme (G-EQUAS) for biological materials. Measurement imprecision was assessed by within-batch and between-batch coefficients of variation, which were <3% and 5–6% respectively. The limit of detection (LOD) was 0.03 ng/ml. All PFNA, PFOA and PFOS concentrations were above the LOD. There were two PFDA samples (0.14%) and 46 PFHxS samples (3.13%) below the LOD. These values were assumed to be 0.015 ng/ml.
2.3. Serum Lipid Measurements
We measured non-fasting TC, HDL-C, LDL-C, and TG in serum obtained from cord blood at birth and from blood samples collected at the 18-month and nine-year assessments. Serum samples were analyzed on a Cobas 8000 analyzer (Roche Diagnostics, Basel Switzerland). Our detection method was accredited under ISO 15189 and used enzymatic colorimetrical assays with absorption. In terms of imprecision, coefficients of variation were ≤ 3% for TC, ≤ 6% for HDL-C, ≤ 6% for LDL-C, and ≤ 5% for TG. Regarding accuracy, bias estimates were ≤ 6.5% for TC, ≤ 11.6% for HDL-C, ≤ 11.9% for LDL-C and ≤ 8% for TG.
2.4. Statistical Analyses
We evaluated within-visit and across-visit PFAS correlations using Spearman correlations. Differences in PFAS concentrations by population sub-groups were assessed using Wilcoxon rank sum and Kruskal-Wallis tests, and differences in lipid concentrations by sex were evaluated using Wilcoxon rank sum tests.
We estimated associations between serum PFAS concentrations and lipid concentrations using multivariable linear regression models. We modeled cross-sectional associations between PFAS and lipid concentrations at birth, 18 months and nine years as well as longitudinal associations between PFAS and subsequent lipid concentrations. PFAS concentrations were natural log-transformed to account for their right-skewed distribution and to minimize the impact of outlier values. Lipids were modeled as continuous variables, and TG concentrations were natural log-transformed to ensure normally distributed residuals. Each regression model had a single PFAS exposure variable. We report the estimated association from each model as the expected difference in lipid concentration (mmol/L) for a doubling in PFAS concentration. Associations between PFASs and TG are reported as the percent difference in TG per doubling in the PFAS concentration, due to the log-transformation of TG. We evaluated effect modification by sex in a second set of models that included an interaction term between sex and PFAS concentrations. These interaction models were used to estimate sex-specific PFAS associations and evaluate whether differences by sex were significant (p-interaction < 0.1).
Models were adjusted a priori for confounders that have been identified in prior literature as associated with PFAS concentrations in pregnancy and childhood (Bjerregaard-Olesen et al., 2016; Brantsæter et al., 2013; Harris et al., 2017; Kingsley et al., 2018; Mørck et al., 2015; Shu et al., 2018; Ye et al., 2018) and with lipid profiles (Ayer et al., 2011; Gaillard et al., 2014; Jaddoe et al., 2008; Jellinger et al., 2017; Owen et al., 2002). All models were adjusted for child sex and maternal education (low, medium, high) as a proxy for socio-economic status. Models with PFAS exposure measured at birth, 18 months, and five years were also adjusted for the following covariates related to prenatal PFAS exposures: maternal smoking during pregnancy (yes, no), maternal pre-pregnancy BMI (kg/m2), and parity (primiparous, multiparous). We did not adjust for birth-related covariates in our models for age-nine PFAS exposures, as the half-lives of most PFASs are less than nine years. Our final directed acyclic graphs (DAGs) are included in the Supplementary Material as Figures S2–S3.
As a secondary analysis, we formally tested the importance of exposure period using multiple informant models of repeated PFAS exposures and age-nine lipid outcomes. Multiple informant models were originally developed to evaluate data from different sources that relate to the same underlying construct, but have been adapted to the setting of repeated exposure measures for the same individual (Horton et al., 1999; Sánchez et al., 2011). For each PFAS-lipid combination, we used generalized estimating equations (GEEs) with a working independence correlation matrix to jointly estimate the PFAS-lipid association in the four repeated PFAS exposure periods. The GEE models were adjusted for child sex, maternal education, maternal smoking during pregnancy, maternal pre-pregnancy BMI, and parity. We tested whether associations differed by exposure period by including an interaction term between PFAS concentration and an indicator variable of child age. We compared this interaction model to the nested equivalent model without an interaction term using a multivariate Wald test, and considered exposure period to be significant for p-values <0.1 (Halekoh et al., 2006). Finally, we allowed estimated effects to also vary by sex by including a three-way interaction between PFAS concentration, exposure period and sex.
We conducted several sensitivity analyses to assess the robustness of our results. Several dietary sources of PFASs, including pilot whale consumption and breastfeeding, may also impact serum-lipid concentrations. We conducted a sensitivity analysis where we adjusted for the following PFAS sources: maternal whale consumption during pregnancy (yes, no; included in models with PFAS concentrations measured at birth, 18 months and five years), duration of exclusive breastfeeding (months; included in models with PFAS concentrations measured at 18 months and five years), and child whale consumption at nine years (yes, no; included in models with PFAS concentrations measured at nine years). Second, we assessed the impact of potential selection bias in our study due to attrition, as some participants may be more likely to return for follow-up visits than others. We calculated stabilized inverse probability weights (IPWs) as the inverse probability of attending each visit, given each participant’s baseline values for relevant covariates (maternal age, maternal smoking and alcohol consumption during pregnancy, maternal pre-pregnancy BMI, maternal education, parity, and child sex) and used these weights in our linear models. We also evaluated our assumption of a linear association between PFAS concentrations and lipids using generalized additive models (GAMs) with penalized thin-plate splines for each PFAS term to allow for nonlinear associations, and compared the fit of the nonlinear models to their linear counterparts using Akaike Information Criterion (AIC) values (Wood, 2004). Finally, while each model covariate had less than 3% of missing data (Table S1), we used multiple imputation by chained equations (MICE) to impute missing covariate values and re-ran our primary linear models using these imputed datasets (Rubin, 2004; van Buuren and Groothuis-Oudshoorn, 2011). Imputations were generated 30 times.
