Abstract
BACKGROUND:
Life expectancy for long-term survivors of allogeneic hematopoietic stem cell transplant (alloHSCT), defined as those living ≥5 years post-transplant, is significantly lower compared to that of the age-matched general population despite a relatively low primary disease relapse rate >2 years post-transplant. Among several factors, patient sex is increasingly recognized as a prognostic indicator of long-term survival.
OBJECTIVE:
We examined the influence of patient sex and donor-recipient sex matching on overall survival in a landmark analysis of long-term survivors.
STUDY DESIGN:
Using our institutional database supplemented with individual patient record review, we retrospectively investigated the relative influence of recipient sex and donor-recipient sex matching on outcomes of long-term survivors receiving alloHSCT between 1994 – 2014.
RESULTS:
Over this 20-year period, 247 met inclusion criteria for analysis; males and females had similar demographic and treatment characteristics. However, significantly more deaths after the 5-year landmark occurred in male recipients. Interestingly, donor sex did not have a significant impact on overall survival in multivariate analysis, and differences in overall survival of donor-recipient sex pairs was driven by recipient sex. In addition to recipient sex, only cGVHD retained significance as a covariate with impact on overall survival in multivariate analysis. Men experienced slightly higher, but non-significant, rates and increased severity of cGVHD, and a greater percentage of cGVHD-related mortality as compared to females.
CONCLUSION:
In this long-term survival analysis of alloHSCT adult patients, one of the only to include follow-up to 15 years, our results show that women survive significantly longer than men irrespective of their age at transplant. This outcome is independent of other common pre-transplant prognostic indicators such as donor sex or performance status at transplant. Inferior survival for males is consistent with survival outcomes described in transplant literature. Gathering evidence suggests a biologic basis for long-term sex-determined outcomes, possibly due to differing rates or severity of cGVHD or sustained alloimmune tolerance in females. Larger studies are warranted to validate these retrospective clinical results.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a potentially curative treatment for many hematologic malignancies but carries significant long-term risk for patients. Retrospective studies have shown that despite a low primary disease relapse rate >2 years post-transplant, long-term survivors of alloHSCT still have a 20% probability of delayed mortality over the next 15 to 20 years post-transplant at a rate up to 9 times higher than their age-matched peers1. Long-term complications, including infection, organ failure, secondary malignancies, and chronic graft-versus-host-disease (cGVHD), contribute to non-relapse mortality in this patient population2.
Several pre-transplant factors influence survival outcomes following alloHSCT3. Of these, the influence of donor sex, recipient sex, and donor-recipient sex pairings has been studied across preclinical models and fully risk-annotated disease cohorts, and has emerged as a critical determinant of transplant outcomes4–14. Early studies repeatedly demonstrated worse outcomes following alloHSCT of a female donor to a male recipient (F→M), mostly due to greater rates of cGVHD6,7,11,14,15. Female donor T cells targeting male recipient minor histocompatibility antigens encoded on the Y chromosome are thought to cause this effect5,6,15,16. The increased graft-versus-leukemia (GVL) anti-tumor effect does not outweigh fatality from cGVHD; as such, the modified European Group for Blood and Marrow Transplantation Risk Score now includes F→M as a negative prognostic indicator 11,17,18.
More recent studies in the modern transplantation era have argued that outcomes disparities are driven solely by recipient sex, with less influence of donor sex10,14. In one of the largest retrospective cohorts to date, multivariate analysis demonstrated more deaths in male recipients regardless of donor sex to a follow up period of five years (M→F reference group, F→M OS HR 1.14, P=0.0004, M→M OS HR 1.1, P=0.0032, F→F OS HR 1.02, P=0.64)14. However, the influence of sex on long-term outcomes remains an area of active investigation, as new data in modern cohorts with longer follow-up time mature. We performed a single-center, retrospective analysis on the impact of recipient sex and donor-recipient sex matching on outcomes of long-term survivors of alloHSCT to address these knowledge gaps. Our primary goal was to examine the influence of patient sex and donor-recipient sex matching on overall survival in a landmark analysis of long-term survivors.