All statistical analyses were conducted in R software version 4.0.2 (R Core Team, 2020). DAGs were drawn using the packages “dagitty” version 0.3–0 and “ggdag” version 0.2.2 (Barrett, 2020; Textor et al., 2016). Generalized estimating equation models were run using the “geepack” package, version 1.3–2 (Halekoh et al., 2006). Non-linear models were run using the “mgcv” package, version 1.8–31 (Wood, 2019). Multiple imputation was completed using the “mice” package, version 3.11.0 (van Buuren and Groothuis-Oudshoorn, 2011).
3. RESULTS
Of the 490 original cohort members, 475 had baseline covariate data. Of these, 459 had lipid and PFAS concentrations measured in cord serum, 334 had lipids and PFASs measured at 18 months, and 366 had lipids and PFASs measured at nine years (Figure S1). Maternal education was the covariate with the most missing data (2.7%; Table S1). The distribution of outcomes and exposures were similar for individuals with missing versus complete covariate information (Figure S4 and S5). Baseline covariates and PFAS concentrations at birth were also similar for children who missed one or more subsequent visits (Figure S6 and S7).
Child and maternal characteristics for the study population are shown in Table 1. Our study population at birth had more males (240, 52.3%) than females (219, 47.7%), and most children had older siblings (324, 70.6%). The median duration of exclusive breastfeeding was five months. While study characteristics were generally similar between males and females (Table S2), a higher percentage of boys at age nine ate whale (47.6%) than girls (36.6%).
Table 1:
Characteristics of the Faroese mother-child pairs at birth (n = 459), 18 months (n = 334) and nine years (n = 366).
| Visit | Characteristic | N (%) or Median [IQR] |
|---|---|---|
|
| ||
| Birth | Sex | |
| Female | 219 (47.7) | |
| Male | 240 (52.3) | |
| Maternal education | ||
| Low | 151 (32.9) | |
| Medium | 119 (25.9) | |
| High | 189 (41.2) | |
| Maternal smoking | ||
| Yes | 70 (15.3) | |
| No | 389 (84.7) | |
| Parity | ||
| Primiparous | 135 (29.4) | |
| Multiparous | 324 (70.6) | |
| Maternal BMI | 23.66 [21.23, 25.93] | |
| Maternal whale consumption | ||
| Yes | 89 (19.7) | |
| No | 362 (80.3) | |
|
| ||
| 18 months | Breastfeed duration (months) | 5.00 [4.00, 6.00] |
|
| ||
| Nine years | Child whale consumption | |
| Yes | 154 (42.3) | |
| No | 210 (57.7) | |
PFAS concentrations were generally higher in childhood than at birth (Figure 1). While serum concentrations of PFDA, PFNA and PFHxS were highest at five years, serum concentrations of PFOA and PFOS were highest at 18 months. Distributions of PFAS concentrations were similar for males and females in our study, although the age-nine concentrations of PFOS and PFHxS were higher for males (Figure S8). PFAS concentrations at birth varied by several maternal characteristics, including parity and smoking status. Concentrations at 18 months varied by breastfeeding duration, and concentrations at nine years varied by the child’s whale consumption (Table S3). Within-visit correlations of the different PFASs were high for PFNA and PFDA (e.g., spearman correlation (rs) = 0.87 at the age-nine visit), and PFOS and PFHxS (e.g., rs = 0.77 at the age-nine visit). PFOS concentrations were strongly correlated across visits (e.g., rs = 0.82 between age-five and age-nine visits), as were PFHxS concentrations (e.g., rs = 0.75 between age-five and age-nine visits) (Figure S9).
Figure 1:
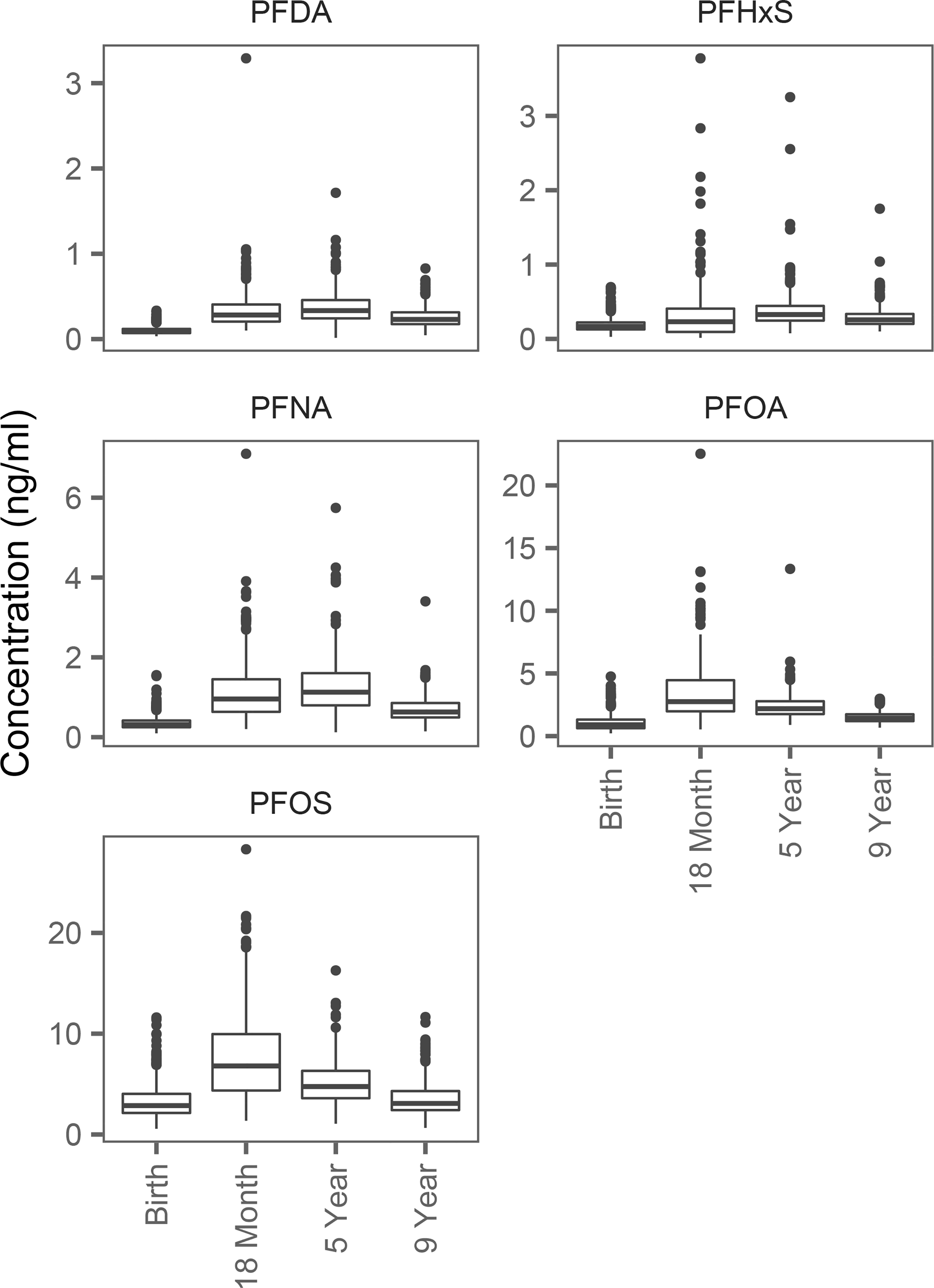
Distribution of serum PFAS concentrations by visit.
Median lipid concentrations were highest at age nine for all lipids except TG, which was highest at 18 months (Table 2). Girls had higher LDL-C and TC concentrations than boys at all visits. They also had higher TG concentrations at the age-18-month and age-nine visits (Table S4).
Table 2:
Median lipid concentrations (mmol/L) at birth, 18 months and nine years.
| Visit | Lipid | Median [IQR] |
|---|---|---|
|
| ||
| Birth | HDL-C | 0.71 [0.59, 0.89] |
| LDL-C | 0.62 [0.48, 0.81] | |
| TG | 0.43 [0.33, 0.56] | |
| TC | 1.68 [1.37, 2.00] | |
|
| ||
| 18 months | HDL-C | 0.72 [0.54, 0.86] |
| LDL-C | 1.89 [1.46, 2.36] | |
| TG | 1.09 [0.79, 1.50] | |
| TC | 3.48 [2.92, 4.11] | |
|
| ||
| 9 years | HDL-C | 1.45 [1.26, 1.65] |
| LDL-C | 2.24 [1.93, 2.65] | |
| TG | 0.88 [0.65, 1.27] | |
| TC | 4.24 [3.82, 4.70] | |
3.1. Associations between PFAS and Lipid Concentrations
We observed strong positive associations between age-nine PFAS and age-nine lipid concentrations, where increased serum concentrations of PFDA, PFNA and PFOS were associated with significant increases in TC, HDL-C and LDL-C (Figure 2 and Table S5). For example, a doubling of PFDA at age nine was associated with a 0.19 mmol/L increase in TC (95% CI: 0.07, 0.32), a 0.10 mmol/L increase in HDL-C (95% CI: 0.05, 0.15) and a 0.12 mmol/L increase in LDL-C (95% CI: 0.02, 0.22). The magnitude of association was similar across all three PFASs. We also found similar associations between PFASs measured at five years and TC measured at nine years, although some associations were only marginally significant. Earlier PFAS exposures (birth and 18 months) were not consistently associated with lipids at age nine.
Figure 2:
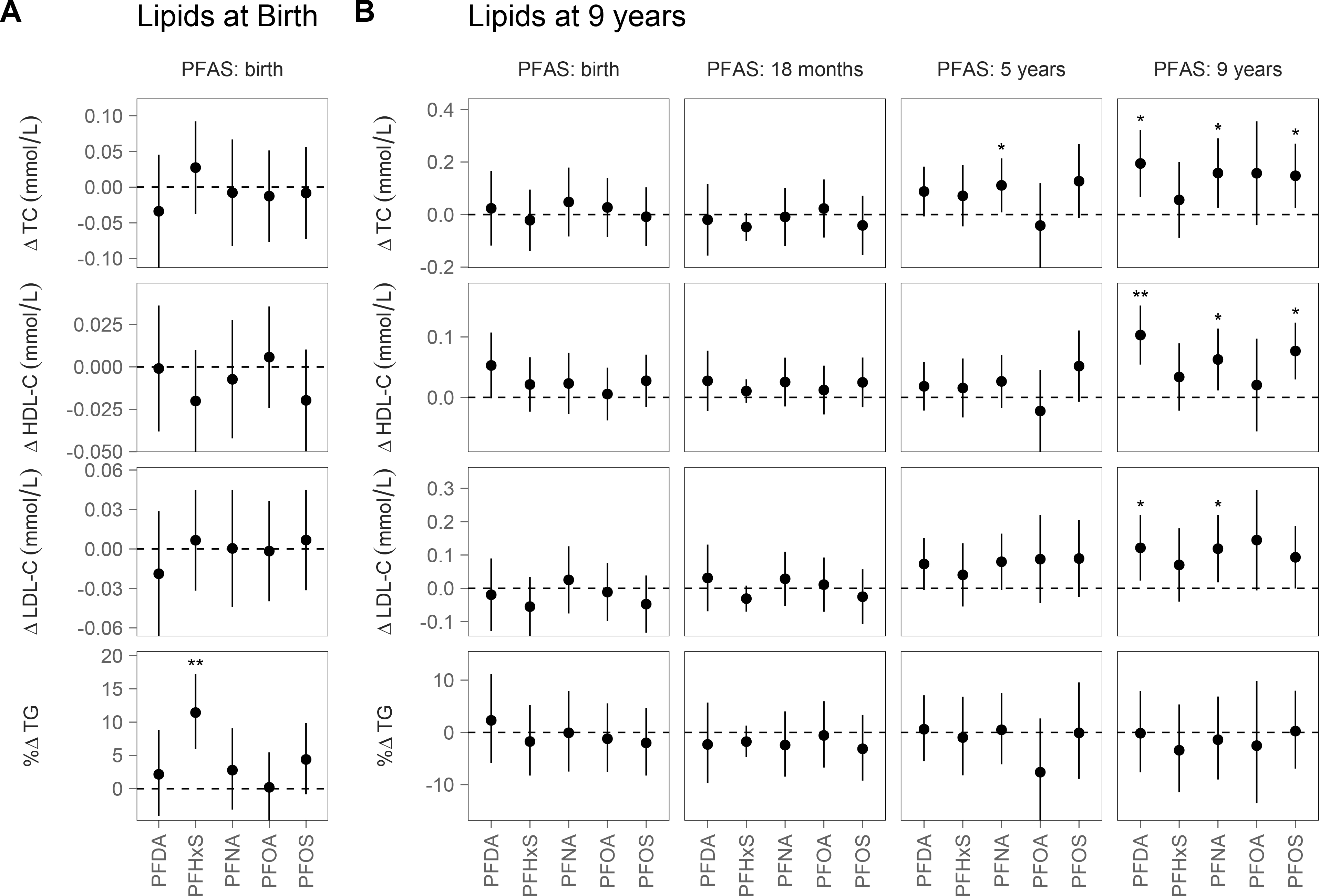
Associations between PFAS exposures and (A) lipid concentrations at birth and (B) lipid concentrations at 9 years, presented as the estimated difference in serum lipid concentrations for a doubling in serum PFAS concentrations. All models were adjusted for child sex and maternal education. Models for PFASs at birth, 18 months and 5 years were also adjusted for smoking, education, parity, and pre-pregnancy BMI.