METHODS
Study Design
We performed a landmark retrospective analysis using Duke University’s institutional Adult Blood and Marrow Transplant database, supplemented by center-specific data review from the Center for International Blood and Marrow Transplant Research (CIBMTR), individual patient chart review, and public records review. Inclusion criteria for this study cohort consisted of long-term survivors who underwent first alloHSCT, excluding syngeneic donors, between 1995 – 2014 for a hematologic malignancy. A long-term survivor was defined as having been alive with documented follow-up to at least five years following alloHSCT.
Statistical Analysis
Patient characteristics were summarized as count (%) for categorical variables and median (interquartile range) for continuous variables. Fisher’s exact tests or t-tests were used to compare difference between groups. Overall survival was estimated using the Kaplan-Meier method and right-censored at 15 years. Cox proportional hazard model was used for multivariate analysis. SAS version 9.4 (SAS Institute, Cary, NC) was used to perform statistical analyses.
RESULTS
Patient Population
Over this 20-year period, 1103 patients underwent alloHSCT, with 247 (22%) meeting inclusion criteria. Of these 247, 111 (44.9%) were female and 136 (55.1%) were male patients. Most patients were white (201/81.4%). Approximately a third of females (38/34.2%) and males (48/35.3%) received matched-related allogeneic grafts. Approximately another third of females (39/35.1%) and males (37/27.2%) received matched-unrelated allogeneic grafts. A minority of all patients received mismatched allogeneic and haploidentical grafts. Twenty-two (19.8%) female patients and 31 (22.8%) male patients received dual cord blood allogeneic grafts. Forty-three (38.7%) women and 46 (33.8%) men in this cohort had experienced acute graft-versus-host disease ≥Grade 2 post-transplant. Seventy-five (55.1%) men experienced cGVHD while 54 (48.6%) women did; the majority of cGVHD cases in both sexes were classified as mild on the NIH Global Severity Scale (23.5% of men and 21.6% of women). Seventy-seven (69.4%) women had children (Table 2). Female and male groups were well-balanced for age, performance status and disease status at transplant, preparative regimen, and median follow-up time (Table 1).
Table 2.
Female Parity Baseline Characteristics
| All Female N=111 (100.0%) |
No Children N=34 (30.6%) |
With Children N=77 (69.4%) |
P-Value | |
|---|---|---|---|---|
| Age | ||||
| Median (IQR) | 46 (37 – 53) | 39 (23 – 54) | 47 (40 – 53) | 0.0006 |
| Number of Children | ||||
| Median (IQR) | 2 (0 – 2) | 0 (0 – 0) | 2 (2 – 2) | <.0001 |
| Donor Sex | ||||
| Cord | 4 (3.6%) | 2 (5.9%) | 2 (2.6%) | 0.6931 |
| Female | 47 (42.3%) | 14 (41.2%) | 33 (42.9%) | |
| Male | 60 (54.1%) | 18 (52.9%) | 42 (54.5%) | |
| Race | ||||
| Asian | 2 (1.8%) | 1 (2.9%) | 1 (1.3%) | 0.5146 |
| Black | 16 (14.4%) | 6 (17.6%) | 10 (13.0%) | |
| White | 93 (83.8%) | 27 (79.4%) | 66 (85.7%) | |
| Hispanic | 2 (1.8%) | 1 (2.9%) | 1 (1.3%) | |
| History of Chronic Graft-versus-Host Diseases | ||||
| No | 57 (51.4%) | 22 (64.7%) | 35 (45.5%) | 0.0614 |
| Yes | 54 (48.6%) | 12 (35.3%) | 42 (54.5%) | |
Table 1.