* p-value < 0.05; ** p-value < 0.001
Associations between PFAS concentrations and lipids measured at birth and 18 months were generally null, with a few exceptions (Figure 2 and Figure S10). TG concentrations at birth were positively associated with cord-serum PFHxS, where a doubling of cord-serum PFHxS was associated with an 11.44% increase in TG (95% CI: 5.93, 17.24).
Several PFAS-lipid associations were modified by sex, with the strongest modification seen for associations between childhood PFAS concentrations (ages five and nine) and lipid concentrations at age nine (Figure 3). While associations between age-nine TC and LDL-C concentrations and both age-five and age-nine PFAS concentrations were positive for females, they were generally null for males. In contrast, associations between age-nine HDL-C concentrations and age-five and age-nine PFASs were generally positive for males, but weakly positive or null for females. For example, a doubling of age-five PFNA exposures in females was associated with a 0.22 mmol/L increase in age-nine TC (95% CI: 0.07, 0.36) and a 0.20 mmol/L increase in age-nine LDL-C (95% CI: 0.08, 0.31), but was not associated with a significant difference in TC or LDL-C in males. However, the same increase in age-five PFNA was associated with a 0.07 mmol/L increase in age-nine HDL-C in males (95% CI: 0.00, 0.13), but no difference in females. The effect modification of sex was statistically significant in each case (p-interaction: 0.04 for TC, <0.01 for LDL-C and 0.08 for HDL-C). Estimates of effect modification for all models are included in the Supplementary Material as Table S5 and Figure S11.
Figure 3:
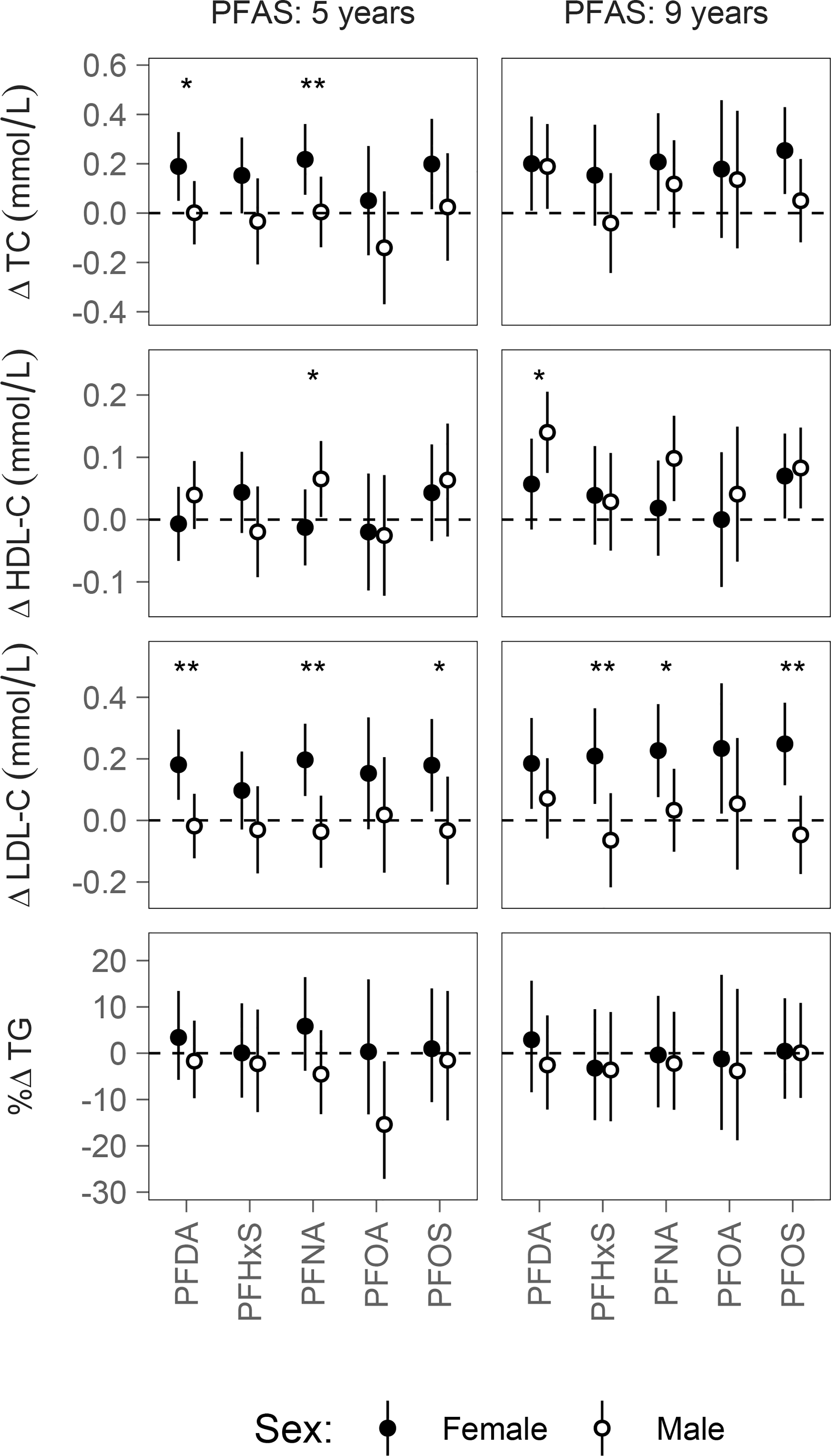
The estimated difference in age-nine lipid concentrations for a doubling in PFAS concentrations, calculated from models that include an interaction term between PFAS exposure and sex.