Patient Baseline Characteristics
| All Patients | Female | Male | ||
|---|---|---|---|---|
| N=247 (100.0%) | N=111 (44.9%) | N=136 (55.1%) | P-Value | |
| Age | ||||
| Median (IQR) | 46 (38 – 54) | 46 (37 – 53) | 47 (38 – 54.5) | 0.38 |
| Follow-Up Years | ||||
| Median (IQR) | 8 (6 – 10) | 8 (6 – 10) | 8 (6 – 10) | 0.89 |
| Race | ||||
| Asian | 5 (2.0%) | 2 (1.8%) | 3 (2.2%) | 0.87 |
| Black | 38 (15.4%) | 16 (14.4%) | 22 (16.2%) | |
| White | 201 (81.4%) | 91 (82.0%) | 110 (80.9%) | |
| Hispanic | 3 (1.2%) | 2 (1.8%) | 1 (0.7%) | |
| Donor Sex | ||||
| *Sex-mismatched Dual Cord Blood | 16 (6.5%) | 4 (3.6%) | 12 (8.8%) | 0.25 |
| Female | 103 (41.7%) | 47 (42.3%) | 56 (41.2%) | |
| Male | 128 (51.8%) | 60 (54.1%) | 68 (50.0%) | |
| Transplant Year | ||||
| 1995–2000 | 20 (8.1%) | 5 (4.5%) | 15 (11.0%) | 0.04 |
| 2001–2005 | 43 (17.4%) | 17 (15.3%) | 26 (19.1%) | |
| 2006–2010 | 102 (41.3%) | 56 (50.5%) | 46 (33.8%) | |
| 2011–2014 | 82 (33.2%) | 33 (29.7%) | 49 (36.0%) | |
| Conditioning | ||||
| Myeloablative | 146 (59.1%) | 68 (61.3%) | 78 (57.4%) | 0.35 |
| Non-myeloablative | 95 (38.5%) | 42 (37.8%) | 53 (39.0%) | |
| Other/Unknown | 6 (2.4%) | 1 (0.9%) | 5 (3.7%) | |
| Karnofsky Performance Status at Transplant | ||||
| 80–100 | 208 (84.2%) | 92 (82.9%) | 116 (85.3%) | 0.38 |
| ≤70 | 27 (10.9%) | 15 (13.5%) | 12 (8.8%) | |
| Unknown | 12 (4.9%) | 4 (3.6%) | 8 (5.9%) | |
| History of Chronic Graft-versus-Host Disease | ||||
| No | 118 (47.8%) | 57 (51.4%) | 61 (44.9%) | 0.37 |
| Yes | 129 (52.2%) | 54 (48.6%) | 75 (55.1%) | |
| NIH Global Severity Score of Chronic Graft-versus-Host Disease | ||||
| None | 118 (47.8%) | 57 (51.4%) | 61 (44.9%) | 0.43 |
| Mild | 56 (22.7%) | 24 (21.6%) | 32 (23.5%) | |
| Moderate | 47 (19.0%) | 22 (19.8%) | 25 (18.4%) | |
| Severe | 26 (10.5%) | 8 (7.2%) | 18 (13.2%) | |
| History of Acute Graft-versus-Host Disease ≥ Grade 2 | ||||
| No | 158 (64.0%) | 68 (61.3%) | 90 (66.2%) | 0.43 |
| Yes | 89 (36.0%) | 43 (38.7%) | 46 (33.8%) | |
| Highest Grade of Acute Graft-versus-Host Disease | ||||
| 0 | 114 (46.2%) | 48 (43.2%) | 66 (48.5%) | 0.58 |
| 1 | 44 (17.8%) | 20 (18.0%) | 24 (17.6%) | |
| 2 | 52 (21.1%) | 28 (25.2%) | 24 (17.6%) | |
| 3 | 36 (14.6%) | 15 (13.5%) | 21 (15.4%) | |
| 4 | 1 (0.4%) | 0 (0.0%) | 1 (0.7%) | |
| Disease Status at Transplant | ||||
| Complete Response | 162 (65.6%) | 75 (67.6%) | 87 (64.0%) | 0.59 |
| Partial Response, Stable Disease, or Unknown | 85 (34.4%) | 36 (32.4%) | 49 (36.0%) | |
| Allogeneic Graft Source | ||||
| Allo-Matched Related | 86 (34.8%) | 38 (34.2%) | 48 (35.3%) | 0.30 |
| Allo-Matched Unrelated | 76 (30.8%) | 39 (35.1%) | 37 (27.2%) | |
| Allo-Mismatched | 8 (3.2%) | 5 (4.5%) | 3 (2.2%) | |
| Dual Cord Blood | 53 (21.5%) | 22 (19.8%) | 31 (22.8%) | |
| Haploidentical | 24 (9.7%) | 7 (6.3%) | 17 (12.5%) | |
Percentage of patients who received sex-mismatched units of cord blood graft, i.e. M+F. Patients who received matched sex cord blood units are not included in this category.
Mortality
Most patients died of secondary malignancy or relapsed disease (Table 3). The third most common cause of non-relapse mortality was cGVHD, which was a more frequent cause of death in men than women (3.7% of men versus 0.9% of women), though this result was not statistically significant (p = 0.23, Table 3).
Table 3.
Cause of Death
| Cause of Death | All Deaths N= 42 / 247 (17.0%) |
Female Deaths N= 10 / 111 (9.0%) |
Male Deaths N= 32 / 136 (23.5%) |
P-value |
|---|---|---|---|---|
| Secondary Malignancy | 11 (4.5%) | 3 (2.7%) | 8 (5.9%) | 0.35 |
| Other Medical Condition | 9 (3.6%) | 2 (1.8%) | 7 (5.1%) | 0.19 |
| Relapsed or persistent disease | 8 (3.2%) | 2 (1.8%) | 6 (4.4%) | 0.30 |
| Chronic GVHD | 6 (2.4%) | 1 (0.9%) | 5 (3.7%) | 0.23 |
| Infection | 6 (2.4%) | 1 (0.9%) | 5 (3.7%) | 0.23 |
| Unknown | 2 (0.8%) | 1 (0.9%) | 1 (0.7%) | 0.99 |
Impact of Recipient Sex on Survival
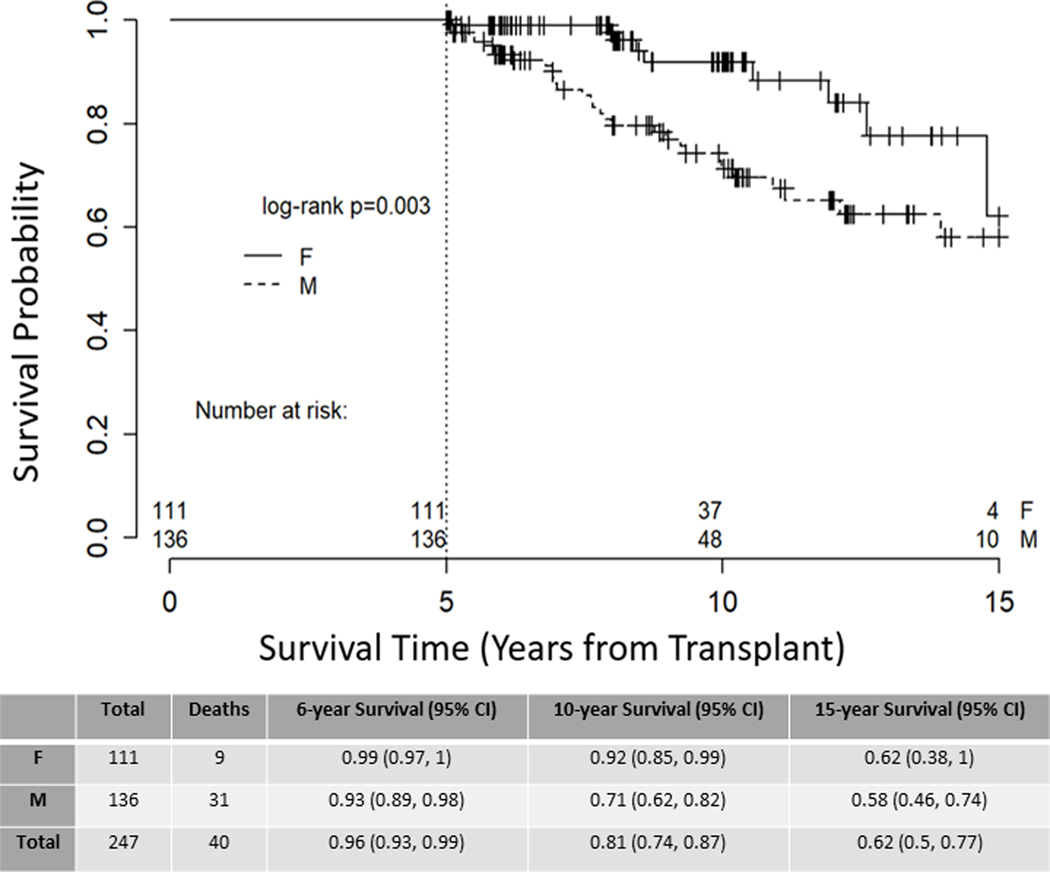
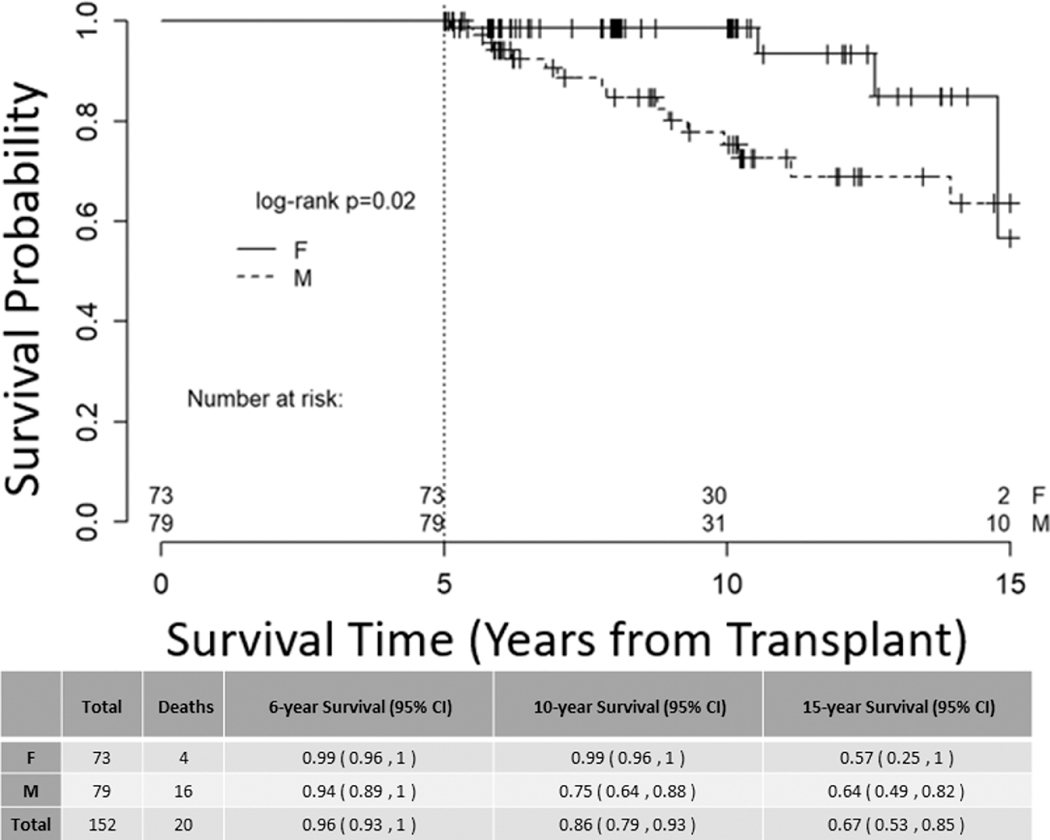
Significantly more deaths occurred in male recipients following the 5-year landmark (Figure 1, p value=0.003). In multivariate analysis, male sex had a three-fold hazard ratio for death as compared to female sex (HR 3.106 (95% CI 1.471 – 6.561), p value = 0.003, Table 4). To estimate whether this was due to the general population-wide shorter life expectancy for males, we performed Kaplan-Meier estimates of survival for patients aged < 50 years at transplant with similar survival results (Figure 2, p value=0.02). To