* p-interaction < 0.1; ** p-interaction < 0.05
3.2. Identifying Significant Exposure Periods
Results from our GEE multiple informant models were similar but not identical to our primary model results. In general, effect estimates were slightly attenuated, but also had narrower confidence intervals (Figure S12 and S13). This change is likely due to the fact that GEE models account for the correlation of repeated exposures within individuals.
In the multiple informant models, estimated associations between repeated PFAS exposures and age-nine lipids differed depending on the time period of exposure. We found significant or marginally significant interactions between PFDA, PFNA and PFOS concentrations and exposure period for TC (p-values of 0.08, 0.05, and 0.07 respectively), LDL-C (p-values of 0.09, 0.11, and 0.07), and HDL-C (p-values of 0.02, 0.11, and <0.01). These are the same exposure-outcome combinations with consistently significant associations in our primary models. Looking at the period-specific effect estimates, we generally found stronger associations with exposures at age five and nine than at birth or 18 months (Figure S12). For example, the associations between TC, HDL-C and LDL-C and age-five and age-nine PFDA exposures were significantly higher than associations with PFDA exposures at birth; a doubling in PFDA at age nine was associated with an increase of 0.08 in TC (95% CI: 0.01, 0.15), while a doubling in PFDA at birth was associated with an increase of 0.04 (95% CI: 0.00, 0.08). Similarly, the associations between PFNA exposures and TC and LDL-C were the greatest for exposures at 5 years and 9 years. When we evaluated sex-specific results using a three-way interaction between exposure, child age and child sex, we found several models where both exposure window and sex were significant effect modifiers. For example, in models of PFDA, PFNA and PFOS exposures and age-nine LDL-C concentrations, females had positive associations specifically for age-five and age-nine exposures, while associations for males and for female exposures at birth were generally null. Associations between PFDA and PFNA and HDL-C concentrations also varied significantly by both sex and exposure period, with the greatest association seen for males at the 9-year period (Figure S13).
3.3. Sensitivity Analyses
The estimated associations between serum PFAS and lipid concentrations remained similar after adjustment for additional PFAS sources (maternal whale consumption during pregnancy, breastfeeding duration and childhood whale consumption; Figure S14). Results from the models that used inverse probability weighting to account for potential selection bias were similar to our primary models (Figure S15). Models that used multiple imputation to account for missing covariate data were also similar to our primary complete case models, although a few effect estimates went from marginally significant to significant, likely due to the slightly larger sample size (Figure S16).
In our nonlinear GAMs, 83% of the penalized splines for the PFAS-lipid association were fit with less than two degrees of freedom. When we compared the nonlinear models to our primary linear models, most (85%) showed a better fit for the linear model, as judged from the AIC. Only three of the models with better nonlinear fits estimated a significant association between PFAS and lipid concentrations. The plots of these three splines are included as Figure S17.
4. DISCUSSION
In this prospective birth cohort in the Faroe Islands, we found that increased childhood PFAS concentrations were associated with increased childhood concentrations of TC, LDL-C and HDL-C, with the strongest associations seen for exposures of PFDA, PFNA and PFOS at age nine. We also observed differences in associations by sex. In females, increases in age-five and age-nine concentrations of PFDA, PFNA and PFOS were associated with increases in age-nine TC and LDL-C. In contrast, increases in males’ age-nine PFDA, PFNA and PFOS concentrations were associated with increases in age-nine HDL-C. Associations between lipids and PFASs measured at birth and at 18 months were less consistent. Multiple informant models confirmed that associations between age-nine lipids and repeated measures of PFOA, PFNA and PFOS varied significantly by the exposure period, where age-five and age-nine exposures generally had stronger associations with the outcomes than exposures at birth or 18 months.
The PFAS concentrations measured in our cohort are comparable to concentrations reported by previous studies. For example, a study from Dayton, OH reported an average PFOA concentration of 0.99 ± 0.45 ng/ml (mean ± SD) and an average PFOS concentration of 2.79 ± 2.10 ng/ml (Khalil et al., 2018), which are similar to our mean age-nine concentrations of PFOA (1.50 ± 0.42 ng/ml) and PFOS (3.62 ± 1.78 ng/ml). However, PFAS concentrations have changed from previous Faroese children cohorts. A study conducted within the Faroe Islands Cohort 3, which also collected PFAS concentrations at age 5 (2002 – 2005), found higher serum concentrations of PFOA and PFOS than our study, but lower serum concentrations of PFDA and PFNA (Grandjean et al., 2012). These changes align with changes in PFAS production, as PFOA and PFOS have been phased out of production but other PFAS remain in production and commercial use (Land et al., 2018).
This study is one of the few to measure PFAS exposures longitudinally through early childhood. In general, PFAS concentrations were higher in early childhood than at birth or in late childhood. This reflects the fact that individuals experience a high PFAS exposure relative to body weight as infants and toddlers (EFSA 2020). Important PFAS sources during this period include breastmilk, consumer products, and dust from increased hand-to-mouth contact, although there is limited data comparing the contribution of different exposure pathways within the same population (Winkens et al., 2017). While serum concentrations of PFDA, PFNA and PFHxS were the highest at five years, PFOA and PFOS were highest at 18 months. This suggests that breastfeeding may be a major contribution to serum concentrations of legacy PFASs, although decreases in their environmental concentrations over time may also play a role (Mogensen et al., 2015).