determine when this survival difference between sexes is first observed in a patient’s transplant course, we ran a separate survival analysis on the entire cohort of 1103 patients transplanted between 1995 – 2014 and found that survival begins to diverge at approximately 6 months post-alloHSCT (Supplementary Figure 1). Among women, recipient parity had minimal influence on long-term survival (Supplementary Figure 2).
Figure 1. Overall Survival.
Kaplan-Meier estimate of overall survival for all long-term survivors, stratified by patient sex, right-censored at 15 years, p=0.003.
Table 4.
Cox Proportional Hazard Model for Overall Survival
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Patient Sex | |||
| Female | -REF- | 0.003 | |
| Male | 3.106 (1.471 – 6.561) | 0.003 | |
| Donor Sex | |||
| Male | -REF- | 0.146 | |
| Sex-Matched Dual Cord Blood Graft | 0.346 (0.080 – 1.486) | 0.153 | |
| Female | 0.584 (0.298 – 1.145) | 0.117 | |
| Age at Transplant | |||
| 1.04 (1.008 – 1.073) | 0.014 | 0.014 | |
| History of Chronic Graft-versus-Host Disease | |||
| No | -REF- | 0.016 | |
| Yes | 2.303 (1.168 – 4.539) | 0.016 |
Figure 2. Overall Survival, patients aged <50 at transplant.
Kaplan-Meier estimate of overall survival stratified by patient sex for all long-term survivors aged <50 years at transplant, right-censored at 15 years, p = 0.02.
Impact of Donor-Recipient Sex Matching on Survival
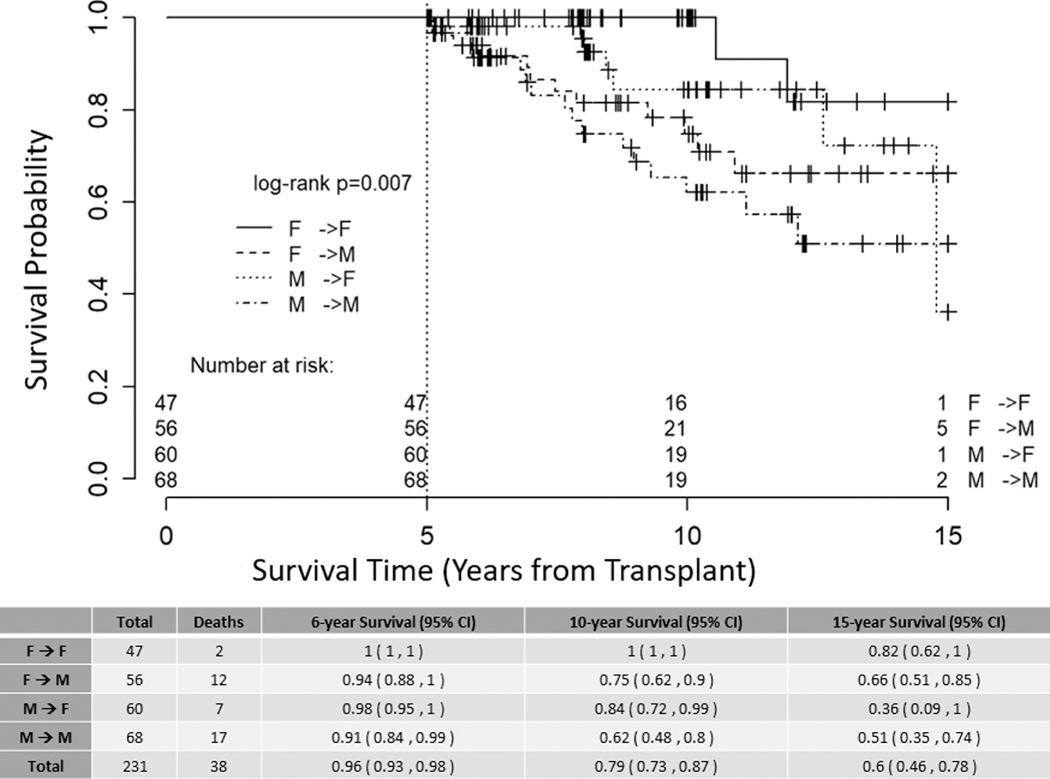
Donor sex did not significantly impact survival in multivariate analysis when adjusted for age, patient sex, and history of cGVHD (Table 4). Kaplan-Meier estimates of survival demonstrated that survival differences between donor-recipient sex pairs was driven by recipient sex (Figure 3, p value =0.007). Recipients of sex mis-matched dual cord blood units were excluded from this analysis for consistency.
Figure 3. Overall Survival donor-recipient sex pairs.
Kaplan-Meier estimate of overall survival stratified by donor-recipient sex pairs. Patients who received sex-mis-matched dual cord blood allografts were excluded from this analysis (16 patients excluded); right-censored at 15 years, p = 0.007.
Impact of Chronic Graft Versus Host Disease on Survival
In addition to recipient sex and age at transplant, only cGVHD impacted overall survival in multivariate analysis (HR 2.303 (95% CI 1.168 – 4.539, p value = 0.016, Table 4). Men experienced cGVHD at a rate of 55.1%, compared to 48.6% of females, though these findings were not statistically significant (Table 1). In women, 54.5% of parous recipients compared with 35.3% of non-parous recipients developed cGVHD (p = 0.06, Table 2).