Previous studies have found mixed results for associations between prenatal PFAS exposures and lipid concentrations in infancy and childhood. A recent study of PFAS and lipid concentrations measured in cord serum found significant associations between TG and PFOA and PFHxS (Spratlen et al., 2019); in this study, a 1% increase in PFHxS was associated with a 0.13% increase in TG (95% CI: 0.038, 0.23). In our cohort, we found that a 1% increase in PFHxS was associated with a similar 0.16% increase in TG (95% CI: 0.08, 0.23). Other studies have estimated associations between lipid concentrations and maternal PFAS concentrations during pregnancy. Of these, two found no associations (Manzano-Salgado et al., 2017; Mora et al., 2018), one found associations between prenatal PFOA and childhood concentrations of TC and LDL-C in females (Maisonet et al., 2015), and one found associations between PFNA and PFDA and age-18-month TC concentrations (Jensen et al., 2020). However, when comparing these results into ours, it is important to consider that maternal PFAS concentrations during pregnancy not only determine the child’s prenatal PFAS exposures, but also exposures during breastfeeding. Mothers with higher body-burdens of PFASs will transfer more to their offspring via breastmilk, depending on the breastfeeding duration (Kingsley et al., 2018). Notably, however, the Danish study (Jensen et al., 2020) found sex-specific associations between prenatal PFAS concentrations and infancy lipids that are similar to the sex-specific associations found in our study for later childhood, where PFAS exposures were positively associated with LDL-C concentrations in females and HDL-C concentrations in males.
Five studies have evaluated cross-sectional associations between PFAS and lipid concentrations in childhood. A close comparison of these studies to our results reveals many similarities. Four of the five childhood cross-sectional studies identified positive significant associations between childhood concentrations of PFOA and PFOS and LDL-C and TC (Geiger et al., 2014; Khalil et al., 2018; Koshy et al., 2017; Zeng et al., 2015), although the association between PFOS and TC in the Ohio study was only marginally significant (Khalil et al., 2018). Similarly, we found positive and significant cross-sectional associations between age-nine PFOS and TC, marginally significant associations between PFOS and LDL-C, and positive but non-significant associations between age-nine PFOA and TC and LDL-C. While the cross-sectional analysis in the Boston study (Mora et al., 2018) did not identify significant associations between PFOS or PFOA and TC and LDL-C in childhood in their overall cohort, it did identify positive associations between PFOA and PFOS and TC specifically in females, which agrees with our sex-specific results. Most studies that also measured PFNA and PFDA found positive associations with these PFASs and TC and LDL-C, as we did (Khalil et al., 2018; Koshy et al., 2017; Zeng et al., 2015). For example, in the World Trade Center study (Koshy et al., 2017), a doubling in the concentration of PFNA at the average age of 14 years was associated with a 2.9 mg/dl increase in LDL-C (95% CI: 0.06, 5.74) measured at the same visit. This is similar to our results, where a doubling in PFNA concentration at nine years was associated with a 4.6 mg/dl (0.12 mmol/L) increase in LDL-C at the same visit (95% CI: 0.7, 8.5). Two other studies also identified positive associations between PFDA and HDL-C (Koshy et al., 2017; Mora et al., 2018). Taken together, we see strong agreement between these cross-sectional studies and our results, where childhood PFAS concentrations are most consistently associated with TC and LDL-C.
In vitro and in vivo studies have demonstrated that PFAS exposures affect several biological pathways related to the regulation of blood lipids. Many PFASs are ligands of the peroxisome proliferator-activated receptor alpha (PPARα), a nuclear receptor that plays an essential role in lipid and lipoprotein metabolism. PFASs may also activate additional targets related to metabolism and lipid regulation, including the nuclear receptors PPARγ, constitutive androstane receptor (CAR), pregnane-X receptor (PXR), and estrogen receptor a (ERα) (ATSDR 2018; EFSA 2020). A recent study conducted in mice with humanized PPARα (hpparα) livers found PFOA exposures to be associated with increased serum cholesterol. Exposures were also associated with changes in the expression of genes involved in cholesterol metabolism and homeostasis, with some significant differences in gene expression by sex (Schlezinger et al., 2020). Additional research is needed to elucidate the role of these different pathways. In addition, it is unclear how changes in metabolism through childhood may determine periods of increased vulnerability to PFAS exposures.
Our study has several limitations. First, residual confounding by socioeconomic status, diet, or other unaccounted covariates may affect our results. However, the Faroese setting suggests that these factors are of limited importance, and our sensitivity analysis adjusting for several dietary PFAS sources found only marginal changes in our estimated associations. Second, the cross-sectional associations that we identified at age nine could theoretically be due to reverse causation (Dhingra et al., 2017; Steenland et al., 2009). Still, we found similar associations between age-five PFASs and age-nine lipids in both our primary and in our GEE models, which take into account correlation within individuals. These results are less likely due to reverse causation. Finally, our study also included multiple models for PFASs and lipids at different time points, which may have led to some statistically significant associations by chance. Given that our GEE models for age-nine lipid outcomes found similar results as our primary models, and the strong agreement in results across PFASs and exposure periods, we believe that the significant associations between several childhood PFAS exposures and lipid levels are not due to chance but reflect a true association.
This study also has several strengths. We leveraged a large prospective birth cohort to evaluate associations between PFAS concentrations and lipids at different periods of sensitivity extending from birth to mid-childhood. To our knowledge, this is the first study using multiple informant models to formally test the importance of PFAS exposure periods for childhood lipid outcomes. We also measured PFAS concentrations directly from cord blood rather than relying on maternal serum concentrations, which may not accurately reflect fetal exposures. In addition, we were able to account for potential confounders, including characteristics of maternal diet during pregnancy and child diet. Finally, we evaluated the role of sex and found significant differences in associations, indicating that its role as an effect modifier should be considered in future studies.