DISCUSSION
In this single-institution retrospective landmark analysis of patients surviving beyond five years post-alloHSCT, we demonstrated that men experience more deaths as compared to women to a follow up time of 15 years (Figure 1), and that this result is independent of donor sex (Table 4). Furthermore, we demonstrated that the only other independent variables to negatively impact survival were age at transplant and presence of cGVHD in multivariate analysis (Table 4). Lastly, we demonstrated that this outcome was independent of the general population-wide longer life expectancy for women, given that younger men aged <50 at transplant also experienced more deaths than age-matched female patients (Figure 2). Our cohort is one of the first to demonstrate sustained survival differences at fifteen years, spanning two decades of data in the modern transplantation era.
Importantly, there is gathering evidence to suggest a biological basis for sex-determined survival. In one of the largest retrospective studies from CIBMTR to date, Kim et al demonstrated worse survival for men compared to women regardless of donor-sex in a cohort of 11,797 patients14. As with our findings, this study demonstrated increased rates of cGVHD among male recipients; however, this finding was only significant for F→M transplants14. Comparatively, M→M transplants experienced higher rates of relapse-mortality14. Earlier studies have argued that minor histocompatibility Y chromosome antigens contribute to higher rates and severity of cGVHD and consequently increased rates of non-relapse mortality in F→M transplants; however, this hypothesis alone does not explain why sex-matched male recipients still experience both worse relapse and non-relapse related mortality compared to their female counterparts6,7,15,16,19,20.
In our cohort, we redemonstrated a correlation with higher rates of non-relapse mortality from either cGVHD or secondary malignancy in men, suggesting a possible immune basis for disparate survival (Table 2)5–7,11,14,16,21. While our cohort did not show a statistically significant difference in the rates of cGVHD between men and women, there was a trend towards men having higher rates of cGVHD (Table 1). Females are thought to undergo alloimmunization during pregnancy through exposure to fetal antigens, which can increase the risk of developing cGVHD and is thought to account for the historically higher rates of cGVHD among recipients of parous female donors8. Comparatively, recipient parity was found to have no impact on rates of cGVHD or survival among women in a large retrospective cohort8. While information on donor parity was not available for our analysis, recipient parity did correlate with higher rates of cGVHD among parous women in our analysis, albeit with no impact on survival (p = 0.06, Table 2, Supplementary Figure 2), raising the possibility that residual recipient immunity following myeloablative conditioning may interact with donor-acquired immunity.
The third most common cause of death for both men and women was disease relapse, with men experiencing relapse-related death at a higher rate than women (Table 3). Interestingly, Kim and colleagues also reported a statistically significant 12% relative increased risk of relapse in male recipients of a male donor when compared to other recipient and donor sex combination groups during a follow-up of 4 years, suggesting higher relapse rates may contribute to more deaths in men14. However, the literature does not consistently report increased relapse risk in male long-term survivors22,23.
Our results support earlier cohort analyses of sex-based survival discrepancies, and report similar findings to more recent analyses stressing the importance of recipient sex over that of the donor4,6,7,9–11,14,20. In one of the largest fully-annotated retrospective analyses to date, Stern et al showed that survival differences in F→M alloHSCT first differentiated at approximately 6 months post-transplant and was sustained to a follow up time of 5 years15. Here, we demonstrate that this survival difference is likely due to recipient sex and is sustained through longer follow-up. An immune-based mechanism underlying this observation is likely, though further preclinical studies are needed to confirm.
There are several limitations to this study. First, as a retrospective study it is inherently subject to bias and confounding variables. Data were extracted retrospectively through our institutional database and supplemented by individual chart review and public records; as such, some data may be missing. As a tertiary referral center, our patient population is geographically and socioeconomically diverse, with many patients engaged in longitudinal follow up with local providers outside our medical record system. We have attempted to minimize any missing or discrepant data through rigorous cross-review of multiple sources. Furthermore, our small sample size limits our statistical power. Lastly, our single-institution reporting may be subject to bias through administration of institutional-specific protocols, conditioning regimens, follow-up practices, among others; however, our institution does follow accepted treatment guidelines, and our analysis compares similarly with published multi-institutional studies13–15.
CONCLUSION
In this long-term survival analysis of allo-HCT adult patients, one of the only to include follow-up to 15 years, our results show that women survive significantly longer than men irrespective of their age at transplant. Inferior survival for males is consistent with survival outcomes described in the transplant literature, including bone marrow and solid organ transplants such as lung and kidney. Gathering evidence suggests a biologic basis for sex-influenced outcomes, possibly due to differing rates or severity of cGVHD, sustained alloimmune tolerance in females, or another mechanism yet to be determined. Importantly, sex remains only one of many important prognostic factors, such as patient age, comorbidities, performance status, disease risk and availability of a suitable allograft. Men should be counseled on their increased long-term risk and need for regular follow-up care. Larger retrospective studies and preclinical research are warranted to validate these single-center clinical results.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institute on Aging grant P30-AG028716-13 Mini#6 (A.D.S.); ASH Scholar Award (A.D.S.). There are no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Battiwalla M, Tichelli A, Majhail NS. Long-Term Survivorship after Hematopoietic Cell Transplantation: Roadmap for Research and Care. Biol Blood Marrow Transplant 2017;23:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther 2017;10:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shouval R, Fein JA, Shouval A, et al. External validation and comparison of multiple prognostic scores in allogeneic hematopoietic stem cell transplantation. Blood Adv 2019;3:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gratwohl A, Hermans J, Niederwieser D, et al. Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J 2001;2:363–70. [DOI] [PubMed] [Google Scholar]
- 5.Spierings E, Vermeulen CJ, Vogt MH, et al. Identification of HLA class II-restricted H-Y-specific T-helper epitope evoking CD4+ T-helper cells in H-Y-mismatched transplantation. Lancet 2003;362:610–5. [DOI] [PubMed] [Google Scholar]
- 6.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood 2004;103:347–52. [DOI] [PubMed] [Google Scholar]
- 7.Gahrton G, Iacobelli S, Apperley J, et al. The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: reduced relapse risk in female to male transplants. Bone Marrow Transplant 2005;35:609–17. [DOI] [PubMed] [Google Scholar]
- 8.Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006;12:758–69. [DOI] [PubMed] [Google Scholar]
- 9.Gahrton G. Risk assessment in haematopoietic stem cell transplantation: impact of donor-recipient sex combination in allogeneic transplantation. Best Pract Res Clin Haematol 2007;20:219–29. [DOI] [PubMed] [Google Scholar]
- 10.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012;120:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gratwohl A. The EBMT risk score. Bone Marrow Transplant 2012;47:749–56. [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014;123:3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhan S, Peres E, Pelland D, et al. Impact of Gender: Female Related Donor Versus Male Matched Unrelated Donor on Peripheral Blood Allogeneic Stem Cell Transplant for Male Recipients. Blood 2014;124:5878-. [Google Scholar]
- 14.Kim HT, Zhang MJ, Woolfrey AE, et al. Donor and recipient sex in allogeneic stem cell transplantation: what really matters. Haematologica 2016;101:1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern M, Brand R, de Witte T, et al. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant 2008;8:2149–57. [DOI] [PubMed] [Google Scholar]
- 16.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood 2005;105:2973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YJ, Zhao XY, Huang XJ. Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease. Front Immunol 2018;9:3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato M, Kurata M, Kanda J, et al. Impact of graft-versus-host disease on relapse and survival after allogeneic stem cell transplantation for pediatric leukemia. Bone Marrow Transplant 2019;54:68–75. [DOI] [PubMed] [Google Scholar]
- 19.Gratwohl A, Dohler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet 2008;372:49–53. [DOI] [PubMed] [Google Scholar]
- 20.Terwey TH, Hemmati PG, Martus P, et al. A modified EBMT risk score and the hematopoietic cell transplantation-specific comorbidity index for pre-transplant risk assessment in adult acute lymphoblastic leukemia. Haematologica 2010;95:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popli R, Sahaf B, Nakasone H, Lee JY, Miklos DB. Clinical impact of H-Y alloimmunity. Immunol Res 2014;58:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copelan EA, Crilley PA, Szer J, et al. Late mortality and relapse following BuCy2 and HLA-identical sibling marrow transplantation for chronic myelogenous leukemia. Biol Blood Marrow Transplant 2009;15:851–5. [DOI] [PubMed] [Google Scholar]
- 23.Pant S, Hamadani M, Dodds AJ, et al. Incidence and reasons for late failure after allogeneic haematopoietic cell transplantation following BuCy2 in acute myeloid leukaemia. Br J Haematol 2010;148:623–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.