5. CONCLUSIONS
This longitudinal study adds to a growing body of evidence that PFAS exposures in childhood are associated with elevated lipid concentrations. The present report is only the second study to evaluate PFAS concentrations at birth, infancy and childhood, and it demonstrates that specific early-life periods may be more vulnerable to the metabolic effects of PFAS exposure. Because serum cholesterol concentrations in childhood constitute a well-known risk factor for subsequent hyperlipidemia and CVD in adulthood (Daniels and Greer, 2008), understanding the life-time impacts of developmental PFAS exposures on lipids and metabolic outcomes is critical to protecting public health.
Supplementary Material
FUNDING SOURCES
This work was supported by the National Institute of Environmental Health Sciences [grant numbers ES026596 and ES027706]; and the Danish Environmental Protection Agency as part of the environmental program Danish Cooperation for Environment in the Arctic (DANCEA). The authors are solely responsible for all results and conclusions, which do not necessarily reflect the position of any of the funding agencies.
ABBREVIATIONS:
- PFAS
per- and polyfluoroalkyl substance
- TC
total cholesterol
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- TG
triglyceride
- PFOS
perfluorooctanesulfonic acid
- PFOA
perfluorooctanoic acid
- PFHxS
perfluorohexanesulfonic acid
- PFNA
perfluorononanoic acid
- PFDA
perfluorodecanoic acid
- LOD
limit of detection
- LC-MS/MS
high-pressure liquid chromatography with tandem mass spectrometry
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr. Philippe Grandjean has served as a health expert in lawsuits on environmental PFAS exposures. All other authors have no competing interests to declare, financial or otherwise.
ETHICS
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Harvard T.H. Chan institutional review board and Faroese ethical review committee approved the study protocol (IRB16-1225), and written informed consent was obtained from all mothers.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agency for Toxic Substances and Disease Registry (ATSDR), 2018. Toxicological profile for Perfluoroalkyls. (Draft for Public Comment). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Ayer JG, Belousova E, Harmer JA, David C, Marks GB, Celermajer DS, 2011. Maternal cigarette smoking is associated with reduced high-density lipoprotein cholesterol in healthy 8-year-old children. European Heart Journal. 32, 2446–2453. 10.1093/eurheartj/ehr174. [DOI] [PubMed] [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ, 2012. Developmental origins of non-communicable disease: Implications for research and public health. Environmental Health. 11, 42. 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett M, 2020. ggdag: Analyze and Create Elegant Directed Acyclic Graphs. R package version 0.2.2. [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bech BH, Nohr EA, et al. , 2016. Determinants of serum levels of perfluorinated alkyl acids in Danish pregnant women. Int J Hyg Environ Health. 219, 867–875. 10.1016/j.ijheh.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, et al. , 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environment International. 54, 74–84. 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nature reviews. Endocrinology. 13, 161–173. 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SR, Greer FR, 2008. Lipid Screening and Cardiovascular Health in Childhood. Pediatrics. 122, 198–208. 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K, 2017. A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Environmental Health Perspectives. 125, 416–421. 10.1289/EHP273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2018. Minutes of the expert meeting on perfluorooctane sulfonic acid and perfluorooctanoic acid in food assessment. Parma, Italy. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, et al. , 2020. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal. 18, 391. 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, et al. , 2013. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PloS ONE. 8, e56969–e56969. 10.1371/journal.pone.0056969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, et al. , 2019. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environment International. 130, 104874. 10.1016/j.envint.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. , 2010. Perfluorooctanoic Acid, Perfluorooctanesulfonate, and Serum Lipids in Children and Adolescents: Results From the C8 Health Project. Archives of Pediatrics & Adolescent Medicine. 164, 860–869. 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D, 2009. Perfluorinated compounds – Exposure assessment for the general population in western countries. International Journal of Hygiene and Environmental Health. 212, 239–270. 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Rurangirwa Akashi A, Williams Michelle A, Hofman A, Mackenbach Johan P, Franco Oscar H, et al. , 2014. Maternal Parity, Fetal and Childhood Growth, and Cardiometabolic Risk Factors. Hypertension. 64, 266–274. 10.1161/HYPERTENSIONAHA.114.03492. [DOI] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Ducatman A, Frisbee S, Innes K, Shankar A, 2014. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere. 98, 78–83. 10.1016/j.chemosphere.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Mølbak K, Weihe P, et al. , 2012. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA. 307, 391–397. 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A, et al. , 2017. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. Journal of Immunotoxicology. 14, 188–195. 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halekoh U, Højsgaard S, Yan J, 2006. The R package geepack for generalized estimating equations. Journal of statistical software. 15, 1–11. 10.18637/jss.v015.i02. [DOI] [Google Scholar]
- Halldorsson TI, Rytter D, Haug Line S, Bech Bodil H, Danielsen I, Becher G, et al. , 2012. Prenatal Exposure to Perfluorooctanoate and Risk of Overweight at 20 Years of Age: A Prospective Cohort Study. Environmental Health Perspectives. 120, 668–673. 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, et al. , 2017. Predictors of Per- and Polyfluoroalkyl Substance (PFAS) Plasma Concentrations in 6–10 Year Old American Children. Environmental Science & Technology. 51, 5193–5204. 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G, 2009. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. Journal of Chromatography A. 1216, 385–393. 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Horton NJ, Laird NM, Zahner GEP, 1999. Use of multiple informant data as a predictor in psychiatric epidemiology. International Journal of Methods in Psychiatric Research. 8, 6–18. 10.1002/mpr.52. [DOI] [Google Scholar]
- Jaddoe VWV, de Ridder MAJ, van den Elzen APM, Hofman A, Uiterwaal CSPM, Witteman JCM, 2008. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: A 27-years follow-up study. Atherosclerosis. 196, 42–48. 10.1016/j.atherosclerosis.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A, 2018. Associations between lipid/lipoprotein levels and perfluoroalkyl substances among US children aged 6–11 years. Environmental Pollution. 243, 1–8. 10.1016/j.envpol.2018.08.060. [DOI] [PubMed] [Google Scholar]
- Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. , 2017. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocrine Practice. 23, 1–87. 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- Jensen RC, Andersen Marianne S, Larsen Pia V, Glintborg D, Dalgård C, Timmermann Clara Amalie G, et al. , 2020. Prenatal Exposures to Perfluoroalkyl Acids and Associations with Markers of Adiposity and Plasma Lipids in Infancy: An Odense Child Cohort Study. Environmental Health Perspectives. 128, 077001. 10.1289/EHP5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, et al. , 2018. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: A pilot study. Environmental Research. 160, 314–321. 10.1016/j.envres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Kelsey KT, Calafat AM, Ehrlich S, Lanphear BP, et al. , 2018. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environmental research. 165, 247–257. 10.1016/j.envres.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy TT, Attina TM, Ghassabian A, Gilbert J, Burdine LK, Marmor M, et al. , 2017. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environment International. 109, 128–135. 10.1016/j.envint.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Birnbaum LS, 2011. Childhood Obesity and Environmental Chemicals. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 78, 22–48. 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M, de Wit CA, Bignert A, Cousins IT, Herzke D, Johansson JH, et al. , 2018. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environmental Evidence. 7, 4. 10.1186/s13750-017-0114-y. [DOI] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL, 2011. Polyfluorinated Compounds: Past, Present, and Future. Environmental Science & Technology. 45, 7954–7961. 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Näyhä S, Lawlor DA, Marcus M, 2015. Prenatal exposures to perfluoroalkyl acids and serum lipids at ages 7 and 15 in females. Environment International. 82, 49–60. 10.1016/j.envint.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Makri A, Goveia M, Balbus J, Parkin R, 2004. Children’s susceptibility to chemicals: a review by developmental stage. Journal of Toxicology and Environmental Health, Part B. 7, 417–435. 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa M-J, Ballester F, Iñiguez C, Martinez D, et al. , 2017. Prenatal Exposure to Perfluoroalkyl Substances and Cardiometabolic Risk in Children from the Spanish INMA Birth Cohort Study. Environmental Health Perspectives. 125, 097018. 10.1289/EHP1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla-Santander N, Valvi D, Lopez-Espinosa M-J, Manzano-Salgado CB, Ballester F, Ibarluzea J, et al. , 2017. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environmental Health Perspectives. 125, 117004. 10.1289/EHP1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcmillen IC, Robinson JS, 2005. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming. Physiological Reviews. 85, 571–633. 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E, 2015. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environmental Science & Technology. 49, 10466–10473. 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Fleisch AF, Rifas-Shiman SL, Woo Baidal JA, Pardo L, Webster TF, et al. , 2018. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environment International. 111, 1–13. 10.1016/j.envint.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morck TA, Nielsen F, Nielsen JKS, Siersma VD, Grandjean P, Knudsen LE, 2015. PFAS concentrations in plasma samples from Danish school children and their mothers. Chemosphere. 129, 203–209. 10.1016/j.chemosphere.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG, 2002. Infant Feeding and Blood Cholesterol: A Study in Adolescents and a Systematic Review. Pediatrics. 110, 597–608. 10.1542/peds.110.3.597. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing,, Vienna, Austria. [Google Scholar]
- Rubin DB, 2004. Multiple imputation for nonresponse in surveys. John Wiley & Sons, Hoboken. [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM, 2011. Statistical Methods to Study Timing of Vulnerability with Sparsely Sampled Data on Environmental Toxicants. Environmental Health Perspectives. 119, 409–415. 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF, 2020. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicology and Applied Pharmacology. 405, 115204. 10.1016/j.taap.2020.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MM, Barofsky DF, Field JA, 2003. Fluorinated Alkyl Surfactants. Environmental Engineering Science. 20, 487–501. 10.1089/109287503768335959. [DOI] [Google Scholar]
- Shu H, Lindh CH, Wikstrom S, Bornehag C-G, 2018. Temporal trends and predictors of perfluoroalkyl substances serum levels in Swedish pregnant women in the SELMA study. PloS one. 13, e0209255–e0209255. 10.1371/journal.pone.0209255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlen MJ, Perera FP, Lederman SA, Robinson M, Kannan K, Herbstman J, et al. , 2019. The Association Between Perfluoroalkyl Substances and Lipids in Cord Blood. The Journal of Clinical Endocrinology & Metabolism. 105, 43–54. 10.1210/clinem/dgz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, et al. , 2014. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environment International. 62, 104–112. 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V, 2009. Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate With Serum Lipids Among Adults Living Near a Chemical Plant. American Journal of Epidemiology. 170, 1268–1278. 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG, 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of Exposure Science & Environmental Epidemiology. 29, 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT, 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. International journal of epidemiology. 45, 1887–1894. 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2011. mice: Multivariate Imputation by Chained Equations in R. Journal of statistical software. 45, 67. 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- Weihe P, Bjerregaard P, Bonefeld-Jorgensen E, Dudarev AA, Halling J, Hansen S, et al. , 2016. Overview of ongoing cohort and dietary studies in the Arctic. International Journal of Circumpolar Health. 75, 33803. 10.3402/ijch.v75.33803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkens K, Vestergren R, Berger U, Cousins IT, 2017. Early life exposure to per- and polyfluoroalkyl substances (PFASs): A critical review. Emerging Contaminants. 3, 55–68. 10.1016/j.emcon.2017.05.001. [DOI] [Google Scholar]
- Wood S, 2019. mgcv: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. R package version 1.8–31. [Google Scholar]
- Wood SN, 2004. Stable and Efficient Multiple Smoothing Parameter Estimation for Generalized Additive Models. Journal of the American Statistical Association. 99, 673–686. 10.1198/016214504000000980. [DOI] [Google Scholar]
- Ye X, Kato K, Wong L-Y, Jia T, Kalathil A, Latremouille J, et al. , 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. International journal of hygiene and environmental health. 221, 9–16. 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X-W, Qian Z, Emo B, Vaughn M, Bao J, Qin X-D, et al. , 2015. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Science of The Total Environment. 512-513, 364–370. 10.1016/j.scitotenv.2015.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


